Abstract
Gastric cancer is not a top ten malignancy in the United States, but represents one of the most common causes of cancer death worldwide. Biological differences between tumors from Eastern and Western countries add to the complexity of identifying standard of care therapy based on international trials. Systemic chemotherapy, radiotherapy, surgery, immunotherapy, and targeted therapy all have proven efficacy in gastric adenocarcinoma and therefore multidisciplinary treatment is paramount to treatment selection. Triplet chemotherapy for resectable gastric cancer is now accepted and could represent a plateau of standard cytotoxic chemotherapy for localized disease. Classification of gastric cancer based on molecular subtypes is providing an opportunity for personalized therapy. Biomarkers, in particular microsatellite instability (MSI), programmed cell death ligand 1 (PD-L1), human epidermal growth factor receptor 2 (HER2), tumor mutation burden (TMB), and Epstein-Barr virus (EBV), are increasingly driving systemic therapy approaches and allowing for identification of populations most likely to benefit from immunotherapy and targeted therapy. Significant research opportunities remain for the less differentiated histologic subtypes of gastric adenocarcinoma and those without markers of immunotherapy activity.
Keywords: gastric cancer, adenocarcinoma, stomach cancer, immunotherapy, molecular subtypes
Introduction
Although gastric cancer is not in the top 10 malignancies ranked by either incidence or mortality in the United States, it does represent the 2nd most common cause of cancer death worldwide.1, 2 Therefore, the advances we make in gastric cancer treatment, even in low incidence countries, can have global implications. Caution must be exercised however, in applying findings from Eastern countries to Western countries, as there are likely differences in biology. Gastric cancers from Eastern countries, such as Japan and Korea, have lower proportions with signet ring histology and proximal stomach involvement.3–6 Due to the lower proportion of cases with these adverse factors, most large randomized trials from the East demonstrate survival rates that are 30–40% higher than trials from the West.7, 8 There are also some basic science studies documenting a difference in tumor biology between regions.9
Risk factors for gastric cancer include many non-modifiable variables such as age, gender, and race/ethnicity. Other risk factors are controllable such as infection with Helicobacter pylori bacteria, smoking, and diets high in nitrates and nitrites.10 There are also several relatively rare risk factors such as a history of mucosa-associated lymphoid tissue lymphoma, previous stomach surgery, and pernicious anemia. Having a first-degree family member with gastric cancer is also a risk factor. There are several known inherited cancer syndromes that are associated with gastric cancer. The strongest association is found in Hereditary Diffuse Gastric Cancer (CDH1) syndrome in which approximately 80% of patients will develop gastric cancer.11 Others with a much lower risk include Lynch, hereditary breast and ovarian cancer (BRCA), Li-Fraumeni, Familial Adenomatous Polyposis, and Peutz-Jegher syndromes.12
Diagnosis and Staging
Patients with newly diagnosed gastric cancer often present with an upper endoscopy report performed for symptoms including dyspepsia and reflux, but also with symptoms or signs that may indicate advanced disease such as dysphagia, weight loss, gastrointestinal bleeding, anemia, and emesis.13 Clear measurements of the extent of the primary tumor are often lacking, and repeat endoscopy with endoscopic ultrasound (EUS) can provide additional clinical staging. Endoscopic ultrasound is most beneficial in identifying the rare early tumor (T1) that may benefit from endoscopic resection or upfront surgery. Most tumors are T2–4, however, and there are known limitations in the ability of EUS, and imaging, to accurately identify nodal metastases.14 Standard chest/abdomen/pelvic CT scans are often sufficient for imaging, but FDG-PET/CT scans can be considered for specific clinical indications such as further evaluation of indeterminate lesions. Tumors with poorly differentiated, signet ring cell type histology, or those without mucinous features, often do not demonstrate increased uptake on PET-CT imaging.15
Staging laparoscopy with peritoneal washings are a critical component of the initial workup as carcinomatosis is identified in approximately 20% of patients without imaging evidence of peritoneal disease.16 In addition, positive peritoneal cytology only is identified in approximately another 10% of patients, which also represents stage IV disease.16 Although only assigned a Category 2B recommendation in current National Comprehensive Cancer Network (NCCN) Guidelines (which indicates there is not uniform NCCN consensus that the procedure is appropriate such as found in a 2A recommendation), the procedure is considered standard at many cancer centers due to the obvious implications of identifying stage IV radiologically occult disease at diagnosis.17 The risks of staging laparoscopy are low and the outpatient procedure can also be combined with port placement for prompt initiation of systemic therapy. If the staging laparoscopy is deferred until the time of attempted resection after preoperative therapy, the identification of stage IV disease represents progression of disease when it was possibly present at the time of diagnosis.18 Appropriate initial staging at diagnosis has clear systemic therapy implications, can prevent unnecessary delays while awaiting surgery, and can also promote access to clinical trials. Most importantly, however, is that patients deserve a clear understanding of the extent of their disease and potential for cure as early as possible in their treatment plan.
A relatively new development in the workup of patients with potentially resectable disease is consideration of micro-satellite instability (MSI) testing at diagnosis. There are several studies providing a signal that patients with MSI-high cancers may have an adverse oncologic outcome when treated with standard systemic chemotherapy approaches.19 In a secondary post hoc analysis of the MAGIC trial, compared to patients with MSS/MSI-low tumors, those with MSI-high tumors had improved survival with surgery alone and inferior survival with perioperative chemotherapy plus surgery.20 Similarly, a post-hoc analysis of the CLASSIC trial showed that patients with resected MSI-high tumors did not have a disease-free survival (DFS) benefit with adjuvant chemotherapy.21 In a pooled meta-analysis of four randomized controlled trials of resected gastric cancer, MSI-high status was associated with longer overall survival (OS) and lack of benefit with perioperative or adjuvant chemotherapy.19 Some centers are recommending upfront surgery for patients with MSI-high tumors and even consideration of preoperative immunotherapy for advanced locoregional disease. The role of perioperative immune checkpoint blockade or omission of chemotherapy in operable MSI-H tumors has not been prospectively assessed. KEYNOTE-585 is a phase 3 randomized double-blind study assessing the addition of pembrolizumab to perioperative chemotherapy in resectable gastroeosphageal cancers (NCT03221426), and this may provide data on the role of checkpoint blockade in the subset of patients with MSI-H cancers. These complex cases are best approached in a multidisciplinary fashion with input from medical, radiation, and surgical oncology.
National guidelines recommend a multidisciplinary team approach for therapeutic decisions for patients with gastric cancer.17 At least once a week meetings are encouraged with individuals from relevant disciplines to include gastroenterology, radiology, pathology, and medical, surgical, and radiation oncology. Review of pathology and imaging is often helpful and not infrequently identifies findings that can change treatment or require further workup. In addition, review of patient outcomes and novel studies can provide an excellent source of continued medical education.
Management – Localized Disease
Randomized clinical trials provide evidence that combined modality therapy is effective for patients with non-metastatic gastric and gastroesophageal adenocarcinoma. Perioperative chemotherapy or postoperative chemotherapy plus chemoradiation are listed as preferred approaches in current guidelines, although postoperative chemotherapy is also an option following an adequate lymph node dissection.17 Studies of large databases, such as the National Cancer Database, demonstrate an increase in the application of preoperative therapy but it appears 1/3 to 1/4 of patients still undergo a surgery upfront approach.22
Perioperative Chemotherapy
For potentially resectable patients with clinical T2N0 or greater disease, neoadjuvant/perioperative therapy is typically administered rather than upfront surgery followed by adjuvant therapy. While there are no randomized trials comparing these approaches, the former approach has a greater likelihood of maximum systemic therapy delivery. Neoadjuvant chemotherapy may also result in downstaging of a locally advanced tumor, address micrometastatic disease, and improve identification of patients for whom surgery may not offer a survival benefit due to disease progression during neoadjuvant therapy.
The MAGIC trial was a seminal study that established the survival benefit of perioperative chemotherapy plus surgery versus surgery alone in patients with operable gastroesophageal adenocarcinoma (5-year survival 36% vs. 23%).23 Perioperative chemotherapy consisted of a three-drug combination of epirubicin, cisplatin, and fluorouracil (ECF). The anthracycline epirubicin is now thought to add additional toxicity without benefit and no longer is used in modern perioperative regimens.24 In support of this conclusion is a phase 3 trial that compared surgery with or without perioperative chemotherapy (cisplatin and fluorouracil) and found a similar 5-year OS benefit of 38% vs. 24% in favor of perioperative chemotherapy.25
Most recently, the phase 2/3 FLOT4-AIO trial compared perioperative FLOT (fluorouracil plus leucovorin, oxaliplatin, and docetaxel) to ECF (or ECX where X refers to capecitabine) in patients with resectable gastroesophageal adenocarcinoma. Perioperative FLOT resulted in superior OS compared to ECF/ECX (median OS 50 months vs. 35 months).26 Notably, patients in the FLOT arm had a 9% improvement in 5-year OS rates (45% vs 36%). Although there are concerns over the comparative arm including epirubicin, which has doubtful efficacy in gastric cancer, FLOT is a new standard of care. In less fit patients, we prefer perioperative therapy with a fluoropyrimidine plus platinum doublet.
The roles of HER2 targeted agents and VEGF inhibition are established in the metastatic setting and are currently being explored in the perioperative setting using a FLOT backbone. In the randomized phase II PETRARCA trial, the addition to trastuzumab and pertuzumab to perioperative FLOT improved pathologic complete response (pCR) (35% vs. 12%) and nodal negativity rates (68% vs. 39%) in patients with HER2 positive resectable gastroesophageal adenocarcinoma. Despite the negative results of the JACOB trial, these striking results warrant further investigation of perioperative trastuzumab plus pertuzumab in the phase 3 setting.27 In the randomized phase 2 portion of the RAMSES/FLOT7 trial, the addition of ramucirumab to FLOT improved R0 (no residual cancer) resection rates (97% vs. 83%) but did not impact pathologic response.28
Adjuvant Chemotherapy
In patients with gastric cancer who undergo upfront surgery and have pathological T3 or T4 lesions, or node positive disease, adjuvant therapy is recommended. The CLASSIC trial established the benefit of adjuvant capecitabine and oxaliplatin in patients who undergo curative-intent gastrectomy with D2 (extended) lymph node dissection.29 As this trial was performed in South Korea, China, and Taiwan, the previously mentioned issues regarding biologic differences between East Asia and U.S./European gastric cancers apply. Three year disease-free survival was 74% in the adjuvant chemotherapy group versus 59% in the surgery only group. In countries where the oral fluoropyrimidine S-1 is approved, adjuvant S-1 monotherapy or S-1 plus docetaxel can also be considered. In the randomized phase 3 ACTS-GC trial, adjuvant S-1 for one year demonstrated a survival benefit compared to surgery alone (5-year OS 72% vs. 61%).30 In the phase 3 JACCRO GC-07 trial, pathologic stage 3 gastric cancer patients who underwent curative intent surgery with D2 lymphadenectomy were randomized to S-1 plus docetaxel or S1 alone.31 At interim analysis, three-year recurrence-free survival was better in the combination group (66% vs. 50%).
Adjuvant Chemoradiotherapy
The role of adjuvant radiotherapy is less certain. The INT 0116 trial showed a nine month OS benefit patients in favor of adjuvant chemoradiation versus observation in patients with gastroesophageal adenocarcinoma who underwent curative intent surgery.32 However this study was limited by the fact that only 10% of patients underwent D2 lymphadenectomy. Thus, adjuvant chemoradiation may have compensated for an inadequate surgery and it was questioned if this benefit would actually persist if appropriate D2 lymphadenectomy were performed. Subsequent trials have compared adjuvant chemotherapy with or without adjuvant chemoradiotherapy with conflicting results.33–35 Per the National Comprehensive Cancer Network (NCCN) guidelines, adjuvant chemoradiation can be given for patients after R1 (microscopic residual cancer) or R2 (macroscopic residual cancer) resection. It also represents a category 1 recommendation as part of adjuvant therapy in patients with pT3-T4 or pN+ (node positive) disease if less than D2 nodal dissection is performed.17 Studies utilizing the National Cancer Database demonstrate an increasing use of perioperative chemotherapy with a decreasing use of postoperative chemoradiotherapy, likely due to improved tolerance for preoperative approaches, concerns over toxicity with postoperative chemoradiotherapy, and the increasingly recognized importance of D2 lymph node dissection.22
Preoperative Chemoradiotherapy
Preoperative chemoradiation is a category 2B (based upon lower-level evidence) treatment option for patients undergoing a preoperative therapy or total neoadjuvant treatment approach.17, 36 Regimens included in current guidelines are based on phase III randomized controlled trials including gastroesophageal junction tumors or smaller non-randomized phase II studies.37–40
Endoscopic Resection
Thin, early stage gastric cancers are infrequently detected in Western populations to allow for endoscopic resection. The criteria for safe and appropriate endoscopic resection are extensive: well to moderately differentiated tumor histology, size less than or equal to 2 cm, without invasion of the deep submucosa, and without lymphovascular invasion.17 Of critical importance, clear negative lateral and deep margins must be obtained. As these lesions are rare within the U.S. population, it is often difficult for endoscopic practitioners to obtain and maintain the proficiency in advanced techniques such as endoscopic mucosal or submucosal resection.
Surgical Resection
Surgical options for gastric cancer are primarily subtotal or total gastrectomy. There are several reasons to exercise caution in considering non-anatomic wedge type resections or limited proximal gastrectomy. First, approximately 75% of tumors in Western populations are poorly differentiated and therefore spread in a diffuse fashion that requires wide resection to ensure negative margins.41 Second, lymph node involvement is found in approximately 10%, 34%, and 44% of T1a, T1b and T2 tumors, respectively.42 Third, proximal gastrectomy with resection of the vagus nerve branches may predispose patients to severe chronic reflux. Lastly, to ensure an adequate D2 lymph node dissection requires anatomic resection and it is unclear if more limited resections adversely impact cancer outcomes.
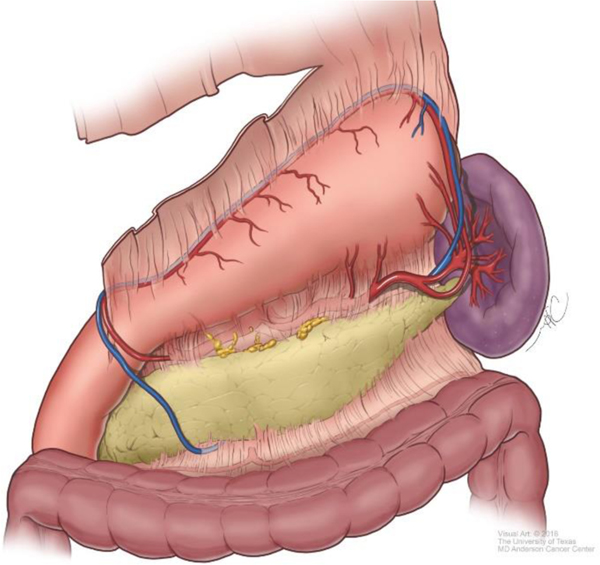
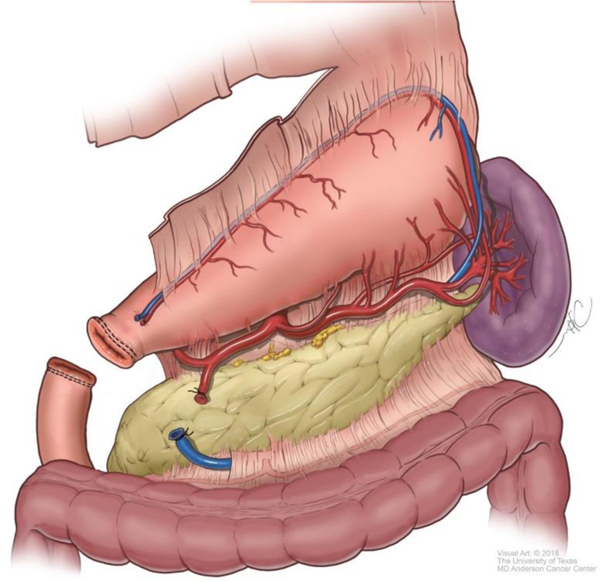
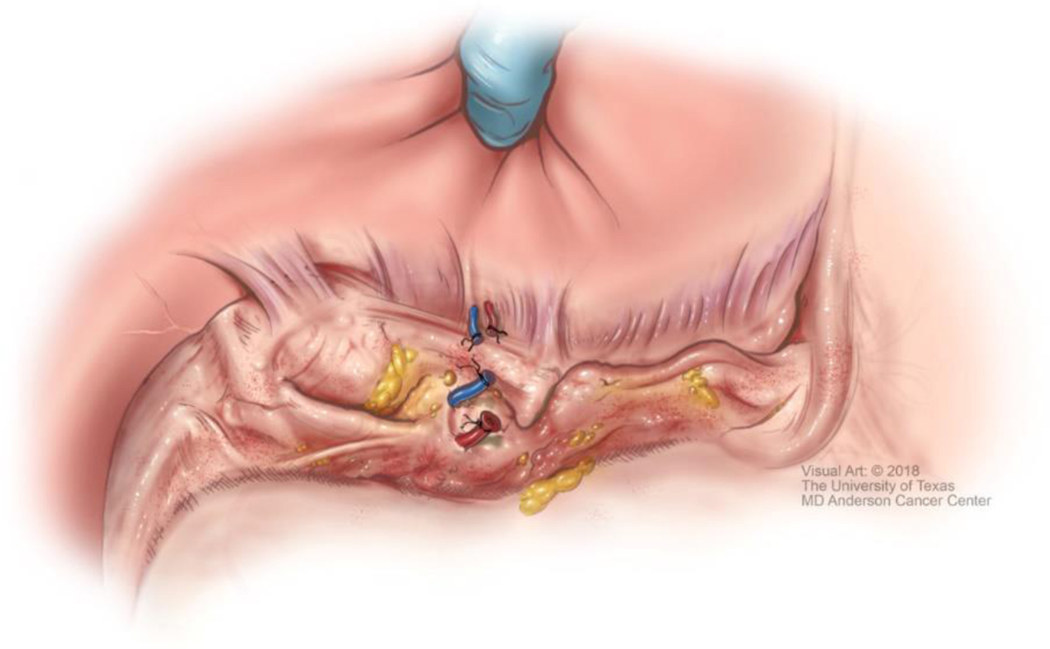
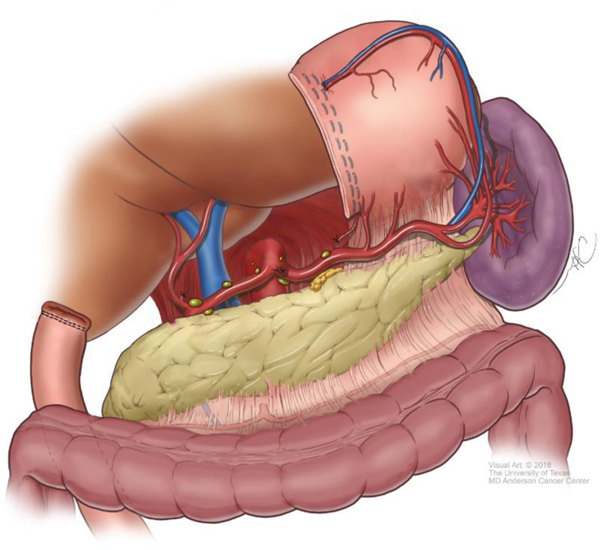
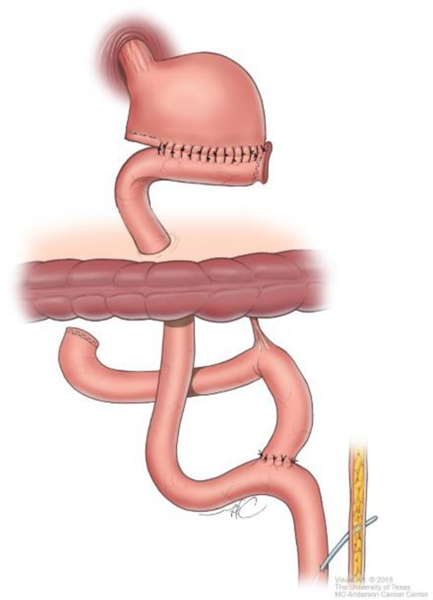
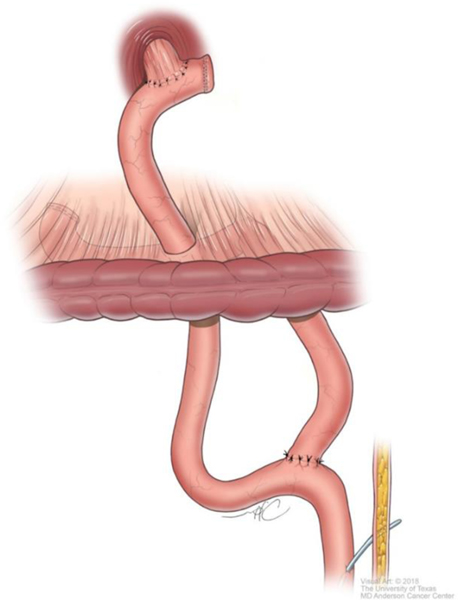
Although technical aspects can vary among various approaches, the following steps will help illustrate the anatomical details of gastrectomy. The greater omentum is separated from the transverse mesocolon as demonstrated in Figure 1. The right gastroepiploic and gastric vessels and duodenum are transected (Figure 2). Then the left gastric vessels are cut (Figure 3) prior to transection of the stomach for subtotal gastrectomy (Figure 4). Reconstruction for subtotal gastrectomy is illustrated in Figure 5. For tumors extending more proximally, the short gastric vessels are also transected and reconstruction is performed similar to Figure 6.
Figure 1.
Separation of the greater omentum from the transverse colon and mesocolon.
Figure 2.
Transection of the right gastroepiploic vessels and duodenum.
Figure 3.
Ligation and transection of the left gastric vessels
Figure 4.
Removal of distal portion of stomach in preparation for subtotal gastrectomy.
Figure 5.
Reconstruction after subtotal gastrectomy.
Figure 6.
Reconstruction after total gastrectomy.
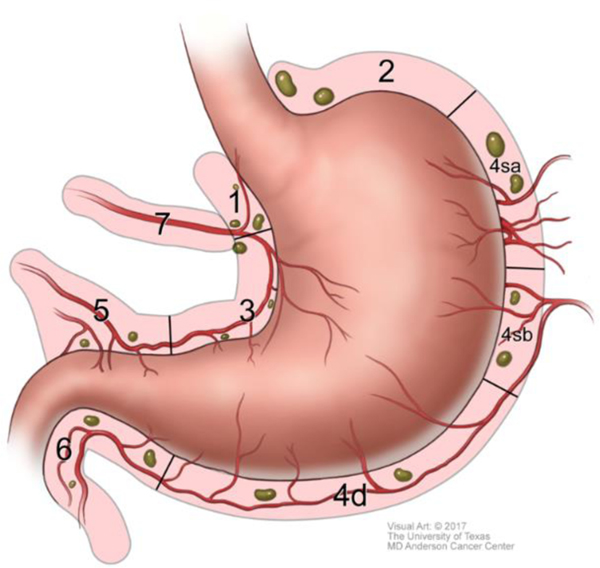
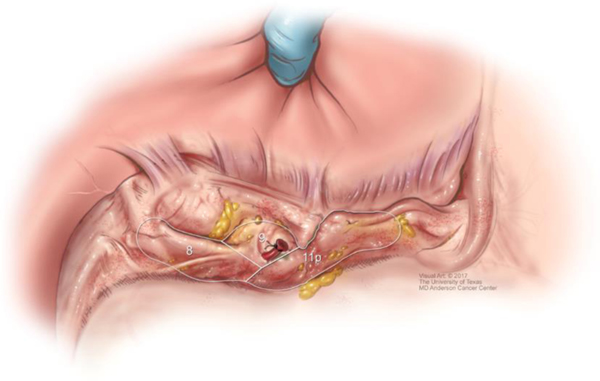
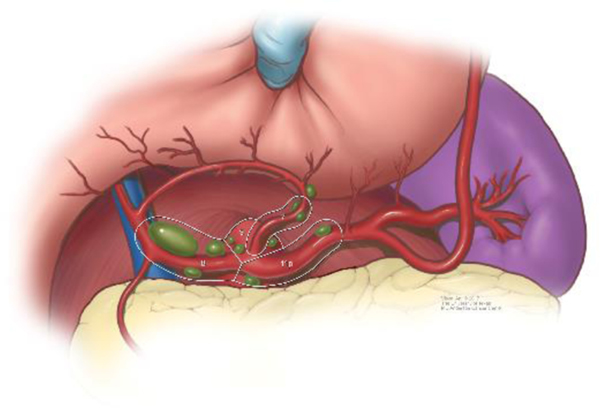
The extent of D1, or regional, lymphadenectomy is shown in Figure 7. Extended, or D2 lymphadenectomy, is essentially removal of lymph nodes along the branches of the celiac trunk as shown in Figures 8 & 9. A Japanese gastric cancer staging system exists that classifies individual areas of lymph nodes according to a numerical system, partly shown in Figures 8 & 9.43
Figure 7.
Illustration of D1 or regional lymph node dissection.
Figure 8.
Extra-regional, or D2, lymph node dissection.
Figure 9.
Branches of celiac trunk and D2 lymph node dissection.
Treatment of Metastatic and Unresectable Gastric Cancer
A number of cytotoxic agents are active in advanced gastric cancer including fluoropyrimidines, platinums, taxanes, and irinotecan. Choice of treatment is dependent upon patient performance status and medical comorbidities as well as toxicity profile of the regimen. Combination regimens offer higher response rates and improved survival compared to single agent therapy. Treatment goals are typically palliative in intent and are aimed at controlling symptoms, controlling disease and extending life. While there is no universal standard first line therapy, a fluoropyrimidine and platinum doublet is typically the preferred backbone regimen for most patients. Oxaliplatin is considered as effective as cisplatin and is the choice platinum in most modern regimens.44 In very fit patients who are willing to sacrifice toxicity for higher response rates and potentially longer progression-free survival (PFS), a triplet regimen combining a fluoropyrimidine, oxaliplatin and docetaxel, can be considered.45 There is no role for epirubicin in contemporary regimens for advanced disease.46 In patients who are not candidates for intensive therapy, single agent therapy with a fluoropyrimidine, irinotecan, or taxane can be considered. In patients with overexpression or amplification of human epidermal growth factor receptor (HER2; also known as ERBB2), trastuzumab should be added to cytotoxic first line chemotherapy, as will be reviewed in detail below. In patients with programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥5, nivolumab should be added to first line chemotherapy, as will be discussed.
In the second line treatment for metastatic gastric cancer, cytotoxic chemotherapy agents not already used in the first line can be attempted. Several years ago, ramucirumab was added to the armamentarium of active agents in this disease. Ramucirumab is a monoclonal antibody that binds to vascular endothelial growth factor receptor-2 (VEGFR-2), blocking receptor activation. In the phase 3 REGARD trial, ramucirumab was shown to have a 1.4 month survival benefit compared to placebo in the second line treatment of advanced gastric adenocarcinoma.47 Subsequently, the phase 3 RAINBOW trial demonstrated that paclitaxel plus ramucirumab was superior to paclitaxel plus placebo in the second line setting with an overall survival (OS) of 9.6 versus 7.4 months.48 In fit patients, paclitaxel plus ramucirumab is a preferred second line regimen after progression on a fluoropyrimidine and platinum doublet. Otherwise single agent cytotoxic chemotherapy or ramucirumab monotherapy can be considered. The oral cytotoxic agent trifluridine-tipiracil combining an antimetabolite trifluridine with a thymidine phosphorylase inhibitor tipiracil, has been shown in the phase 3 setting to have a survival benefit over placebo (5.7 vs. 3.6 months) in treatment refractory gastric cancer and is now an approved third line regimen.49 The role of immunotherapy and targeted therapies in gastric cancer is discussed further below, with an emphasis on recent progress and biomarkers including microsatellite instability high (MSI-H), PD-L1, tumor mutation burden (TMB), Epstein-Barr virus (EBV), and HER2.
Immunotherapy in Gastric Cancer
In the last decade, immune checkpoint blockade has emerged as an exciting treatment strategy across a spectrum of malignancies. This includes monoclonal antibodies that inhibit programmed cell death protein 1 (PD-1), PD-L1, and cytotoxic T-lymphocyte antigen 4 (CTLA-4).
High Microsatellite Instability/Mismatch Repair-Deficient Tumors
The Cancer Genome Atlas (TCGA) Research Network performed a comprehensive molecular characterization of 295 untreated gastric adenocarcinomas and categorized gastric cancer into four subtypes – MSI-H tumors, EBV positive tumors, tumors exhibiting chromosomal instability (CIN), and genomically stable tumors.50 In this analysis of untreated tumors, 22% were MSI-H, however the incidence in metastatic disease has been reported to be much lower, only 3% in a recent cohort of patients with stage IV disease.51
Mismatch repair (MMR) genes are responsible for fixing errors that occur during deoxyribonucleic acid (DNA) replication. Tumors with defects in the mismatch repair system (MMR deficient, dMMR) harbor significantly more mutations than tumors with intact mismatch repair machinery (MMR proficient, pMMR). Mismatch repair deficient tumors are vulnerable to mutations in microsatellites, repetitive sequences of nucleotide bases found throughout the genome, leading to high levels of microsatellite instability. Across tumor types, patients with dMMR cancers are more likely to respond to PD-1 blockade than are pMMR cancers.52 In part, this is due to high levels of neoantigens and PD-L1 positive T cell infiltration in dMMR tumors.
Pembrolizumab is a humanized monoclonal antibody that inhibits PD-1 activity by binding to PD-1 receptors on T-cells, thereby blocking PD-1 ligands (PD-L1 and PD-L2) from binding. PD-1 blockade results in removal of the physiologic brake on an active immune system and induces antitumor response. KEYNOTE-158 was a phase 2 trial that enrolled patients with treatment refractory non-colorectal MSI-H/dMMR cancers to receive pembrolizumab.53 Of the 24 patients with gastric cancer, there were 11 responses (including four complete responses) and median PFS was 11 months. This trial ultimately led to the tissue-agnostic FDA approval of pembrolizumab for patients with unresectable or metastatic MSI-H or dMMR of any solid tumor type, including gastric cancer, that progressed following prior treatment and who have no satisfactory alternative treatment.54
Prospective tumor sequencing of patients with metastatic gastroesophageal adenocarcinoma has demonstrated patients with MSI-H tumors are chemotherapy resistant and more likely to obtain durable responses to immunotherapy.51 An analysis of patients with MSI-H gastric cancers enrolled to KEYNOTE-59, KEYNOTE-061, and KEYNOTE-062, found that both OS and PFS were prolonged with pembrolizumab monotherapy compared to chemotherapy and that pembrolizumab is more effective than chemotherapy in the first line setting.55
Immunotherapy Trials in Gastric Adenocarcinoma
KEYNOTE-059 was a phase 2 trial of pembrolizumab therapy in patients with advanced gastric cancer and disease progression after two or more lines of therapy. Overall, the objective response rate (ORR) was 11.6% with median duration of response (DoR) of 8.4 months. However in PD-L1 positive (CPS ≥1) patients, ORR was 15.5% with a 16.3 month median DoR. These results were the basis of the United States Food and Drug Administration (FDA) approval of pembrolizumab for third line treatment of PD-L1 positive (CPS ≥1) gastric adenocarcinoma.
Pembrolizumab was compared to paclitaxel in the second-line treatment of advanced gastric adenocarcinoma in the randomized phase III KEYNOTE-061 trial.56 In an updated analysis, pembrolizumab did not significantly improve survival compared to paclitaxel in the second line setting. However, pembrolizumab numerically prolonged OS and showed increasing benefit with higher PD-L1 scores, with fewer treatment-related adverse events.
KEYNOTE-062 was a phase 3 trial comparing pembrolizumab with or without chemotherapy versus chemotherapy for first line treatment of PD-L1 positive (CPS ≥1) gastric or gastroesophageal junction adenocarcinoma. Compared to chemotherapy, pembrolizumab was noninferior for OS in CPS ≥1.57 In CPS ≥10, pembrolizumab improved OS compared to chemotherapy, however this difference was not statistically tested. Pembrolizumab plus chemotherapy did not improve OS or PFS in CPS ≥1 or ≥10.
Nivolumab is another humanized monoclonal antibody that inhibits PD-1. In the phase 3 ATTRACTION-2 trial, there was a survival benefit of nivolumab compared to placebo in heavily pretreated patients with advanced gastric adenocarcinoma (5.3 vs. 4.1 months).58 This study was performed in an Asian population and did not select for PD-L1 expression. Nivolumab is now approved in Japan for advanced gastric cancer refractory to conventional chemotherapy, regardless of PD-L1 expression. Nivolumab alone and in combination with ipilimumab (a monoclonal antibody inhibiting CTLA-4) has been studied in Western populations with chemotherapy refractory gastroesophageal adenocarcinoma and has been shown to have encouraging anti-tumor activity with an acceptable toxicity profile.59 CheckMate-649 is a phase 3 trial investigating nivolumab plus chemotherapy or nivolumab plus ipilimumab versus chemotherapy alone in the first line treatment of metastatic HER2 negative gastric cancer.60 In initial results, patients with PD-L1 CPS ≥5 receiving nivolumab plus chemotherapy compared to chemotherapy alone had improved OS (14.4 vs. 11.1 months) at a pre-specified interim analysis and PFS (7.7 vs. 6.1 months) at final analysis.61 An OS benefit was also seen in the all-randomized population. This is a practice changing study that establishes chemotherapy plus nivolumab as a new standard of care for first-line treatment of HER2 negative gastric cancer with PD-L1 CPS ≥5. However, this regimen is not yet FDA approved nor is it known what CPS cut-off will be used.
Unfortunately, a survival benefit was not shown for the PD-L1 inhibitor avelumab compared to clinicians’ choice therapy in the third line setting.62
Tumor Mutation Burden
Gastric cancer is a heterogeneous group of diseases with variable responsiveness to immunotherapy. Many biomarkers have been examined to identify susceptibility to PD-1 blockade including MSI status and PD-L1 expression as discussed. Tumor mutation burden is another biomarker currently under investigation. TMB quantifies the number of somatic mutations per coding area of a genome. It has been hypothesized that a heavily mutated tumor can produce a large number of neoantigens resulting in T-cell infiltration and potentially increased responsiveness to checkpoint blockade.
In June 2020, the FDA granted accelerated approval for treatment of patients with unresectable or metastatic TMB-high (TMB-H, ≥10 mutations/megabase (mt/Mb)) solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options. This was based upon a prospectively-planned retrospective analysis of previously treated patients with advanced solid tumors and TMB-H enrolled to KEYNOTE-158. In this non-randomized trial, of 790 evaluable patients, 102 (13%) were TMB-H and had an ORR of 29%, with a median DoR not reached.63
In an exploratory analysis from KEYNOTE-061, there was a positive association between TMB determined by FoundationOne CDx and clinical outcomes in patients with gastric cancer treated with pembrolizumab, but not paclitaxel.64 In patients with TMB ≥10 mut/Mb, pembrolizumab demonstrated an OS benefit compared to paclitaxel, and this benefit persisted even when MSI-H patients were excluded. These findings are hypothesis generating. In contrast, a retrospective analysis of genomically profiled gastroesophageal adenocarcinomas found that while survival was associated with increasing TMB, this association was lost after multivariate analysis and exclusion of MSI-H patients.65
Epstein Barr Virus
EBV is a human herpes virus implicated in a number of malignancies including gastric adenocarcinoma. EBV positive gastric cancer is a distinct subset of gastric cancer identified by TCGA and is associated with a rich CD8+ T-cell infiltrate and increased PD-L1 and PD-L2 expression, which may potentially make it more susceptible to PD-1 blockade.50, 66 Several reports have described robust responses of EBV positive tumors to immune checkpoint blockade, however this needs to be prospectively studied.51, 67
HER2 Positive Gastric Cancer
Approximately 15–20% of advanced gastric and gastroesophageal junction adenocarcinomas have overexpression or amplification of HER2. HER2 positivity is more commonly seen in intestinal-type compared to diffuse- or mixed-type cancers, in the TCGA CIN subtype, and in cancers arising from the gastroesophageal junction compared to the body of the stomach.50, 68
Trastuzumab is a humanized monoclonal antibody that targets the HER2 receptor, inhibits downstream signal activation, and induces antibody-dependent cellular toxicity. The pivotal phase 3 ToGA trial established the addition of trastuzumab to chemotherapy as the standard of care in the first line treatment of advanced HER2 positive gastric adenocarcinoma.69 Trastuzumab plus chemotherapy improved median overall survival (mOS) compared to chemotherapy alone, particularly in a post-hoc analysis of patients with HER2 immunohistochemistry (IHC) score 3+ or HER2 IHC 2+ with fluorescent in situ hybridization [FISH]-positive tumors (16.0 vs. 11.8 months).69 The level of ERRB2 amplification quantified by next generation sequencing is correlated with PFS on trastuzumab, with higher ERRB2 amplification levels associated with longer PFS on first line trastuzumab.51 Conversely, co-occurring alterations in the RTK-RAS-PI3K pathway are associated with shorter time to progression on first line trastuzumab based therapy.51
Numerous subsequent attempts to target HER2 have been disappointing. Lapatinib, a tyrosine kinase inhibitor affecting both HER2 and epidermal growth factor receptor (EGFR), does not improve survival when combined with chemotherapy in both first and second line settings in metastatic HER2 positive gastric adenocarcinoma.70, 71 Trastuzumab emtansine, an antibody-drug conjugate of trastuzumab bound to the tubulin inhibitor emtansine, does not prolong OS in the second line treatment of HER2 positive patients.72 Pertuzumab, a humanized monoclonal antibody that binds to a different epitope on the HER2 receptor, in addition to trastuzumab and chemotherapy, also failed to show a survival benefit in the first line JACOB trial.73 Finally, trastuzumab beyond progression has not been shown to improve survival. In patients who progressed on first line trastuzumab plus chemotherapy, trastuzumab plus paclitaxel did not improve PFS compared to paclitaxel alone.74 However, this trial just mandated HER2 positivity before first-line therapy and reconfirmation of HER2 positive status prior to continuing trastuzumab was not required. Exploratory analysis revealed that HER2 positivity was lost after first-line chemotherapy in 11 of 16 of evaluable patients. Given potential for loss of HER2 expression over time, second line trials targeting HER2 should require re-demonstration of HER2 positivity.
There is enthusiasm surrounding a number of novel HER2-targeted agents. ZW25 has been shown to be well tolerated with single-agent activity in a heavily pretreated group of HER2 positive malignancies.75 Margetuximab has also demonstrated tolerability and anti-tumor activity in HER2 positive cancers.76 Most promising at this point in time is trastuzumab deruxtecan, a humanized monoclonal anti-HER2 antibody attached to a cytotoxic topoisomerase I inhibitor via a cleavable linker. DESTINY-Gastric01 was a randomized phase 2 trial that evaluated trastuzumab deruxtecan versus chemotherapy in a refractory population of patients with HER2 positive gastric and gastroesophageal adenocarcinoma who had progressed on at least two prior therapies including trastuzumab.77 Trastuzumab deruxtecan showed improvements in OS (12.5 vs. 8.4 months) and response rate (RR) (51% vs. 14%) compared to chemotherapy.77 Side effects were notable for myelosuppression and interstitial lung disease.
Immunotherapy has also been successfully added to HER2 directed therapy. A phase 2 trial demonstrated that pembrolizumab could be safely combined with trastuzumab plus chemotherapy in HER2 positive metastatic gastroesophageal adenocarcinoma.78 Notably, there was an impressive 91% RR and mOS of 27.3 months, which is much higher than what is seen with chemotherapy plus trastuzumab (47% RR), suggesting that there may be a synergistic benefit of combining checkpoint blockade with standard trastuzumab plus chemotherapy.69 Efficacy is currently being evaluated in the randomized double-blind phase 3 KEYNOTE-811 trial (NCT03615326).
Anti-Angiogenic Therapy
As discussed above, ramucirumab, a monoclonal antibody against VEGFR-2, has a proven survival benefit in the second line treatment of gastric cancer, both as monotherapy, and in combination with paclitaxel.47, 48 Lenvatinib and regorafenib, both multikinase inhibitors of angiogenic (including VEGF receptor) and oncogenic receptor tyrosine kinases, have been investigated in combination with immunotherapy in East Asian populations. Lenvatinib has been safely combined with pembrolizumab with a 69% RR in the first and second line treatment of advanced gastric cancer.79 The addition of regorafenib to nivolumab has also been shown to be safe with encouraging anti-tumor activity in the phase 1 setting.80 We look forward to exploring the efficacy of combined VEGF inhibition and PD-1 blockade in larger cohorts of patients.
Investigational Biomarkers and Future Therapies
Targeting epidermal growth factor receptor (EGFR) is a therapeutic strategy in development in gastric cancer. While EGFR inhibitors are active in a number of cancers, these drugs have not shown efficacy in the phase 3 setting in unselected patients. In REAL-3 (first line chemotherapy with or without panitumumab), EXPAND (first line chemotherapy with or without cetuximab), and COG (second line gefitinib versus placebo), EGFR inhibition failed to improve survival.81–83 In a prospective cohort, patients with metastatic gastroesophageal adenocarcinoma were screened for EGFR amplification and 6% (8/140) were identified, of which 7 patients received anti-EGFR therapy.84 The ORR was 58% (4/7) and disease control rate was 100% (7/7), suggesting that EGFR inhibition should be further studied in selected patients.
Claudin18.2, a protein expressed by a subset of gastric cancers, is a novel target for drug development. Zolbetuximab, a chimeric monoclonal antibody that binds to Claudin-18.2, is tolerable with antitumor activity both as monotherapy and in combination with chemotherapy in patients with Claudin18.2 positive gastroesophageal adenocarcinoma and is being further investigated in the phase 3 setting.85, 86
In addition to drug development aimed at targeting specific biomarkers, systemic therapy can also be guided and informed by advanced imaging techniques. One of the challenges of biomarker driven therapy is intra- and inter-tumoral heterogeneity, which can lead to varying responsiveness to targeted therapies. Positron emission tomography (PET) using novel tracers such as radiolabelled trastuzumab may help assess and monitor tumor heterogeneity over time and is an area of active investigation.87
Recent Progress in Surgery
As the peritoneum is the most common site of metastatic disease at diagnosis, but also the most common site of recurrence after potentially curative surgery, it is a good target for novel therapeutic approaches.16, 88 Existing systemic chemotherapy has been shown to improve survival for peritoneal disease, but only at a median of 4 months according to population-based studies.89 There has been some enthusiasm for applying heated intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer, based on the improved survival in peritoneal disease from other primary sites such as appendiceal mucinous tumors, ovarian cancer, and mesothelioma. There is only one completed and published randomized controlled trial of HIPEC in gastric cancer patients with peritoneal disease.90 This small study, from China, demonstrated improved survival for patients undergoing cytoreduction and HIPEC compared to cytoreduction alone. However, the survival rates were modest and not all patients received systemic therapy prior to surgery in this trial. A recent multi-institutional registry report from Europe also compared patients undergoing cytoreduction to cytoreduction and HIPEC.91 Notably, the patients that underwent HIPEC demonstrated a long-term survival rate of 20%. A prospective randomized controlled trial with a similar design to look at the benefits of HIPEC in addition to cytoreduction, the GASTRIPEC trial, was closed early but should provide randomized controlled data regarding HIPEC.92 Another study from Europe, the PERISCOPE II trial, will answer perhaps the most important question in comparing standard of care systemic chemotherapy to HIPEC.93
An older body of literature exists regarding HIPEC in patients with gastric cancers at high risk of developing peritoneal disease such as T3 and T4 category lesions, albeit exclusively from Chinese and Japanese centers.94 These studies are dated and adjuvant HIPEC is not a standard of care in Eastern or Western centers. Most studies of peritoneal disease in Japan currently focus on the efficacy of intraperitoneal in combination with systemic paclitaxel.95 However, the role of adjuvant HIPEC in Western populations is an active question and should be answered by the ongoing GASTRICHIP randomized controlled trial.96
Novel Approaches to Detect Recurrence and Future Directions
Despite the advances made in the multimodality treatment of gastric cancer, recurrences are common. Current research is aimed at identifying patients at risk for recurrence after definitive therapy, with the hopes of intervening and potentially improving outcomes. In patients with cancer, circulating tumor DNA (ctDNA) can be detected in the bloodstream and can be a marker of minimal residual disease if detected after definitive therapy. A liquid biopsy can capture the spectrum of alterations present in a heterogeneous tumor compared to a traditional tissue biopsy. However, this DNA needs to be distinguished from cell-free DNA (cfDNA) alterations that exist from clonal hematopoiesis. Recently reported was an analysis of samples from patients in the CRITICS trial, a study that investigated perioperative therapies in patients with resectable gastric cancer. Circulating tumor DNA was identified after filtering alterations from matched white blood cells and predicted recurrence after treatment.97 In another 1630 patient cohort of ctDNA results, genomic alterations were correlated with clinicopathologic characteristics and outcomes and provided prognostic and predictive information.98 Future research should be aimed at prospectively collecting ctDNA to confirm these findings. Presence of persistent ctDNA after curative intent treatment of gastric cancer may be a marker of minimal residual disease and trials are currently underway to determine if additional adjuvant therapy can result in clearance of ctDNA. Specifically, adjuvant pembrolizumab is being investigated in MSI-tumors (NCT03832569) and adjuvant adjuvant trastuzumab plus pembrolizumab vs. trastuzumab is being studied in HER2+ tumors (NCT04510285).
Funding:
The No Stomach for Cancer Research Fund; The Holly Clegg Gastric Cancer Research Fund.
Footnotes
Conflicts of Interest: None.
References
- 1.World Health Organization, International Agency for Research on Cancer, GLOBOCAN 2018: Estimated Cancer Incidence, Mortality and Prevalence Worldwide, http://globocan.iarc.fr. Accessed June 16, 2020.
- 2.The American Cancer Society, Cancer Statistics Center, https://cancerstatisticscenter.cancer.org; accessed 6/16/2020.
- 3.Russo AE, Strong VE. Gastric Cancer Etiology and Management in Asia and the West. Annu Rev Med. Jan 27 2019;70:353–367. doi: 10.1146/annurev-med-081117-043436 [DOI] [PubMed] [Google Scholar]
- 4.Chon HJ, Hyung WJ, Kim C, et al. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Annals of surgery. May 2017;265(5):946–953. doi: 10.1097/SLA.0000000000001793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Annals of surgery. Apr 2010;251(4):640–6. doi: 10.1097/SLA.0b013e3181d3d29b [DOI] [PubMed] [Google Scholar]
- 6.Noguchi Y, Yoshikawa T, Tsuburaya A, Motohashi H, Karpeh MS, Brennan MF. Is gastric carcinoma different between Japan and the United States? Cancer. Dec 1 2000;89(11):2237–46. [PubMed] [Google Scholar]
- 7.Badgwell B. Multimodality Therapy of Localized Gastric Adenocarcinoma. Journal of the National Comprehensive Cancer Network : JNCCN. Oct 2016;14(10):1321–1327. [DOI] [PubMed] [Google Scholar]
- 8.Badgwell B, Das P, Ajani J. Treatment of localized gastric and gastroesophageal adenocarcinoma: the role of accurate staging and preoperative therapy. J Hematol Oncol. Aug 15 2017;10(1):149. doi: 10.1186/s13045-017-0517-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SJ, Gagnon-Bartsch JA, Tan IB, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. Nov 2015;64(11):1721–31. doi: 10.1136/gutjnl-2014-308252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.wcrf.org/dietandcancer/stomach-cancer. American Institute for Cancer Research, World Cancer Research Fund. Accessed November 24, 2020. [Google Scholar]
- 11.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. Apr 2015;1(1):23–32. doi: 10.1001/jamaoncol.2014.168 [DOI] [PubMed] [Google Scholar]
- 12.Lott PC, Carvajal-Carmona LG. Resolving gastric cancer aetiology: an update in genetic predisposition. Lancet Gastroenterol Hepatol. Dec 2018;3(12):874–883. doi: 10.1016/S2468-1253(18)30237–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World journal of gastroenterology : WJG. Feb 28 2008;14(8):1149–55. doi: 10.3748/wjg.14.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikoma N, Lee JH, Bhutani MS, et al. Preoperative accuracy of gastric cancer staging in patient selection for preoperative therapy: race may affect accuracy of endoscopic ultrasonography. Journal of gastrointestinal oncology. Dec 2017;8(6):1009–1017. doi: 10.21037/jgo.2017.04.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada K, Patnana M, Wang X, et al. Low metabolic activity in primary gastric adenocarcinoma is associated with resistance to chemoradiation and the presence of signet ring cells. Surg Today. May 14 2020;doi: 10.1007/s00595-020-02018-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikoma N, Blum M, Chiang YJ, et al. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Annals of surgical oncology. Jul 6 2016;doi: 10.1245/s10434-016-5409-7 [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network Guidelines Version 2.2020, Gastric Cancer, available at www.nccn.org. Accessed June 15, 2020. [Google Scholar]
- 18.Thiels CA, Ikoma N, Fournier K, et al. Repeat staging laparoscopy for gastric cancer after preoperative therapy. Journal of surgical oncology. Jul 2018;118(1):61–67. doi: 10.1002/jso.25094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrantonio F, Miceli R, Raimondi A, et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Dec 10 2019;37(35):3392–3400. doi: 10.1200/JCO.19.01124 [DOI] [PubMed] [Google Scholar]
- 20.Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. Sep 01 2017;3(9):1197–1203. doi: 10.1001/jamaoncol.2016.6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi YY, Kim H, Shin SJ, et al. Microsatellite Instability and Programmed Cell Death-Ligand 1 Expression in Stage II/III Gastric Cancer: Post Hoc Analysis of the CLASSIC Randomized Controlled study. Annals of surgery. Aug 2019;270(2):309–316. doi: 10.1097/sla.0000000000002803 [DOI] [PubMed] [Google Scholar]
- 22.Ikoma N, Cormier JN, Feig B, et al. Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: Analysis of the National Cancer Data Base, 2006–2014. Cancer. Mar 1 2018;124(5):998–1007. doi: 10.1002/cncr.31155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. Clinical Trial, Phase III Comparative Study Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t. The New England journal of medicine. Jul 6 2006;355(1):11–20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 24.Elimova E, Janjigian YY, Mulcahy M, et al. It Is Time to Stop Using Epirubicin to Treat Any Patient With Gastroesophageal Adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Feb 2017;35(4):475–477. doi: 10.1200/JCO.2016.69.7276 [DOI] [PubMed] [Google Scholar]
- 25.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. May 01 2011;29(13):1715–21. doi: 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 26.Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. May 11 2019;393(10184):1948–1957. doi: 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 27.Hofheinz RD, Haag GM, Ettrich TJ, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: Final results of the PETRARCA multicenter randomized phase II trial of the AIO. Journal of Clinical Oncology. 2020;38(15_suppl):4502–4502. doi: 10.1200/JCO.2020.38.15_suppl.4502 [DOI] [Google Scholar]
- 28.Al-Batran S-E, Hofheinz RD, Schmalenberg H, et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7): Results of the phase II-portion—A multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. Journal of Clinical Oncology. 2020;38(15_suppl):4501–4501. doi: 10.1200/JCO.2020.38.15_suppl.4501 [DOI] [Google Scholar]
- 29.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. Jan 28 2012;379(9813):315–21. doi: 10.1016/s0140-6736(11)61873-4 [DOI] [PubMed] [Google Scholar]
- 30.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Nov 20 2011;29(33):4387–93. doi: 10.1200/JCO.2011.36.5908 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida K, Kodera Y, Kochi M, et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol. May 20 2019;37(15):1296–1304. doi: 10.1200/jco.18.01138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. Clinical Trial Comparative Study Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S. The New England journal of medicine. Sep 6 2001;345(10):725–30. doi: 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. Jan 20 2012;30(3):268–73. doi: 10.1200/jco.2011.39.1953 [DOI] [PubMed] [Google Scholar]
- 34.Park SH, Zang DY, Han B, et al. ARTIST 2: Interim results of a phase III trial involving adjuvant chemotherapy and/or chemoradiotherapy after D2-gastrectomy in stage II/III gastric cancer (GC). Journal of Clinical Oncology. 2019;37(15_suppl):4001–4001. doi: 10.1200/JCO.2019.37.15_suppl.4001 [DOI] [Google Scholar]
- 35.Yu C, Yu R, Zhu W, Song Y, Li T. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. Journal of cancer research and clinical oncology. Feb 2012;138(2):255–9. doi: 10.1007/s00432-011-1085-y [DOI] [PubMed] [Google Scholar]
- 36.Badgwell B, Blum M, Estrella J, et al. Predictors of Survival in Patients with Resectable Gastric Cancer Treated with Preoperative Chemoradiation Therapy and Gastrectomy. Journal of the American College of Surgeons. Jul 2015;221(1):83–90. doi: 10.1016/j.jamcollsurg.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 37.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t. The New England journal of medicine. May 31 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 38.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. Clinical Trial Research Support, Non-U.S. Gov’t. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Feb 20 2005;23(6):1237–44. doi: 10.1200/JCO.2005.01.305 [DOI] [PubMed] [Google Scholar]
- 39.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. Clinical Trial Multicenter Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Jul 15 2004;22(14):2774–80. doi: 10.1200/JCO.2004.01.015 [DOI] [PubMed] [Google Scholar]
- 40.Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. Clinical Trial, Phase II Multicenter Study Research Support, N.I.H., Extramural. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Aug 20 2006;24(24):3953–8. doi: 10.1200/JCO.2006.06.4840 [DOI] [PubMed] [Google Scholar]
- 41.Allen CJ, Blumenthaler AN, Smith GL, et al. Chemotherapy Versus Chemotherapy Plus Chemoradiation as Preoperative Therapy for Resectable Gastric Adenocarcinoma: A Propensity Score-Matched Analysis of a Large, Single-Institution Experience. Annals of surgical oncology. Jul 21 2020;doi: 10.1245/s10434-020-08864-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikoma N, Blum M, Chiang YJ, et al. Race Is a Risk for Lymph Node Metastasis in Patients With Gastric Cancer. Annals of surgical oncology. Apr 2017;24(4):960–965. doi: 10.1245/s10434-016-5645-x [DOI] [PubMed] [Google Scholar]
- 43.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. Jan 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. Jan 03 2008;358(1):36–46. doi: 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 45.Al-Batran SE, Pauligk C, Homann N, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer. Mar 2013;49(4):835–42. doi: 10.1016/j.ejca.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 46.Oba K, Paoletti X, Bang YJ, et al. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer. May 2013;49(7):1565–77. doi: 10.1016/j.ejca.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 47.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. Jan 04 2014;383(9911):31–9. doi: 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 48.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. Oct 2014;15(11):1224–35. doi: 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 49.Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. Nov 2018;19(11):1437–1448. doi: 10.1016/s1470-2045(18)30739-3 [DOI] [PubMed] [Google Scholar]
- 50.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. Sep 11 2014;513(7517):202–9. doi: 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janjigian YY, Sanchez-Vega F, Jonsson P, et al. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer discovery. Jan 2018;8(1):49–58. doi: 10.1158/2159-8290.Cd-17-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. Jun 25 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. Jan 1 2020;38(1):1–10. doi: 10.1200/jco.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. U.S. Food and Drug Administration. Accessed November 30, 2020.
- 55.Chao J, Fuchs CS, Shitara K, et al. Pembrolizumab (pembro) in microsatellite instability-high (MSI-H) advanced gastric/gastroesophageal junction (G/GEJ) cancer by line of therapy. Journal of Clinical Oncology. 2020;38(4_suppl):430–430. doi: 10.1200/JCO.2020.38.4_suppl.430 [DOI] [Google Scholar]
- 56.Fuchs CS, Özgüroğlu M, Bang Y-J, et al. Pembrolizumab versus paclitaxel for previously treated patients with PD-L1–positive advanced gastric or gastroesophageal junction cancer (GC): Update from the phase III KEYNOTE-061 trial. Journal of Clinical Oncology. 2020;38(15_suppl):4503–4503. doi: 10.1200/JCO.2020.38.15_suppl.4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. Oct 1 2020;6(10):1571–1580. doi: 10.1001/jamaoncol.2020.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boku N, Kang YK, Satoh T, et al. 617OA Phase 3 Study of nivolumab (Nivo) in previously treated advanced gastric or gastroesophageal junction (G/GEJ) cancer: Updated results and subset analysis by PD-L1 expression (ATTRACTION-02). Annals of Oncology. 2017;28(suppl_5):mdx369.001-mdx369.001. doi: 10.1093/annonc/mdx369.001 [DOI] [Google Scholar]
- 59.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol. Oct 1 2018;36(28):2836–2844. doi: 10.1200/jco.2017.76.6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moehler MH, Janjigian YY, Adenis A, et al. CheckMate 649: A randomized, multicenter, open-label, phase III study of nivolumab (NIVO) + ipilimumab (IPI) or nivo + chemotherapy (CTX) versus CTX alone in patients with previously untreated advanced (Adv) gastric (G) or gastroesophageal junction (GEJ) cancer. Journal of Clinical Oncology. 2018;36(4_suppl):TPS192-TPS192. doi: 10.1200/JCO.2018.36.4_suppl.TPS192 [DOI] [Google Scholar]
- 61.Moehler M, Shitara K, Garrido M, et al. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Annals of Oncology. 2020;31((suppl_4)):S1142–S1215. [Google Scholar]
- 62.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. Oct 1 2018;29(10):2052–2060. doi: 10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. Sep 10 2020;doi: 10.1016/s1470-2045(20)30445–9 [DOI] [PubMed] [Google Scholar]
- 64.Shitara K, Özgüroğlu M, Bang Y-J, et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. Journal of Clinical Oncology. 2020;38(15_suppl):4537–4537. doi: 10.1200/JCO.2020.38.15_suppl.4537 [DOI] [Google Scholar]
- 65.Greally M, Chou JF, Chatila WK, et al. Clinical and Molecular Predictors of Response to Immune Checkpoint Inhibitors in Patients with Advanced Esophagogastric Cancer. Clin Cancer Res. Oct 15 2019;25(20):6160–6169. doi: 10.1158/1078-0432.Ccr-18-3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derks S, Liao X, Chiaravalli AM, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. May 31 2016;7(22):32925–32. doi: 10.18632/oncotarget.9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nature medicine. Sep 2018;24(9):1449–1458. doi: 10.1038/s41591-018-0101-z [DOI] [PubMed] [Google Scholar]
- 68.Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. Jul 2015;18(3):476–84. doi: 10.1007/s10120-014-0402-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. Aug 28 2010;376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 70.Hecht JR, Bang Y-J, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2–Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. Journal of Clinical Oncology. 2016;34(5):443–451. doi: 10.1200/jco.2015.62.6598 [DOI] [PubMed] [Google Scholar]
- 71.Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. Jul 01 2014;32(19):2039–49. doi: 10.1200/JCO.2013.53.6136 [DOI] [PubMed] [Google Scholar]
- 72.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. The Lancet Oncology. 18(5):640–653. doi: 10.1016/S1470-2045(17)30111-0 [DOI] [PubMed] [Google Scholar]
- 73.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. Oct 2018;19(10):1372–1384. doi: 10.1016/s1470-2045(18)30481-9 [DOI] [PubMed] [Google Scholar]
- 74.Makiyama A, Sukawa Y, Kashiwada T, et al. Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). Journal of Clinical Oncology. 2020;38(17):1919–1927. doi: 10.1200/jco.19.03077 [DOI] [PubMed] [Google Scholar]
- 75.Meric-Bernstam F, Beeram M, Mayordomo JI, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. Journal of Clinical Oncology. 2018;36(15_suppl):2500–2500. doi: 10.1200/JCO.2018.36.15_suppl.2500 [DOI] [Google Scholar]
- 76.Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. Apr 1 2017;28(4):855–861. doi: 10.1093/annonc/mdx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shitara K, Bang Y-J, Iwasa S, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. New England Journal of Medicine. 2020;382(25):2419–2430. doi: 10.1056/NEJMoa2004413 [DOI] [PubMed] [Google Scholar]
- 78.Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. Jun 2020;21(6):821–831. doi: 10.1016/s1470-2045(20)30169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. Aug 2020;21(8):1057–1065. doi: 10.1016/s1470-2045(20)30271-0 [DOI] [PubMed] [Google Scholar]
- 80.Fukuoka S, Hara H, Takahashi N, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. Jun 20 2020;38(18):2053–2061. doi: 10.1200/jco.19.03296 [DOI] [PubMed] [Google Scholar]
- 81.Okines AF, Ashley SE, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol. Sep 01 2010;28(25):3945–50. doi: 10.1200/jco.2010.29.2847 [DOI] [PubMed] [Google Scholar]
- 82.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. May 2013;14(6):490–9. doi: 10.1016/s1470-2045(13)70102-5 [DOI] [PubMed] [Google Scholar]
- 83.Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. Jul 2014;15(8):894–904. doi: 10.1016/s1470-2045(14)70024-5 [DOI] [PubMed] [Google Scholar]
- 84.Maron SB, Alpert L, Kwak HA, et al. Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma. Cancer discovery. Jun 2018;8(6):696–713. doi: 10.1158/2159-8290.Cd-17-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. Sep 1 2019;30(9):1487–1495. doi: 10.1093/annonc/mdz199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Batran S-E, Schuler MH, Zvirbule Z, et al. FAST: An international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. Journal of Clinical Oncology. 2016;34(18_suppl):LBA4001-LBA4001. doi: 10.1200/JCO.2016.34.18_suppl.LBA4001 [DOI] [Google Scholar]
- 87.Mondaca S, Janjigian YY. Application of positron emission tomography imaging to personalize esophagogastric cancer care. Cancer. Apr 15 2019;125(8):1214–1217. doi: 10.1002/cncr.31940 [DOI] [PubMed] [Google Scholar]
- 88.Ikoma N, Chen HC, Wang X, et al. Patterns of Initial Recurrence in Gastric Adenocarcinoma in the Era of Preoperative Therapy. Annals of surgical oncology. Mar 22 2017;doi: 10.1245/s10434-017-5838-y [DOI] [PubMed] [Google Scholar]
- 89.Thomassen I, Bernards N, van Gestel YR, et al. Chemotherapy as palliative treatment for peritoneal carcinomatosis of gastric origin. Acta Oncol. Mar 2014;53(3):429–32. doi: 10.3109/0284186X.2013.850740 [DOI] [PubMed] [Google Scholar]
- 90.Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Clinical Trial, Phase III Randomized Controlled Trial Research Support, Non-U.S. Gov’t. Annals of surgical oncology. Jun 2011;18(6):1575–81. doi: 10.1245/s10434-011-1631-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonnot PE, Piessen G, Kepenekian V, et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. May 14 2019:JCO1801688. doi: 10.1200/JCO.18.01688 [DOI] [PubMed] [Google Scholar]
- 92.Beckert S, Konigsrainer A. [Oligometastases in gastric and esophageal cancer : Current clinical trials and surgical concepts]. Chirurg. Jul 2018;89(7):505–509. Oligometastasierung beim Magen- und Osophaguskarzinom : Aktuelle Studienlage und chirurgische Konzepte. doi: 10.1007/s00104-018-0645-y [DOI] [PubMed] [Google Scholar]
- 93.Koemans WJ, van der Kaaij RT, Boot H, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC cancer. May 6 2019;19(1):420. doi: 10.1186/s12885-019-5640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Meta-Analysis Research Support, Non-U.S. Gov’t Review. Annals of surgical oncology. Oct 2007;14(10):2702–13. doi: 10.1245/s10434-007-9487-4 [DOI] [PubMed] [Google Scholar]
- 95.Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. Mar 2017;20(Suppl 1):128–134. doi: 10.1007/s10120-016-0684-3 [DOI] [PubMed] [Google Scholar]
- 96.Glehen O, Passot G, Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC cancer. Mar 14 2014;14:183. doi: 10.1186/1471-2407-14-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leal A, van Grieken NCT, Palsgrove DN, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nature communications. Jan 27 2020;11(1):525. doi: 10.1038/s41467-020-14310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maron SB, Chase LM, Lomnicki S, et al. Circulating Tumor DNA Sequencing Analysis of Gastroesophageal Adenocarcinoma. Clin Cancer Res. Dec 1 2019;25(23):7098–7112. doi: 10.1158/1078-0432.Ccr-19-1704 [DOI] [PMC free article] [PubMed] [Google Scholar]