Abstract
Knowledge of the reproductive strategy is a key prerequisite to predict population dynamics and potential invasiveness of both native and non-indigenous outbreak-forming species. In 2014 the Lessepsian upside-down jellyfish Cassiopea andromeda reached the harbor of Palermo (NW Sicily, Thyrrenian Sea), to date its established westernmost outpost in the Mediterranean Sea. To predict C. andromeda reproductive success in its novel habitat, gonad histology was carried out to record the number and size of mature and immature oocytes. Both male and female simultaneously presented gametes at all stages of development suggesting an asynchronous, yet apparently continuous, reproduction strategy. Indeed, oogenesis was observed throughout the year from pre-vitellogenic, vitellogenetic, and late-vitellogenetic to mature oocytes suggesting multiple reproductive events, as known in other Mediterranean Rhizostomeae. Oocytes were found from May to December, with two seasonal peaks of abundance (late spring = 392 and autumn = 272), suggesting imminent spawning events. Further, jellyfish size varied significantly throughout the year, with maximum diameter (up to 24 cm) in summer, and minimum diameter (6 cm) in winter. Small-sized jellyfish in winter belong to the new cohort, most probably arising from intense summer strobilation of polyps. Late spring fertilization, planula development, and metamorphosis, followed by polyp strobilation in the summer months, may explain the late appearance of a new jellyfish cohort, likely coincident with that recorded throughout winter.
Introduction
Alien species are considered nowadays one of the major threats for biodiversity and ecosystem functioning [1]. The Mediterranean Sea is considered a hotspot of marine bioinvasions [2, 3] where physical and environmental conditions vary greatly at geographical scale, making possible the entrance of non-indigenous species (NIS) from the Red Sea and the Atlantic Ocean following anthropogenic and natural dispersion patterns [4, 5]. Introduction of marine NIS in the Mediterranean Sea has been facilitated by ships’ fouling and ballast water, aquaculture activities, aquarium trade, and particularly by the progressive enlargement of the Suez Canal [6, 7]. Global warming and species translocation are driving the so called tropicalization of the Mediterranean Sea, with the increasing occurrence of warm-water biota in the basin [8]. On March 2022, 751 validated NIS were considered established and spreading westward, especially from the Levant Sea [9, 10].
Generally, these non-indigenous species become invasive, severely impacting local biodiversity by displacing native species and altering community and ecosystem structures and functioning. As a consequence, such perturbations may transform the ecosystem services and human well-being [11–15]. NIS may have also positive effects on ecosystem functioning through the creation of novel habitats (acting as ecosystem engineers), providing food and shelter to other species or helping in the regulation of the ecosystem functioning in stressed or degraded habitats [14, 16]. The balance between positive and negative effects is sometimes complicated to calibrate, being some features such as the biological cycle, key questions that may help to elucidate such problems.
Cnidarians include a non-neglectable group of new colonizers of the Mediterranean basin [17], whose impact depend both on the species traits and the ecological features of the new habitat [18]. Changes in the relative abundance of jellyfish have socio-economic consequences on coastal human activities and on ecosystems functioning [19–23] by exerting top-down and bottom-up controls on the marine food web, through competition and predation relationships with coexisting organisms [24–27]. Cassiopea andromeda (Rhizostomeae, Scyphozoa) is a photosymbiotic tropical jellyfish, originally described from the Red Sea (Forskål 1775) that has invaded the Mediterranean basin. Outside the Mediterranean, it has been recorded across the Indo-Pacific and Atlantic waters but considered as NIS in Hawaii [28] and Brazil [29]. It has an epibenthic (so called upside-down) lifestyle, and it is commonly found lying on seagrass beds, mangrove habitats and lagoons [30, 31]. First discovered in Cyprus in 1903 [32], C. andromeda colonized the entire Levant Sea [33–35], and spread over the Central Mediterranean in 2009, approaching the Maltese islands [36] and, in 2014, it was found for the first time in Italian waters, specifically in the Palermo “Cala” Harbour, in Sicily [37, 38]. Temperature and several environmental parameters in the Mediterranean Sea are markedly different from tropical seas; nonetheless, C. andromeda seems to be rapidly spreading thanks to its high asexual proliferative potential through planuloid bud production [39], wide thermal tolerance (18–29°C), and physiological adaptive plasticity to changes of nutrient and light conditions [29, 40–42]. Cassiopea spp. in their type localities are mostly associated with mangrove-dominated habitats [28]. Shallow-water anthropogenic areas, such as small bays and harbors (characterized by high nutrient concentrations and high temperature) might facilitate their spread, size and recurrent outbreaks [41, 43–46], supported by plastic symbionts that maintain a high photosynthetic efficiency even when facing a rapid change in light exposure [42]. Available evidence shows that outbreaks (>10 jellyfish/m2) of Cassiopea can lead to variations in the community structure, e.g., reducing the seagrass abundance and the meadows faunal densities [47]. In addition, Cassiopea can locally modify nutrient cycling through ammonium absorption and phosphate uptake [48, 49]; thus, investigating jellyfish biology and ecology may become an essential element for a sound ecosystem management.
One of the main features defining a successful biological invader is represented by its reproductive strategy [50]. A combination of traits, like high fecundity, rapid sexual maturation, high reproductive output, and asexual reproduction and hermaphroditism can sustain such successful invasion [50]. Jellyfish can use different strategies to cope with environmental variability, reduce the risks of local extinction, and opportunistically capitalize into population outbreaks [51] such as: a) species with a complex (planula-polyp-medusa) life cycle, employ a combination of a single, seasonal sexual reproduction event and of multiple, alternative modes of asexual reproduction and overwintering stages [39, 52–54]; b) species with holoplanktonic life cycle with continuous reproduction [55, 56]. Global warming and the rise of seawater temperature may boost jellyfish reproduction, through increased strobilation or budding formation [20] or extend the timing of gonad maturation [56]. This fact, combined especially with overfishing, boosted the medusae proliferation in many areas of the world, including the Mediterranean Sea [20].
In spite of its distinctive symbiotic association and prevalently epibenthic lifestyle, C. andromeda shares with most Scyphomedusae a typical three-stage life cycle (planula larva, polyp, medusa) and gonochorism [57–59]. Males of C. andromeda freely release sperms in the water and fertilization take place in the gastrovascular cavity of females, which will store embryos in special brooding vesicles in the center of the oral disc [59, 60]. Nearly 3–4 days post fertilization, competent ciliated planula larvae are released, actively searching for suitable settlement sites, where metamorphosis into the benthic polyp stage eventually occurs [60–62]. Budding of free-swimming planuloids or lateral polyps, and strobilation, are asexual processes regulated by external factors as temperature, salinity, and symbiont presence [62–64]. Whereas bud morphogenesis, settlement, and metamorphosis of C. andromeda have been widely assessed in scientific literature [65, 66], the detailed characterization of the temporal pattern of sexual reproduction is still missing. In this framework, classical morphometric and histological methods were used here to investigate gametogenesis of C. andromeda over an annual cycle, by recording the number and size of differentiating oocytes in female gonads. This study was carried out in NW Sicily, i.e., so far, the westernmost location of an established C. andromeda population in the Mediterranean Sea. Filling this information gap might be important to forecasting further spread of the upside-down jellyfish, especially in recently invaded habitats, and for a better understanding of the mechanisms of outbreaks formation.
Materials and methods
Sample collection and processing
Specimens of Cassiopea andromeda were sampled by hand net in the Cala harbor of Palermo (NW Sicily; 38° 07.22′ N, 13° 22.09′ E) [46], from 15th of May 2017 to the 20th of April 2018, for a total of 16 collecting dates. Measurements of surface water temperature and salinity were taken at every sampling date with a multiparametric probe (Hanna, HI98194) (S1 Table). Jellyfish were transported to the laboratory and morphological analyses were immediately performed. Diameter of each organism was recorded, and the jellyfish bell was cut off and fixed in 4% formaldehyde solution in seawater. One week before the histological analyses, samples were washed (5 times with non-filtered seawater and once/twice with fresh water) and stored in ethanol 70%.
No permits were required because the sampling site is part of the public maritime domain. Moreover, samplings were carried out by boat and members are either members of the national environmental authority (ISPRA) belonging to the Italian Ministry of Environment, or to bodies of the Ministry of Research and Education, such as the Stazione Zoologica and the universities of Palermo and Lecce.
Histological analysis
Specimens (n = 64: 16 males and 48 females) of Cassiopea andromeda were dissected under a stereomicroscope (Leica MZ6). Two pieces of gonads per organism (total 128 gonads pieces) were cut away and dehydrated using a series of ethanol with increasing concentrations (80% to 100%), cleared in Xylene (histological grade) and impregnated in BioPlast paraffin (Bio-Optica, melting point 56–58°C). The tissue was then embedded in paraffin, sectioned at 7 μm with a Leica microtome (RM 2155), and stained with hematoxylin and eosin. Histological sections were examined under Zeiss Primo Star optic microscope equipped with ZEN software.
Oocytes count and measurement
Five sections per piece of female gonad was used for oocytes count, 10 sub-replicates per each specimen (n = 48), with a total of 470 examined sections. For female specimens, all visible oocytes were counted with a manual counter. Each count was made in ⁓15 mm2 of the ovary. In each section, 10 oocytes were measured along a linear transect (100 oocytes per specimen), choosing a random starting point in the field of view of the microscope. The mean diameter (d) was calculated according to Szafranski et al. [67] by using the following formula:
where “l” is the length of oocytes (i.e., the major diameter) measured in (μm), and “w” is the width of oocytes (i.e., minor diameter) measured in μm.
In the case of male specimens (n = 16), spermatic sacs were counted in 10 sections (five per piece of gonad) per specimen and the diameter of the sacs was also measured.
Statistical analysis
Temporal pattern and autocorrelation of jellyfish diameter were tested using a generalized additive mixed model (GAMM) with an autoregressive moving average (ARMA) structure to characterize the trend and autocorrelations within the time series. Moreover, as different portions of the gonad were analyzed for each individual, a random effect structure on jellyfish specimens’ ID was introduced to avoid individual pseudo replication problem [68]. The same statistical structure was used to analyzed variation in oocytes number and diameter with jellyfish bell diameter. The validation of the used models was based on the analysis of the normality of the residuals, on the absence of trends in the residuals, and on the inspection of the correlograms, through the ACF function. All analyses were undertaken in R statistical software (R Core Team 2020) using the car [69], mgcv [70], and forecast [71] packages.
Results
Oogenesis and spermatogenesis
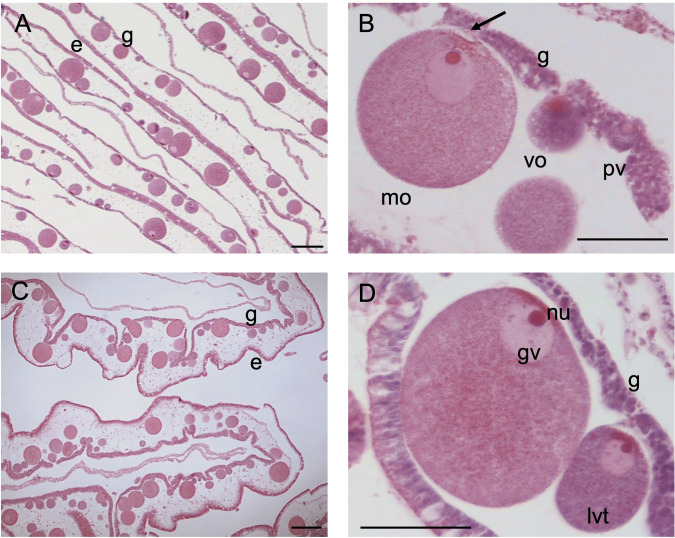
Female germ cells (oogonia) rise from the gastrodermis (Fig 1A). Oogenesis starts with the mitotic division of oogonia that will then develop in pre-vitellogenic oocytes (pv) (Fig 1B). Pre-vitellogenic oocytes are still embedded in the gastrodermis and are characterized by a round shape with a darker ooplasm in histological sections, due to the presence of free ribosomes causing basophilia. During development, oocytes increase their size by accumulating yolk in the ooplasm and are progressively displaced into the mesoglea (Fig 1A and 1C). Vitellogenic oocytes are characterized by a round shape, intermediate level of basophilia, some yolk granules and a conspicuous germinal vesicle (that face the gastrodermis) containing a nucleolus. When oocytes have reached ⁓60 μm they are almost entirely embedded in the mesoglea, but always maintaining direct contact with the gastrodermis. Late-vitellogenic oocytes are characterized by an increased yolk granules content that makes them lighter in histological sections and a large germinal vesicle (Fig 1D). Oocytes at the final stage of maturation are squeezed out in the genital sinus through a pit where they were attached through specialized gastrodermal cells termed trophocytes [72] (Fig 1B).
Fig 1. Histological section of a female gonad.
(A, C) Ovary (B, D) Development of oocytes. g gastrodermis, e endodermis, pv pre-vitellogenic oocyte embedded within gastrodermis, vo vitellogenic oocyte, lvt late-vitellogenic oocyte mo mature oocyte, arrow residual linkage with trophocytes (paraovular body), nu nucleolus, gv germinal vesicle. Scale bars: A, C = 100 μm; B, D = 50 μm.
Based on diameters, oocytes were grouped in 4 size classes: <20 μm (oocytes in pre-vitellogenesis), 20–60 μm (oocytes in vitellogenesis), 60–100 μm (late-vitellogenic oocytes), >100 μm (mature oocytes)
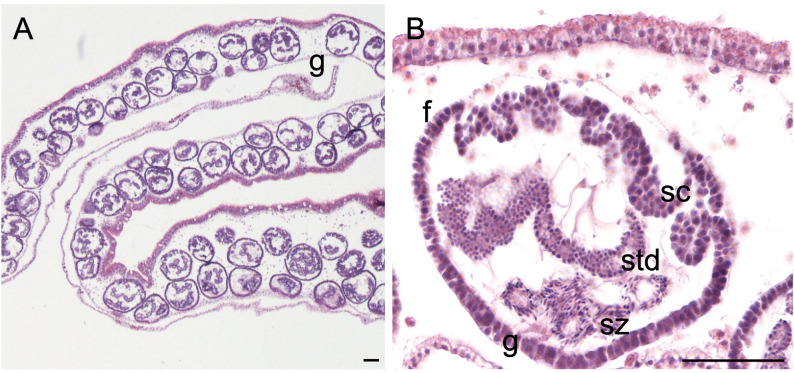
Spermatogenesis occurs inside follicles, which are distributed evenly in the male gonads (Fig 2A). The process starts from male germ cells (spermatogonia) that going through multiple mitosis give rise to the sperm follicles that have mostly a round/ovoidal shape. Spermatogonia will then develop into primary spermatocytes which will move inward in the follicle. Through meiosis, secondary spermatocytes will be formed, characterized by a high chromatin quantity. With the second meiosis, secondary spermatocytes will form spermatids that will then develop a flagellum and become spermatozoans (Fig 2B). At the final stage of spermatogenesis, the aboral part of the follicle (facing gastrodermis) will be filled by mature spermatozoan with a visible flagellum, packed in sperm bundles called spermatozeugmata [73] (Fig 2B). At this point, they will be released through a pore in the genital sinus and then in the water column. Once male has spawned, follicles are characterized by free space in the lumen and the beginning of a new spermatogenic cycle underline by the presence of new cellular divisions in spermatogonia (Fig 2).
Fig 2. Histological section of a male gonad.
(A) Structure of testis (B) Sperm follicle. f follicle, sc spermatocytes, std spermatids, sz spermatozoa, g gastrodermis. Sperm follicles ranged 93–115 μm during the sampling period. Scale bar = 50 μm.
Temporal pattern of reproduction
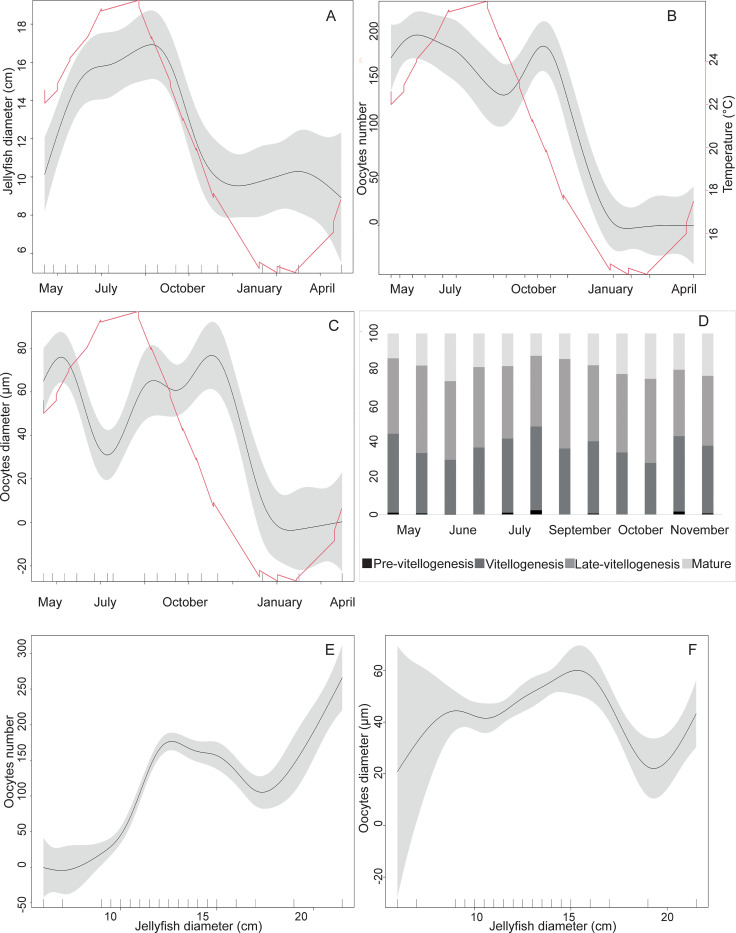
Sampled jellyfish ranged 6–21.5 cm in bell diameter, changing significantly (Table 1). The largest jellyfish were observed in summer months (> 16 cm diameter) while from December to April, the jellyfish diameter was consistently below 10 cm (Fig 3A).
Table 1. Statistical result table.
To the left of the tilde (~) symbol the response variable, and to the right the independent variable. "s" = natural cubic spline smoother; p = autocorrelation term (AR); q = moving average term (MA); med (medusa ID) and gon (gonad ID nested in med) used as random intercept. Jellyfish_D = jellyfish diameter.
| Formula | p | q | random part | Fvalue | pvalue |
|---|---|---|---|---|---|
| Oocyte Diameter ~ s(time) | 1 | 2 | med = ~1; gon = ~1 | 27.43 | <0.001 |
| Oocytes number ~ s(time) | 1 | 1 | med = ~1; gon = ~1 | 31.38 | <0.001 |
| Oocyte Diameter ~ s(Jellyfish_D) | 1 | 2 | med = ~1; gon = ~1 | 5.157 | <0.001 |
| Oocytes number ~ s(Jellyfish_D) | 1 | 1 | med = ~1; gon = ~1 | 21.35 | <0.001 |
| Jellyfish Diameter ~ s(time) | 1 | 1 | 10.52 | <0.001 |
Fig 3. Representation of the temporal pattern of reproduction.
(A) Jellyfish diameter (in cm) during the sampling period from May to April (B) Oocytes number per section area (mm2) during the sampling period showing two seasonal peaks (late spring and autumn) and seasonal temperatures (C) Oocytes diameter during the sampling period and seasonal temperatures (D) Percentage of each oocyte maturation stage during the whole sampling period. There are two columns for each sampling date (E) Oocytes number in relation to the jellyfish diameter (F) Oocytes diameter in relation to jellyfish diameter. Graphs A, B, C, E, F: The black line represents the mean value while grey areas represent the confidence interval. Temperature is plotted in red.
Female gonads contained oocytes from May to December, in all stage of development. Two oocytes abundance peaks can be observed over the year, the first in spring-summer (June) and the second one in autumn (October), with a maximal number of oocytes of 392 and 272 (per 15 mm2) respectively, while from January to April gonads were empty (Fig 3B). Oocytes ranged from pre-vitellogenic (<20 μm) to mature (>100 μm). Gonads were prevalently filled with pre-vitellogenic and vitellogenic oocytes in July and November (Fig 3C and 3D), and with mature oocytes in June and at the end of October (Fig 3D).
Smaller jellyfish (<10 cm) were found with empty gonads (Fig 3E, S1 Fig); when the bell diameter ranged between 10 and 15 cm the number of oocytes increased and subsequent stabilized, while larger specimens (>17 cm) showed the highest number of oocytes (>100/15mm2). The mean oocyte diameter increased when jellyfish bell diameter ranged between 10 and 15 cm, while when jellyfish diameter was between 15 and 20 cm, oocytes diameter dropped (down to 14 μm) (Fig 3F).
Changes in water temperature and salinity
During late spring (in May) sea water temperature was 22.3°C and increased to 26.35°C at the end of July and up to 26.65 early September. Autumn and winter were characterized by cooler temperatures: with 17.7°C at the end of November and 14.45° C in January. Temperature raised back to 16.25°C in spring months (April) of the following year (Fig 3B). Salinity ranged from 33.95 to 35.7 throughout the whole sampling period.
Discussion
In the present study, sexual reproduction and gonadal output of the Red Sea alien jellyfish Cassiopea andromeda collected in Sicily (Central Mediterranean Sea) were investigated through histological analysis. Specimens sampled in the Palermo harbor showed to be strictly gonochoric over the year, as previously reported from the Red Sea [59]. In contrast, C. andromeda medusae showed the potential for a transient simultaneous hermaphroditism in an artificial coastal lagoon in the Hawaii islands [73]. The triggering factor for a bisexual gonad maturation in the Hawaii medusae still remains to be clarified. However, given the limited success of sexual reproduction from hermaphroditic medusae, the gonochoric condition seems the most effective reproductive strategy for C. andromeda, as for most scyphozoans [57].
Both oocytes and sperm follicles were clearly detectable in the gonads of C. andromeda in mature specimens (size > 10 cm in this study). As in other rhizostome species (Lychnorhiza lucerna [74], Cotylorhiza tuberculata [75]), oogenesis starts in the gastrodermis, with developing oocytes nourished by gastrodermal trophocytes and, as they increase in size, progressively bulging into the mesoglea. This process is typical for rhizostomes, while absent in coronates except for the deep-sea species Peryphilla peryphilla [76, 77]. Fertilization in C. andromeda is thought to occur within the female gastrovascular cavity that will act as brooding chamber for embryos [59]. As in other rhizostomes, it is hypothesized that oocytes are released outside the ovarian epithelium passing through pits formed in the gastrodermis, possibly where they are in contact with trophocytes [74].
Spermatogenesis occurs inside sperm follicles, as reported for other jellyfish species [57]. No clear timeline of spermatogenesis has been reported for Cassiopea but in other rhizostome medusae, as Nemopilema nomurai, the entire maturation process (from spermatogonia to spermatozoans) seems to take place–at least—in a couple of days, when jellyfish are kept in a net (at 1m depth) [78]. Rhizostome jellyfish belonging to Kolpophorae (including Mastigiidae, Cepheidae and Cassiopeidae families) have been reported to differentiate sperms clustered by a secretion of lipid nature (spermatozeugmata) [74, 79]. Histological sections of C. andromeda from Palermo confirm the observation of mature spermatozoans within spermatozeugmata packages (Fig 2).
To analyze sexual differentiation in scyphomedusae, different approaches can be used [56, 80, 81]. Female specimens can be classified as mature or immature based on the presence of post-fertilization sexual products, i.e., as embryos or larvae; differently, the progress of gametogenesis can be investigated morphologically and biochemically, assessing the state of gamete differentiation [52]. In the second case, oocytes are classified by yolk density and diameter (oocytes size distribution) whereas the maturity of male sperm follicles can be assessed by the relative abundance of spermatogonia, spermatocytes, spermatids, and of functional sperms [56, 81]. In the present work, using histological analyses, the morphological approach was applied, with the definition of new size classes (pre-vitellogenic, early vitellogenic, late vitellogenic, and mature oocytes) for oocyte differentiation, specifically applicable to C. andromeda. Finally, the occurrence of fully developed gametes is regarded as a sexual maturity proxy, related to potentially imminent spawning events [74].
Both male and female specimens collected in Palermo harbor simultaneously possess gametes at different level of differentiation, from early vitellogenic to mature oocytes and from spermatids to mature sperms, suggesting an asynchronous reproduction strategy, as observed in other rhizostomes [53, 82]. From May to December female specimens always had oocytes at different stages of differentiation, including mature oocytes, but these were found with two seasonal peaks of abundance (late spring and autumn), probably linked to the imminence of bimodal spawning events, and suggesting an iteroparous reproduction (as in other outbreak-forming Mediterranean scyphozoans [56, 83]). The same pattern is observed in native Mediterranean jellyfish, such as Pelagia noctiluca, characterized by spring and autumn spawning events [56]. Therefore, C. andromeda can be considered a seasonal species in the Mediterranean, tuning its increasing growth and sexual reproduction with higher water temperature. In fact, larger specimens (diameter > 16 cm) were found in early September, when water reached the highest temperature (26.6°C) and jellyfish showed the highest number of oocytes in the gonads (> 20 oocytes per mm2 of gonad tissues), disclosing the achievement of full sexual maturity.
Accordingly, minimum jellyfish bell diameters were found in January. The presence of small jellyfish (< 10cm) from January to April suggests the recruitment of new cohort following a summer strobilation, that can be observed in Cassiopea at temperatures > 17–18°C [64] but with optimal condition ≥ 25°C [62]. This is in line with the reproductive strategy of other scyphozoans in the Mediterranean Sea: in late summer, the newly born jellyfish spread into the water column taking advantage of the renewed trophic conditions, after the stratification occurred in the warm period [84].
Oocyte diameter changed over time following the same trend. In particular, the late-spring and autumn peaks were characterized also by a higher proportion of mature oocytes, meaning that by the expected successive fertilization, polyp formation and strobilation a new cohort may arise, likely corresponding to that found during winter months. This is also supported by the observation that, in the winter period, gonads were empty. This observation is also paralleled by a drop in the jellyfish diameter (<10 cm) supporting the hypothesis we deal with new, still immature cohort. The two spring and autumn peaks do not present a difference in term of oocyte diameter, which represent a key factor for animal reproductive strategies [85]. Cassiopea andromeda from this study had smaller oocytes than reported by literature (140–170 μm; [59]) but larger oocytes than other rhizostomes as Rhizostoma pulmo (personal observations, up to 68 μm for mature oocytes). Oocyte diameter is known to be influenced by both biotic and abiotic factors; for instance, a direct positive relationship exist with temperature [86, 87]. In fact, C. andromeda oocytes were larger in the warm periods, when the higher temperatures boost oocyte differentiation. Another major driver of gonadal input is the food source availability [84] since sexual reproduction requires a large investment of energy for gonadal development and gamete differentiation [88]. As a mixotrophic species with a flexible nutritional mode, C. andromeda might be greatly favored when facing changing environmental conditions [42, 45, 50]. In spring, it may take advantage of available seston and organic matter through a classical heterotrophic feeding; in autumn, the jellyfish may use a reservoir of photosynthates produced throughout the high-irradiance summer period, coincident with the reduction of heterotrophic resources. The harbor of Palermo, as other coastal confined habitats in the Mediterranean Sea (like the Salini lagoon in Malta, see [89]), seem to be ideal spots for C. andromeda establishment and persistence [46]. In fact, these coastal protected environments are characterized by nutrient-enriched, calm waters and a reduced number of predators, compared to the natural, more exposed coastal habitats. This condition may favor the growth and reproduction of C. andromeda, originally from the protected habitats of mangrove lagoons in the Indo-Pacific area [28]. The high reproductive potential of the upside-down jellyfish, coupled with, and seemingly sustained by, a high nutritional flexibility and photosynthetic plasticity [42], and wide eurythermal tolerance [45], may appoint C. andromeda as a potential winner in the current warming scenario of the Mediterranean Sea.
Supporting information
A gonads during the winter months B close up on gonads containing only germ cells. Scale bar = 50 μm.
(DOCX)
Temperature (°C) and salinity data during the sampling period.
(DOCX)
Acknowledgments
The Authors are grateful to dr. Patrizia Pagliara for her valuable advices on histological protocols, dr. Franco De Nuccio for technical suggestions on the use of the microtome use and for staining, and prof. Giuseppe Nicolardi for granting access to M.M. to the facilities of the Laboratory of Human Anatomy at the Università del Salento (Lecce).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Roy HE, Bacher S, Essl F, Adriaens T, Aldridge DC, Bishop JDD et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob Change Bio. 2009; 25(3): 1032–1048. doi: 10.1111/gcb.14527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuttelod A, García N, Abdul Malak D, Temple H, Katariya V. The Mediterranean: A biodiversity hotspot under threat. In: Vié J-C, Hilton-Taylor C, Stuart SN, editors. The 2008 review of the IUCN red list of threatened species: IUCN Gland, Switzerland. 2008. [Google Scholar]
- 3.Coll M, Piroddi C, Steenbeek J, Kaschner K, Lasram FBR, Aguzzi J, et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE. 2010; 5(8). doi: 10.1371/journal.pone.0011842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehring S. International shipping–a risk for aquatic biodiversity in Germany. Biological invasions–from ecology to control. Neobiota. 2005; 6: 125–143. [Google Scholar]
- 5.Hulme PE. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J Appl Ecol. 2009; 46(1): 10–18. doi: 10.1111/j.1365-2664.2008.01600.x [DOI] [Google Scholar]
- 6.Katsanevakis S, Zenetos A, Belchior C, Cardoso AC. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast Manag. 2013; 76: 64–74. doi: 10.1016/j.ocecoaman.2013.02.024 [DOI] [Google Scholar]
- 7.Galil BS, Boero F, Fraschetti S, Piraino S, Campbell ML, Hewitt CL, et al. The enlargement of the Suez Canal and introduction of non-indigenous species to the Mediterranean Sea. Limnol Oceanogr Bull. 2015; 24: 43–45. doi: 10.1002/lob.10036 [DOI] [Google Scholar]
- 8.Bianchi CN, Morri C. Global sea warming and “tropicalization” of the Mediterranean Sea: biogeographic and ecological aspects. Biogeographia–The Journal of Integrative Biogeography. 2003; 24. doi: 10.21426/b6110129 [DOI] [Google Scholar]
- 9.Zenetos A, Çinar ME, Crocetta F, Golani D, Rosso A, Servello, et al. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar Coast Shelf Sci. 2017; 191: 171–187. Academic Press. doi: 10.1016/j.ecss.2017.03.031 [DOI] [Google Scholar]
- 10.Zenetos A, Albano PG, López Garcia E, Stern N, Tsiamis K, Galanidi M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr Mar Sci. 2022; 23(1). doi: 10.12681/mms.29106 [DOI] [Google Scholar]
- 11.Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, et al. The population biology of invasive species. 2001. www.annualreviews.org [Google Scholar]
- 12.Molnar JL, Gamboa RL, Revenga C, Spalding MD. Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ. 2008; 6(9): 485–492. doi: 10.1890/070064 [DOI] [Google Scholar]
- 13.Vilà M, Basnou C, Pysek P, Josefsson M, Genovesi P, Gollasch S, et al. Hulme PE. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross taxa assessment. Front Ecol Environ. 2010; 8 (3): 135e144. [Google Scholar]
- 14.Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, et al. Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol. 2013; 28(1): 58–66. doi: 10.1016/j.tree.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 15.Boudouresque CF, Verlaque M. Nature conservation, Marine Protected Areas, sustainable development and the flow of invasive species to the Mediterranean Sea. Sci Rep Port-Cros natl Park. 2005; 21: 29–54. [Google Scholar]
- 16.Schlaepfer MA, Sax DF, Olden J. Del Valor de Conservación Potencial de Especies No Nativas. Biol Conserv. 2011; 25(3): 428–437. doi: 10.1111/j.1523-1739.2010.01646.x [DOI] [PubMed] [Google Scholar]
- 17.Gravili C, Rossi S. Who’s next? Non-indigenous cnidarian and ctenophoran species approaching to the italian waters. Water. 2021; 13: 8. doi: 10.3390/w13081062 [DOI] [Google Scholar]
- 18.Tsiamis K, Azzurro E, Bariche M, Çinar ME, Crocetta F, de Clerck O, et al. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquat Conserv: Mar Freshw Ecosyst. 2020; 30(4): 794–845. doi: 10.1002/aqc.3267 [DOI] [Google Scholar]
- 19.Graham WM, Bayha KM. Biological invasions by marine jellyfish. In: Nentwig W, editor. Ecological studies, Vol 193, biological invasions. Springer-Verlag, Berlin, 2007. pp 240–255. [Google Scholar]
- 20.Purcell JE, Uye SI, Lo WT. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar Ecol Prog Ser. 2007; 350: 153–174. doi: 10.3354/meps07093 [DOI] [Google Scholar]
- 21.Boero F, Bouillon J, Gravili C, Miglietta MP, Parsons T, Piraino S. Gelatinous plankton: Irregularities rule the world (sometimes). Mar Ecol Prog Ser. 2008; 356: 299–310. doi: 10.3354/meps07368 [DOI] [Google Scholar]
- 22.Boero F Review of jellyfish blooms in the Mediterranean and Black Sea. GFCM Studies and Reviews. 2013; 92: 53. [Google Scholar]
- 23.Brotz L, Cheung WWL, Kleisner K, Pakhomov E, Pauly D. Increasing jellyfish populations: Trends in Large Marine Ecosystems. Hydrobiologia. 2012; 690: 3–20. doi: 10.1007/s10750-012-1039-7 [DOI] [Google Scholar]
- 24.Purcell JE, Arai MN. Jellyfish Blooms: Ecological and Societal Importance. Springer science and business media. 2001. doi: 10.1007/978-94-010-0722-1 [DOI] [Google Scholar]
- 25.Purcell JE, Sturdevant MV. Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Mar Ecol Prog Ser. 2001; 210: 67–83. doi: 10.3354/meps210067 [DOI] [Google Scholar]
- 26.Milisenda G, Rosa S, Fuentes VL, Boero F, Guglielmo L, Purcell JE, et al. Jellyfish as Prey: Frequency of Predation and Selective Foraging of Boops boops (Vertebrata, Actinopterygii) on the Mauve Stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PLoS ONE. 2014; 9(4): e94600. doi: 10.1371/journal.pone.0094600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milisenda G, Rossi S, Vizzini S, Fuentes VL, Purcell JE, Tilves U, et al. Seasonal variability of diet and trophic level of the gelatinous predator Pelagia noctiluca (Scyphozoa). Sci Rep. 2018; 8(1). doi: 10.1038/s41598-018-30474-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland BS., Dawson MN, Crow GL, Hofmann DK. Global phylogeography of Cassiopea (Scyphozoa: Rhizostomeae): Molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar Biol. 2004; 145: 1119–1128. doi: 10.1007/s00227-004-1409-4 [DOI] [Google Scholar]
- 29.Morandini A.C., Stampar S.N., Maronna M.M., Da Silveira FL. All non-indigenous species were introduced recently? the case study of Cassiopea (Cnidaria: Scyphozoa) in Brazilian waters. J Mar Biol Assoc. United Kingdom. 2017; 97: 321–328. doi: 10.1017/S0025315416000400 [DOI] [Google Scholar]
- 30.Ohdera AH, Abrams MJ, Ames CL, Baker DM, Suescún-Bolívar LP, Collins AG, et al. Upside-down but headed in the right direction: Review of the highly versatile Cassiopea xamachana system. Front Ecol Evol. 2018; 6: 1–15. doi: 10.3389/fevo.2018.00035 [DOI] [Google Scholar]
- 31.Thé J, de Sousa Barroso Hortê, Mammone M, Viana M, Batista Melo CS, Mies M, et al. Aquaculture facilities promote populational stability throughout seasons and increase medusae size for the invasive jellyfish Cassiopea andromeda. Mar Environ Res. 2020; 162: 105398. doi: 10.1016/j.marenvres.2020.105161 [DOI] [PubMed] [Google Scholar]
- 32.Maas O (1903) Die Scyphomedusen der Siboga Expedition. Siboga Expedition. 1901. 11; 1–91, 12 pls. [Google Scholar]
- 33.Goy J, Lakkis S and Zeidane R. Les Meduses de la Mediterranee Orientale. Rapports et Procès-Verbaux des Réunions Conseil Internationale pour l’Exploration de la Mer. 1988; 31: 299. [Google Scholar]
- 34.Galil B, Spanier E, Ferguson W. The Scyphomedusae of the Mediterranen coast of Israel, including two Lessepsian migrants new to the Mediterranean. Zool Meded. 1990; 64: 95–105. [Google Scholar]
- 35.Özgür E, Öztürk B. Open accea population of the alien jellyfish, Cassiopea andromeda (Forsskål, 1775) (Cnidaria: Scyphozoa: Rhizostomea) in the Ölüdeniz Lagoon, Turkey. Aquat Invasions. 2008; 3: 423–428. doi: 10.3391/ai.2008.3.4.8 [DOI] [Google Scholar]
- 36.Schembri PJ, Deidun A, Vella PJ. First record of Cassiopea andromeda (Scyphozoa: Rhizostomeae: Cassiopeidae) from the central Mediterranean Sea. Mar Biodivers Rec. 2010; 3: 1–2. doi: 10.1017/s1755267209990625 [DOI] [Google Scholar]
- 37.Cillari T, Andaloro F, Castriota L. First documented record of Cassiopea andromeda (Cnidaria: Scyphozoa) in Italian waters. Cah Biol Mar. 2018; 59: 193–195. doi: 10.21411/CBM.A.1037AA [DOI] [Google Scholar]
- 38.Maggio T, Allegra A, Bosch-Belmar M, Cillari T, Cuttitta A, Falautano M et al. Molecular identity of the non-indigenous Cassiopea sp. from Palermo Harbour (central Mediterranean Sea). J Mar Biol Assoc. United Kingdom. 2019; 99: 1765–1773. doi: 10.1017/S0025315419000924 [DOI] [Google Scholar]
- 39.Schiariti A, Morandini AC, Jarms G, Von Glehn Paes R, Franke S, Mianzan H. Asexual reproduction strategies and blooming potential in Scyphozoa. Mar Ecol Prog Ser. 2014. 510; 241–253. doi: 10.3354/meps10798 [DOI] [Google Scholar]
- 40.Fitt WK, Kristin C. The role of temperature in survival of the polyp stage of the tropical rhizostome jellyfish Cassiopea xamachana. J Exp Mar Biol Ecol. 1998; 222(1–2): 79–91. [Google Scholar]
- 41.Stoner EW, Layman CA, Yeager LA, Hassett HM. Effects of anthropogenic disturbance on the abundance and size of epibenthic jellyfish Cassiopea spp. Mar Pollut Bull. 2001; 62: 1109–1114. doi: 10.1016/j.marpolbul.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 42.Mammone M, Ferrier-Pages C, Lavorano S, Rizzo L, Piraino S, Rossi S. High photosynthetic plasticity may reinforce invasiveness of upside-down zooxanthellate jellyfish in Mediterranean coastal waters. PloS ONE. 2021; 16: 1–17. doi: 10.1371/journal.pone.0248814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoner EW, Archer SK, Layman CA. Increased nutrient availability correlates with increased growth of the benthic jellyfish Cassiopea spp. Food Webs. 2022; 31: e00231. doi: 10.1016/j.fooweb.2022.e00231 [DOI] [Google Scholar]
- 44.Arai MN. Pelagic coelenterates and eutrophication: a review. Hydrobiologia. 2001; 69: 69e87. [Google Scholar]
- 45.Aljbour SM, Zimmer M, Kunzmann A. Cellular respiration, oxygen consumption, and trade-offs of the jellyfish Cassiopea sp. in response to temperature change. J. Sea Res. 2017; 128: 92–97. doi: 10.1016/j.seares.2017.08.006 [DOI] [Google Scholar]
- 46.Cillari T, Allegra A, Berto D, Bosch-Belmar M, Falautano M, Maggio T, et al. Snapshot of the Distribution and Biology of Alien Jellyfish Cassiopea andromeda (Forsskål, 1775) in a Mediterranean Touristic Harbour. Biology. 2022; 11(2). doi: 10.3390/biology11020319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoner EW, Yeager LA, Sweatman JL, Sebilian SS, Layman CA. Modification of a seagrass community by benthic jellyfish blooms and nutrient enrichment. J Exp Mar Bio Ecol. 2014; 461: 185–192. doi: 10.1016/j.jembe.2014.08.005 [DOI] [Google Scholar]
- 48.Todd BD, Thornhill DJ, Fitt WK. Patterns of inorganic phosphate uptake in Cassiopea xamachana: A bioindicator species. Mar Pollut Bull. 2006; 52: 515–521. doi: 10.1016/j.marpolbul.2005.09.044 [DOI] [PubMed] [Google Scholar]
- 49.Jantzen C, Wild C, Rasheed M, El-Zibdah M, Richter C. Enhanced pore-water nutrient fluxes by the upside-down jellyfish Cassiopea sp. in a Red Sea coral reef. Mar Ecol Progr Ser. 2010; 411: 117–125. doi: 10.3354/meps08623 [DOI] [Google Scholar]
- 50.Bayha KM, Graham WM. Nonindigenous Marine Jellyfish: Invasiveness, Invasibility, and Impacts. doi: 10.1007/978-94-007-7015-7 [DOI] [Google Scholar]
- 51.Schnedler-Meyer NA, Kiørboe T, Mariani P. Boom and Bust: Life History, Environmental Noise, and the (un)Predictability of Jellyfish Blooms. Front Mar Sci. 2018; 31. doi: 10.3389/fmars.2018.00257 [DOI] [Google Scholar]
- 52.Lucas CH. Population dynamics of Aurelia aurita (Scyphozoa) from an isolated brackish lake, with particular reference to sexual reproduction. J Plankton Res. 1996; 18: 987–1007. [Google Scholar]
- 53.Pitt KA, Kingsford MJ. Reproductive biology of the edible jellyfish Catostylus mosaicus (Rhizostomeae). Mar Biol. 2000; 137: 791–799. doi: 10.1007/s002270000399 [DOI] [Google Scholar]
- 54.Hubot N, Lucas CH, Piraino S. Environmental control of asexual reproduction and somatic growth of Aurelia spp. (Cnidaria, Scyphozoa) polyps from the Adriatic Sea. PLoS ONE. 2017; 12(6): e0178482. doi: 10.1371/journal.pone.0178482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucas CH, Reed AJ. Gonad morphology and gametogenesis in the deep-sea jellyfish Atolla wyvillei and Periphylla periphylla (Scyphozoa: Coronatae) collected from Cape Hatteras and the Gulf of Mexico. J Mar Biol Assoc. United Kingdom. 2010; 90: 1095–1104. doi: 10.1017/S0025315409000824 [DOI] [Google Scholar]
- 56.Milisenda G, Martinez-Quintana A, Fuentes VL, Bosch-Belmar M, Aglieri G, Boero F, et al. Reproductive and bloom patterns of Pelagia noctiluca in the Strait of Messina, Italy. Estuar Coast Shelf Sci. 2018; 201: 29–39. doi: 10.1016/j.ecss.2016.01.002 [DOI] [Google Scholar]
- 57.Arai MN. A Functional Biology of Scyphozoa. Chapman & Hall, London. 1997. pp 316. [Google Scholar]
- 58.Mayer AG. Medusae of the World: The Scyphomedusae. Carnegie Institution of Washington, Washington. 1910. pp 735. [Google Scholar]
- 59.Gohar HAF, Eisawy AM. The development of Cassiopea andromeda. Publ Mar Biol Stat. 1960; 11: 148–190. [Google Scholar]
- 60.Müller WA, Leitz T. Metamorphosis in the Cnidaria. Can J Zool. 2014; 80 (10). doi: 10.1139/z02-130 [DOI] [Google Scholar]
- 61.Bigelow RP. The anatomy and development of Cassiopea xamachana. Boston Society of Natural History. 1900; 751–785. [Google Scholar]
- 62.Hofmann DK, Neumann R, Henne K. Strobilation, budding and initiation of scyphistoma morphogenesis in the rhizostome Cassiopea andromeda (Cnidaria: Scyphozoa). Mar Biol. 1978; 47: 161–176. doi: 10.1007/BF00395637 [DOI] [Google Scholar]
- 63.Heins A, Glatzel T, Holst S. Revised descriptions of the nematocysts and the asexual reproduction modes of the scyphozoan jellyfish Cassiopea andromeda (Forskål, 1775). Zoomorphology. 2015; 134: 351–366. doi: 10.1007/s00435-015-0263-x [DOI] [Google Scholar]
- 64.Rahat M, Adar O. Effect of symbiotic zooxanthellae and temperature on budding and strobilation in Cassiopeia andromeda (Eschscholz). Biol Bull. 1980; 159: 394–401. doi: 10.2307/1541102 [DOI] [Google Scholar]
- 65.Hofmann DK, Honegger TG. Bud formation and metamorphosis in Cassiopea andromeda (Cnidaria-Scyphozoa)- a developmental and ultrastructural study. Mar Biol. 1990; 105: 509–518. doi: 10.1007/BF01316322 [DOI] [Google Scholar]
- 66.Neumann R. Bacterial induction of settlement and metamorphosis in theplanula larvae of Cassiopea andromeda (Cnidaria- Scyphozoa, Rhizostomeae). Mar Ecol Prog Ser. 1979; 1: 21–28. doi: 10.3354/meps001021 [DOI] [Google Scholar]
- 67.Szafranski KM, Gaudron SM, Duperron S. Direct evidence for maternal inheritance of bacterial symbionts in small deep-sea clams (Bivalvia: Vesicomyidae). Naturwissenschaften. 2014; 101(5): 373–383. doi: 10.1007/s00114-014-1165-3 [DOI] [PubMed] [Google Scholar]
- 68.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015; 67: 1–48. [Google Scholar]
- 69.Fox J, Weisberg S. An {R} Companion to Applied Regression, Third Edition. Thousand Oaks CA: Sage. 2019. https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [Google Scholar]
- 70.Wood SN. Generalized Additive Models: An Introduction with R (2nd edition). Chapman and Hall/CRC. 2017. [Google Scholar]
- 71.Hyndman R, Athanasopoulos G, Bergmeir C, Caceres G, Chhay L, O’Hara-Wild M, et al. Forecast: Forecasting functions for time series and linear models. R package version 8.16. 2022. https://pkg.robjhyndman.com/forecast/. [Google Scholar]
- 72.Eckclbarger KJ, Larson R. Ultrastructure of the ovary and oogenesis in the jellyfish Linuche unguiculata and Stomolophus meleagris, with a review of ovarian structure in the Scyphozoa. Mar Biol. 1992; 114. [Google Scholar]
- 73.Hofmann DK, Hadfield MG. Hermaphroditism, gonochorism, and asexual reproduction in Cassiopea sp.—an immigrant in the islands of hawaii. Invertebrate Reproduction and Development. 2002; 41(1–3): 215–221. doi: 10.1080/07924259.2002.9652754 [DOI] [Google Scholar]
- 74.Schiariti AÍ, Christiansen E, Morandini AC, da silveira FL, Giberto A, Mianzan HW. Reproductive biology of Lychnorhiza lucerna (Cnidaria: Scyphozoa: Rhizostomeae): Individual traits related to sexual reproduction. Mar Biol Res. 2012; 8(3): 255–264. doi: 10.1080/17451000.2011.616897 [DOI] [Google Scholar]
- 75.Kikinger R. Cotylorhiza tuberculata (Cnidaria: Scyphozoa)- life history of a stationary population. Mar Ecol. 1992; 13(4): 333–362. [Google Scholar]
- 76.Péron F, Lesueur CA. Tableau des caractères génériques et spécifiques de toutes les espèces de méduses connues jusqu’à ce jour. Annales du Muséum d’Histoire Naturelle. 1810; 325–366. [Google Scholar]
- 77.Tiemann H, Gerhard J. Organ-like gonads, complex oocyte formation, and long-term spawning in Periphylla periphylla (Cnidaria, Scyphozoa, Coronatae). Mar Biol. 2010; 157(3): 527–535. doi: 10.1007/s00227-009-1338-3 [DOI] [Google Scholar]
- 78.Hikeda H, Ohtsu K, Uye S. Fine structure, histochemistry, and morphogenesis during excystment of the podocysts of the giant Jellyfish “Nemopilema nomurai” (Scyphozoa, Rhizostomeae). Bio Bull. 2011; 221: 248–260. [DOI] [PubMed] [Google Scholar]
- 79.Tiseo GR. Gonadal structure, histochemistry and ultrastructural characterization of male gonad and sperm of jellyfish species (Cubozoa and Scyphozoa, Medusozoa, Cnidaria). 2016. [Google Scholar]
- 80.Toyokawa M, Shimizu A, Sugimoto K, Nishiuchi K, Yasuda T. Seasonal changes in oocyte size and maturity of the giant jellyfish, Nemopilema nomurai. Fish Sci. 2009; 76: 55–62. doi: 10.1007/s12562-009-0187-9 [DOI] [Google Scholar]
- 81.Avian M, Sandrini LR. Oocyte development in four species of scyphomedusa in the northern Adriatic Sea. Hydrobiologia. 1991; 216: 189–195. doi: 10.1007/BF00026461 [DOI] [Google Scholar]
- 82.Iguchi N. Reproduction of the giant jellyfish, Nemopilema nomurai (Scyphozoa: Rhizostomeae), in 2006–2008 as peripherally-transported populations. Ocean Sci J. 2010; 45(2): 129–138. [Google Scholar]
- 83.Canepa A, Fuentes V, Sabates A, Piraino S, Boero F, Gili J.-M. Pelagia noctiluca in the Mediterranean Sea. In: Pitt K.A., Lucas C.H., editors. Jellyfish Blooms. Springer, Netherlands. 2014; pp. 237e266. doi: 10.1007/978-94-007-7015-7_11 [DOI] [Google Scholar]
- 84.Rossi S, Gravili C, Milisenda G, Bosch-Belmar M, Vito D. de, Piraino S. Effects of global warming on reproduction and potential dispersal of Mediterranean Cnidarians. Eur Zool J. 2019; 86(1): 255–271. doi: 10.1080/24750263.2019.1631893 [DOI] [Google Scholar]
- 85.Olive P. Physiological adaptations and the concepts of optimal reproductive strategy and physiological constraints in marine invertebrates. Symposia of the society for experimental Biology. 1985. [PubMed] [Google Scholar]
- 86.Kokita T. Potential latitudinal variation in egg size and number of a geographically widespread reef fish, revealed by common-environment experiments. Mar Biol. 2003; 143: 593e601. doi: 10.1007/s00227-003-1104-x [DOI] [Google Scholar]
- 87.Laptikhovsky V. Latitudinal and bathymetric trends in egg size variation: a new look at Thorson’s and Rass’s rules. Mar Ecol. 2006; 27.1: 7–14. doi: 10.1111/j.1439-0485.2006.00077.x [DOI] [Google Scholar]
- 88.Rossi S, Coppari M, Viladrich N. Benthic-Pelagic Coupling: New Perspectives in the Animal Forests. In: Rossi S, Bramanti L, Gori A, Orejas C, editors. Marine Animal Forests: the ecology of benthic biodiversity hotspots. Springer, Germany. 2017; 855–886. [Google Scholar]
- 89.Deidun A, Gauci A, Sciberras A, Piraino S. Back with a bang–an unexpected massive bloom of Cassiopea andromeda (Forskaal, 1775) in the Maltese Islands, nine years after its first appearance. Bioinvasions Rec. 2018; 7: 399–404. doi: 10.3391/bir.2018.7.4.07 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A gonads during the winter months B close up on gonads containing only germ cells. Scale bar = 50 μm.
(DOCX)
Temperature (°C) and salinity data during the sampling period.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.