Abstract
The primary defect in cystic fibrosis (CF) is abnormal chloride and bicarbonate transport in the cystic fibrosis transmembrane conductance regulator (CFTR) epithelial ion channel. The apical surface of the respiratory tract is lined by an airway surface liquid layer (ASL) composed of mucin comprising mainly MUC5A and MUC5B glycoproteins. ASL homeostasis depends on sodium bicarbonate secretion into the airways and secretion deficits alter mucus properties leading to airway obstruction, inflammation, and infections. Downstream effects of abnormal ion transport in the lungs include altered intrinsic immune defenses. We observed that neutrophils killed Pseudomonas aeruginosa more efficiently when it had been exposed to sodium bicarbonate, and formation of neutrophil extracellular traps (NETs) by neutrophils was augmented in the presence of increasing bicarbonate concentrations. Physiological levels of bicarbonate sensitized P. aeruginosa to the antimicrobial peptide cathelicidin LL-37, which is present in both lung ASL and in NETs. Sodium bicarbonate has various uses in clinical medicine and in the care of CF patients, and could be further explored as a therapeutic adjunct against Pseudomonas infections.
Keywords: cystic fibrosis, bicarbonate, mucus, pneumonia, bacterial infection, neutrophil, cathelicidin, neutrophil extracellular traps
Introduction
Cystic fibrosis (CF) is one of the most common life-shortening genetic diseases affecting about 31,000 people in the United States and an additional 70,000 people worldwide. While life expectancy continues to rise due to earlier detection and recent treatment advances, the median age of death is calculated to be 34.1 years old in the CF Foundation Patient Registry 2020 Annual Data Report.1 CF is caused by a mutation in the gene encoding the CF transmembrane conductance regulator (CFTR) and inherited through an autosomal recessive pattern. CFTR is a transmembrane ATP-binding cassette ion transporter that is required for chloride and bicarbonate secretion on epithelial surfaces, and loss-of-function mutations lead to severe reduction or absence of these anions in the CF lung. Over 2000 CFTR mutations have been identified2 with the most common mutation being deletion of phenylalanine at the 508 position, referred to as Phe508del or ΔF508, which is present in 70% of CFTR alleles worldwide.3 As a multi-organ systemic disease, CF can manifest as chronic sinusitis, chronic respiratory infections, pancreatic exocrine insufficiency, diabetes mellitus, meconium ileus, cirrhosis, and infertility due to obstruction of the vas deferens.4 Vast heterogeneity exists in the clinical presentation and severity of the disease depending on the underlying CFTR mutation(s), combination of alleles, and other factors such as modifier genes, environment, and lifestyle.5,6
Cystic fibrosis and altered innate lung defenses
Hallmark characteristics of CF involve a vicious cycle of mucus-obstructed airways, inflammation, and recurrent pulmonary infections. Signs of early lung disease are detected even in young infants with CF. Multiple studies have demonstrated proinflammatory mediators in the CF lung even in the absence of (and prior to) the development of lung infections.7,8 However, infection is no doubt a trigger and accelerator of the chronic inflammatory process.9 The first lower respiratory tract pathogens acquired in CF are typically Staphylococcus aureus and Haemophilus influenzae. Later on, patients can acquire the Gram-negative opportunistic pathogen Pseudomonas aeruginosa, which is the most common colonizer of the CF respiratory tract, with 45% of patients having culture-positive respiratory samples. The median age of first infection is 1 year,10 and once acquired, CF lung infections are almost impossible to eradicate despite aggressive and repeated antibiotic regimens. Antibiotic resistance rates are high and almost a quarter of strains are multidrug-resistant (MDR).1 Pseudomonas infections in particular are associated with a decline in lung function and poor outcomes in CF.11
The host immune system mounts a response to bacterial infections by recruiting key effector leukocytes, which provoke a cascade of airway inflammation. Circulating neutrophils are signaled to migrate to the site of infection via microbial pathogen-associated molecular patterns. Once activated, neutrophils generate reactive oxygen and nitrogen species (ROS and RNS, respectively), release proteases, and perform phagocytic functions. Stimulated neutrophils can also be activated to release elaborate web-like structures called neutrophil extracellular traps (NETs) through a specialized cell death process termed NETosis. NETs are formed via the extravasation of chromatin structures, ie, nuclear or mitochondrial DNA and histones, into the extracellular space and have the ability to ensnare and kill invading pathogens.12,13
Found within NET complexes are antimicrobial peptides (AMPs) that are naturally occurring endogenous defense molecules. AMPs are also expressed in neutrophil granules, macrophages, and epithelial cells, including those in the airway—examples include lactoferrin, lysozyme, surfactant B, cationic defensins, and cathelicidin LL-37. AMPs have a measure of direct antimicrobial effect on a broad spectrum of microorganisms,14–16 which has received attention toward clinical translation against antibiotic-resistant strains of S. aureus and P. aeruginosa.17,18 These host defense peptides can also have synergistic and additive properties when combined with conventional antibiotics, which may be of particular significance in the current era of expanding drug resistance.19–21 A prevalent and well-studied AMP, human cathelicidin LL-37, exemplifies the killing activity of this class of defense molecules, specifically targeting the lipid bilayer of bacterial cell membranes to form transmembrane pores leading to bacterial lysis.22 In addition to their antimicrobial functions, NETs and AMPs also contribute to immune cell differentiation, chemoattraction of neutrophils and monocytes, and cytokine release.23,24 Furthermore, these immune defense factors can play an immunomodulatory role in autoimmune diseases,25–27 and can promote re-epithelialization in wound healing.28
Chronic inflammation in CF is driven by an underlying dysfunctional immune response. Neutrophils are activated in this highly inflammatory environment in the airways, but are unable to effectively clear bacterial infections due to innate defects in apoptosis, phagocytosis, and the generation of ROS.29–32 Excessive neutrophil degranulation occurs in acidic cytosolic pH levels, leading to the release of primary granules that contain enzymes such as myeloperoxidase (MPO) and neutrophil elastase (NE).33–36 Indeed, NE can serve as a longitudinal biomarker for lung function in CF and is inversely associated with a decline in lung function.37,38 It has been proposed that imbalanced levels of inflammatory NE proteases cause breakdown of lung parenchyma, leading to lung damage.39
Excessive activation of NETosis and the release of NE and extracellular DNA have been associated with ex vivo CF patient neutrophils and in vivo CF pig neutrophils in response to clinical isolates of P. aeruginosa.40–42 Using bicarbonate to increase the pH to an alkaline level also boosted ROS production, histone cleavage, and bacterially induced NETosis. P. aeruginosa, S. aureus, and the Gram-negative cell wall component lipopolysaccharide (LPS) contributed to increased NETs in an alkaline pH.43 Differences in NETs induced by P. aeruginosa have been differentiated between “early” bacterial isolates (obtained from CF patients from 3 months to 11 years) and “late” bacterial isolates (obtained 5–20 years later than the earlier sample) with more NETs formed in response to the early isolates.41 Acquired resistance to NET-mediated killing is associated with late stage P. aeruginosa isolates and their mucoid phenotype.42 Given the early age of acquisition of P. aeruginosa and our understanding of NET-killing with late clinical isolates, the CF Foundation recommends the use of recombinant deoxyribonuclease or rDNase, an inhaled enzyme medication that catalyzes the breakdown of DNA, starting at the age of 6. While CF inflammation secondary to neutrophil and related NET formation is well-supported in literature, anti-inflammatory treatment options are not routinely used as we have yet to consolidate our experimental findings with the clinical response in human patients.
Mucus and bicarbonate within the airway surface liquid
Airway mucus is composed mainly of water and a much smaller fraction of mucin glycoproteins. There are 21 types of mucins, which are encoded by corresponding MUC genes, and they are subcategorized between gel-forming and membrane-bound mucins. Gel-forming mucins MUC5AC and MUC5B are the primary structural components of airway mucus; MUC5B is predominantly expressed in the healthy airway and MUC5AC is upregulated in response to inflammatory conditions including CF.44 Goblet cells secrete mucus in a process that is dependent on functional CFTR and bicarbonate.45,46 Treatment of bronchial epithelial cells with the Th-2 cytokine IL-4 led to goblet cell hyperplasia, increased bicarbonate permeability and secretion, and further augmented release of mucus.47 Native mucus and isolated mucin-glycans dispersed bacterial cells within a mature biofilm into the planktonic state and prevented bacterial attachment to human cells. As such, mucins are innately equipped to regulate bacterial virulence phenotypes.48 A thin layer of mucus covers the apical (luminal) surface of ciliated respiratory epithelium that comprises the airway surface liquid (ASL). As a major driving factor of airway homeostasis, the ASL regulates ciliary function and mucociliary transport to move entrapped microbes and foreign particles towards the pharynx to be expelled or removed. It also contains innate immune AMPs that contribute to airway defenses.
Normal function of the ASL and airway host defenses depend on bicarbonate transport into the airway lumen.45,49–52 Small airways concomitantly secrete and absorb bicarbonate to precisely maintain its steady state concentration within the thin ASL layer.53 Comparing airway epithelia from CF, non-CF, and CFTR heterozygous individuals in the piglet model, CFTR expression had a direct relationship to bicarbonate secretion and bacterial killing of S. aureus, ASL pH, and ASL viscosity.54 Dysfunctional bicarbonate secretion leads to ASL acidification and changes in ASL volume and ionic concentrations. Increased sodium reabsorption draws water away from the ASL and contributes to the low volume/dehydration hypothesis of CF lung disease.51 A landmark observation was made by Quinton about the role of bicarbonate secretion on CF “mucoviscidosis”. Mucin glycoproteins have large negative repulsive charges but remain in compacted granules through cationic Ca2+ interactions. When mucin is released in the presence of normal bicarbonate levels, the bicarbonate anion (HCO3−) binds Ca2+ and unshields mucin. This exposed electronegative repulsion allows for rapid mucin expansion.55 In line with these findings, gastrointestinal tract mucus in CF mice was thick and adherent to the epithelium but was restored to normal mucus when secreted into 100mM sodium bicarbonate buffer.45 Clinically, thickened and inspissated mucus in the airways causes airway inflammation, resulting in progressive airway disease with structural lung changes. Ultimately this process drives the significant morbidity and mortality in the CF population.55–57
Bicarbonate is a robust buffer that maintains a tightly regulated physiologic pH in the blood. The renal system reabsorbs sodium bicarbonate when the blood acid-base system becomes acidotic. A pH of 7.40 is ideal for aerobic cellular respiration and other biochemical processes. Physiologic concentrations of bicarbonate vary depending on the organ and designated function. The pancreas is a major bicarbonate secretor, and the pancreatic ductular fluid concentration is maintained at 150mM. Bicarbonate in the blood and salivary glands are 24mM and 60mM, respectively. In vivo measurements of ASL bicarbonate concentrations have varied widely due to technical challenges such as effects of the measuring electrode on transport equilibrium and CO2 variations during breathing,58,59 but there is a general consensus that pH is more alkaline in the lower airways (eg, bronchi) than in the upper airways (eg, nasal mucosa).58,60 ASL pH has also been estimated in vitro using monolayers of the human bronchial adenocarcinoma cell line Calu3, which exhibit cyclic adenosine monophosphate (cAMP)-stimulated, CFTR-dependent bicarbonate transport. Calu3 model estimates of ASL bicarbonate concentrations have ranged from 10–20mM61 to 25–30mM,62,63 likely reflecting a measure of dynamic responsiveness to experimental conditions. However, from both in vivo and in vitro experimental studies, the pH and bicarbonate buffering capacity calculated in normal respiratory ASL (CFTR function intact) has always been higher than in CF ASL.64–66 For example, one study reported a normal ASL pH of 7.18 and a CF ASL pH of 6.57,64 and another investigation calculated the bicarbonate buffering capacity of ASL from CFTR-deficient Calu3 monolayers dropping by more than 50% compared to that from normal Calu3 cells.67
Bicarbonate administration in clinical medicine
In patient care settings, sodium bicarbonate is given intravenously in cases of severe sepsis or metabolic acidosis to raise the blood pH if it becomes exceedingly acidotic.68,69 Treatment with sodium bicarbonate in patients with chronic kidney disease and chronic metabolic acidosis delays further progression of disease and prevents contrast-induced kidney disease when undergoing contrast procedures.70,71 Sodium bicarbonate is an antidote for cardiac arrhythmias caused by antidepressants and other sodium channel blocker medications.72 Bicarbonate also reverses toxic ingestions of salicylate and methanol ingestion.73 There have been studies on improving performance levels of severe intensity exercises with sodium bicarbonate supplementation. The use of sodium bicarbonate as an antibacterial agent was initially described against periodontal pathogens,74,75 and has been implicated for dental hygiene use.76 The varied interest in the use of bicarbonate can be attributed to being widely accessible, having a favorable side effect profile, low toxicity, and low cost.
Inhaled bicarbonate use has been safe and tolerated as a therapeutic agent in the care of CF patients. The anion can be given via inhalation as a mucolytic in CF and possibly in other mucus-obstructed airway diseases such as chronic obstructive pulmonary disease and non-CF bronchiectasis. A small prospective clinical study was recently published on the use of twice daily inhaled sodium bicarbonate. There were no adverse events associated with taking the aerosolized agent. Following a 10-week administration period, sputum pH increased as well as sputum rheology moduli (elasticity, viscosity, viscoelasticity) and this correlated favorably to lung function and quality of life.77 Other clinical trials using inhaled sodium bicarbonate in CF patients are in progress. These studies are measuring the effect of sodium bicarbonate on mucociliary clearance, sputum pH, and pre- and post-treatment pulmonary function tests.78,79 Successful case study reports of inhaled sodium bicarbonate in COVID-19 have also prompted clinical trials investigating this urgent area of study.80,81
Direct and indirect antimicrobial effects of bicarbonate
Sodium bicarbonate can function as an antimicrobial agent either alone82 or synergistically with other antimicrobials.83,84 Bicarbonate was first shown to inhibit the growth of various aerobic and anaerobic microorganisms, such as Escherichia coli, Pseudomonas species, Lactobacillus plantarum, and Saccharomyces yeast species, and later extended to S. aureus.82,83 Subsequent studies validated its effectiveness in artificial sputum media that more closely mimics physiologic conditions.85 Aminoglycosides demonstrated synergistic growth inhibition of E. coli in the presence of bicarbonate.83 Tobramycin, an aminoglycoside commonly used in CF clinical care, synergistically killed P. aeruginosa in the planktonic form and further antagonized biofilm formation.84
The transition from planktonic bacteria to biofilms is a sign of chronic colonization in the CF respiratory airways. Early bacterial aggregates stimulate neutrophil activation, but are able to evade neutrophil killing and continue to form biofilms.86–88 Mature biofilms self-produce extracellular matrix components such as alginate, polysaccharide synthesis locus (Psl), and pellicle (Pel) that protect them from host defenses. The biofilm bacteria become tolerant and eventually resistant to antibiotics. Ongoing inflammation and recurrent infections lead to challenges in diagnosis and clinical treatment of patients.89,90 Interestingly, P. aeruginosa biofilm formation can be impeded in 100mM sodium bicarbonate via increased production of intracellular cAMP production in P. aeruginosa, independent of osmolality or the ionic strength of the solution.91
Selected experimentation
Performing studies that mimic CF airways remains challenging. Greater knowledge regarding the antimicrobial and innate immune system response to bicarbonate in the context of CF pathogens and their interactions with neutrophils inspired the short, focused set of research studies below.
Bacterial strains and growth conditions
P. aeruginosa strains used include the widely studied reference strain PAO1 initially isolated from a human wound, P4, an MDR human clinical lung isolate obtained from a tertiary academic hospital in the New York metropolitan area, and two clinical strains (101 and 103) cultured from the sputum of CF patients at Rady Children’s Hospital San Diego. Bacterial strains were routinely streaked from glycerol stocks onto Luria Broth (LB) plates biweekly and overnight cultures in LB were diluted 1:10 in LB +/− 25mM sodium bicarbonate and incubated at 37 °C with shaking to mid-log phase (OD600 = 0.4).
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments. Under a UC San Diego approved IRB protocol (approval #131002, annual approval latest Jun 15, 2022) with informed consent, healthy adult human donors provided venous blood collected with heparin as an anticoagulant.
P. aeruginosa exposed to bicarbonate are more efficiently killed by neutrophils
Neutrophils were isolated from human blood with PolymorphPrep (Axis-Shield) per manufacturer’s instructions, diluted to 1×107 cells/mL in Hank’s balanced salt solution, and seeded into a 2 mL Eppendorf tube at 3×106 cells/mL. Sodium bicarbonate buffers of varying concentration were prepared from a 1 M stock solution (Sigma-Aldrich, Cat # S6014) in Roswell Park Memorial Institute medium (RPMI, Gibco, Cat # 11835030) + 10% LB added to Eppendorf tubes. PAO1 bacteria were added at a multiplicity of infection (MOI) of 0.01 bacteria/neutrophil, tubes were rotated at 37 °C for 1 hour, then neutrophils were lysed by sonication and dilution plated on LB to enumerate bacterial colony forming units (CFU). P. aeruginosa grown in physiologic bicarbonate (25mM) were more efficiently killed by human neutrophils compared with bacteria grown in the absence of bicarbonate (Figure 1A).
Figure 1. Effects of bicarbonate concentration on Pseudomonas aeruginosa innate immune susceptibility and virulence.
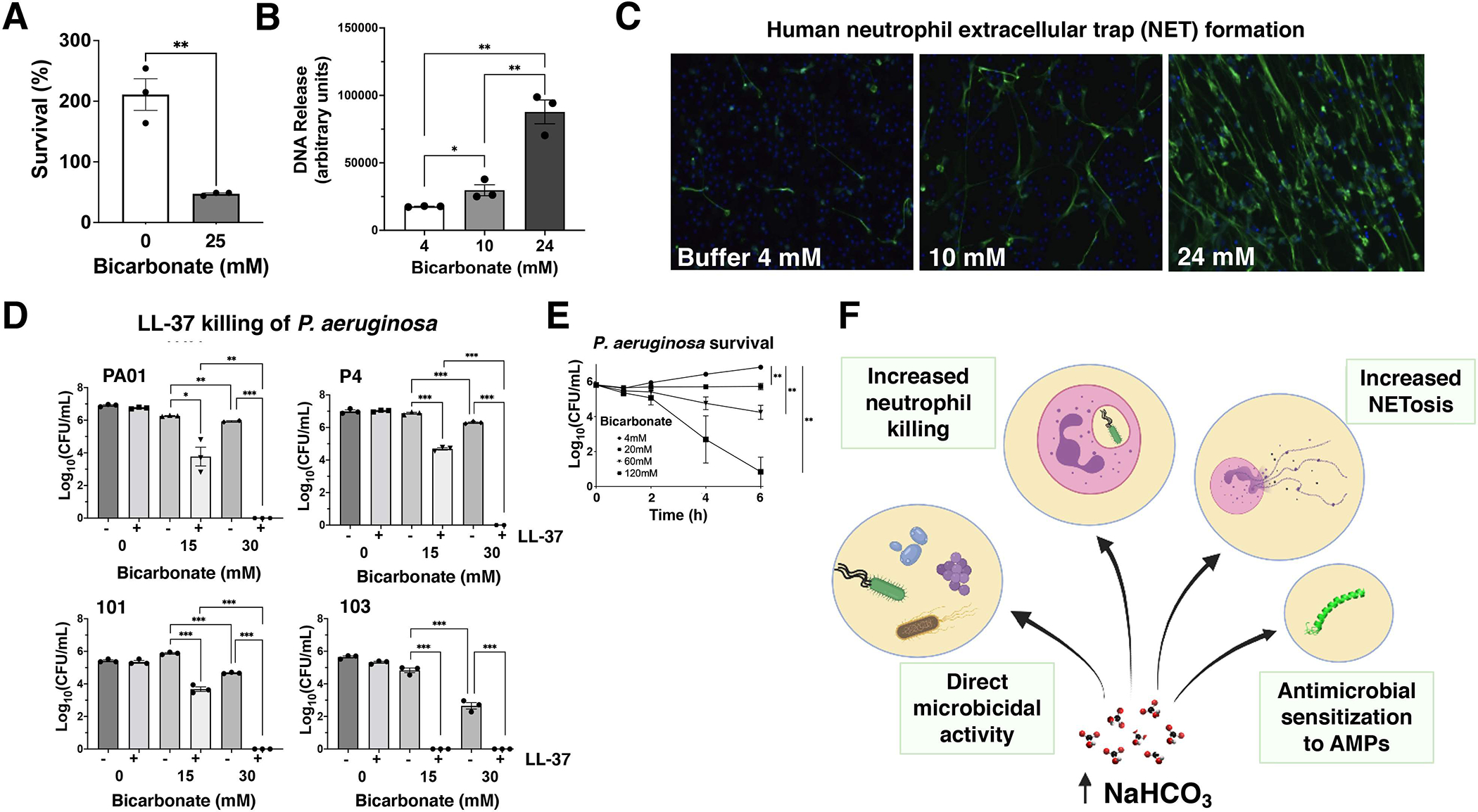
A: Neutrophil killing of P. aeruginosa strain P4 grown in the presence or absence of 25mM bicarbonate in the overnight culture. B: PicoGreen quantification of PMA-induced human neutrophil extracellular trap (NET) DNA release in different bicarbonate concentrations. C: Representative images of PMA-induced NETs in different bicarbonate concentrations by anti-human myeloperoxidase antibody and AlexaFluor488-conjugated secondary antibody. D: Survival of P. aeruginosa strains in the presence (+) or absence (−) of 4 μM LL-37 at the indicated bicarbonate concentrations. E: Killing kinetics of P. aeruginosa in increasing concentrations of bicarbonate. F: Schematic summary of the effects of bicarbonate on P. aeruginosa innate immune susceptibility and virulence. Unpaired parametric t-tests (A, B) and 2-way ANOVA (D, E) were performed; *P < 0.05, **P < 0.01, *** P < 0.005. AMPs: antimicrobial peptides; CFU: colony forming units; NET: neutrophil extracellular trap; PMA: phorbol 12-myristate 13-acetate.
Bicarbonate enhances NET formation
ASL is the site of complex ionic interactions that are perturbed in CF. We previously showed that NETosis was reduced in the absence of extracellular chloride, the first deficient anion discovered in CF.92 To study the effect of bicarbonate on NET formation, we seeded human neutrophils at 2×105 cells/well onto 48-well plates with sodium bicarbonate at 4mM (buffer alone), 10mM (low) and 24mM (physiologic) concentrations. Phorbol 12-myristate 13-acetate (PMA, 25nM) was added to stimulate NET formation at 37 °C + 5% CO2 for 3 hours. After this, micrococcal nuclease was added at 500 mU to digest extracellular DNA and the reaction was stopped with 5mM ethylenediaminetetraacetic acid (EDTA). Samples were centrifuged at 200×g for 8 minutes. The supernatant was transferred to a separate 96-well flat bottom plate and extracellular DNA was quantified using the Quant-iT PicoGreen assay kit (Life Technologies). As shown in Figure 1B, we observed a significant difference in the production of NETs between the lowest concentration at 4mM and both the 10mM and 24mM concentrations (P = 0.04, P = 0.001, respectively); more NETs were produced at 24mM compared to 10mM (P = 0.004).
NETs were visualized per a protocol previously described by von Köckritz-Blickwede et al.93,94 Briefly, slides were fixed by adding paraformaldehyde at a final concentration of 4%. Slides were blocked by adding 2% bovine serum albumin (BSA)-phosphate buffered saline (PBS)+2% goat serum. Slides were washed with PBS 3 times between each step. Rabbit anti-human MPO (Dako #A0398) was added and after 1 hour incubation, the second antibody AlexaFluor488 goat anti-rabbit IgG (Invitrogen #A11070) was added. Slides were then embedded in ProlongGold antifade + DAPI (Invitrogen #P36931). Samples were examined using an inverted confocal laser-scanning 2-photon Olympus Fluoview FV1000 microscope with Fluoview Spectral Scanning Technology (Olympus) and representative images are shown in Figure 1C.
Sodium bicarbonate enhances the intrinsic activity of innate AMP LL-37
LL-37 facilitates the formation and stabilization of NETs,95 and an optimal ionic environment is crucial for AMP activity, as pH buffering changes can influence their native cationic charge and killing properties.96,97 We performed kinetic killing curves for LL-37 against P. aeruginosa strains over a range of bicarbonate concentrations, using bicarbonate concentrations of 30mM as a representative of bicarbonate concentration in the normal lung and 15mM as a reduced concentration approximating a secretion defect as might be found in the CF lung. Log phase bacteria at OD600 = 0.4 were washed twice and diluted with RPMI with 10% LB to an initial inoculum of 2×106 CFU/mL. LL-37 (Bachem, Cat # 4042456) and a 1M sodium bicarbonate stock solution were diluted in the same media to the desired concentrations as indicated. Assays were conducted in a flat 96-well plate with a total volume of 200 μL and each condition was tested in triplicate. Plates were placed into a 37 °C incubator with shaking and removed after 4 hours. Aliquots were taken from the plate and serially diluted for CFU enumeration. We found that AMP activity of LL-37 could be augmented with sodium bicarbonate and was greater at higher (physiologic) concentrations vs. lower (CF) concentrations (Figure 1D).
Direct antimicrobial effect of bicarbonate on P. aeruginosa
Airway cells cultured from CF patients possess inherent defects in AMP activity compared to cells from non-CF patients, and aerosolizing high-dose sodium bicarbonate (100mM) into the airway ASL of CF pigs enhanced killing of S. aureus-coated grids.66 Prior reports of direct antimicrobial activity of bicarbonate against P. aeruginosa tested concentrations of 100mM or greater.85,91 Using the MDR human lung clinical P. aeruginosa isolate P4, we found dose-dependent reduction in P. aeruginosa CFUs occurred at bicarbonate concentrations of 60mM or 120mM, suggesting the potential of the aerosolized agent to exert direct antimicrobial activity against the pathogen (Figure 1E).
Statistical analyses
Statistical analyses were performed using GraphPad Prism, v8.1.2 (GraphPad Software Inc., La Jolla, CA, USA). Unpaired parametric t-tests and 2-way ANOVA were performed and P < 0.05 was considered statistically significant.
Conclusions and areas for future study
A defect in bicarbonate secretion into the airways in CF causes pathophysiologic changes in mucus biology, the ASL, and components within the ASL. Here we have outlined our understanding of sodium bicarbonate as an antimicrobial agent against various microorganisms, and specifically MDR and CF clinical isolates of P. aeruginosa, and bicarbonate in concert with ASL antimicrobial peptides including cathelicidin LL-37. The antimicrobial effect seen at higher concentrations of bicarbonate is not due to osmolality or ionic strength.91 This suggests that increased concentrations may contribute as a defense mechanism against bacterial infection and chronic colonization in the lungs. Obstructive CF sputum consists of extracellular DNA98–100 and clinical treatment targets the breakdown of NETs. We present a novel perspective on how alterations in sodium bicarbonate affect the innate immune system. We found that sodium bicarbonate stimulates NETosis, and pretreating P. aeruginosa with sodium bicarbonate enhanced bacterial killing effects of neutrophils. Sodium bicarbonate appears to increase bacterial susceptibility to killing through increasing intracellular bacterial cAMP levels and disrupting the pH gradient of the proton motive force across the cytoplasmic membrane of Gram-negative and Gram-positive bacteria.91,101 It is possible that commensal lung bacteria may also be impacted with augmented levels of bicarbonate. The extent of such effects likely depends on the condition of the lung, since the physiologic level of bicarbonate is dynamic in nature. Introduction of bacteria due to an acute infection may result in a precipitous decrease in bicarbonate levels. Treatment with sodium bicarbonate can theoretically raise bicarbonate to levels those pathogenic bacteria are not accustomed to, and thus perhaps more susceptible to, in comparison to commensal bacteria of the upper respiratory tract. The multifaceted potential contributions of bicarbonate to airway host defense are summarized schematically in Figure 1F.
Given the above considerations, the use of inhaled bicarbonate continues to be investigated as a potential therapeutic in CF and other respiratory diseases. The development of CFTR modulators within the last decade has created landmark shifts in the treatment and outcome of patients with CF. These treatments have led to increased lung function, weight gain, reduction of pulmonary exacerbations, and improvement in quality of life. Gene-directed therapies are currently available for 82%–90% of CF patients worldwide, as a result of the inclusion of one copy of DF508 as a qualifying mutation.102 These small molecules target CFTR mutations, alter the malfunctioning protein, and restore functional expression. Drugs are classified based on their effect on the gene and are either termed “potentiators”, which increase the gating of the CFTR chloride-bicarbonate ion channel at the cell surface, or “correctors”, which act to rescue post-translational folding, processing, and trafficking of the protein. In the presence of CFTR modulating drugs, restored chloride conductance can result in normalization of sweat chloride levels. Bicarbonate transport activity was rescued in cells expressing p.F508del-CFTR. By calculating HCO3- influx from the pH, a HCO3- influx of 17 – 25.8mM occurred in the presence of one corrector and 45.9mM with two correctors.103 Since CFTR modulators treat the underlying defect, the downstream effects of an abnormal CFTR are mitigated. For 10%–18% of patients or more in countries where the prevalence of ΔF508 is higher and who do not have therapeutic options, the use of sodium bicarbonate as a therapeutic may be more relevant. The safety and tolerability profile of bicarbonate makes it an agreeable medication to be considered for long-term use. As such, its therapeutic application can be considered a step towards restoring the depressed bicarbonate levels characteristic of CF airways. Long-term inhaled bicarbonate therapy may create an environment in which colonized P. aeruginosa is constantly exposed to sodium bicarbonate, which renders it more sensitized to the defenses of the immune system. There likely also exists a synergistic effect of pretreated bacteria with additional host innate immune factors that can be further investigated.
Acknowledgements
The authors would like to extend our gratitude to Dr. Paul Quinton for engaging our curiosity in this field and inspiring us to answer the questions outlined in this manuscript.
Funding:
This work was supported by NIH training fellowships 5T32HD08798 and 5K12HL141956 to RS and NIH research grant R01AI145310 to VN. The funders had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: The authors reported no conflicts of interest.
References
- 1.Cystic Fibrosis Foundation. Patient Registry 2020 Annual Data Report. Available from: https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf. Published November 2021. Accessed May 20, 2022
- 2.Cystic Fibrosis Mutation Database. Available from: http://www.genet.sickkids.on.ca/. Accessed July 7, 2021.
- 3.Gentzsch M, Mall MA. Ion channel modulators in cystic fibrosis. Chest 2018;154(2):383–393. 10.1016/j.chest.2018.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunzelmann K, Schreiber R, Hadorn HB. Bicarbonate in cystic fibrosis. J Cystic Fibros 2017;16(6):653–662. 10.1016/j.jcf.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Soave D, Miller MR, et al. Unraveling the complex genetic model for cystic fibrosis: pleiotropic effects of modifier genes on early cystic fibrosis-related morbidities. Hum Genet 2014;133(2):151–161. 10.1007/s00439-013-1363-7. [DOI] [PubMed] [Google Scholar]
- 6.Corvol H, Blackman SM, Boëlle PY, et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun 2015;6:8382. 10.1038/ncomms9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tirouvanziam R, Khazaal I, Péault B. Primary inflammation in human cystic fibrosis small airways. Am J Physiol Lung Cell Mol Physiol 2002;283(2):L445–L451. 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 8.Fritzsching B, Zhou-Suckow Z, Trojanek JB, et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med 2015;191(8):902–913. 10.1164/rccm.201409-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong DS, Hook SM, Jamsen KM, et al. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 2005;40(6):500–510. 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 2005;293(5):581–588. 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 11.Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev 2017;4(4):CD004197. 10.1002/14651858.CD004197.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkmann V Neutrophil extracellular traps in the second decade. J Innate Immun 2018; 10(5–6):414–421. 10.1159/000489829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303(5663):1532–1535. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 14.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001;414(6862):454–457. 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 15.Nizet V Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol 2006;8(1):11–26. 10.21775/cimb.008.011. [DOI] [PubMed] [Google Scholar]
- 16.Chromek M, Slamová Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 2006;12(6):636–641. 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 17.Shurko JF, Galega RS, Li C, Lee GC. Evaluation of LL-37 antimicrobial peptide derivatives alone and in combination with vancomycin against S. aureus. J Antibiot 2001;414(6862):454–457. 10.1038/s41429-018-0090-7. [DOI] [PubMed] [Google Scholar]
- 18.Dosler S, Karaaslan E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides 2014;62:32–37. 10.1016/j.peptides.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Zucca M, Savoia D. The post-antibiotic era: promising developments in the therapy of infectious diseases. Int J Biomed Sci 2010;6(2):77–86. [PMC free article] [PubMed] [Google Scholar]
- 20.Kumaraswamy M, Lin L, Olson J, et al. Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against MDR Stenotrophomonas maltophilia. J Antimicrob Chemother 2016;71(5):1264–1269. 10.1093/jac/dkv487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Nonejuie P, Munguia J, et al. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant gram-negative bacterial pathogens. EBiomedicine 2015; 2(7):690–698. 10.1016/j.ebiom.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CC, Sun Y, Qian S, Huang HW. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J 2011;100(7):1688–1696. 10.1016/j.bpj.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 2011;216(3):322–333. 10.1016/j.imbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, Chen Q, Schmidt AP, et al. LLl-37, the neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (Fprl1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 2000;192(7):1069–1074. 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol 2013;191(10):4895–4901. 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lande R, Ganguly D, Facchinetti V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3(73):73ra19. 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007;449(7162):564–569. 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 28.Tokumaru S, Sayama K, Shirakata Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol 2005;175(7):4662–4668. 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 29.Painter RG, Valentine VG, Lanson NA Jr, et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006;45(34):10260–10269. 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonfield TL, Hodges CA, Cotton CU, Drumm ML. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol 2012;92(5):1111–1122. 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray RD, Hardisty G, Regan KH, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax 2018;73(2):134–144. 10.1136/thoraxjnl-2017-210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moriceau S, Lenoir G, Witko-Sarsat V. In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: evidence for an innate neutrophil disturbance. J Innate Immun 2010;2(3):260–266. 10.1159/000295791. [DOI] [PubMed] [Google Scholar]
- 33.Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med 1999;160(5 Pt 2):S49–S52. 10.1164/ajrccm.160.supplement_1.13. [DOI] [PubMed] [Google Scholar]
- 34.Koller DY, Urbanek R, Götz M. Increased degranulation of eosinophil and neutrophil granulocytes in cystic fibrosis. Am J Respir Crit Care Med 1995;152(2):629–633. 10.1164/ajrccm.152.2.7633718. [DOI] [PubMed] [Google Scholar]
- 35.Taggart C, Coakley RJ, Greally P, Canny G, O’Neill SJ, McElvaney NG. Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-α and interleukin-8. Am J Physio Lung Cell Mol Physiol 2000;278(1):L33–L41. 10.1152/ajplung.2000.278.1.L33. [DOI] [PubMed] [Google Scholar]
- 36.Pohl K, Hayes E, Keenan J, et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood 2014;124(7):999–1009. 10.1182/blood-2014-02-555268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer-Hamblett N, Aitken ML, Accurso FJ, et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med 2007;175(8):822–828. 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagel SD, Sontag MK, Wagener JS, Kapsner RK, Osberg I, Accurso FJ. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J Pediatr 2002;141(6):811–817. 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- 39.Margaroli C, Garratt LW, Horati H, et al. Elastase exocytosis by airway neutrophils Is associated with early lung damage in children with cystic fibrosis. Am J Respir Crit Care Med 2019;199(7):873–881. 10.1164/rccm.201803-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Law SM, Gray RD. Neutrophil extracellular traps and the dysfunctional innate immune response of cystic fibrosis lung disease: a review. J Inflamm 2017;14:29. 10.1186/s12950-017-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase–DNA and neutrophil elastase–DNA complexes. Immunol Lett 2014;160(2):186–194. 10.1016/j.imlet.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Young RL, Malcolm KC, Kret JE, et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One 2011;6(9):e23637. 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan MA, Philip LM, Cheung G, et al. Regulating NETosis: Increasing pH promotes NADPH oxidase-dependent NETosis. Front Med 2018;5:19. 10.3389/fmed.2018.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J, Rubin BK, Voynow JA. Mucins, mucus, and goblet cells. Chest 2018;154(1):169–176. 10.1016/j.chest.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson JK, Ermund A, Ambort D, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 2012;209(7):1263–1272. 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia MAS, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 2009;119(9):2613–2622. 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorrieri G, Scudieri P, Caci E, et al. Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep 2016; 6:36016. 10.1038/srep36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wheeler KM, Cárcamo-Oyarce G, Turner BS, et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat Microbiol 2019;4(12):2146–2154. 10.1038/s41564-019-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joo NS, Krouse ME, Wu JV, et al. HCO3− transport in relation to mucus secretion from submucosal glands. JOP 2001;2(4 Suppl):280–284. [PubMed] [Google Scholar]
- 50.Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol Lung Cell Mol Physiol 2002;283(2 27–2). 10.1152/ajplung.00277.2001. [DOI] [PubMed] [Google Scholar]
- 51.Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol 1998;274(2):L258–L263. 10.1152/ajplung.1998.274.2.L258. [DOI] [PubMed] [Google Scholar]
- 52.Cooper JL, Quinton PM, Ballard ST. Mucociliary transport in porcine trachea: Differential effects of inhibiting chloride and bicarbonate secretion. Am J Physiol Lung Cell Mol Physiol 2013;230(3):L184–90. 10.1152/ajplung.00143.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shamsuddin AKM, Quinton PM. Concurrent absorption and secretion of airway surface liquids and bicarbonate secretion in human bronchioles. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L953–L960. 10.1152/ajplung.00545.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah VS, Ernst S, Tang XX, et al. Relationships among CFTR expression, HCO3− secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc Natl Acad Sci U S A 2016;113(19):5382–5387. 10.1073/pnas.1604905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 2008;372(9636):415–417. 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 56.Chen EYT, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 2010;299:542–549. 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinton PM. The neglected ion: HCO3−. Nat Med 2001;7(3):292–293. 10.1038/85429. [DOI] [PubMed] [Google Scholar]
- 58.Zajac M, Dreano E, Edwards A, Planelles G, Sermet-Gaudelus I. Airway surface liquid pH regulation in airway epithelium current understandings and gaps in knowledge. Int J Mol Sci 2021;22(7):3384. 10.3390/ijms22073384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benedetto R, Centeio R, Ousingsawat J, Schreiber R, Janda M, Kunzelmann K. Transport properties in CFTR−/− knockout piglets suggest normal airway surface liquid pH and enhanced amiloride-sensitive Na+ absorption. Pflugers Arch 2020;472(10):1507–1519. 10.1007/s00424-020-02440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McShane D, Davies JC, Davies MG, Bush A, Geddes DM, Alton EWFW. Airway surface pH in subjects with cystic fibrosis. Eur Respir J 2003;21(1):37–42. 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 61.Borowitz D CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr Pulmonol 2015;50(S40): S24–S30. 10.1002/ppul.23247. [DOI] [PubMed] [Google Scholar]
- 62.Shan J, Liao J, Huang J, et al. Bicarbonate-dependent chloride transport drives fluid secretion by the human airway epithelial cell line Calu-3. J Physiol 2012;590(21):5273–5297. 10.1113/jphysiol.2012.236893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garnett JP, Hickman E, Burrows R, et al. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem 2011;286(47):41069–41082. 10.1074/jbc.M111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol 2006;290(3):C741–C749. 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Tang XX, Vargas Buonfiglio LG, et al. Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense. Am J Physiol Lung Cell Mol Physiol 2016;310(7):L670–L679. 10.1152/ajplung.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012;487(7405):109–113. 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim D, Liao J, Hanrahan JW. The buffer capacity of airway epithelial secretions. Front Physiol 2014;5:188. 10.3389/fphys.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaber S, Paugam C, Futier E, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet 2018;392(10141):31–40. 10.1016/S0140-6736(18)31080-8. [DOI] [PubMed] [Google Scholar]
- 69.Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal 2014;2014:627673. 10.1155/2014/627673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iorio BRD, Di Iorio BR, The UBI Study Group, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol 2019;32(6):989–1001. 10.1007/s40620-019-00656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang B, Liang L, Chen W, Liang C, Zhang S. The efficacy of sodium bicarbonate in preventing contrast-induced nephropathy in patients with pre-existing renal insufficiency: a meta-analysis. BMJ Open 2015;5(3):e006989. 10.1136/bmjopen-2014-006989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruccoleri RE, Burns MM. A literature review of the use of sodium bicarbonate for the treatment of QRS widening. J Med Toxicol 2016;12(1):121–129. 10.1007/s13181-015-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mirrakhimov AE, Ayach T, Barbaryan A, Talari G, Chadha R, Gray A. The role of sodium bicarbonate in the management of some toxic ingestions. Int J Nephrol 2017;2017:7831358. 10.1155/2017/7831358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newbrun E, Hoover CI, Ryder MI. Bactericidal action of bicarbonate ion on selected periodontal pathogenic microorganisms. J Periodontol 1984;55(11):658–667. 10.1902/jop.1984.55.11.658. [DOI] [PubMed] [Google Scholar]
- 75.Cerra MB, Killoy WJ. The effect of sodium bicarbonate and hydrogen peroxide on the microbial flora of periodontal pockets. A preliminary report. J Periodontol 1982;53(10):599–603. 10.1902/jop.1982.53.10.599. [DOI] [PubMed] [Google Scholar]
- 76.Drake D Antibacterial activity of baking soda. Compend Contin Educ Dent Suppl. 1996;17(19). [PubMed] [Google Scholar]
- 77.Gomez CCS, Parazzi PLF, Clinckspoor KJ, et al. Safety, tolerability, and effects of sodium bicarbonate inhalation in cystic fibrosis. Clin Drug Investig 2020;40(2):105–117. 10.1007/s40261-019-00861-x. [DOI] [PubMed] [Google Scholar]
- 78.Effects of inhaled bicarbonate on airway pH in cystic fibrosis. In: ClinicalTrials.gov [database online]. Available from: https://clinicaltrials.gov/ct2/show/NCT03391414. Updated January 5, 2018. Accessed September 6, 2021.
- 79.Inhaled bicarbonate therapy in cystic fibrosis. In: ClinicalTrials.gov [database online]. Available from: https://clinicaltrials.gov/ct2/show/NCT00177645. Updated February 17, 2016. Accessed September 6, 2021.
- 80.Wardeh A, Conklin J, Ko M. Case reports of observed significant improvement in patients with ARDS due to COVID-19 and maximum ventilatory support after inhalation of sodium bicarbonate. J Clin Intensive Care Med 2020;5:016–019. 10.29328/journal.jcicm.1001029. [DOI] [Google Scholar]
- 81.The role of sodium bicarbonate as an adjuvant treatment of computed tomography identified COVID-19 pneumonia. In: ClinicalTrials.gov [database online]. Available from: https://clinicaltrials.gov/ct2/show/NCT04374591. Updated: October 6, 2020. Accessed September 6, 2021.
- 82.Corral LG, Post LS, Montville TJ. Antimicrobial activity of sodium bicarbonate: a research note. J Food Sci 1988;53(3):981–982. 10.1111/j.1365-2621.1988.tb09005.x. [DOI] [Google Scholar]
- 83.Gutiérrez-Huante M, Martínez H, Bustamante VH, Puente JL, Sánchez J. Bicarbonate enhances the in vitro antibiotic activity of kanamycin in Escherichia coli. Lett Appl Microbiol 2015;60(5):440–446. 10.1111/lam.12388. [DOI] [PubMed] [Google Scholar]
- 84.Kaushik KS, Stolhandske J, Shindell O, Smyth HD, Gordon VD. Tobramycin and bicarbonate synergise to kill planktonic Pseudomonas aeruginosa, but antagonise to promote biofilm survival. NPJ Biofilms Microbiomes 2016;2:16006. 10.1038/npjbiofilms.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaikumpun P, Ruksakiet K, Stercz B, et al. Antibacterial effects of bicarbonate in media modified to mimic cystic fibrosis sputum. Int J Mol Sci 2020;21(22):8614. 10.3390/ijms21228614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pestrak MJ, Chaney SB, Eggleston HC, et al. Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathog 2018;14(2):e1006842. 10.1371/journal.ppat.1006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mishra M, Byrd MS, Sergeant S, et al. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell Microbiol 2012;14(1):95–106. 10.1111/j.1462-5822.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jensen PØ, Bjarnsholt T, Phipps R, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 2007;153(Pt 5):1329–1338. 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 89.Ciofu O, Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front Microbiol 2019;10:913. 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moser C, Jensen PØ, Thomsen K, et al. Immune responses to Pseudomonas aeruginosa biofilm onfections. Front Immunol 2021;12:625597. 10.3389/fimmu.2021.625597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dobay O, Laub K, Stercz B, et al. Bicarbonate inhibits bacterial growth and biofilm formation of prevalent cystic fibrosis pathogens. Front Microbiol 2018;9:2245. 10.3389/fmicb.2018.02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akong K, Chow O, Hazen S, Nizet V. Neutrophil extracellular traps require hypochlorite production via myeloperoxidase. Am J Respir Crit Med 2012;185:A1368. 10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A1368. [DOI] [Google Scholar]
- 93.von Köckritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med 2009;87(8):775–783. 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.von Köckritz-Blickwede M, Chow O, Ghochani M, Nizet V. Visualization and functional evaluation of phagocyte extracellular traps. Methods Microbiol 2010;37:139–160. 10.1016/S0580-9517(10)37007-3. [DOI] [Google Scholar]
- 95.Neumann A, Berends ETM, Nerlich A, et al. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem J 2014;464(1):3–11. 10.1042/BJ20140778. [DOI] [PubMed] [Google Scholar]
- 96.Dorschner RA, Lopez-Garcia B, Peschel A, et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J 2006;20(1):35–42. 10.1096/fj.05-4406com. [DOI] [PubMed] [Google Scholar]
- 97.Abou Alaiwa MH, Reznikov LR, Gansemer ND, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci U S A 2014;111(52):18703–18708. 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papayannopoulos V, Staab D, Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One 2011;6(12):e28526. 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marcos V, Zhou Z, Yildirim AO, et al. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med 2010;16(9):1018–1023. 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 100.Dubois AV, Gauthier A, Bréa D, et al. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am J Respir Cell Mol Biol 2012;47(1):80–86. 10.1165/rcmb.2011-0380OC. [DOI] [PubMed] [Google Scholar]
- 101.Farha MA, French S, Stokes JM, Brown ED. Bicarbonate alters bacterial susceptibility to antibiotics by targeting the proton motive force. ACS Infect Dis 2018;4(3):382–390. 10.1021/acsinfecdis.7b00194. [DOI] [PubMed] [Google Scholar]
- 102.Southern KW, Patel S, Sinha IP, Nevitt SJ. Correctors (specific therapies for class II CFTR mutations) for cystic fibrosis. Cochrane Database Syst Rev 2018;8(8):CD010966. 10.1002/14651858.CD010966.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fiore M, Picco C, Moran O. Correctors modify the bicarbonate permeability of F508del-CFTR. Sci Rep 2020;10(1):8440. 10.1038/s41598-020-65287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


