Abstract
Substance use disorder (SUD) is characterized, in part, by lack of control over drug seeking and taking. The prefrontal cortex (PFC) is highly involved in control of behavior and deficits in PFC structure and function have been demonstrated in clinical and preclinical studies of SUD. Of the various classes of drugs associated with the development of SUD, inhalants are among the least studied despite their widespread use among adolescents and children. In this work, we review what is currently known regarding the sites and mechanisms of action of inhalants with a focus on the volatile solvent toluene that is contained in a wide variety of legal and easily obtained products. We then describe how inhalants including toluene affect various behaviors with an emphasis on those associated with PFC function and how chronic use of inhalants alters brain structure and neuronal signaling. Findings from these studies highlight advances made in recent years that have expanded our understanding of the effects of inhalants on brain structure and reinforce the need for continued work in this field.
Keywords: Solvents, set-shifting, probabilistic discounting
1. Introduction
The term “inhalant” refers to any volatile, non-combusted substance that is inhaled to experience a euphoric high [1,2]. Human inhalant users commonly achieve this high by either “sniffing” fumes directly from a canister or concentrating the substance in a bag or cloth and inhaling or “huffing” the vapor (3000 – 15,000 ppm), several times over the course of 15 minutes to several hours [3]. Inhalants are chemically and pharmacologically diverse and include anesthetics, alkyl nitrites, nitrous oxide, and volatile organic solvents such as toluene (methylbenzene) [1,3,4] that is the main focus of this review. Whereas intoxicating illicit substances are often difficult or expensive to obtain, volatile organic solvents are found in many common and accessible household products (e.g. spray paints, cleaners, and adhesives). Despite the potential for harmful effects following acute or chronic use [1] of inhalants and the prevalence of their use among adolescents and children, only a handful of NIH funded grants are devoted to their study (NIH Reporter, https://reporter.nih.gov).
2. Inhalant Use: General Features
2.1. Epidemiology
The National Survey of Drug Use and Health reports that over 26 million Americans aged 12 and older have used inhalants (includes volatile solvents, gases and nitrites) at least once in their lives [5] with 2.4 million individuals reporting use in the past year [5]. This number is likely an underestimate as it does not account for delinquent and homeless individuals that are particularly susceptible to inhalant use. The highest percentage of inhalant users are adolescents aged 12–17 and unlike most other drugs, inhalant use declines with age [5]. Inhalant use in the United States peaked in the 1970’s but among adolescents has remained relatively constant over the period from 2015–2020 [5]. There are also sex-related differences in inhalant use (reviewed by Crossin and Arunogiri) [6]. For example, in American middle schools, girls have a higher lifetime prevalence than boys, but this is reversed by the end of high school [7]. In Europe, while lifetime prevalence in male adolescents has historically been higher than females, the opposite finding is reported in certain countries (e.g. Estonia, Croatia, Latvia) [8] although these gender specific differences are relatively small.
Inhalant use is a global phenomenon although usage patterns vary widely. Other well-developed nations including Canada, Japan and several Western European countries report comparable or lower lifetime rates of inhalant use compared to the United States [8–10]. A particular burden, however, is placed on impoverished or isolated communities [11]. For example, groups of young Native Americans have been found “bagging” gasoline in rural Alaska [12]. In India, 35% of homeless children reported huffing toluene-containing whitener [13] and 91% of children living on the streets of Upper Egypt have been reported to use inhalants [14]. Native populations in the United states [15,16], Australia [17] and eastern Slovakia [18] also report some of the highest rates of inhalant use. These findings extend to the most impoverished countries of Europe, as high lifetime prevalence has been reported in Croatia (25%), Slovenia (14%), Greece (13%), Estonia (13%), Georgia (12%) and Austria (10%) [8]. A similar trend is observed across the United States where some of the poorest states (Alabama, Arkansas, Mississippi, West Virginia) report the highest lifetime prevalence (all > 9.9%) of inhalant use [19]. An important sequela of inhalant use is a high lifetime prevalence of mood, personality or anxiety disorders with female inhalant users being more likely to develop multiple psychiatric disorders than males [20]. In addition, early onset of inhalant use is associated with an increased risk of developing a substance use disorder including that for alcohol, cannabis, nicotine and psychostimulants [21].
2.2. Pharmacokinetics of Toluene
Organic solvents are perhaps the most accessible inhalant as illustrated by U.S. Poison Control data which describes cases involving over 3000 different solvent containing products [22]. Despite the overall modest amount of basic neuroscience research on inhalants, there are a number of reports on toluene (methylbenzene) including those examining the toxicity of toluene-containing products like adhesives, paints and paint thinners [23–27] and those focused on industry workers exposed to chronic, low-level concentrations of vapor [28–33]. Following inhalation of toluene, the majority of vapor is exhaled unchanged. The rest enters the bloodstream through the alveoli and distributes through the body [34]. Blood concentration in rats reaches 60% of the peak concentration about 10 minutes following inhalation and declines to 30% about 40 minutes following inhalation [35]. Only about 3% of inhaled toluene reaches the brain [35] and it is mostly eliminated from the central nervous system 30 min following inhalation [36].
2.3. Pharmacodynamics of Toluene: Ion Channels
Toluene modulates the function of a diverse array of neuronal ion channels with effects ranging from inhibition to excitation to no effect (Figure 1) [3,37]. Toluene enhances the activity of recombinant and neuronal γ-aminobutyric acid (GABAA) receptors [38–40] as well as glycine receptors [38] and serotonin-gated 5HT3 receptors [41]. However, it inhibits nicotinic acetylcholine receptors [42], L-type neuronal calcium channels [43,44], gap junction connexin channels [45], and BK and GIRK potassium channels [46]. Toluene’s effect on ATP-gated P2X receptors is subtype-dependent as it potentiates P2X2, P2X4 and P2X2/X4 receptors while inhibiting currents of P2X3 receptors [47]. Finally, toluene inhibits skeletal (Nav1.4) and cardiac (Nav1.5) voltage-gated sodium channels [48,49] but has little direct effect on those expressed in neurons in the hippocampus or mPFC [39,40].
Figure 1.
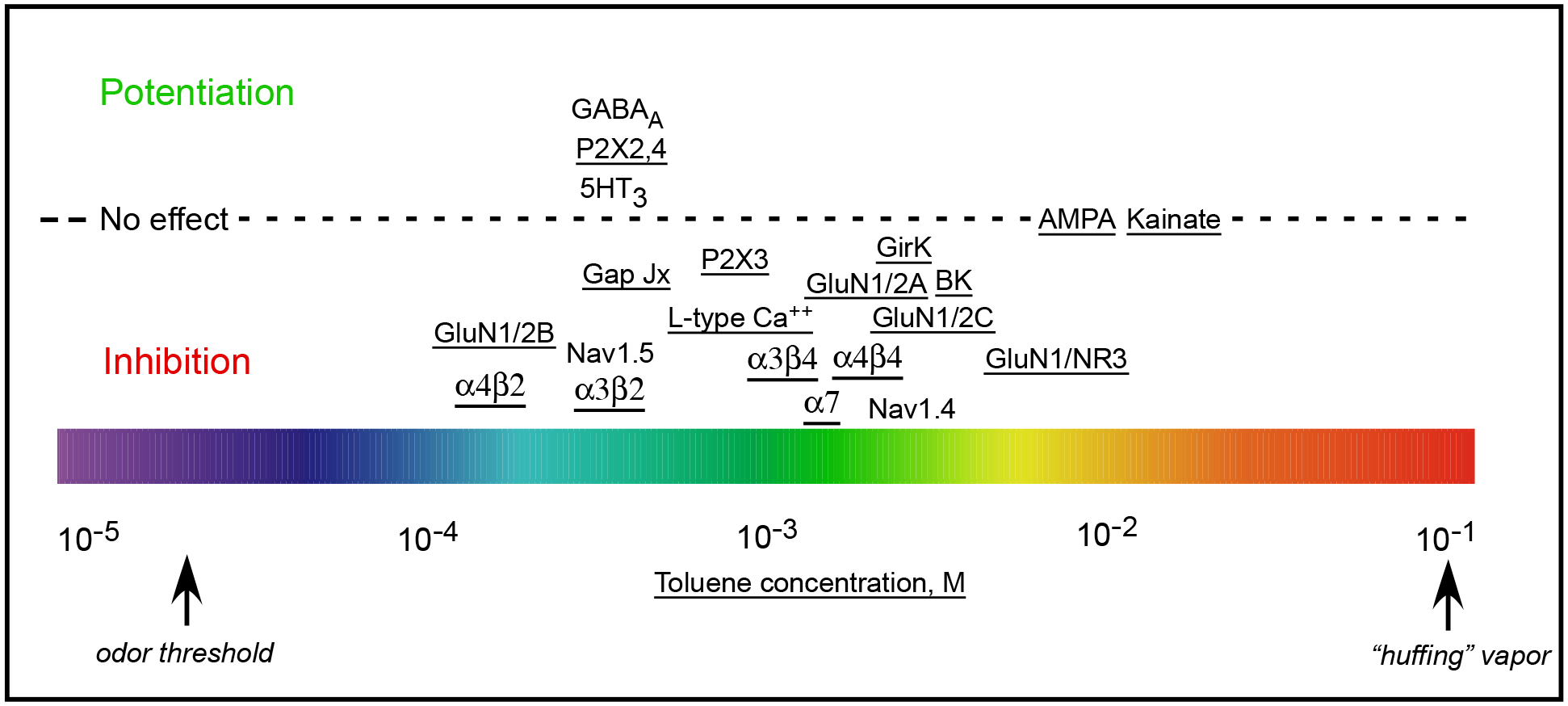
Summary of toluene’s effect on selected recombinant ion channels. Color bar at bottom shows toluene concentrations ranging from odor threshold (left) to that associated with voluntary inhalation (right). Position of the channel subtype along the color bar indicates degree of sensitivity to toluene while position relative to the dotted horizontal line indicates inhibition (below), potentiation (above) or no effect (on the line). Underlined channel indicates data from the author’s laboratory. Figure updated from Beckley and Woodward, 2013 and used with permission.
Toluene dose-dependently inhibits recombinant N-methyl-D-aspartate receptors (NMDAR) at concentrations that do not affect membrane integrity and these effects are rapid, reversible, and subunit selective, with GluN1/2B receptors being more sensitive than GluN1/2A or GluN1/2C [50]. Toluene also inhibits native NMDAR channels expressed by neurons in the rodent medial prefrontal cortex (mPFC), nucleus accumbens, and hippocampus [40,51,52]. Toluene’s site of action on NMDARs is not precisely known although transmembrane domain mutations that reduce the inhibitory actions of alcohol on NMDARs have no effect on toluene inhibition suggesting different sites of action for these compounds [53]. In addition, a recent study found that pretreatment of mice with the NMDAR agonist N,N-dimethylglycine prior to toluene exposure reduced toluene-induced memory impairments [54]. These protective effects were prevented by co-treatment with the glycine site antagonist 7-chlorokynurenic acid suggesting that the NMDAR glycine binding site may be a critical mediator of toluene’s action on NMDARs.
In contrast to NMDARs, toluene has little direct effect on recombinant AMPA (2-amino-3-(3-hydroxy5-methyl-isoxazol-4-yl) propionic acid or kainate receptors [50,51]. However, in mPFC NAc neurons, and BLA neurons innervated by the mPFC, toluene induces a slowly developing inhibition of AMPA-mediated excitatory post-synaptic currents (EPSCs) that persists following washout of toluene [40,55,56]. This effect resembled that of endocannabinoids (ECs) that reduce the release of glutamate by activating cannabinoid type I receptors (CB1R) on presynaptic terminals [57]. The toluene inhibition of AMPA EPSCs in mPFC and NAc neurons was blocked by a CB1R antagonist as well as a chelator of intracellular calcium suggesting that toluene induces the formation of ECs in these neurons [40,55]. Interestingly, in the NAc, the toluene-EC mediated inhibition of AMPA-mediated EPSCs was observed only in D2 medium spiny neurons [52] similar to that found for EC-mediated long-term depression of AMPA EPSCs [58].
3. Behavioral Effects of Inhalants
3.1. Human Studies
Intoxication occurs after inhaling concentrated vapors (3000 – 15,000 ppm) of inhalants several times over the course of 15 minutes to several hours [3]. Individuals report positive effects including euphoria, disinhibition, and excitement that lasts 15 – 60 minutes following use of the drug [59,60]. While the addictive potential of inhalants was first posited in the mid-1900s [61,62], a specific disorder related to inhalant use was underdiagnosed through the early 2000s, with one meta-analysis classifying only 8% of inhalant users as individuals with a disorder [63]. With the adoption of the DSM-5, inhalant misuse and dependence is now defined using similar diagnostic criteria as traditional drugs of misuse including symptoms such as strong desire to use the inhalant (craving), continued use despite negative physical or interpersonal problems and tolerance to the effects of the inhalant [64]. Comparison of two similarly sourced populations shows that approximately 46% of individuals who use inhalants now meet criteria for an inhalant use disorder [65], up from 35% in 2006 [66].
3.2. Animal Studies
3.2.1. Locomotor Effects
Inhalation of toluene vapor causes biphasic effects on goal-directed behavior that is typical of central nervous system (CNS) depressants: low concentrations of toluene increase and high concentrations decrease fixed-rate responding for a food reward in mice, rats and pigeons [67–69]. Toluene generates an inverted-U dose-response curve on locomotion like that produced by other CNS depressants [70–73]. Brief, repeated schedules of high-concentration toluene vapor exposures designed to model human consumption patterns also causes locomotor sensitization [74–76]. Behavioral sensitization has also been reported to vary across several strains of mice, with the ethanol-avoiding DBA/2J strain being particularly susceptible [77]. Toluene shows cross-sensitization to the locomotor effects of other drugs including diazepam and cocaine [78] and in a drug discrimination task, toluene substitutes for CNS depressants including ethanol and pentobarbital [79,80]. Similar to locomotor effects, toluene also cross-sensitizes with cocaine and substitutes for amphetamine in drug-discrimination studies [81,82]. While toluene can suppress CNS activity, these findings suggest that toluene is not strictly a depressant, supporting clinical observations and its mixed pharmacological profile in studies of human solvent users [59,60].
3.2.2. Conditioned Place Preference and Intracranial Self-Stimulation
The conditioned place preference (CPP) test is used with animals to measure the association of a rewarding experience with the context in which it was experienced. Like all other drugs of misuse, toluene generates this hedonic association in rodents [83,84]. In addition to CPP, intracranial self-stimulation (ICSS) can be used to assess the effect of drugs on reward-processing. To conduct this task, rodents are implanted with an electrode in the median forebrain bundle and then trained to operantly respond for different frequencies of activation. Stimulation of this tract is highly reinforcing, as it activates limbic structures involved in reward [85]. The response rate in the presence of a drug compared to baseline is used to measure how primed the reward neurocircuitry is compared to baseline with lower rates indicating that the animal needs less stimulation to feel reward. Animals treated with amphetamine, methamphetamine, MDMA, cocaine, nicotine, diazepam, or caffeine have lower ICSS response rates [85–87]. Surprisingly, initial reports showed that glue vapors containing 25% toluene increased ICSS threshold [88]. Studies using pure toluene vapor, however, have demonstrated the expected decreases in ICSS response rate, an effect that requires mGlu2/3 and GABAA activity [89–92].
3.2.3. Inhalant Self-Administration
In operant-based drug self-administration, animals learn to self-administer a drug (usually orally or intravenously via a chronically implanted catheter) for multiple hours per day over the course of several weeks. This is usually followed by extinction training where no cues or drug are presented followed by reinstatement or relapse trials to detect changes in drug seeking or craving. The development of an animal model of inhalant self-administration has lagged behind other drugs presumably due to the difficulty in controlling inhalant concentrations and the possible aversive effects associated with solvent odor. In early studies, a small number of monkeys fitted with a nasal catheter [93] or a custom inhalation helmet [67] were trained to self-administer lacquer thinner, nitrous oxide or toluene vapor. Response patterns were largely consistent with reward-based behavior although there was considerable variability between subjects. A subsequent study using a yoked control procedure in mice involved intravenous administration of a toluene-containing solution elicited via a nosepoke at the front of the chamber [94]. Mice whose injections were contingent upon the nosepoke made more responses than those who received the same dose in a non-contingent manner suggesting that responses were reward driven. More recently, our group developed a rat model of toluene self-administration in which a nosepoke in the active port delivered a brief exposure to toluene vapor while a response in the other port had no consequence [95]. During training, animals first underwent sessions where they were exposed to low vapor concentrations (~100 ppm) to acclimate them to the odor of toluene followed by sessions involving regularly timed non-contingent infusions accompanied by a cue light and sound. During subsequent free choice sessions, toluene-trained rats demonstrated a preference for the active nosepoke and achieved vapor concentrations that produce conditioned place preference (Figure 2). Responding for the active nosepoke showed a burst during initial extinction trials (no vapor or cues provided) followed by a gradual reduction in activity. Following extinction, responding for the active nosepoke was elicited by the introduction of cues previously associated with toluene vapor (cue-induced reinstatement) but not by a single re-exposure to the toluene vapor (drug-induced reinstatement). The failure of toluene to induce responding likely indicates that the dose (500 ppm) and duration (15 seconds) of the vapor cue were sub-optimal and that additional exposures should be tested. However, this finding could also reflect a differential loss of drug versus cue-induced reinstatement such as that reported for methamphetamine [96], albeit over a shorter time frame. In addition, prefrontal circuits underlying drug and cue-induced reinstatement appear to differ [97,98] and as reviewed below, toluene-induced alterations in mPFC neural activity are projection and layer specific [99] suggesting a possible mechanism underlying the dissociation between cue and drug-induced reinstatement following toluene self-administration.
Figure 2.
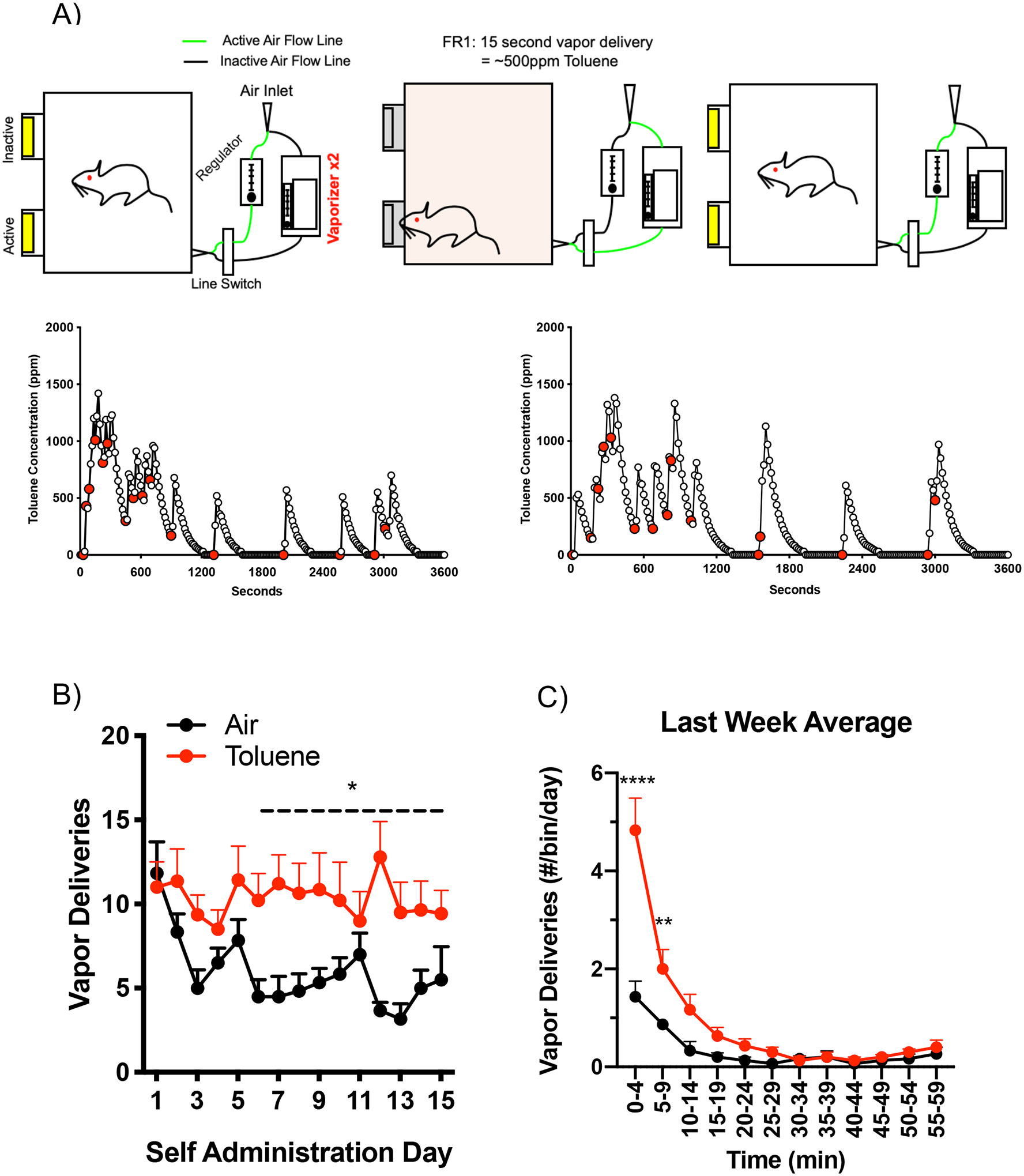
Self-administration of toluene vapor by rats. A) Schematic shows setup of vapor inhalation chamber with active and inactive nosepokes and vaporizers supplying toluene vapor. Entry into the active nosepoke delivers 15 sec of toluene vapor from vaporizers. Traces below show examples of toluene vapor self-administration by different animals with vapor concentration (ppm) on the y-axis and session time (sec) on the x-axis. Open circles indicate toluene vapor concentration during the session with red-filled circles indicating active nosepokes. B) Daily average of self-administration of toluene or air (mean ± SEM). C) Time course of self-administration of toluene or air during a 1 hr session. Data are mean (± SEM) from the last week of self-administration. Figure adapted from Braunscheidel et al., 2020 and used with permission.
4. The Prefrontal Cortex
4.1. Executive Function & Top-Down Control of Behavior
The prefrontal cortex (PFC), a specialized sub-division of the frontal lobe, is a hub for executive function and exerts control over a variety of sub-cortical areas [100,101]. In a top-down executive control model, the PFC engages in executive functions by maintaining patterns of activity that represent goals [102]. This model also suggests subdivisions of the PFC based not only on anatomy, but also subcortical (especially limbic) projections. Under this model, the rodent PFC is broken down into the following subdivisions, each of which have human homologues [100]: dorsomedial (anterior cingulate and precentral), ventromedial (prelimbic, infralimbic, medial orbitofrontal), lateral (anterior insula and lateral orbitofrontal), and ventral (ventral orbitofrontal and ventrolateral orbitofrontal). The ventromedial PFC is particularly relevant for toluene-induced alterations since this region appears to be involved in maladaptive goal-based decision making seen in substance use disorders [103,104].
Cognitive “top-down” control of executive function refers to the ability to regulate cognitive activity and behaviors needed to achieve a central goal, especially in the presence of intermediate steps, alternate rewards or distractions [105,106]. This process includes monitoring interactions with the world, evaluating the results of actions, and modifying behaviors to reach the intended goal. Thus, executive control includes functions such as working memory, temporal processing, planning, flexibility, and decision making. Pathological failures of cognitive control spans many psychiatric illnesses [107–109] including substance use disorders [110], where drug relapse is common despite known negative consequences and expressly wanting to quit.
4.2. Effect of addictive substances including inhalants on PFC function
4.2.1. Human Studies
Chronic intake of rewarding substances impairs PFC function consistent with the notion of weakened top-down control in individuals with an SUD. For instance, reduced PFC reactivity to negative reinforcers predicts marijuana and cocaine use in humans [111] and activating the PFC can reduce drug craving in humans [112]. Descending cortico-striatal pathways seem especially important [113,114] as human imaging studies have demonstrated that activity in the PFC is linked to striatal function, and is inversely associated with sensitivity to hedonic effects of psychostimulants [111,115]. Further, reduced expression or function of striatal D2Rs is linked to decreased activity in the dorsolateral PFC in human subjects with SUD [116]. Moreover, the function of the PFC in individuals with an SUD has been shown to predict clinical outcomes, with disrupted connectivity between PFC and striatal regions being a consistent finding among individuals addicted to various drug classes [117]. Recently, Jain and colleagues measured BOLD fMRI responses in response to drug-associated cues to compare craving among inhalant users and control subjects [118]. In inhalant users, activity in the dorsal striatum. a dopamine-rich region posited to be important for habit formation, was increased by inhalant cues. These results are an important finding in the pursuit of treatments for inhalant use in humans. For instance, it was reported that substitution therapy using lavender oils and perfume reduced cravings in inhalant abusers although these comparisons were only made against baseline cravings and thus do not control for a potential placebo effect [119].
4.2.2. Animal Studies
Studies in animals corroborate the importance of the PFC and its descending striatal projections in regulating drug reward related behaviors [103,120–122]. For instance, mPFC activity is reduced in cocaine seeking rodents, and optogenetic excitation of the mPFC during abstinence reduced drug seeking [123]. In line with human studies, specific frontostriatal pathways are involved in drug-seeking behaviors in rodents. For instance, prelimbic mPFC neurons that project to the nucleus accumbens core (PL-NAcc) promote, while infralimbic neurons that project to the nucleus accumbens shell (IL-NAcs) block psychostimulant seeking behavior [97,120,121,124]. A similar effect was observed for toluene as the expression of toluene CPP was blocked when IL-NAcs neurons were chemogenetically silenced [84]. However, these effects may be drug-specific, as reinstatement of heroin seeking was enhanced by activation of IL-NAcs neurons [125,126] and reinstatement of alcohol self-administration was facilitated by inactivation of PL-NAcc neurons [127]. Nonetheless, these data strongly support the involvement of PFC-NAc circuitry in regulating substance use-related behaviors in rodents, although the specific sub-circuitry does not completely generalize among different rewarding substances.
At the cellular level, the effect of toluene vapor inhalation on mPFC-NAc signaling is circuit specific. Following a single binge-like exposure to toluene, current-evoked firing of deep layer PL-NAcs neurons is reduced while that of deep layer PL-NAcc neurons is enhanced [99]. These changes in excitability were transient and age-dependent as they were present 24h following the exposure but not after 7 days and were observed when animals were exposed during mid-late adolescence (PN41–44 days) but not during adulthood (PN97–100 days). The mPFC also impacts reward-related circuitry as pharmacological enhancement of mPFC activity via intra-mPFC infusion of picrotoxin blocked the toluene-induced increase in the AMPA/NMDA ratio of VTA DA neurons that project to the NAc [55]. Conversely, pharmacologically inhibiting the mPFC allowed a previously sub-threshold dose of toluene vapor to increase the AMPA/NMDA ratio of NAc-projecting VTA DA neurons [55]. Interestingly, this effect was circuit selective as toluene inhalation had no effect on the AMPA/NMDA ratio of VTA DA neurons that project to the prelimbic mPFC. These studies provide strong evidence that toluene alters the function of specific mPFC connected networks that may underlie maladaptive decision making in inhalant users.
5. Inhalant-induced Cognitive Dysfunction
5.1. Human Studies-Behavior
The sequelae of inhalant misuse extend beyond changes in reward value processing and cue reactivity. Early studies on recreational inhalant users mention impairments in memory, attention and judgements compared with control and even polydrug users [27,128]. Impaired processing speeds can even be detected after a single drug experience [129]. Other studies have demonstrated reductions in IQ, working memory, behavioral flexibility, attention, and response inhibition in inhalant users [130–132]. Some toluene-induced impairments in cognition appear to recover (e.g. paired-associations, response inhibition) while others (e.g. visual motor speed, learning and memory, and executive control) can persist despite a protracted period of drug abstinence [133,134].
Lasting deficits may reflect damage to white matter fiber tracts tissue that are particularly susceptible to toluene due to their high lipid content and toluene’s high lipophilicity. Toluene damages the corpus callosum and other areas of cortical white matter essential for executive function [135–138] with the degree of white matter injury correlated with the severity of cognitive impairment [139]. In humans, prolonged inhalant use can also produce toluene leukoencephalopathy, a form of dementia [27,135,137,139,140].
5.2. Animal Studies
5.2.1. Learning and Memory
In line with human studies, chronic exposure of rodents to toluene also causes a wide range of impairments in cognitive behaviors including reduced novel object recognition, spatial learning deficits, and altered inhibitory avoidance when tested following little or no drug abstinence [64,75,141,142]. Toluene-treated rats also showed insensitivity to contingency degradation as responding in a food-reward task was similar between degraded and non-degraded levers [143]. Repeated exposures to toluene increases the expression of CYP2E1 and promotes production of reactive oxygen species [144]. Subsequent astrogliosis and inflammation, which is also evident following acute toluene exposure, could lead to cell death and dysfunction in the prefrontal cortex and other areas and contribute to learning and memory deficits [141,145,146]. In fact, Cruz et al. 2020 found that co-treatment of rats with toluene and the anti-inflammatory compound minocycline across several days of treatment abolished toluene-induced deficits in temporal order memory and passive avoidance. Minocycline also prevented glial activation, and partially rescued toluene-induced changes in NLRP3 and IL-1β in the prelimbic cortex. These findings suggest that persistent deficits in cognitive function following chronic toluene exposure may involve both proinflammatory actions as well as effects on ion channel function and neurotransmitter signaling.
5.2.2. Delay Discounting and Strategy Set-Shifting
Significant increases in delay discounting, where animals are tasked with choosing to wait progressively longer periods of time for a large food reward, have also been observed after a history of toluene inhalation [143]. This finding is in contrast to other rewarding drugs that normally cause a leftward shift in delay discounting indicating that rodents are unwilling or unable to wait longer periods of time for a large reward [147]. A similar finding is observed in humans [148,149] and is usually interpreted as increased impulsivity. The rightward shift in delay discounting produced by toluene was accompanied by increased inhibitory GABAAα1 protein in the prelimbic mPFC. Rightward shifts in delayed discounting have also been observed following pharmacological lesioning of the mPFC [150,151] and recent reports show that subsets of PL-mPFC neurons encode reward value during this task [152]. Interestingly, Bowen and colleagues previously reported that performance in a waiting-for-reward task was altered in mice chronically exposed to toluene vapor. In this task of inhibitory control, animals can earn “free” food rewards by waiting progressively longer periods of time after making a lever press. Mice chronically treated with toluene vapor earned significantly fewer free rewards than control mice and this effect persisted for several weeks following cessation of the toluene exposure [153].
When strategy set-shifting was tested, rats with a prior history of adolescent toluene exposure required fewer trials than controls to achieve criteria using a between-session protocol but not during a within-session test [154]. This was hypothesized to reflect a toluene-induced reduction in the ability to access the previously learned rule possibly via effects on the hippocampus. These effects may be selective for different forms of memory as a similar adolescent toluene exposure protocol had no effect on spatial memory learning in adults [155]. Regarding effects of toluene on motivation to obtain a reward in an operant model, Furlong et. al (2016) noted an increase in progressive ratio breakpoint in animals with a history of toluene inhalation [143] while other studies have reported no difference in this measure [154,155].
5.2.3. Risky Choice Decision Making
Additional studies have examined the effects of toluene vapor on decision making using a paradigm that incorporates an element of risk or uncertainty. In one example, food-restricted rats undergo sessions where one lever predicts a small but always delivered reward (safe choice) while the other lever yields a large reward under an increasing or decreasing probabilistic schedule (risky choice). Animals typically shift their preference towards the risky choice as the odds of receiving it increase (and vice versa). Work by Floresco and colleagues demonstrated that flexible changes in choice behavior requires areas of the prefrontal and orbitofrontal cortex and downstream targets including the BLA and NAc [156,157]. In a study from the author’s lab using the probabilistic risk task, rats briefly exposed to toluene vapor 30 min before the test session showed a dose-dependent rightward shift in the preference for the risky lever as reward probabilities decreased as compared to air controls. This effect was observed in both males and females and was accompanied by changes in choice strategy reflected as a decrease in lose-shift behavior (Figure 3) [158]. While these findings initially suggested that toluene vapor increases risky decision making, toluene-exposed rats were slower to shift their preference towards the larger reward when reward probability went from low to high. Together, these findings are more consistent with a loss of behavioral flexibility manifested as impaired updating of the probability of obtaining the large reward as the task progressed. A similar finding was reported in rats administered amphetamine [159,160] prior to testing suggesting the involvement of altered dopaminergic signaling in the mPFC and connected structures such as the BLA [161]. The toluene-induced impairment of flexible decision making reported by Braunscheidel et al. was accompanied by alterations in mPFC neural activity as measured by in vivo fiber photometry. In air-exposed animals, mPFC GCaMP6f calcium signals during reward consumption scaled with the size of the reward while those in toluene-exposed animals were similar for small or large rewards (Figure 3). This might reflect a toluene-induced deficit in the ability of mPFC neurons to properly assign value to a new reward that could then affect subsequent decision making. Notably, no lasting changes in probabilistic decision making were observed in rats treated with toluene during adolescence and then tested in adulthood in the absence of drug. In these adult animals, an acute exposure just prior to the test produced similar effects on risk behavior in toluene naïve and adolescent-exposed animals [158].
Figure 3.
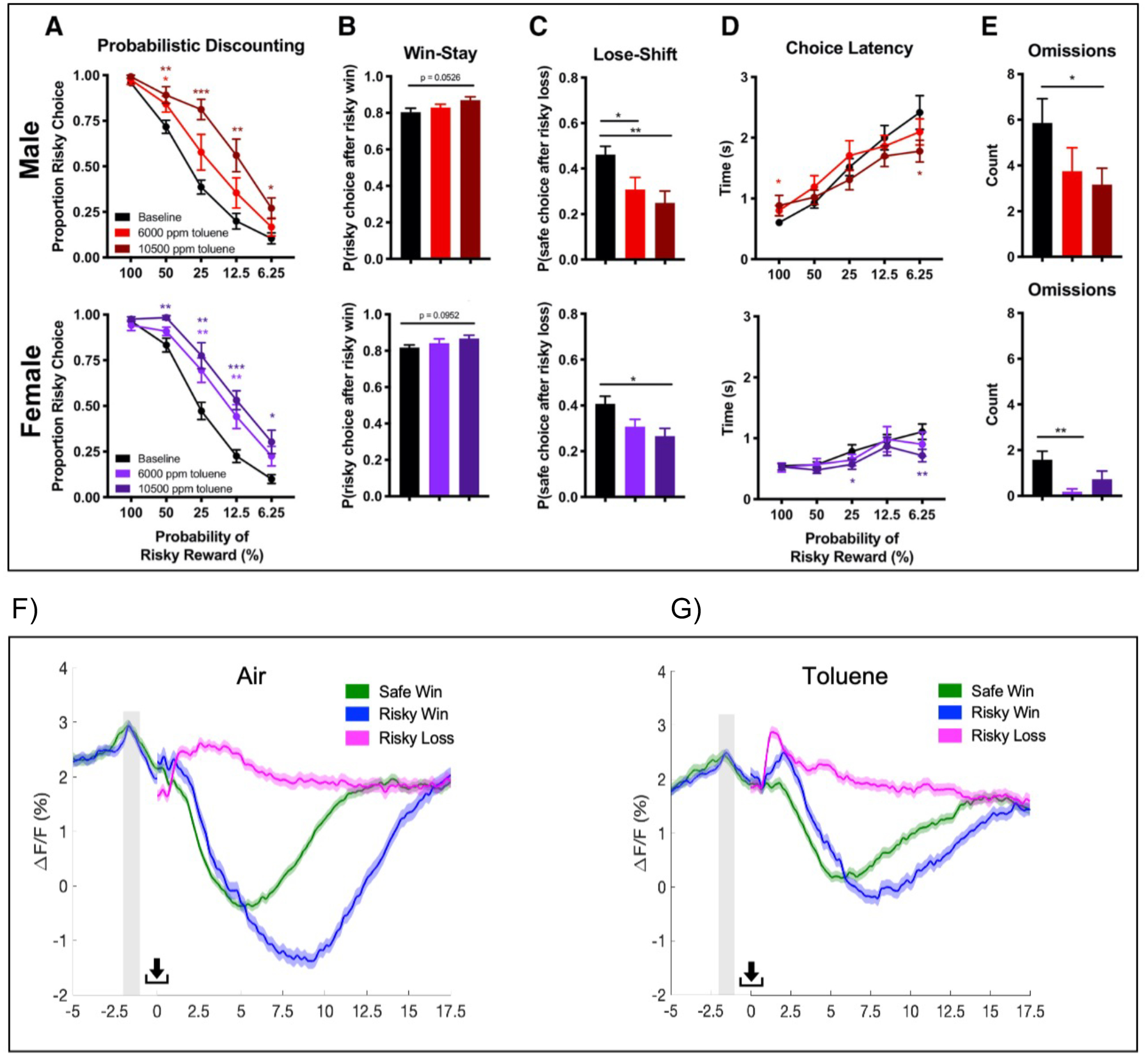
Effects of toluene vapor on decision making by male and female rats during a probabilistic risk task. Top panel show performance of males (top) and females (bottom) on (A) proportion of risky choice during descending odds protocol; (B) proportion of risky choice after risky win (“Win-Stay”); (C) proportion of safe choice after risky loss (“Lose-Shift”); (D) choice latency after cue; (E) omissions. Data are mean ± SEM. Changes in mPFC neuronal calcium measured by GCaMP6f fiber photometry during probabilistic risk task in air (F) and toluene (G) exposed rats. Note increase in signal during deliberation phase just prior to choice selection and change in activity during reward consumption. Unlike controls, toluene exposed animals showed no discrimination between a safe win (green trace) and a risky win (blue trace). Figure adapted from Braunscheidel et al., 2019 and used with persmission.
5.2.4. Decision Making: Role of Endocannabinoids
As mentioned earlier, slice electrophysiology studies showed that toluene induces the formation of endocannabinoids to produce a CB1 receptor-dependent long-term depression of glutamatergic signaling in mPFC pyramidal neurons, D2 medium spiny neurons in the nucleus accumbens and BLA neurons innervated by the mPFC [40,52,56]. Given the role of these areas in decision making, Braunscheidel et al. investigated whether CB1Rs including those in the mPFC are involved in risk/reward decision making and if they mediate toluene-induced impairment of flexible decision making. Systemic administration of the CB1R antagonist AM281 had no effect on choice behavior in air-exposed rats and did not prevent the toluene-induced shift in risky choice preference [162]. AM281 did reduce the likelihood of an animal choosing the risky lever following a risky win (termed win-stay behavior) but this was only observed in air-treated animals and there was no effect on lose-shift behavior in either group. Intra-mPFC administration of the CB1R antagonist AM251 also did not affect choice behavior in air exposed animals and did not prevent the shift in risk preference following toluene vapor exposure. A similar lack of effect on choice behavior was observed following intra-mPFC infusion of the CB1R agonist WIN55,212–2 in control animals. However, other studies have reported that CB1R agonists can affect reversal learning [163] and memory recall [164] suggesting that effects of cannabinoid based signaling on behavior are task-dependent. While findings from the Braunscheidel et al. study did not support the hypothesis that release of ECs mediated toluene’s effects on risk behavior, other factors may be involved. For example, the slice electrophysiology studies illustrating toluene/EC long-term depression of AMPA receptor signaling were done with adolescent rats while animals used in the behavioral risk experiments were of adult age before testing due to the extensive training required to learn the task. Whether toluene-induced release of ECs is age-dependent is not currently known but other effects of toluene including locomotor stimulation and modulation of mPFC neural activity have been shown to more pronounced in adolescent animals than adults [75,99]. More studies are needed to establish whether age or other factors are important in linking toluene/endocannabinoid long-term depression of excitatory signaling and changes in cognitive and/or reward-based behavior.
5.2.5. Long-term Effects of Inhalant Use
Reports of persistent behavioral effects following exposure to inhalants are mixed: deficits in learning and memory have been noted, but more complicated tasks such as outcome devaluation and Pavlovian-to-instrumental transfer do not appear to be significantly altered [143,154,155]. However, rats treated chronically with toluene vapor during adolescence and tested in adulthood required more trials to achieve criteria during initial simple operant conditioning [154,155]. This deficit was transient however, and performance of toluene-treated animals matched those of control animals in subsequent phases of training.
In contrast with results from human studies, leukoencephalopathy was not observed in animals that experienced a near lifetime exposure to toluene vapor [165]. However, treating rodents with chronic, intermittent toluene vapor binges does result in region-specific white matter abnormalities, with deficits in the anterior commissure noted following a month of exposure [166]. Rearing (vertical plane exploration) deficits were also identified, but they preceded any changes in white matter abnormalities and persisted following white matter recovery suggesting separate underlying mechanisms. Rearing more likely reflects either fear and anxiety or non-specific increases in motivation and arousal and not higher ordered executive function [167]. As such, and in agreement with the general lack of an effect on complicated decision making in other preclinical studies, the corpus callosum was not affected in this study while other white matter deficits showed recovery following 8 weeks of drug abstinence [166]. At the molecular level, chronic exposure of adolescent rats to toluene vapor produces changes in expression of NMDAR subunits (GluN2A, GluN2B) and PSD95 in the hippocampus that persist well after the last exposure [143,168]. Toluene-induced changes in GABA receptor expression have also been noted although the direction of these changes appears to be region-dependent [143,168].
6. Concluding Remarks
Much of our understanding on the pharmacology and neurobiology of volatile organic solvents comes from studies of toluene, a methylated form of benzene found in a variety of paints, paint thinners, and glues. While these studies have identified potential sites and mechanisms of action for inhalants on brain reward and control circuitry, inhalants remain an understudied class of drugs despite their worldwide use and potential for harmful use especially among children and adolescents. Maladaptive decision making in substance use disorders is driven in part by drug-induced changes in the prefrontal cortex, a key brain area involved in top-down control over complex decision making. The studies cited in this review highlight an emerging literature on the effects of toluene on cognitive function and the physiology of the prefrontal cortex and its down-stream circuitry. Understanding how toluene vapor inhalation produces these changes is critically important for developing targeted treatment strategies for individuals diagnosed with an inhalant use disorder.
Acknowledgements:
This work was supported by grants from the National Institutes of Health (5R01DA013951 to JJW; F31DA045485 and F32DA054802 to KMB). Portions of this work appear in an academic dissertation authored by KMB.
References
- 1.Balster RL, The Pharmacology of Inhalants, in Principles of Addiction Medicine, Miller SA, Fiellin DA, Rosenthal RN, Saitz R, Editor. 2019, Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia. p. 263–272. [Google Scholar]
- 2.Johnston LD, et al. , Monitoring the Future National Survey Results on Drug Use 1975–2020: Overview, Key Findings on Adolescent Drug Use. 2021, University of Michigan: Ann Arbor: Institute for Social Research. p. 136. [Google Scholar]
- 3.Cruz SL and Bowen SE, The last two decades on preclinical and clinical research on inhalant effects. Neurotoxicol Teratol. 87 (2021) 106999 DOI: 10.1016/j.ntt.2021.106999. [DOI] [PubMed] [Google Scholar]
- 4.Beckley JT and Woodward JJ, Volatile solvents as drugs of abuse: focus on the cortico-mesolimbic circuitry. Neuropsychopharmacology. 38 (2013) 2555–67 DOI: 10.1038/npp.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SAMHSA Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 6.Crossin R and Arunogiri S, Harms associated with inhalant misuse in adolescent females - a review of the pre-clinical and clinical evidence. Drug Alcohol Depend. 216 (2020) 108232 DOI: 10.1016/j.drugalcdep.2020.108232. [DOI] [PubMed] [Google Scholar]
- 7.Miech RA, et al. , Monitoring the Future National Survey Results on Drug Use 1975–2021: Volume I: Secondary School Students. 2021, University of Michigan: Ann Arbor: Institute for Social Research. [Google Scholar]
- 8.ESPAD, ESPAD Report 2015: Results from the European School Survey Project on Alcohol and Other Drugs. 2016, Publications Office of the European Union: Luxembourg. [Google Scholar]
- 9.Kikuchi A and Wada K, Factors associated with volatile solvent use among junior high school students in Kanto, Japan. Addiction. 98 (2003) 771–84 DOI: [DOI] [PubMed] [Google Scholar]
- 10.Adlaf E, Begin P, and Sawka E, Canadian Addiction Survey (CAS): A national survey of Canadian’s use of alcohol and other drugs: Prevalence of use and related harms: Detailed report. 2004: Ottawa: Canadian Centre on Substance Abuse. [Google Scholar]
- 11.Perron BE and Howard MO, Adolescent inhalant use, abuse and dependence. Addiction. 104 (2009) 1185–92 DOI: 10.1111/j.1360-0443.2009.02557.x. [DOI] [PubMed] [Google Scholar]
- 12.Eggertson L, Children as young as six sniffing gas in Pikangikum. CMAJ. 186 (2014) 171–2 DOI: 10.1503/cmaj.109-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Praveen D, et al. , Determinants of inhalant (whitener) use among street children in a South Indian city. Subst Use Misuse. 47 (2012) 1143–50 DOI: 10.3109/10826084.2011.644844. [DOI] [PubMed] [Google Scholar]
- 14.Elkoussi A and Bakheet S, Volatile substance misuse among street children in Upper Egypt. Subst Use Misuse. 46 Suppl 1 (2011) 35–9 DOI: 10.3109/10826084.2011.580202. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman A, Gasoline sniffing among children in a Pueblo Indian village. Pediatrics. 51 (1973) 1060–4 DOI: [PubMed] [Google Scholar]
- 16.Beauvais F, et al. , Inhalant abuse among American Indian, Mexican American, and non-Latino white adolescents. Am J Drug Alcohol Abuse. 28 (2002) 171–87 DOI: 10.1081/ada-120001287. [DOI] [PubMed] [Google Scholar]
- 17.Cairney S, et al. , The neurobehavioural consequences of petrol (gasoline) sniffing. Neurosci Biobehav Rev. 26 (2002) 81–9 DOI: [DOI] [PubMed] [Google Scholar]
- 18.Vazan P, et al. , Chronic toluene misuse among Roma youth in Eastern Slovakia. Subst Use Misuse. 46 Suppl 1 (2011) 57–61 DOI: 10.3109/10826084.2011.580213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kann L, et al. , Youth Risk Behavior Surveillance - United States, 2015. MMWR Surveill Summ. 65 (2016) 1–174 DOI: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- 20.Wu LT and Howard MO, Psychiatric disorders in inhalant users: results from The National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 88 (2007) 146–55 DOI: 10.1016/j.drugalcdep.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu LT, Howard MO, and Pilowsky DJ, Substance use disorders among inhalant users: results from the National Epidemiologic Survey on alcohol and related conditions. Addict Behav. 33 (2008) 968–73 DOI: 10.1016/j.addbeh.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsolek MR, White NC, and Litovitz TL, Inhalant abuse: monitoring trends by using poison control data, 1993–2008. Pediatrics. 125 (2010) 906–13 DOI: 10.1542/peds.2009-2080. [DOI] [PubMed] [Google Scholar]
- 23.Malm G and Lying-Tunell U, Cerebellar dysfunction related to toluene sniffing. Acta Neurol Scand. 62 (1980) 188–90 DOI: 10.1111/j.1600-0404.1980.tb03021.x. [DOI] [PubMed] [Google Scholar]
- 24.King MD, et al. , Solvent encephalopathy. Br Med J (Clin Res Ed). 283 (1981) 663–5 DOI: 10.1136/bmj.283.6292.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streicher HZ, et al. , Syndromes of toluene sniffing in adults. Ann Intern Med. 94 (1981) 758–62 DOI: 10.7326/0003-4819-94-6-758. [DOI] [PubMed] [Google Scholar]
- 26.Fornazzari L, et al. , Cerebellar, cortical and functional impairment in toluene abusers. Acta Neurol Scand. 67 (1983) 319–29 DOI: 10.1111/j.1600-0404.1983.tb03149.x. [DOI] [PubMed] [Google Scholar]
- 27.Hormes JT, Filley CM, and Rosenberg NL, Neurologic sequelae of chronic solvent vapor abuse. Neurology. 36 (1986) 698–702 DOI: 10.1212/wnl.36.5.698. [DOI] [PubMed] [Google Scholar]
- 28.Apostoli P, et al. , Biomonitoring of occupational toluene exposure. Int Arch Occup Environ Health. 50 (1982) 153–68 DOI: 10.1007/BF00378077. [DOI] [PubMed] [Google Scholar]
- 29.Foo SC, et al. , Biological monitoring for occupational exposure to toluene. Am Ind Hyg Assoc J. 52 (1991) 212–7 DOI: 10.1080/15298669191364613. [DOI] [PubMed] [Google Scholar]
- 30.Morata TC, et al. , Effects of occupational exposure to organic solvents and noise on hearing. Scand J Work Environ Health. 19 (1993) 245–54 DOI: 10.5271/sjweh.1477. [DOI] [PubMed] [Google Scholar]
- 31.Neubert D, et al. , Multicenter field trial on possible health effects of toluene. I. Toluene body burdens in workers of the rotogravure industry. Toxicology. 168 (2001) 139–57 DOI: 10.1016/s0300-483x(01)00406-1. [DOI] [PubMed] [Google Scholar]
- 32.Neubert D, et al. , Multicenter field trial on possible health effects of toluene. II. Cross-sectional evaluation of acute low-level exposure. Toxicology. 168 (2001) 159–83 DOI: 10.1016/s0300-483x(01)00407-3. [DOI] [PubMed] [Google Scholar]
- 33.Guo D, et al. , Alternations in neural oscillation related to attention network reveal influence of indoor toluene on cognition at low concentration. Indoor Air. 32 (2022) e13067 DOI: 10.1111/ina.13067. [DOI] [PubMed] [Google Scholar]
- 34.ATSDR, Agency for Toxic Substances and Disease Registry. Toxicological profile for toluene 2000: Atlanta: US Public Health Service. [Google Scholar]
- 35.Benignus VA, et al. , Toluene levels in blood and brain of rats during and after respiratory exposure. Toxicol Appl Pharmacol. 61 (1981) 326–34 DOI: [DOI] [PubMed] [Google Scholar]
- 36.Gerasimov MR, et al. , Study of brain uptake and biodistribution of [11C]toluene in non-human primates and mice. Life Sci. 70 (2002) 2811–28 DOI: 10.1016/s0024-3205(02)01542-4. [DOI] [PubMed] [Google Scholar]
- 37.Woodward JJ and Beckley JT, The Effects of Abused Inhalants on Neurons Within the Addiction Neurocircuitry of the Brain, in Neuropathology of Drug Addictions and Substance Misuse. Stimulants, Club and Dissociative Drugs, Hallucinogens, Steroids, Inhalants and International Aspects, Preedy VR, Editor. 2016, Academic Press, Elsevier. [Google Scholar]
- 38.Beckstead MJ, et al. , Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 57 (2000) 1199–205 DOI: [PubMed] [Google Scholar]
- 39.MacIver MB, Abused inhalants enhance GABA-mediated synaptic inhibition. Neuropsychopharmacology. 34 (2009) 2296–304 DOI: 10.1038/npp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckley JT and Woodward JJ, The abused inhalant toluene differentially modulates excitatory and inhibitory synaptic transmission in deep-layer neurons of the medial prefrontal cortex. Neuropsychopharmacology. 36 (2011) 1531–42 DOI: 10.1038/npp.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopreato GF, et al. , Inhaled drugs of abuse enhance serotonin-3 receptor function. Drug Alcohol Depend. 70 (2003) 11–5 DOI: [DOI] [PubMed] [Google Scholar]
- 42.Bale AS, Smothers CT, and Woodward JJ, Inhibition of neuronal nicotinic acetylcholine receptors by the abused solvent, toluene. Br J Pharmacol. 137 (2002) 375–83 DOI: 10.1038/sj.bjp.0704874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kofke WA, et al. , Striatal extracellular dopamine levels are not increased by hyperglycemic exacerbation of ischemic brain damage in rats. Brain Res. 633 (1994) 171–177 DOI: [DOI] [PubMed] [Google Scholar]
- 44.Tillar R, Shafer TJ, and Woodward JJ, Toluene inhibits voltage-sensitive calcium channels expressed in pheochromocytoma cells. Neurochem Int. 41 (2002) 391–7 DOI: [DOI] [PubMed] [Google Scholar]
- 45.Del Re AM and Woodward JJ, Inhibition of gap junction currents by the abused solvent toluene. Drug Alcohol Depend. 78 (2005) 221–4 DOI: 10.1016/j.drugalcdep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Del Re AM, Dopico AM, and Woodward JJ, Effects of the abused inhalant toluene on ethanol-sensitive potassium channels expressed in oocytes. Brain Res. 1087 (2006) 75–82 DOI: 10.1016/j.brainres.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 47.Woodward JJ, Nowak M, and Davies DL, Effects of the abused solvent toluene on recombinant P2X receptors expressed in HEK293 cells. Brain Res Mol Brain Res. 125 (2004) 86–95 DOI: 10.1016/j.molbrainres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Cruz SL, et al. , Inhibition of cardiac sodium currents by toluene exposure. Br J Pharmacol. 140 (2003) 653–60 DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gauthereau MY, Salinas-Stefanon EM, and Cruz SL, A mutation in the local anaesthetic binding site abolishes toluene effects in sodium channels. Eur J Pharmacol. 528 (2005) 17–26 DOI: [DOI] [PubMed] [Google Scholar]
- 50.Cruz SL, et al. , Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 286 (1998) 334–40 DOI: [PubMed] [Google Scholar]
- 51.Bale AS, et al. , Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 130 (2005) 197–206 DOI: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 52.Beckley JT, et al. , Phenotype-dependent inhibition of glutamatergic transmission on nucleus accumbens medium spiny neurons by the abused inhalant toluene. Addict Biol. 21 (2016) 530–46 DOI: 10.1111/adb.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smothers CT and Woodward JJ, Differential effects of TM4 tryptophan mutations on inhibition of N-methyl-d-aspartate receptors by ethanol and toluene. Alcohol. 56 (2016) 15–19 DOI: 10.1016/j.alcohol.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsieh CP, et al. , N,N-dimethylglycine prevents toluene-induced impairment in recognition memory and synaptic plasticity in mice. Toxicology. 446 (2020) 152613 DOI: 10.1016/j.tox.2020.152613. [DOI] [PubMed] [Google Scholar]
- 55.Beckley JT, et al. , Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J Neurosci. 33 (2013) 804–13 DOI: 10.1523/jneurosci.3729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braunscheidel KM, Effects of the abused inhalant on mPFC-dependent cognitive behaviors and associated neural activity. 2020, Medical University of South Carolina: MUSC Theses and Dissertations. 66. p. 195. [Google Scholar]
- 57.Melis M, et al. , Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 24 (2004) 53–62 DOI: 10.1523/JNEUROSCI.4503-03.2004 24/1/53 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grueter BA, Brasnjo G, and Malenka RC, Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 13 (2010) 1519–25 DOI: nn.2685 [pii] 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flanagan RJ and Ives RJ, Volatile substance abuse. Bull Narc. 46 (1994) 49–78 DOI: [PubMed] [Google Scholar]
- 60.Anderson CE and Loomis GA, Recognition and prevention of inhalant abuse. Am Fam Physician. 68 (2003) 869–74 DOI: [PubMed] [Google Scholar]
- 61.Clinger OW and Johnson NA, Purposeful inhalation of gasoline vapors. Psychiatr Q. 25 (1951) 557–67 DOI: 10.1007/BF01584303. [DOI] [PubMed] [Google Scholar]
- 62.Glaser HH and Massengale ON, Glue-sniffing in children. Deliberate inhalation of vaporized plastic cements. JAMA. 181 (1962) 300–3 DOI: 10.1001/jama.1962.03050300020004. [DOI] [PubMed] [Google Scholar]
- 63.Wu LT and Ringwalt CL, Inhalant use and disorders among adults in the United States. Drug Alcohol Depend. 85 (2006) 1–11 DOI: 10.1016/j.drugalcdep.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DSM-5, Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013: American Psychiatric Association. [Google Scholar]
- 65.Ridenour TA, Halliburton AE, and Bray BC, Does DSM-5 nomenclature for inhalant use disorder improve upon DSM-IV? Psychol Addict Behav. 29 (2015) 211–7 DOI: 10.1037/adb0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ridenour TA, Bray BC, and Cottler LB, Reliability of use, abuse, and dependence of four types of inhalants in adolescents and young adults. Drug Alcohol Depend. 91 (2007) 40–9 DOI: 10.1016/j.drugalcdep.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss B, Wood RW, and Macys DA, Behavioral toxicology of carbon disulfide and toluene. Environ Health Perspect. 30 (1979) 39–45 DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glowa JR, Some effects of sub-acute exposure to toluene on schedule-controlled behavior. Neurobehav Toxicol Teratol. 3 (1981) 463–5 DOI: [PubMed] [Google Scholar]
- 69.Wood RW, Rees DC, and Laties VG, Behavioral effects of toluene are modulated by stimulus control. Toxicol Appl Pharmacol. 68 (1983) 462–72 DOI: 10.1016/0041-008x(83)90291-0. [DOI] [PubMed] [Google Scholar]
- 70.Kjellstrand P, et al. , Effects of organic solvents on motor activity in mice. Toxicology. 35 (1985) 35–46 DOI: 10.1016/0300-483x(85)90130-1. [DOI] [PubMed] [Google Scholar]
- 71.Wood RW and Colotla VA, Biphasic changes in mouse motor activity during exposure to toluene. Fundam Appl Toxicol. 14 (1990) 6–14 DOI: [DOI] [PubMed] [Google Scholar]
- 72.Riegel AC and French ED, Acute toluene induces biphasic changes in rat spontaneous locomotor activity which are blocked by remoxipride. Pharmacol Biochem Behav. 62 (1999) 399–402 DOI: [DOI] [PubMed] [Google Scholar]
- 73.Lo PS, et al. , Acute neurobehavioral effects of toluene: involvement of dopamine and NMDA receptors. Toxicology. 265 (2009) 34–40 DOI: S0300–483X(09)00467–3 [pii] 10.1016/j.tox.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Himnan DJ, Tolerance and reverse tolerance to toluene inhalation: effects on open-field behavior. Pharmacol Biochem Behav. 21 (1984) 625–31 DOI: 10.1016/s0091-3057(84)80048-9. [DOI] [PubMed] [Google Scholar]
- 75.Batis JC, Hannigan JH, and Bowen SE, Differential effects of inhaled toluene on locomotor activity in adolescent and adult rats. Pharmacol Biochem Behav. 96 (2010) 438–48 DOI: 10.1016/j.pbb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paez-Martinez N, et al. , Environmental enrichment reduces behavioural sensitization in mice previously exposed to toluene: The role of D1 receptors. Behav Brain Res. 390 (2020) 112624 DOI: 10.1016/j.bbr.2020.112624. [DOI] [PubMed] [Google Scholar]
- 77.Bowen SE, Kimar S, and Irtenkauf S, Comparison of toluene-induced locomotor activity in four mouse strains. Pharmacol Biochem Behav. 95 (2010) 249–57 DOI: 10.1016/j.pbb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiley JL, Bale AS, and Balster RL, Evaluation of toluene dependence and cross-sensitization to diazepam. Life Sci. 72 (2003) 3023–33 DOI: 10.1016/s0024-3205(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 79.Rees DC, et al. , Toluene, halothane, 1,1,1-trichloroethane and oxazepam produce ethanol-like discriminative stimulus effects in mice. J Pharmacol Exp Ther. 243 (1987) 931–7 DOI: [PubMed] [Google Scholar]
- 80.Rees DC, et al. , Discriminative stimulus properties of toluene in the mouse. Toxicol Appl Pharmacol. 88 (1987) 97–104 DOI: 10.1016/0041-008x(87)90273-0. [DOI] [PubMed] [Google Scholar]
- 81.Beyer CE, et al. , Repeated exposure to inhaled toluene induces behavioral and neurochemical cross-sensitization to cocaine in rats. Psychopharmacology (Berl). 154 (2001) 198–204 DOI: 10.1007/s002130000614. [DOI] [PubMed] [Google Scholar]
- 82.Bowen SE, et al. , The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 28 (2006) 636–47 DOI: S0892–0362(06)00112–7 [pii] 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 83.Funada M, et al. , Evaluation of rewarding effect of toluene by the conditioned place preference procedure in mice. Brain Res Brain Res Protoc. 10 (2002) 47–54 DOI: S1385299X02001824 [pii]. [DOI] [PubMed] [Google Scholar]
- 84.Wayman WN and Woodward JJ, Chemogenetic Excitation of Accumbens-Projecting Infralimbic Cortical Neurons Blocks Toluene-Induced Conditioned Place Preference. J Neurosci. 38 (2018) 1462–1471 DOI: 10.1523/JNEUROSCI.2503-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Negus SS and Miller LL, Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 66 (2014) 869–917 DOI: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bauer CT, et al. , Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 168 (2013) 850–62 DOI: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bauer CT, Banks ML, and Negus SS, The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology (Berl). 231 (2014) 2461–70 DOI: 10.1007/s00213-013-3405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yavich L, Patkina N, and Zvartau E, Experimental estimation of addictive potential of a mixture of organic solvents. Eur Neuropsychopharmacol. 4 (1994) 111–8 DOI: 10.1016/0924-977x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 89.Chan MH, et al. , Sarcosine attenuates toluene-induced motor incoordination, memory impairment, and hypothermia but not brain stimulation reward enhancement in mice. Toxicol Appl Pharmacol. 265 (2012) 158–65 DOI: S0041–008X(12)00434–6 [pii] 10.1016/j.taap.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan MH, et al. , The group II metabotropic glutamate receptor agonist LY379268 reduces toluene-induced enhancement of brain-stimulation reward and behavioral disturbances. Psychopharmacology (Berl). 232 (2015) 3259–68 DOI: 10.1007/s00213-015-3973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tracy ME, Banks ML, and Shelton KL, Negative allosteric modulation of GABAA receptors inhibits facilitation of brain stimulation reward by drugs of abuse in C57BL6/J mice. Psychopharmacology (Berl). 233 (2016) 715–25 DOI: 10.1007/s00213-015-4155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu CY, et al. , Attenuation of toluene-induced brain stimulation reward enhancement and behavioral disturbances by N-acetylcysteine in mice. Toxicology. 408 (2018) 39–45 DOI: 10.1016/j.tox.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 93.Yanagita T, et al. , Volunatry inhalation of volatile anesthetics and organic solvents by monkeys. Jpn. J. Clin. Pharmacol 1 (1970) 13–16 DOI: [Google Scholar]
- 94.Blokhina EA, et al. , Intravenous self-administration of abused solvents and anesthetics in mice. Eur J Pharmacol. 485 (2004) 211–8 DOI: [DOI] [PubMed] [Google Scholar]
- 95.Braunscheidel KM, et al. , Self-Administration of Toluene Vapor in Rats. Front Neurosci. 14 (2020) 880 DOI: 10.3389/fnins.2020.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan Y, et al. , Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res. 177 (2007) 261–8 DOI: 10.1016/j.bbr.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 97.Augur IF, et al. , Chemogenetic Activation of an Extinction Neural Circuit Reduces Cue-Induced Reinstatement of Cocaine Seeking. J Neurosci. 36 (2016) 10174–80 DOI: 10.1523/JNEUROSCI.0773-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mesa JR, et al. , The roles of rat medial prefrontal and orbitofrontal cortices in relapse to cocaine-seeking: A comparison across methods for identifying neurocircuits. Addict Neurosci. 4 (2022) DOI: 10.1016/j.addicn.2022.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wayman WN and Woodward JJ, Exposure to the Abused Inhalant Toluene Alters Medial Prefrontal Cortex Physiology. Neuropsychopharmacology. 43 (2018) 912–924 DOI: 10.1038/npp.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kesner RP and Churchwell JC, An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 96 (2011) 417–31 DOI: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 101.Widge AS, Heilbronner SR, and Hayden BY, Prefrontal cortex and cognitive control: new insights from human electrophysiology. F1000Res. 8 (2019) DOI: 10.12688/f1000research.20044.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller EK and Cohen JD, An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24 (2001) 167–202 DOI: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 103.Kalivas PW, The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 10 (2009) 561–72 DOI: [DOI] [PubMed] [Google Scholar]
- 104.Volkow ND, Michaelides M, and Baler R, The Neuroscience of Drug Reward and Addiction. Physiol Rev. 99 (2019) 2115–2140 DOI: 10.1152/physrev.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Botvinick M and Braver T, Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol. 66 (2015) 83–113 DOI: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- 106.Shenhav A, et al. , Toward a Rational and Mechanistic Account of Mental Effort. Annu Rev Neurosci. 40 (2017) 99–124 DOI: 10.1146/annurev-neuro-072116-031526. [DOI] [PubMed] [Google Scholar]
- 107.Gruner P and Pittenger C, Cognitive inflexibility in Obsessive-Compulsive Disorder. Neuroscience. 345 (2017) 243–255 DOI: 10.1016/j.neuroscience.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryman SG, et al. , Impaired Midline Theta Power and Connectivity During Proactive Cognitive Control in Schizophrenia. Biol Psychiatry. 84 (2018) 675–683 DOI: 10.1016/j.biopsych.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Z, et al. , Cognitive Behavioral Therapy Is Associated With Enhanced Cognitive Control Network Activity in Major Depression and Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 3 (2018) 311–319 DOI: 10.1016/j.bpsc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butler K and Le Foll B, Impact of Substance Use Disorder Pharmacotherapy on Executive Function: A Narrative Review. Front Psychiatry. 10 (2019) 98 DOI: 10.3389/fpsyt.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blair MA, et al. , Blunted Frontostriatal Blood Oxygen Level-Dependent Signals Predict Stimulant and Marijuana Use. Biol Psychiatry Cogn Neurosci Neuroimaging. 3 (2018) 947–958 DOI: 10.1016/j.bpsc.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terraneo A, et al. , Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 26 (2016) 37–44 DOI: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winstanley CA, et al. , Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 34 (2010) 1306–18 DOI: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang YY, et al. , Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci. 19 (2015) 439–44 DOI: 10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 115.Volkow ND, et al. , Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 46 (2002) 79–82 DOI: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 116.Volkow ND, et al. , Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol. 23 (2013) 639–48 DOI: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tomasi D and Volkow ND, Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol. 48 (2013) 1–19 DOI: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jain S, et al. , BOLD activation during cue induced craving in adolescent inhalant users. Asian J Psychiatr. 52 (2020) 102097 DOI: 10.1016/j.ajp.2020.102097. [DOI] [PubMed] [Google Scholar]
- 119.Kalayasiri R, Maneesang W, and Maes M, A novel approach of substitution therapy with inhalation of essential oil for the reduction of inhalant craving: A double-blinded randomized controlled trial. Psychiatry Res. 261 (2018) 61–67 DOI: 10.1016/j.psychres.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 120.Peters J, Kalivas PW, and Quirk GJ, Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 16 (2009) 279–88 DOI: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rocha A and Kalivas PW, Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 31 (2010) 903–9 DOI: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen H and Kalivas PW, Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int J Neuropsychopharmacol. 16 (2013) 1165–7 DOI: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen BT, et al. , Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 496 (2013) 359–62 DOI: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 124.Ball KT and Slane M, Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psychopharmacology (Berl). 224 (2012) 377–85 DOI: 10.1007/s00213-012-2762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bossert JM, et al. , Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 14 (2011) 420–2 DOI: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bossert JM, et al. , Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 32 (2012) 4982–91 DOI: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Willcocks AL and McNally GP, The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 37 (2013) 259–68 DOI: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- 128.Korman M, Trimboli F, and Semler I, A comparative evaluation of 162 inhalant users. Addict Behav. 5 (1980) 143–52 DOI: 10.1016/0306-4603(80)90032-5. [DOI] [PubMed] [Google Scholar]
- 129.Stollery BT, Long-term cognitive sequelae of solvent intoxication. Neurotoxicol Teratol. 18 (1996) 471–6 DOI: 10.1016/0892-0362(96)00024-4. [DOI] [PubMed] [Google Scholar]
- 130.Howard MO, et al. , Inhalant use among incarcerated adolescents in the United States: prevalence, characteristics, and correlates of use. Drug Alcohol Depend. 93 (2008) 197–209 DOI: 10.1016/j.drugalcdep.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 131.Lubman DI, Yucel M, and Lawrence AJ, Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol. 154 (2008) 316–26 DOI: bjp200876 [pii] 10.1038/bjp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuncu Z, et al. , Abnormal white matter integrity and impairment of cognitive abilities in adolescent inhalant abusers. Neurotoxicol Teratol. 47 (2015) 89–95 DOI: 10.1016/j.ntt.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 133.Dingwall KM, et al. , Cognitive recovery during and after treatment for volatile solvent abuse. Drug Alcohol Depend. 118 (2011) 180–5 DOI: 10.1016/j.drugalcdep.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 134.Takagi M, et al. , Executive control among adolescent inhalant and cannabis users. Drug Alcohol Rev. 30 (2011) 629–37 DOI: 10.1111/j.1465-3362.2010.00256.x. [DOI] [PubMed] [Google Scholar]
- 135.Rosenberg NL, et al. , Toluene abuse causes diffuse central nervous system white matter changes. Ann Neurol. 23 (1988) 611–4 DOI: 10.1002/ana.410230614. [DOI] [PubMed] [Google Scholar]
- 136.Kornfeld M, et al. , Solvent vapor abuse leukoencephalopathy. Comparison to adrenoleukodystrophy. J Neuropathol Exp Neurol. 53 (1994) 389–98 DOI: 10.1097/00005072-199407000-00011. [DOI] [PubMed] [Google Scholar]
- 137.Filley CM, Halliday W, and Kleinschmidt-DeMasters BK, The effects of toluene on the central nervous system. J Neuropathol Exp Neurol. 63 (2004) 1–12 DOI: [DOI] [PubMed] [Google Scholar]
- 138.Marulanda N and Colegial C, Neurotoxicity of solvents in brain of glue abusers. Environ Toxicol Pharmacol. 19 (2005) 671–5 DOI: 10.1016/j.etap.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 139.Filley CM, Heaton RK, and Rosenberg NL, White matter dementia in chronic toluene abuse. Neurology. 40 (1990) 532–4 DOI: 10.1212/wnl.40.3_part_1.532. [DOI] [PubMed] [Google Scholar]
- 140.Filley CM, Toluene abuse and white matter: a model of toxic leukoencephalopathy. Psychiatr Clin North Am. 36 (2013) 293–302 DOI: 10.1016/j.psc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 141.Baydas G, et al. , Learning and memory deficits in rats induced by chronic thinner exposure are reversed by melatonin. J Pineal Res. 39 (2005) 50–6 DOI: 10.1111/j.1600-079X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 142.Lee MY, et al. , Increased behavioral and neuronal responses to a hallucinogenic drug after adolescent toluene exposure in mice: Effects of antipsychotic treatment. Toxicology. 445 (2020) 152602 DOI: 10.1016/j.tox.2020.152602. [DOI] [PubMed] [Google Scholar]
- 143.Furlong TM, et al. , Toluene inhalation in adolescent rats reduces flexible behaviour in adulthood and alters glutamatergic and GABAergic signalling. J Neurochem. 139 (2016) 806–822 DOI: 10.1111/jnc.13858. [DOI] [PubMed] [Google Scholar]
- 144.Nakajima T and Wang RS, Induction of cytochrome P450 by toluene. Int J Biochem. 26 (1994) 1333–40 DOI: 10.1016/0020-711x(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 145.Cruz SL, et al. , Minocycline prevents neuronal hyperexcitability and neuroinflammation in medial prefrontal cortex, as well as memory impairment caused by repeated toluene inhalation in adolescent rats. Toxicol Appl Pharmacol. 395 (2020) 114980 DOI: 10.1016/j.taap.2020.114980. [DOI] [PubMed] [Google Scholar]
- 146.Svenson DW, et al. , Acute exposure to abuse-like concentrations of toluene induces inflammation in mouse lungs and brain. J Appl Toxicol. 42 (2022) 1168–1177 DOI: 10.1002/jat.4285. [DOI] [PubMed] [Google Scholar]
- 147.Simon NW, Mendez IA, and Setlow B, Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 121 (2007) 543–9 DOI: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Petry NM, Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 63 (2001) 29–38 DOI: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 149.Kirby KN and Petry NM, Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 99 (2004) 461–71 DOI: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 150.Churchwell JC, et al. , Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 123 (2009) 1185–96 DOI: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gill TM, Castaneda PJ, and Janak PH, Dissociable roles of the medial prefrontal cortex and nucleus accumbens core in goal-directed actions for differential reward magnitude. Cereb Cortex. 20 (2010) 2884–99 DOI: 10.1093/cercor/bhq036. [DOI] [PubMed] [Google Scholar]
- 152.Sackett DA, Moschak TM, and Carelli RM, Prelimbic Cortical Neurons Track Preferred Reward Value and Reflect Impulsive Choice during Delay Discounting Behavior. J Neurosci. 39 (2019) 3108–3118 DOI: 10.1523/JNEUROSCI.2532-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bowen SE and McDonald P, Abuse pattern of toluene exposure alters mouse behavior in a waiting-for-reward operant task. Neurotoxicol Teratol. 31 (2009) 18–25 DOI: 10.1016/j.ntt.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Braunscheidel KM, et al. , Persistent cognitive and morphological alterations induced by repeated exposure of adolescent rats to the abused inhalant toluene. Neurobiol Learn Mem. 144 (2017) 136–146 DOI: 10.1016/j.nlm.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dick AL, et al. , Specific impairments in instrumental learning following chronic intermittent toluene inhalation in adolescent rats. Psychopharmacology (Berl). 231 (2014) 1531–42 DOI: 10.1007/s00213-013-3363-7. [DOI] [PubMed] [Google Scholar]
- 156.St Onge JR and Floresco SB, Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 20 (2010) 1816–28 DOI: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- 157.St Onge JR, et al. , Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J Neurosci. 32 (2012) 2886–99 DOI: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Braunscheidel KM, et al. , The Abused Inhalant Toluene Impairs Medial Prefrontal Cortex Activity and Risk/Reward Decision-Making during a Probabilistic Discounting Task. J Neurosci. 39 (2019) 9207–9220 DOI: 10.1523/JNEUROSCI.1674-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.St Onge JR and Floresco SB, Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 34 (2009) 681–97 DOI: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- 160.St Onge JR, Chiu YC, and Floresco SB, Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology (Berl). 211 (2010) 209–21 DOI: 10.1007/s00213-010-1883-y. [DOI] [PubMed] [Google Scholar]
- 161.Jenni NL, Larkin JD, and Floresco SB, Prefrontal Dopamine D1 and D2 Receptors Regulate Dissociable Aspects of Decision Making via Distinct Ventral Striatal and Amygdalar Circuits. J Neurosci. 37 (2017) 6200–6213 DOI: 10.1523/JNEUROSCI.0030-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Braunscheidel KM, et al. , Cannabinoid receptor type 1 antagonists alter aspects of risk/reward decision making independent of toluene-mediated effects. Psychopharmacology (Berl). (2021) DOI: 10.1007/s00213-021-05914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klugmann M, et al. , AAV-Mediated Overexpression of the CB1 Receptor in the mPFC of Adult Rats Alters Cognitive Flexibility, Social Behavior, and Emotional Reactivity. Front Behav Neurosci. 5 (2011) 37 DOI: 10.3389/fnbeh.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuhnert S, Meyer C, and Koch M, Involvement of cannabinoid receptors in the amygdala and prefrontal cortex of rats in fear learning, consolidation, retrieval and extinction. Behav Brain Res. 250 (2013) 274–84 DOI: 10.1016/j.bbr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 165.Ranson MA and Del Bigio MR, Chronic near lifetime toluene exposure in rodents does not replicate solvent abuse leukoencephalopathy in humans. Neurotoxicology. 69 (2018) 260–265 DOI: 10.1016/j.neuro.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 166.Duncan JR, et al. , Adolescent toluene inhalation in rats affects white matter maturation with the potential for recovery following abstinence. PLoS One. 7 (2012) e44790 DOI: 10.1371/journal.pone.0044790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lever C, Burton S, and O’Keefe J, Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 17 (2006) 111–33 DOI: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- 168.Williams JM, Stafford D, and Steketee JD, Effects of repeated inhalation of toluene on ionotropic GABA A and glutamate receptor subunit levels in rat brain. Neurochem Int. 46 (2005) 1–10 DOI: [DOI] [PubMed] [Google Scholar]


