Abstract
Purpose of review:
Malicious or accidental radiation exposure increases risk for the hematopoietic acute radiation syndrome (H-ARS) and the delayed effects of acute radiation exposure (DEARE). Radiation medical countermeasure (MCM) development relies on robust animal models reflective of all age groups and both sexes. This review details critical considerations in murine H-ARS and DEARE model development including divergent radiation responses dependent on age, sex, and genetic diversity.
Recent findings:
Radioresistance increases with murine age from pediatrics through geriatrics. Between sexes, radioresistance is higher in male weanlings, pubescent females, and aged males, corresponding with accelerated myelopoiesis. Jackson diversity outbred (JDO) mice resemble non-human primates in radiation response for modeling human diversity. Weanlings and JDO models exhibit less DEARE than other models.
Summary:
Highly characterized age-, sex- and diversity-conscious murine models of H-ARS and DEARE provide powerful and essential tools in MCM development for all radiation victims.
Keywords: acute radiation syndrome, H-ARS, DEARE, aged, pediatric, hematopoietic stem cell
Introduction
Nuclear accidents and threats of terrorist activity involving radioactive material put humankind at risk for life-threatening acute radiation syndromes (ARS) and the delayed effects of acute radiation exposure (DEARE) in survivors of ARS. The hematopoietic system is comprised of the rare and highly quiescent bone marrow (BM) hematopoietic stem cells (HSC), which give rise to the lineage-committed, mitotically active hematopoietic progenitor cell (HPC) pool, which in turn produces all the formed elements of the blood at a rate as high as half a trillion elements daily [1]. However, less differentiated and actively proliferating cells are the most sensitive to irradiation, as in the Law of Bergonié and Tribondeau [2, 3]. High sensitivity of the hematopoietic (H) system to radiation damage results in H-ARS, where loss of neutrophils and platelets increases susceptibility to infection, hemorrhage, and death within weeks if untreated [4, 5]. The H-ARS subsyndrome begins to manifest at absorbed doses of approximately 0.7–2 Gy for humans [2, 6, 7], with the LD50/60 (lethal radiation dose for 50% of humans at 60 days post-exposure) estimated to be around 4 Gy [8]. Administration of antibiotics and fluids as supportive care can increase the LD50/60 to around 6-7 Gy [9, 10]. Exposure to higher radiation doses damages more radioresistant organ systems, including the gastrointestinal (GI) system (>8 Gy), the cardiovascular system (>20 Gy), and ultimately the central nervous system at extreme doses (>80 Gy) where death is imminent [11].
In the event of a nuclear or radiological emergency, H-ARS would be the most prevalent but also the most treatable of the ARS subsyndromes, with administration of approved and stock-piled medical countermeasures (MCMs) that significantly increase survival via targeted recovery or protection of the exquisitely radiosensitive HPCs. There are currently four hematopoietic growth factors (HGFs) licensed by the Food and Drug Administration (FDA) as MCMs for treatment of lethally irradiated personnel: Neupogen® (G-CSF), Neulasta® (peg-G-CSF), Leukine® (GM-CSF), and Nplate® (TPO receptor agonist). These HGF accelerate recovery of neutrophils or platelets and have demonstrated efficacy to increase survival from H-ARS when administered as radiomitigators at least 24h after lethal radiation exposure [12]. Given the current environment and ongoing threat of malicious use of radioactivity, research and development of MCMs with more favorable administration schedules, storage conditions, shelf lives, efficacy for radioprotection, or efficacy for DEARE continues at an astounding pace. The need for radiation MCMs and large-scale medical preparedness has never been greater, and robust pre-clinical animal models are pivotal to this work [13].
Important considerations for animal models of H-ARS and DEARE
The FDA Animal Rule (AR), introduced in 2002, requires safety and efficacy testing of MCMs in practical and relevant animal models when human testing is unethical, such as for exposures to chemical, biological, radiological, or nuclear substances (21 CFR, parts 314 and 601) [14]. The FDA AR requires efficacy testing in two animal species with mechanisms expected to be predictive of the human response, or one species if that species is sufficiently well-characterized as a model for the human response. In 2015, a supporting guidance document was published [15] that encouraged MCM development to also include safety and efficacy in at-risk populations including pediatric, geriatric, & pregnant mothers. This is particularly relevant since most H-ARS models have been developed in animals of an age representing young adult humans, and often in only one sex. There is good reason for this; young adult animals offer highly uniform biological replicates ideal for establishing MCM efficacy and mechanisms of action, and avoids variables associated with ongoing maturation in pediatric animals and onset of deterioration in aging animals. However, the uniformity of response in young adult animals makes them poor models for at-risk populations.
Considerable variability exists in the radiation response and MCM sensitivity due to age- and sex-dependent differences in body size/composition, physiology, growth rates, and metabolism, among others [16-20]. In animals and humans, the pediatric hematopoietic system is more radiosensitive than that of adults [21-28], possibly due to faster HSC cycling rates in pediatrics and differences in surface marker expression [29, 30]. Rapid development in the tissues and organ systems of children can affect radiation response and pharmacokinetics/pharmacodynamics of candidate MCMs compared to adults [31-33]. Recovery of thymopoiesis from myeloablative therapies has an inverse relationship with advancing age [34-36], and baseline DNA damage accumulates in aging HSCs [37-40]. In addition, advanced age brings diminished ability to respond to hematopoietic stress, altered HSC/HPC niche composition and function, increased clonal hematopoiesis associated with malignant transformation, HSC bias toward the myeloid lineage, and declining immunity against viruses and cancer [41-45]. Furthermore, sex affects radiosensitivity in humans and mice [46-48] and can influence MCM effectiveness [49]. Thus, male and female pediatric, young adult, and aged hematopoietic systems are distinct from one another and warrant separate modeling for development of MCMs effective for all radiation victims.
While H-ARS animals models have been developed in non-human primates [50], canines [51, 52], and mini-pigs [53, 54]), mice are the most common species for MCM efficacy testing due to their favorable economics, existence of historical databases, feasibility of aging studies, and research tools allowing for sophisticated mechanistic studies. Of the myriad mouse strains, C57BL/6 mice are one of the most commonly used strains for radiation studies. The hematopoietic system of C57BL/6 mice is well-characterized, and the availability of congenic strains provides the only true assay for HSC function, the competitive transplantation assay. Important for radiation studies, C57BL/6 are moderately radiosensitive compared to other mouse strains [55] and are one of two strains of mice recommended for testing of radiomitigators and radioprotectants by the Centers for Medical Countermeasures against Radiation in 2010 [56].
Given the genetic homogeneity of C57BL/6 mice, numerous variables can readily affect survival outcomes of lethal radiation studies, and must be strictly controlled. We have documented survival effects from time of day of irradiation, frequency of blood sampling and other stress effects, provision of support such as antibiotics or wet feed during the acute phase, mouse source (vendor, barrier, room), and the dosing volume, frequency, and route for MCMs and vehicle controls [57-61]. We have also observed the importance of controlling for cage effects, acclimation period, identification method, housing (vent rack, barrier cages, social groups), husbandry (food, bedding, enrichment, water pH, temperature, humidity, air changes, light:dark cycles), irradiation (source, dose rate, irradiation apparatus, dosimetry), anesthesia methods, and euthanasia criteria [57, 58]. In addition, water consumption rate was found to change with age in a sex-specific manner, dropping significantly following irradiation, and dropping even lower with provision of wet feed, which is relevant for MCM dosed ad libitum in drinking water [57, 61]. We have shown significantly increased radioresistance of C57BL/6J mice derived from our in-house breeding colony compared to C57BL/6J mice purchased from Jackson laboratories [60], and geriatric mice of substrain C57BL/6JN sourced from the National Institute on Aging (NIA) were significantly more radiosensitive than C57BL/6J aged in-house [61]. These observations are in line with reports of genetic drift in same-strain mice maintained in separate facilities for extended periods [62, 63]. Taken together, these data illustrate the importance of strict control of all possible variables in conducting murine H-ARS studies.
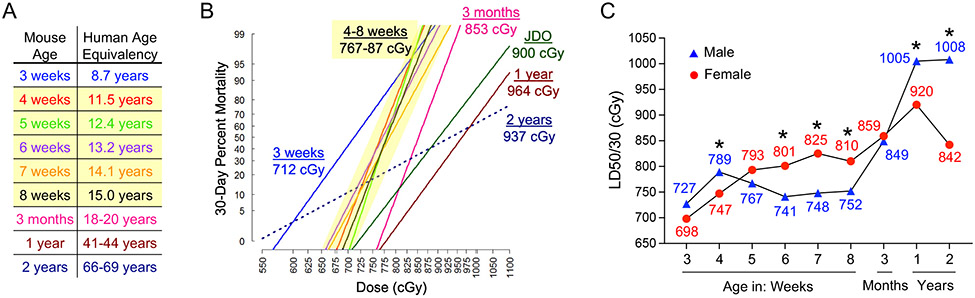
To develop murine H-ARS models relevant for humans of different ages, correlates between mouse age and human age must be established, but this is not straightforward. Towards this goal, efforts to align developmental rates of various organs and tissues between species have been attempted. For example, by epithelial gene expression, the GI system of a 4-week-old mouse is equivalent to a newborn human [64], while the brain of a 5-week-old mouse is developmentally comparable to a 2-year-old human [65]. In our models, we based age equivalency on the growth and maturation rate and sexual maturity of mice according to Flurkey et. al [66] and Richman et. al. [67] as shown in Figure 1A. Note that mouse maturation rate is accelerated at younger ages compared to older, and puberty occurs at the age of 5 weeks in most inbred strains like C57BL/6J [67], which correlates well with the calculated human age of 12–13 years. Nevertheless, age equivalencies in Figure 1A should be interpreted cautiously. In this review, we will focus on well-characterized murine models of H-ARS and DEARE, how they are impacted by age, sex, and genetic diversity, and what we can learn from them about hematopoietic radiation biology and MCM development.
Figure 1. Pediatric, young adult, geriatric, and JDO murine models of H-ARS.
A) Mouse-human age equivalencies used to develop C57BL/6J H-ARS models. B) Groups of C57BL/6 pediatric mice at 3, 4, 5, 6, 7, or 8 weeks old, young adult mice at 3 months old, middle-aged mice at 1 year old, and geriatric mice at 2 years old, or JDO mice at 8 weeks old, were exposed to various doses of TBI, n=200-400 mice per age/strain, and day 30 survival at each dose was used to construct sex-combined DRR probit plots for each group with LD50/30 indicated. Slope and LD50/30 of 4-8-week-old C57BL/6J mice were similar (yellow highlight). C) Sex differences in LD50/30 with age in C57BL/6J mice (*p<0.05 for hazard ratio of death between sexes by Cox regression analysis). Data compiled from [60, 61, 130].
Young adult H-ARS murine model
We first developed a total body irradiation (TBI) young adult murine model of H-ARS in 3-month-old C57BL/6J mice [57], roughly equivalent to 18-20-year-old humans [67, 68] (Figure 1A). The radiation Dose lethality Response Relationship (DRR) was defined using multiple groups of ~20 mice/group, with each group exposed to a different radiation dose spanning 6.5 to 9 Gy in 0.25 Gy increments. Radiosensitivity was similar between sexes in the young adult model, with an LD50/30 of 849 cGy in males and 859 cGy in females. Likely due to their accelerated physiology compared to larger animals, mice survive higher radiation doses than humans but experience H-ARS mortality within a shorter period following exposure. Thus, the lethal dose estimates in mice are based on survival at 30 days (i.e., LD50/30) rather than 60 days (i.e., LD50/60) as in humans. Probit plots of percent 30-day mortality at each radiation dose are used to estimate the LD50/30, or any other LDXX/30 for the model, as seen in Figure 1. A relatively steep slope was observed for the probit plot defining the young adult C57BL/6J model, such that the entire range from LD10/30 to LD90/30 spanned only 1.1 Gy. This steep slope underscores the homogeneity among young adults of this inbred strain, and illustrates the need for tight control of all experimental variables mentioned earlier in this article. For comparison, the LD10/60 to LD90/60 in nonhuman primates spans 2.27 Gy [50]. As acknowledged previously by Cerveny et. al., “the more inbred and homogenous the population, the steeper the slope of the lethality curve” [69].
Kinetics of complete blood cell (CBC) decline and recovery were defined throughout the 30-day acute phase in our young adult C57BL/6J model. Lymphocytes declined most rapidly, reaching their nadir by day 2, followed by neutrophils which reached their nadir around days 6-10, and then erythrocytes and platelets which slowly declined to their nadirs by approximately day 20. All blood cell parameters were mostly recovered by day 30 in surviving mice, except for lymphocytes which were still somewhat depressed [57]. Increased recovery of CBC parameters correlated with increased survival when mice were administered effective MCM: the radiomitigator Neupogen increased survival from 30% to 67% and accelerated CBC recovery, while the radioprotectant Amifostine provided 100% survival with significantly less severe nadirs for all CBC parameters along with faster recovery [57]. This model has been widely used to test other candidate MCMs from the government or private industry [70-77], and supported the licensure of Neulasta for treatment of H-ARS.
Young adult hematopoietic DEARE murine model
The DEARE are a spectrum of chronic illnesses affecting multiple organ systems in survivors of H-ARS, and are believed to be due to chronic oxidative stress, inflammation, fibrosis, and loss of stem cell self-renewal potential. Hematopoietic DEARE is characterized by long-term depression of hematopoiesis and immune function, which becomes more evident under stress [78-82]. We established a DEARE model in C57BL/6J mice surviving H-ARS as young adults using mice from vehicle-treated control groups in MCM efficacy studies [70, 82-84]. Following survival from LD50/30–LD70/30, mice were maintained in our facility until natural death or until scheduled euthanasia for DEARE analyses, typically at 6, 12, and/or 18 months post-irradiation.
H-ARS survivors exhibit significantly shortened lifespan compared to age-matched non-irradiated (NI) controls ([85] and our observations), which makes analyses at the latest time point (18 months) challenging due to limiting numbers of surviving mice. H-ARS survivors exhibited severe lifelong damage to HSCs, characterized by loss of HSC repopulating potential, increased cell cycling, decreased numbers of BM HSCs and HPCs, decreased CBCs, increased incidence of hematologic malignancies, and myeloid skewing, all which persisted for up to 2 years post-TBI [70, 82, 83]. These data illustrate unrecoverable loss of HSC self-renewal and differentiation potential, the two established hallmarks of HSCs [86-92], and suggest that compensatory mechanisms of hematopoietic support cannot overcome the “second hit” imparted by aging in survivors of H-ARS [37, 41, 93, 94]. Similar findings have been reported by others but at earlier time points and/or after sub-lethal irradiation [85, 95].
The T cell compartment was particularly depressed in H-ARS survivors, with significantly fewer naïve T cells in the spleen and peripheral blood, and a decrease in all subsets of immature thymocytes compared to age-matched NI controls [84]. T cell responses in DEARE mice may also be skewed toward pro-inflammatory TH1 polarization [85]. In addition, BM was significantly deficient in lymphoid-primed multipotent progenitors and common lymphoid progenitors, which were only 8-10% of levels in age-matched NI controls. Of interest, alluding to sex differences with age as discussed later in this article, irradiated males experienced significantly better long-term reconstitution of thymocyte subsets and peripheral blood elements compared to females [84]. Unfortunately, no radiomitigators that significantly increase H-ARS survival have been effective at alleviating hematopoietic DEARE [70], underscoring the need for continued and aggressive research for MCMs effective for DEARE and other radiation injuries.
Pediatric H-ARS murine model and sex effects related to puberty
Pediatric H-ARS models were developed in C57BL/6J mice at 3, 4, 5, 6, 7, and 8 weeks of age [60], roughly equivalent to 8-15-year-old humans [67, 68] (Figure 1A). Models were defined in terms of body weight, steady state CBC levels, intestinal development rates, and radiation DRRs. Neulasta significantly increased 30-day survival (85-100% survival) from the LD50/30 in all pediatric age groups [60], validating the model for testing MCM according to the FDA AR and contributing to the licensure of Neulasta for treatment of lethal radiation exposure in children.
The 3-week-old weanling mice were the most radiosensitive age group, as illustrated in the pediatric DRR probit plots (Figure 1B). The LD50/30 of 3-week-old mice was 712 cGy (blue line) compared to 767–787 cGy for 4-8-week-old mice (yellow shaded grouping). All pediatric models were more radiosensitive than the 3-month-old young adult model (pink line). 3-4-week-old weanling mice of strains SAS/4, BALB/c X DBA/2 F1, and New Zealand Black mice [21-27, 60, 96-99] were also observed to be more radiosensitive than other pediatric or young adult ages.
3-week-old mice had the lowest body weight, lowest RBC counts, and most rapidly growing intestinal epithelium (indicating the highest proliferation rate in intestinal crypts) of any age group [60], which may contribute to its high radiosensitivity. Indeed, others have documented more severe damage to the small intestine of 3-4-week-old mice of various strains compared to older mice, indicating initiation of GI-ARS at relatively lower radiation doses in weanlings and correlation with increased radiosensitity [24, 27, 96, 100, 101]. Increased radiosensitivity in weanlings may also be related to the more proliferative fetal-type HSCs present in the youngest mice, as opposed to more quiescent, and thus more radioresistant, HSCs in older C57BL/6J mice [29]. Also, weanling BM has been found to have fewer and more radiosensitive colony forming cells (CFC), with slower CBC recovery compared to adult mice [97]. However, partial BM shielding or hematopoietic cell transplantation are not fully radioprotective in weanling mice as they are in adult mice [24, 27, 99, 101-103] which, along with accelerated timing of mortalities [60], indicates GI effects are likely more involved in weanling radiation deaths in these models.
Sex-specific radiosensitivity was marked in the pediatric models and dependent on pediatric age. Females were more radiosensitive than males before 5 weeks of age, but more radioresistant than males after 5 weeks of age (Figure 1C) [60], coincident with the onset of puberty. Estrogen begins to increase in female C57BL/6J mice around 5-6 weeks of age [104, 105], and sex steroids have been found to influence hematopoietic regeneration [106-110]. In both mice and humans, estrogen receptor α (ERα) is highly expressed on HSCs and ERα signaling increases HSC regeneration following hematopoietic stress [106, 110-112]. One steroid hormone with estrogenic properties, 5-androstenediol, is under development as a radioprotectant [113]. Thus, it is possible that hormones contribute to the sex differences in pediatric radiation responses surrounding puberty. We did not detect significant sex differences in GI histology across pediatric age groups, but sex-specific expression of infection response genes reported in the small intestine and colon of prepubertal mice [114] could relate to greater radiosensitivity of females observed at this age.
Pediatric DEARE
Interestingly, while 3-week-old mice were the most radiosensitive in regard to acute survival, hematopoietic DEARE following LD50/30 was least severe in survivor mice irradiated at 3 weeks old, closely followed by those at 4 weeks old. Three and four week old models showed higher CBCs and increased BM progenitor number and function at 6-months post-TBI, and the least HSC myeloid bias at 12 months post-TBI [60], suggesting very young age at irradiation may confer greater hematopoietic resilience in H-ARS survivors. The fact that 3-week-olds received a lower LD50/30 radiation dose of 712 cGy versus 767–787 cGy for the other pediatric groups may contribute to this observation, though survivors in the 4-week-old group dosed in the higher 767-787 cGy range had similarly mild DEARE. By 12 months post-TBI, CBCs and BM progenitors, which were higher at 6 months in the 3- and 4-week-old groups, succumbed to similarly low levels as the other age groups, illustrating how the added stress of aging can impact hematopoietic DEARE regardless of age at irradiation. We also observed some sex-specific DEARE in the pediatric models, such as decreased body weights 6 months post-TBI in males only, which was most severe in the 3-week-old irradiated group and lessened with increasing age-at-irradiation, while females showed more severe kidney dysfunction than males at 12 months post-TBI, evidenced by increased levels of BUN [60].
Neulasta radiomitigation for H-ARS in pediatric mice again had no apparent effect on the severity of long-term hematopoietic or renal DEARE. While we have not yet characterized cancer incidence in our pediatric H-ARS survivors, increased cancer incidence has been documented after pediatric radiation exposure in humans [115].
Aging/Geriatric H-ARS murine models and significant sex divergence with age
Middle-aged and geriatric H-ARS models were developed in C57BL/6J mice at 1 and 2 years of age [61], roughly equivalent to 41-44-year-old and 66-69-year-old humans, respectively [67, 68] (Figure 1A). The DRRs (Figure 1B) showed decreasing slope with age, reflecting increased variability, and increased hematopoietic radioresistance compared to pediatric and young adult mice, with sex-combined LD50/30s of 964 cGy (1 year) and 937 cGy (2 years). However, striking sex differences were found in radiosensitivity of the aged mice which resulted in a >100 cGy difference in LD50/30 between 2-year-old geriatric males (1008 cGy) and females (842 cGy) (Figure 1C).
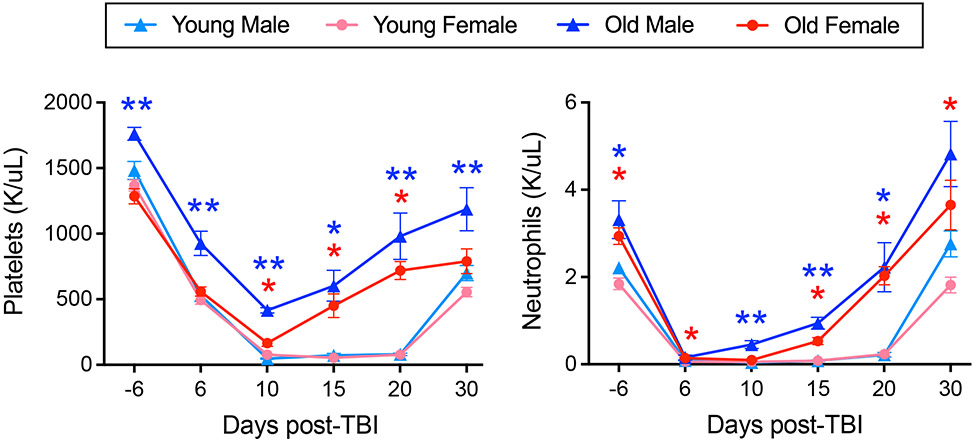
The geriatric model provided a convenient paradigm for studying differential hematopoietic radioresistance. Aged males had higher steady state platelet and neutrophil counts compared to females, while counts in both sexes were increased from their young counterparts, in accordance with reported trends from Jackson laboratories [116, 117]. Blood production is known to become increasingly myeloid-biased with HSC aging [41, 43]. Paradoxically, this hallmark of HSC aging may present an advantage for H-ARS survival, as myeloid components—namely neutrophils and platelets—are essential to prevent death from infection and hemorrhage post-irradiation [4, 5]. When a fixed radiation dose of 650 cGy [61] or 800 cGy (Figure 2) was given to young and old, male and female mice, platelet and neutrophil rebound was accelerated in aged mice and significantly higher in aged males versus females. Interestingly, following exposure to the age- and sex-specific LD70/30 for young adult mice (872 cGy), middle-aged males (1043 cGy) and females (960 cGy), and geriatric males (1124 cGy) and females (947 cGy), despite differences up to 252 cGy, functional myeloid progenitors in the BM decreased to an equivalent nadir of ~50-100 CFCs per mouse after 2 days in all age and sex groups. Thus, this low level of residual CFCs in the BM may represent a threshold for survival of approximately 30% of mice, and illustrates a correlation between the age- and sex-specific LD70/30 and number of radioresistant CFC. In line with these data, when young adult and 24 m/o geriatric male mice were exposed to the same radiation dose, significantly more CFCs remained in old BM compared to young, indicating greater radioresistance at the level of the aged HPC [61].
Figure 2. Platelet and neutrophil recovery post-TBI is accelerated in aged mice and higher in aged males.
Young (3-month-old C57BL/6J from Jackson Laboratories) and old (21-month-old C57BL/6JN from NIA) male and female mice were exposed to 800 cGy on day 0, with peripheral blood CBC analyses performed at the indicated time points using an Element HT5 veterinary hematology analyzer (HESKA Corporation, Loveland, CO); n = 4 for aged males, and n = 9-14 for other groups; one-way ANOVA with Sidak’s test was performed at each time point: *p<0.05 for old versus young male (blue) or female (red) and **p<0.05 for old male versus both young male and old female.
Accelerated CFC recovery from the nadir in aged mice [61] possibly implicated a stronger/earlier influx from upstream HSCs. Aged BM is enriched for HSCs primed for a myelopoietic inflammatory response, marked by increased expression of markers such as CD61 and CD150 [118, 119]. In our hands, expression of both CD61 and CD150 was significantly higher on HSCs in aged males versus females, suggesting a more extreme myeloid-primed aging phenotype that may in part underlie the increased hematopoietic radioresistance observed in aged males. In addition, RNA sequencing identified sex-divergent HSC aging gene signatures related to cholesterol metabolism (increased in males), interferon signaling (increased in females), and GIMAP family members (decreased in males and increased in females with age) for follow-up as potential sex-specific targets in HSC aging and radiomitigation [61].
In contrast to our observation of increased radioresistance of geriatric C57BL/6J mice compared to young adults, Spaulding et. al [21, 120] showed in the 1960s that RF-strain female mice were less radioresistant with age. There are several possible explanations for this difference. In addition to being done half a century earlier in a different lab with different experimental protocols, Mus musculus strains exhibit different degrees of radiosensitivity based on their genetics [55], which can lead to opposing experimental results. In particular, mice of the RF genetic background have significantly lower hematopoietic function compared to C57BL/6J mice [121]. In addition, males were excluded from the RF studies, whereas males were the major source of increased radioresistance with age in our models [61].
Diversity outbred models of H-ARS and DEARE
Jackson Diversity Outbred (JDO) mice are the most genetically diverse strain available, with randomly recombined genetic elements from eight founder strains uniquely comprising each animal for a more accurate model of human genetic diversity [122-125]. The unique genetic makeup of JDO mice also opens the possibility of discovering phenotypes and mechanisms not present in inbred strains [126-129], and represents an exciting model to study radiobiology. Thus, we also developed young adult JDO mouse models of H-ARS and DEARE [130].
The DRR of JDO mice showed a shallower slope of 1.02 in the probit plot compared to that of the inbred C57BL/6J young adult mice (slope = 2.56; Figure 1B), mirroring the shallower slopes of probit plots for non-human primates and humans, which are characteristic of increased variability. In comparison with the C57BL/6J model, JDO mice were more radioresistant, with an LD50/30 of 900 cGy, (Figure 1B). They were more sensitive to levofloxacin radiomitigation than C57BL/6J mice, and similarly responsive to radiomitigation and accelerated CBC recovery with Neulasta [130]. Long-term JDO survivors exhibited less severe hematopoietic DEARE than C57BL/6J mice, with increased recovery of CBCs and CFCs. While long-term JDO H-ARS survivors did show more severe loss of HSC numbers, these JDO HSCs maintained a greater degree of quiescence compared to those from C57BL/6J H-ARS survivors, which is essential for maintaining function [130]. These JDO radiation models are highly advantageous, offering the economics, lifespan, and other beneficial features of small animals, with the genetic diversity of large animals, providing a valuable and potentially more translatable option for MCM development in H-ARS and DEARE.
Conclusions
Development of MCMs against radiation relies on robust animal models, and vast differences in hematopoietic function and radiation response as a consequence of age and sex necessitate individual modeling of age and sex groups for accurate assessment of survival efficacy in at-risk populations. Models must be stringently conducted and all known variables tightly controlled for optimal results.
Funding:
Dr. Orschell received funding from the National Institute of Allergy and Infectious Diseases (NIAID) under contracts HHSN266200500043C and HHSN272201000046C and grants 1U01AI107340-01, 2R44 AI088288-03A1, and UH2/UH3AI128894-04, and National Institute on Aging (NIA) under grant R01AG046246-01, National Institutes of Health (NIH), Department of Health and Human Services (DHHS), and the Department of Defense (DOD) under grants PR140896, PR141527, and PR140433P1.
Footnotes
Conflict of Interest: Christie M. Orschell reports receiving consulting fees from NSRI. Tong Wu and Andrea M. Patterson declare that they have no conflict of interest.
Human and Animal Rights: This article does not contain any studies with human subjects performed by any of the authors. Animal studies performed by the authors (previously published and new data in Figure 2) were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kaushansky K Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354(19):2034–45. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 2.Christensen DM, Iddins CJ, Sugarman SL. Ionizing radiation injuries and illnesses. Emerg Med Clin North Am. 2014;32(1):245–65. doi: 10.1016/j.emc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Bergonie J, Tribondeau L. Interpretation of some results of radiotherapy and an attempt at determining a logical technique of treatment. Radiat Res. 1959;11:587–8. [PubMed] [Google Scholar]
- 4.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, et al. Molecular and cellular biology of moderate-dose (1-10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17-18, 2001. Radiat Res. 2003;159(6):812–34. [DOI] [PubMed] [Google Scholar]
- 5.Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology (Am Soc Hematol Educ Program). 2003:473–96. [DOI] [PubMed] [Google Scholar]
- 6.Lopez M, Martin M. Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother. 2011;16(4):138–46. doi: 10.1016/j.rpor.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC: Acute Radiation Syndrome: A Fact Sheet for Clinicians. https://www.cdc.gov/nceh/radiation/emergencies/arsphysicianfactsheet.htm#1 Accessed June 25, 2022.
- 8.Vriesendorp H, Van Bekkum D. Susceptibility to total-body irradiation. In: Broerse J, MacVittie T, editors. Response of Different Species to Total Body Irradiaton. Amsterdam: Martinus Nijhoff; 1984. [Google Scholar]
- 9.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513. [DOI] [PubMed] [Google Scholar]
- 10.Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing-radiation lethality. Health Phys. 2003;84(5):565–75. [DOI] [PubMed] [Google Scholar]
- 11.Barabanova AV, Bushmanov AJ, Kotenko KV. Acute Radiation Sickness From Chernobyl. Reference Module in Earth Systems and Environmental Sciences. Elsevier; 2019. [Google Scholar]
- 12.DiCarlo AL, Horta ZP, Aldrich JT, Jakubowski AA, Skinner WK, Case CM Jr. Use of Growth Factors and Other Cytokines for Treatment of Injuries During a Radiation Public Health Emergency. Radiat Res. 2019;192(1):99–120. doi: 10.1667/RR15363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiCarlo AL, Homer MJ, Coleman CN. United States medical preparedness for nuclear and radiological emergencies. J Radiol Prot. 2021. doi: 10.1088/1361-6498/ac0d3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford L. New drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. In: Food and Drug Administration UDoHaHS, editor. 105 ed: Federal Register; 2002. p. 37988–98. [PubMed] [Google Scholar]
- 15.FDA-CDER. Product development under the animal rule: Guidance for industry. FDA, Silver Spring, MD; 2015. [Google Scholar]
- 16.Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environmental health perspectives. 1994;102 Suppl 11:107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grahn D, Hamilton KF. Genetic Variation in the Acute Lethal Response of Four Inbred Mouse Strains to Whole Body X-Irradiation. Genetics. 1957;42(3):189–98. doi: 10.1093/genetics/42.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grahn D. Acute Radiation Response of Mice from a Cross between Radiosensitive and Radioresistant Strains. Genetics. 1958;43(5):835–43. doi: 10.1093/genetics/43.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casarett A. Radiation biology. Englewood, New Jersey: Prentice-Hall Inc.; 1968. [Google Scholar]
- 20.Yuhas JM, Yurconic M, Kligerman MM, West G, Peterson DF. Combined use of radioprotective and radiosensitizing drugs in experimental radiotherapy. Radiat Res. 1977;70(2):433–43. [PubMed] [Google Scholar]
- 21.Spalding J, Johnson O, Archuleta R. Acute radio-sensitivity as a function of age in mice. Nature. 1965;208(5013):905–6. [DOI] [PubMed] [Google Scholar]
- 22.Langendorff H, Langendorff M. [Studies on biological radiation protection. 68. Radiation sensitivity and protective effect of serotonin on mice of various ages]. Strahlentherapie. 1966;129(3):425–31. [PubMed] [Google Scholar]
- 23.Kohn HI, Kallman RF. Age, growth, and the LD50 of x-rays. Science. 1956;124(3231):1078-. [DOI] [PubMed] [Google Scholar]
- 24.Rauchwerger JM. Radiation Protection by Tibia-shielding in Adult, Weanling and Suckling Mice. Comparative Protection Studies. International Journal of Radiation Biology. 1972;22(3):269–78. [DOI] [PubMed] [Google Scholar]
- 25.Crosfill ML, Lindop PJ, Rotblat J. Variation of sensitivity to ionizing radiation with age. Nature. 1959;183(4677):1729–30. [DOI] [PubMed] [Google Scholar]
- 26.Lindop PJ, Rotblat J. The age factor in the susceptibility of man and animals to radiation. I. The age factor in radiation sensitivity in mice. Br J Radiol. 1962;35:23–31. [DOI] [PubMed] [Google Scholar]
- 27.Fred SS, Wilson SM, Smith WW. Role of intestinal injury in 30-day radiation lethality of weanling mice. Monogr Nucl Med Biol. 1968;1:413–20. [Google Scholar]
- 28.Adams TG, Sumner LE, Casagrande R. Estimating Risk of Hematopoietic Acute Radiation Syndrome in Children. Health Phys. 2017;113(6):452–7. doi: 10.1097/hp.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 29.Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. The Journal of clinical investigation. 2006;116(10):2808–16. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito T, Tajima F, Ogawa M. Developmental changes of CD34 expression by murine hematopoietic stem cells. Exp Hematol. 2000;28(11):1269–73. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, Kozlak M, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicological sciences : an official journal of the Society of Toxicology. 2002;66(2):185–200. [DOI] [PubMed] [Google Scholar]
- 32.Hattis D, Ginsberg G, Sonawane B, Smolenski S, Russ A, Kozlak M, et al. Differences in pharmacokinetics between children and adults--II. Children's variability in drug elimination half-lives and in some parameters needed for physiologically-based pharmacokinetic modeling. Risk analysis : an official publication of the Society for Risk Analysis. 2003;23(1):117–42. [DOI] [PubMed] [Google Scholar]
- 33.Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying Children's susceptibility to environmental toxicants. Environmental health perspectives. 2000;108 Suppl 1:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875–81. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 35.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. The Journal of clinical investigation. 2005;115(4):930–9. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again. Tissue Antigens. 2012;79(2):83–9. doi: 10.1111/j.1399-0039.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- 37.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 38.Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6(3):e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15(1):37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moehrle BM, Nattamai K, Brown A, Florian MC, Ryan M, Vogel M, et al. Stem Cell-Specific Mechanisms Ensure Genomic Fidelity within HSCs and upon Aging of HSCs. Cell Rep. 2015;13(11):2412–24. doi: 10.1016/j.celrep.2015.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102(26):9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MJ, Kim MH, Kim SA, Chang JS. Age-related Deterioration of Hematopoietic Stem Cells. Int J Stem Cells. 2008;1(1):55–63. doi: 10.15283/ijsc.2008.1.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108(50):20012–7. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verovskaya EV, Dellorusso PV, Passegue E. Losing Sense of Self and Surroundings: Hematopoietic Stem Cell Aging and Leukemic Transformation. Trends Mol Med. 2019;25(6):494–515. doi: 10.1016/j.molmed.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SanMiguel JM, Young K, Trowbridge JJ. Hand in hand: intrinsic and extrinsic drivers of aging and clonal hematopoiesis. Exp Hematol. 2020. doi: 10.1016/j.exphem.2020.09.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones JW, Alloush J, Sellamuthu R, Chua HL, MacVittie TJ, Orschell CM, et al. Effect of Sex on Biomarker Response in a Mouse Model of the Hematopoietic Acute Radiation Syndrome. Health Phys. 2019;116(4):484–502. doi: 10.1097/hp.0000000000000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tatsukawa Y, Misumi M, Yamada M, Masunari N, Oyama H, Nakanishi S, et al. Alterations of body mass index and body composition in atomic bomb survivors. Int J Obes (Lond). 2013;37(8):1123–8. doi: 10.1038/ijo.2012.193. [DOI] [PubMed] [Google Scholar]
- 48.Tonorezos ES, Vega GL, Sklar CA, Chou JF, Moskowitz CS, Mo Q, et al. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatr Blood Cancer. 2012;58(1):31–6. doi: 10.1002/pbc.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. •. Sridharan V, Johnson KA, Landes RD, Cao M, Singh P, Wagoner G, et al. Sex-dependent effects of genetic upregulation of activated protein C on delayed effects of acute radiation exposure in the mouse heart, small intestine, and skin. PLoS One. 2021;16(5):e0252142. doi: 10.1371/journal.pone.0252142. Findings from this study show that sex can influence effectiveness of MCM and DEARE outcomes.
- 50.Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W 3rd, Cohen DM, et al. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103(4):367–82. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacVittie TJ, Farese AM, Jackson W 3rd. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89(5):546–55. [DOI] [PubMed] [Google Scholar]
- 52.MacVittie T, Monroy R, Vigneulle R, Zeman G, Jackson W. The relative biological effectiveness of mixed fission-neutron-gamma radiation on the hematopoitic syndrome in the canine: effect of therapy on survival. Radiat Res. 1991;128(1 Suppl):29–36. [PubMed] [Google Scholar]
- 53.Moroni M, Ngudiankama BF, Christensen C, Olsen CH, Owens R, Lombardini ED, et al. The Gottingen Minipig Is a Model of the Hematopoietic Acute Radiation Syndrome: G-Colony Stimulating Factor Stimulates Hematopoiesis and Enhances Survival From Lethal Total-Body γ-Irradiation. International Journal of Radiation Oncology*Biology*Physics. 2013;86(5):986–92. doi: 10.1016/j.ijrobp.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moroni M, Coolbaugh TV, Lombardini E, Mitchell JM, Moccia KD, Shelton LJ, et al. Hematopoietic radiation syndrome in the Gottingen minipig. Radiat Res. 2011;176(1):89–101. [DOI] [PubMed] [Google Scholar]
- 55.Kallman RF, Kohn HI. The influence of strain on acute x-ray lethality in the mouse. I. LD50 and death rate studies. Radiat Res. 1956;5(4):309–17. [PubMed] [Google Scholar]
- 56.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal Models for Medical Countermeasures to Radiation Exposure. Radiation Research. 2010;173(4):557–78. doi: 10.1667/rr1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, et al. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103(4):343–55. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plett PA, Sampson CH, Chua HL, Jackson W, Vemula S, Sellamuthu R, et al. The H-ARS Dose Response Relationship (DRR): Validation and Variables. Health Physics. 2015;109(5):391–8. doi: 10.1097/hp.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrett J, Sampson CH, Plett PA, Crisler R, Parker J, Venezia R, et al. Characterization and Etiology of Swollen Muzzles in Irradiated Mice. Radiation Research. 2019;191(1):31–42. doi: 10.1667/rr14724.1. [DOI] [PubMed] [Google Scholar]
- 60. •. Patterson AM, Sellamuthu R, Plett PA, Sampson CH, Chua HL, Fisher A, et al. Establishing Pediatric Mouse Models of the Hematopoietic Acute Radiation Syndrome and the Delayed Effects of Acute Radiation Exposure. Radiat Res. 2021;195(4):307–23. doi: 10.1667/RADE-20-00259.1. This work describes well-characterized murine models of H-ARS and DEARE in pediatric mice of 6 different ages (3, 4, 5, 6, 7, and 8 weeks of age).
- 61. •. Patterson AM, Vemula S, Plett PA, Sampson CH, Chua HL, Fisher A, et al. Age and sex divergence in hematopoietic radiosensitivity in aged mouse models of the hematopoietic acute radiation syndrome. Radiation Res. 2022;InPress. This work describes well-characterized murine models of H-ARS in aged mice at 12 and 24 months old, identifying a drastic sex divergence in radioresistance with age correlating to altered hematopoietic factors.
- 62.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477(7364):289–94. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14(7):R82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanford AH, Gong H, Noonan M, Lewis AN, Gong Q, Lanik WE, et al. A direct comparison of mouse and human intestinal development using epithelial gene expression patterns. Pediatric research. 2020;88(1):66–76. doi: 10.1038/s41390-019-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling Transformations of Neurodevelopmental Sequences across Mammalian Species. The Journal of Neuroscience. 2013;33(17):7368–83. doi: 10.1523/jneurosci.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flurkey K CJ, Harrison DE. Mouse Models in Aging Research. In: Fox JGDT, Quimby FW, Barthold SW, Newcomer CE and Smith AL, editor. The Mouse in Biomedical Research (Second Edition). Burlington, MA: Elsevier; 2007. p. 637–72. [Google Scholar]
- 67.Richman C, Kutilek S, Miyakoshi N, Srivastava AK, Beamer WG, Donahue LR, et al. Postnatal and Pubertal Skeletal Changes Contribute Predominantly to the Differences in Peak Bone Density Between C3H/HeJ and C57BL/6J Mice. Journal of Bone and Mineral Research. 2001;16(2):386–97. doi: 10.1359/jbmr.2001.16.2.386. [DOI] [PubMed] [Google Scholar]
- 68.Flurkey K, Currer J, Harrison D. The Mouse in Aging Research. In: Fox JG ea, editor. The Mouse in Biomedical Research 2nd Edition. 2nd ed. Burlington: American College Laboratory Animal Medicine (Elsevier); 2007. p. 637–72. [Google Scholar]
- 69.Cerveny T, MacVittie T, Young R. Acute Radiation Syndrome in Humans. . Vol 2. Falls Church, VA: TTM Publisher; 1989:17–36. Medical Consequences of Nuclear Warfare. Falls Church, VA: Walker RI,. TTM; 1989. [Google Scholar]
- 70.Chua HL, Plett PA, Sampson CH, Katz BP, Carnathan GW, MacVittie TJ, et al. Survival efficacy of the pegylated G-CSF Maxy-G34 and Neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Physics. 2014;106(1):21–38. doi: 10.1097/HP.0b013e3182a4df10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoggatt J, Singh P, Stilger KN, Plett PA, Sampson CH, Chua HL, et al. Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells Mol Dis. 2013;50(3):147–53. doi: 10.1016/j.bcmd.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plett PA, Chua HL, Sampson CH, Katz BP, Fam CM, Anderson LJ, et al. PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Physics. 2014;106(1):7–20. doi: 10.1097/HP.0b013e3182a4dd4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, et al. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). PLoS One. 2012;7(3):e33044. doi: 10.1371/journal.pone.0033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dynlacht JR, Garrett J, Joel R, Lane K, Mendonca MS, Orschell CM. Further Characterization of the Mitigation of Radiation Lethality by Protective Wounding. Radiat Res. 2017;187(6):732–42. doi: 10.1667/RR14725.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faller DV, Castaneda SA, Zhou D, Vedamony M, Newburger PE, White GL, et al. An oral Hemokine(TM), alpha-methylhydrocinnamate, enhances myeloid and neutrophil recovery following irradiation in vivo. Blood Cells Mol Dis. 2017;63:1–8. doi: 10.1016/j.bcmd.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garrett J, Orschell CM, Mendonca MS, Bigsby RM, Dynlacht JR. Subcutaneous wounding postirradiation reduces radiation lethality in mice. Radiat Res. 2014;181(6):578–83. doi: 10.1667/RR13267.1. [DOI] [PubMed] [Google Scholar]
- 77.Patterson AM, Wu T, Chua HL, Sampson CH, Fisher A, Singh P, et al. Optimizing and Profiling Prostaglandin E2 as a Medical Countermeasure for the Hematopoietic Acute Radiation Syndrome. Radiat Res. 2021;195(2):115–27. doi: 10.1667/RADE-20-00181.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Botnick LE, Hannon EC, Hellman S. A long lasting proliferative defect in the hematopoietic stem cell compartment following cytotoxic agents. Int J Radiat Oncol Biol Phys. 1979;5(9):1621–5. doi: 10.1016/0360-3016(79)90785-5. [DOI] [PubMed] [Google Scholar]
- 79.Mauch P, Rosenblatt M, Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood. 1988;72(4):1193–6. [PubMed] [Google Scholar]
- 80.Unthank JL, Miller SJ, Quickery AK, Ferguson EL, Wang M, Sampson CH, et al. Delayed Effects of Acute Radiation Exposure in a Murine Model of the H-ARS: Multiple-Organ Injury Consequent to <10 Gy Total Body Irradiation. Health Phys. 2015;109(5):511–21. doi: 10.1097/HP.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63(17):5414–9. [PubMed] [Google Scholar]
- 82. •. Chua HL, Plett PA, Fisher A, Sampson CH, Vemula S, Feng H, et al. Lifelong residual bone marrow damage in murine survivors of the hematopoietic acute radiation syndrome (H-ARS): a compilation of studies comprising the Indiana University experience. Health Physics. 2019;116(4):546–57. This paper describes hematopoietic DEARE up to 30 months of age in H-ARS survivors from a compilation of 38 similar studies conducted over a seven-year period in the authors’ laboratory, comprising more than 1,500 irradiated young adult C57BL/6 mice and almost 600 day-30 survivors.
- 83.Chua HL, Plett PA, Sampson CH, Joshi M, Tabbey R, Katz BP, et al. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103(4):356–66. doi: 10.1097/HP.0b013e3182666d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu T, Plett PA, Chua HL, Jacobsen M, Sandusky GE, MacVittie TJ, et al. Immune Reconstitution and Thymic Involution in the Acute and Delayed Hematopoietic Radiation Syndromes. Health Phys. 2020;119(5):647–58. doi: 10.1097/HP.0000000000001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. •.Micewicz ED, Iwamoto KS, Ratikan JA, Nguyen C, Xie MW, Cheng G, et al. The Aftermath of Surviving Acute Radiation Hematopoietic Syndrome and its Mitigation. Radiat Res. 2019;191(4):323–34. doi: 10.1667/RR15231.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research. 1961;14:213. [PubMed] [Google Scholar]
- 87.Abramson S, Miller RG, Phillips RA. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. Journal of Experimental Medicine. 1977;145:1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones RJ, Celano P, Sharkis SJ, Sensenbrenner LL. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood. 1989;73(2):397–401. [PubMed] [Google Scholar]
- 89.Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming units. Nature. 1990;347:188–9. [DOI] [PubMed] [Google Scholar]
- 90.Keller G, Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990;171(5):1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Visser JWM, Bauman JGJ, Mulder AH, Eliason JF, DeLeeuw AM. Isolation of murine pluripotent hematopoietic stem cells. Journal of Experimental Medicine. 1984;159:1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hall E. Acute effects of total-body irradiation. In: Hall E, editor. Radiobiolgy for the radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. p. 124–35. [Google Scholar]
- 93.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–96. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 94.Yahata T, Takanashi T, Muguruma Y, Abd Aziz I, Matsuzawa H, Uno T, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. 2011. [DOI] [PubMed] [Google Scholar]
- 95.Simonnet AJ, Nehme J, Vaigot P, Barroca V, Leboulch P, Tronik-Le Roux D. Phenotypic and functional changes induced in hematopoietic stem/progenitor cells after gamma-ray radiation exposure. Stem Cells. 2009;27(6):1400–9. doi: 10.1002/stem.66. [DOI] [PubMed] [Google Scholar]
- 96.Abrams HL. Influence of age, body weight, and sex on susceptibility of mice to the lethal effects of X-radiation. Proc Soc Exp Biol Med. 1951;76(4):729–32. [DOI] [PubMed] [Google Scholar]
- 97.Fred SS, Smith WW. Radiation sensitivity and proliferative recovery of hemopoietic stem cells in weanling as compared to adult mice. Radiation Research. 1967;32(2):314–26. [PubMed] [Google Scholar]
- 98.Morton JI, Siegel BV. Radiation sensitivity of New Zealand black mice and the development of autoimmune disease and neoplasia. Proc Natl Acad Sci U S A. 1971;68(1):124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Genogozian N. Radiation protection studies in young mice. (Abstract) Radiation Research. 1959;11(3):444. [Google Scholar]
- 100.Quastler H. Studies on roentgen death in mice; body weight and sensitivity. The American journal of roentgenology and radium therapy. 1945;54:457. [PubMed] [Google Scholar]
- 101.Lorenz E, Jacobson L, Uphoff D, Sutton H, Schweisthal R. ANL-4451. Argonne National Laboratory; 1950. [Google Scholar]
- 102.Cole LJ, Ellis ME. Age, strain and species factors in post-irradiation protection by spleen homogenates. The American journal of physiology. 1953;173(3):487–94. doi: 10.1152/ajplegacy.1953.173.3.487. [DOI] [PubMed] [Google Scholar]
- 103.Jacobsen L, Marks E, Robson M, Gaston E, Zirkle R. The effect of spleen protection on mortality following x-irradiation. J Lab Clin Med. 1949;34:1538–43. [Google Scholar]
- 104.Hill RA, Wu YWC, Kwek P, Buuse Mvd. Modulatory Effects of Sex Steroid Hormones on Brain-Derived Neurotrophic Factor-Tyrosine Kinase B Expression during Adolescent Development in C57Bl/6 Mice. Journal of Neuroendocrinology. 2012;24(5):774–88. doi: 10.1111/j.1365-2826.2012.02277.x. [DOI] [PubMed] [Google Scholar]
- 105.Kamimura I, Watarai A, Takamura T, Takeo A, Miura K, Morita H, et al. Gonadal steroid hormone secretion during the juvenile period depends on host-specific microbiota and contributes to the development of odor preference. Developmental Psychobiology. 2019;61(5):670–8. doi: 10.1002/dev.21827. [DOI] [PubMed] [Google Scholar]
- 106.Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–8. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khong DM, Dudakov JA, Hammett MV, Jurblum MI, Khong SM, Goldberg GL, et al. Enhanced hematopoietic stem cell function mediates immune regeneration following sex steroid blockade. Stem Cell Reports. 2015;4(3):445–58. doi: 10.1016/j.stemcr.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dudakov JA, Goldberg GL, Reiseger JJ, Vlahos K, Chidgey AP, Boyd RL. Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. J Immunol. 2009;183(11):7084–94. doi: 10.4049/jimmunol.0900196. [DOI] [PubMed] [Google Scholar]
- 109.Hui SK, Sharkey L, Kidder LS, Zhang Y, Fairchild G, Coghill K, et al. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PLoS One. 2012;7(8):e42668. doi: 10.1371/journal.pone.0042668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oguro H, McDonald JG, Zhao Z, Umetani M, Shaul PW, Morrison SJ. 27-Hydroxycholesterol induces hematopoietic stem cell mobilization and extramedullary hematopoiesis during pregnancy. The Journal of clinical investigation. 2017;127(9):3392–401. doi: 10.1172/JCI94027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chapple RH, Hu T, Tseng YJ, Liu L, Kitano A, Luu V, et al. ERalpha promotes murine hematopoietic regeneration through the Ire1alpha-mediated unfolded protein response. Elife. 2018;7. doi: 10.7554/eLife.31159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fananas-Baquero S, Orman I, Becerra Aparicio F, Bermudez de Miguel S, Garcia Merino J, Yanez R, et al. Natural estrogens enhance the engraftment of human hematopoietic stem and progenitor cells in immunodeficient mice. Haematologica. 2021;106(6):1659–70. doi: 10.3324/haematol.2019.233924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu T, Liu W, Fan T, Zhong H, Zhou H, Guo W, et al. 5-Androstenediol prevents radiation injury in mice by promoting NF-kappaB signaling and inhibiting AIM2 inflammasome activation. Biomed Pharmacother. 2020;121:109597. doi: 10.1016/j.biopha.2019.109597. [DOI] [PubMed] [Google Scholar]
- 114.Steegenga WT, Mischke M, Lute C, Boekschoten MV, Pruis MG, Lendvai A, et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol Sex Differ. 2014;5:11. doi: 10.1186/s13293-014-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–43. [DOI] [PubMed] [Google Scholar]
- 116.Physiological Data Summary – Aged C57BL/6J (000664). https://www.jax.org/jax-mice-and-services/find-and-order-jax-mice/most-popular-jax-mice-strains/aged-b6 Accessed January 19 2021.
- 117.Grubb SC, Maddatu TP, Bult CJ, Bogue MA. Mouse phenome database. Nucleic Acids Res. 2009;37(Database issue):D720–30. doi: 10.1093/nar/gkn778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mann M, Mehta A, de Boer CG, Kowalczyk MS, Lee K, Haldeman P, et al. Heterogeneous Responses of Hematopoietic Stem Cells to Inflammatory Stimuli Are Altered with Age. Cell Rep. 2018;25(11):2992–3005 e5. doi: 10.1016/j.celrep.2018.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107(12):5465–70. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spalding JF, Trujillo TT. Radiosensitivity of mice as a function of age. Radiat Res. 1962;16:125–9. [PubMed] [Google Scholar]
- 121.Horland AA, McMorrow L, Wolman SR. Growth of granulopoietic bone marrow cells of RF mice. Exp Hematol. 1980;8(8):1024–30. [PubMed] [Google Scholar]
- 122.Collaborative Cross C. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics. 2012;190(2):389–401. doi: 10.1534/genetics.111.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J, et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36(11):1133–7. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- 124.Roberts A, Pardo-Manuel de Villena F, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007;18(6-7):473–81. doi: 10.1007/s00335-007-9045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, et al. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics. 2012;190(2):437–47. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Logan RW, Robledo RF, Recla JM, Philip VM, Bubier JA, Jay JJ, et al. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes, brain, and behavior. 2013;12(4):424–37. doi: 10.1111/gbb.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Recla JM, Robledo RF, Gatti DM, Bult CJ, Churchill GA, Chesler EJ. Precise genetic mapping and integrative bioinformatics in Diversity Outbred mice reveals Hydin as a novel pain gene. Mamm Genome. 2014;25(5-6):211–22. doi: 10.1007/s00335-014-9508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Niazi MK, Dhulekar N, Schmidt D, Major S, Cooper R, Abeijon C, et al. Lung necrosis and neutrophils reflect common pathways of susceptibility to Mycobacterium tuberculosis in genetically diverse, immune-competent mice. Disease models & mechanisms. 2015;8(9):1141–53. doi: 10.1242/dmm.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morton NM, Beltram J, Carter RN, Michailidou Z, Gorjanc G, McFadden C, et al. Genetic identification of thiosulfate sulfurtransferase as an adipocyte-expressed antidiabetic target in mice selected for leanness. Nat Med. 2016;22(7):771–9. doi: 10.1038/nm.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. •. Patterson AM, Plett PA, Chua HL, Sampson CH, Fisher A, Feng H, et al. Development of a Model of the Acute and Delayed Effects of High Dose Radiation Exposure in Jackson Diversity Outbred Mice; Comparison to Inbred C57BL/6 Mice. Health Phys. 2020;119(5):633–46. doi: 10.1097/HP.0000000000001344. This paper compares well-characterized models of H-ARS and DEARE developed in inbred C57BL/6 mice versus JDO mice, the most genetically diverse mouse strain available for modeling human diversity.