Abstract
The process of photon upconversion promises importance for many optoelectronic applications, as it can result in higher efficiencies and more effective photon management. Upconversion via triplet–triplet annihilation (TTA) occurs at low incident powers and at high efficiencies, requirements for integration into existing optoelectronic devices. Semiconductor nanocrystals are a diverse class of triplet sensitizers with advantages over traditional molecular sensitizers such as energetic tunability and minimal energy loss during the triplet sensitization process. In this Perspective, we review current progress in semiconductor nanocrystal triplet sensitization, specifically focusing on the nanocrystal, the ligand shell which surrounds the nanocrystal, and progress in solid-state sensitization. Finally, we discuss potential areas of improvement which could result in more efficient upconversion systems sensitized by semiconductor nanocrystals. Specifically, we focus on the development of solid-state TTA upconversion systems, elucidation of the energy transfer mechanisms from nanocrystal to transmitter ligand which underpin the upconversion process and propose novel configurations of nanocrystal-sensitized systems.
Keywords: upconversion, nanocrystals, excitons, luminescence, energy transfer
1. Introduction
Manipulation of photon energy is a promising approach toward utilizing the solar spectrum to its full potential. In particular, the generation of higher energy photons from lower energy photons via photon upconversion (UC) has potential to advance photovoltaics (PV)1,2 beyond the detailed-balance limit of single-junction solar cells.3 In addition, UC has potential applications in other disciplines4 such as biological imaging,5 and optogenetics.6 To ensure compliance with fundamental energy conservation laws, a minimum of two lower energy photons are combined to generate the desired higher energy photon. For successful integration into devices and widespread technologies, UC systems which efficiently upconvert light across the electromagnetic spectrum with low incident powers must be designed. These aims have been met most consistently through the process of triplet–triplet annihilation (TTA).7 Since TTA is mediated through a long-lived spin-triplet state, TTA is typically more efficient at lower incident powers than other UC techniques,8 especially those taking advantage of the ladder-like 4f states of lanthanide ions.9 A schematic detailing the process of TTA is shown in Figure 1: the combination of two anticorrelated triplet states results in the population of an excited singlet state in a spin-allowed process. Radiative relaxation of the generated spin-singlet state yields the desired higher energy photon.
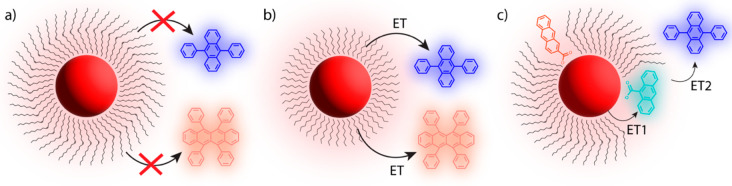
Figure 1.
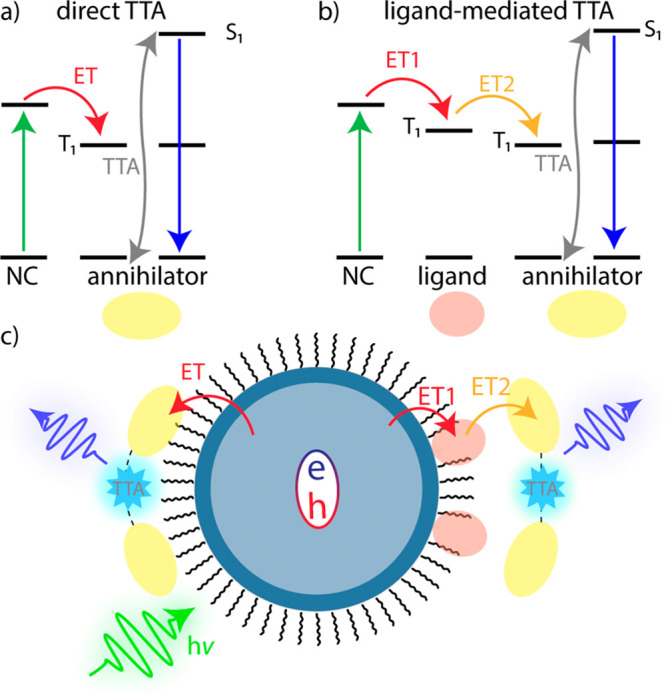
Schematic of NC-sensitized TTA-UC. (a) Direct TTA: Triplet excitons are transferred directly from the NC to an annihilator via triplet energy transfer (ET). TTA yields the high energy singlet state. (b) Ligand-mediated TTA: Triplet excitons are transferred from the NC to a surface-bound transmitter ligand. A second ET step populates the annihilator, enabling TTA. (c) Illustration demonstrating the two triplet sensitization pathways. Direct ET to the annihilator occurs (left) and ligand-mediated population of the annihilator triplet state (right).
The rapid generation of triplet states in TTA-active molecules termed triplet annihilators, or, simply, annihilators, cannot occur directly, since direct singlet-to-triplet absorption is a spin-forbidden process. Thus, a triplet sensitizer capable of populating the triplet state of an organic annihilator by, e.g., energy transfer, is commonly employed.8 Decades after the seminal work by Parker and Hatchard, which reported “delayed fluorescence” from anthracene and phenanthrene,10 early works by the Castellano group11 and Baluschev et al.(12) coupled metal–organic triplet sensitizers with organic annihilators and prompted the rapidly evolving field of sensitized TTA-UC. The development of metal–organic triplet sensitizers paved the way for novel triplet sensitization routes, including direct singlet-to-triplet absorbing sensitizer materials,13 bulk14,15 and two-dimensional (2D) semiconductors,16,17 and semiconductor nanocrystals (NCs).18
NCs have tunable band gap energies due to quantum confinement.19 The development of NCs in the past few decades has realized highly emissive materials with tunable band gap energies throughout the visible and infrared (IR) spectrum.20 High photoluminescence (PL) quantum yields (QYs),21 narrow emission line widths,22 and synthetical band gap tunability23 have enabled their widespread application in biosensing/bioimaging,24 lighting,25 photovoltaics,26 and triplet sensitization.18,27
Put together, NCs can serve as efficient triplet sensitizers capable of populating triplet states in organic molecules. While the scope of this Perspective focuses on NC-sensitized TTA-UC, the broad concept of triplet sensitization can also be used to drive photochemical transformations.28,29 More recently, NC triplet sensitizers have served as photocatalysts, mediated through a triplet–triplet energy transfer process.30,31 Thus, advancements in NC triplet sensitization for use in TTA-UC schemes could yield more efficient NC photocatalysts, and vice versa.32
In this Perspective, we focus specifically on current research which utilizes NC-sensitized triplet generation for TTA-UC. Specifically, we begin with a concise review, discuss current challenges, and address the requirements to improve NC-sensitized TTA-UC.
2. Semiconductor Nanocrystals as Triplet Sensitizers: A Short Review
2.1. Molecular Sensitization and the Development of Semiconductor Nanocrystal Sensitizers
Initially, metal–organic complexes were employed as triplet sensitizers in TTA-UC schemes. The heavy atom effect induces spin–orbit coupling, allowing for facile intersystem crossing (ISC) and, thus, triplet generation in the sensitizer molecule.33 However, the energy gap between singlet and triplet states in metal–organic complexes leads to a large inherent energetic loss typically on the order of 0.3–1 eV,13,34,35 motivating the search for other sensitization routes. The observation that lead chalcogenide NCs can act as triplet acceptors via energy transfer from conjugated organic chromophores,36 in concert with the motivation for lessened exchange losses, paved the way to the first series of NC-sensitized TTA-UC systems.37,38
In all, NCs are promising triplet sensitizers for many reasons but especially due to their electronic fine structure. Direct energy transfer to organic molecular triplet states can occur upon excitation across the band gap, as singlet/triplet splitting (bright/dark state splitting) is on the order of 10 meV, which is significantly less than those of their metal–organic counterparts.39,40 The unique fine excitonic structure of NCs can be studied using both variable temperature and magnetic field in combination with PL spectroscopy characterization.41−43 In addition, low temperature studies can be used to unravel the role of defect sites in triplet energy transfer (TET).44 This direct energy transfer mechanism is shown in detail in Figure 1a, where the triplet state (T1) of an annihilator molecule can be populated through a Dexter-type exchange transfer (ET) of an exciton upon excitation across the band gap.45
In less than a decade, research focused on employing NCs as triplet sensitizers for TTA-UC schemes has rapidly expanded. Early difficulties, namely, the interplay between imperfect NC surface passivation and the long-chain native organic ligands that impede energy transfer, were among the first problems to solve. Passivation strategies and core–shell heterostructures have been utilized to solve the former,46,47 while the use of surface-bound triplet acceptors, typically called transmitter ligands, which serve as a mediator between the sensitizer and annihilator, have mitigated the latter.48 This novel approach, introduced by Tang and co-workers in 201537 is shown in Figure 1b, where a bound photoactive ligand mediates energy transfer between the nanocrystal and the triplet annihilator, enabling TTA-UC upon the population of two excited annihilator triplet states (Figure 1c).
Here, we briefly review the seminal works that have formed the foundation of NC sensitization with three focus directions. First, we discuss the nanocrystal sensitizer itself, in terms of its composition, passivation, and morphology. We then turn to the ligand surface, both native ligand and transmitter ligand added postsynthesis. Finally, we turn our attention to solid-state TTA-UC devices.
2.2. Semiconductor Nanocrystal Sensitizers: Composition, Morphology, and Heterostructures
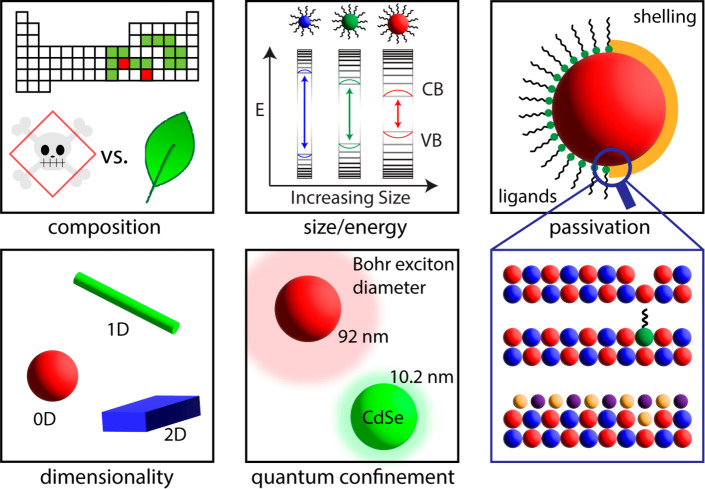
First, we briefly review the developments in NC sensitization focused specifically on the NC, as summarized in the schematic in Figure 2. Specifically, improvements to the NC composition, morphology, size, and surface passivation have resulted in less toxic and more efficient TTA-UC systems. Varying the NC composition can tune the energy while also exploring novel NC compositions that are nontoxic, which could be especially useful in vivo. Likewise, exploring different NC morphologies can endow novel properties on the TTA-UC system resulting from the unique NC shape. Additionally, the NC size can tune the efficiency of energy transfer through the energetic driving force and the degree of electronic coupling between NC and triplet mediator, while the degree of surface passivation can reduce the likelihood of parasitic pathways to energy transfer. Here, we briefly review the developments in these areas.
Figure 2.
Schematic summarizing the approaches of NC-centered modifications that have been implemented toward improving TTA-UC. Top left: emphasis on development of nontoxic NCs. Top middle: the energy of a NC band gap is size dependent. Top right: passivation of the NC surface is often achieved by ligand addition or coating the surface with another material. Bottom left: the number of dimensions in which NCs are confined can be altered. Bottom middle: the Bohr exciton diameter, and therefore the possible extent of quantum confinement, varies between NC compositions. Bottom right: at the surface of the NCs, vacancies can be filled by the binding group of the ligand or by the shell material.
2.2.1. Composition
The chemical composition of the NC is an important determining factor of successful triplet sensitizer functionality within TTA-UC systems. Huang et al. demonstrated NC-sensitized TTA-UC in a seminal work in 2015, where TTA-UC was achieved in both PbSe and CdSe NC-sensitized systems.37 In both UC schemes, organic molecules with accessible and long-lived triplet states were chosen as triplet acceptors/annihilators. Pairing a near-infrared (NIR) absorber, PbSe NCs, with a visible emitter, rubrene, resulted in NIR-to-visible UC; similarly, pairing a green-absorbing CdSe NC with blue-emitting 9,10-diphenylanthracene (DPA) resulted in green-to-blue UC. While the PbSe NCs directly sensitized the triplet state of the rubrene annihilator, the CdSe NCs required a triplet mediating ligand to sensitize the DPA annihilator. Incorporation of 9-anthracene carboxylic acid (9-ACA) as the transmitter ligand for a CdSe NC-sensitized system enabled efficient TTA-UC in DPA. Transmitter ligands for NIR PbS and PbSe NCs have also been developed since this initial work to enable more efficient NIR-to-visible UC.49,50 For more information on transmitter ligands, we refer the reader to section 2.3.
After this seminal work, many attempts have been made to improve NC-sensitized TTA-UC systems18 in three areas, which can be quantified using three figures of merit. The UC QY is a measurement of the efficiency of the UC process as a whole, i.e., the percent of UC photons emitted over the photons absorbed by the sensitizer,8,51 analogous to a standard PL QY measurement. The maximal UC QY is 50%, as TTA-UC is a two-to-one photon conversion process, and most NC-sensitized TTA-UC works have normalized this UC QY to a UC efficiency, multiplying the UC QY by a factor of 2 to normalize to 100%. For clarity, all UC efficiencies reported in this Perspective are absolute UC QY, where some normalized values were divided in half from their originally reported values. Additionally, the power threshold, Ith, describes the power density at which TTA becomes the dominant triplet decay pathway.52,53 Ideally, this power threshold will be as low as possible. Finally, the apparent anti-Stokes shift describes the shift in energy of the UC emission relative to the sensitizer absorption.54 Overall, we can summarize the progress of the works briefly reviewed and summarized here in Table 1, where the overall system compositions (sensitizer/transmitter ligand/annihilator, UC QY, power threshold Ith, sensitizer absorption wavelength, and UC emission wavelength) are given. We note here though that not all values are perfect comparisons, as the power threshold Ith depends on the sample absorbance and the excitation wavelength.51
Table 1. Table Summarizing Various TTA-UC Systems Described in This Perspective as Well as Their Respective Upconversion Quantum Yields (UC QY), the Power Threshold Ith, the Peak Absorption Wavelength of the Sensitizer (λabs), and the Peak UC Emission Wavelength (λem)a.
| TTA-UC system composition | UC QY (%) | Ith (mW/cm2) | λabs (nm) | λem (nm) | ref |
|---|---|---|---|---|---|
| solution-based UC | |||||
| PbSe NC/rubrene | 0.005 | N/A | 980 | 560 | (37) |
| CdSe NC/9-ACA/DPA | 4.5 | N/A | ∼530 | 425 | (37) |
| PbS NC/CPT/rubrene | 0.85 | N/A | 907 | 560 | (49) |
| PbSe NC/CPT/rubrene | 1.07 | N/A | 845 | 560 | (49) |
| PbS/CdS NC/5-CT/rubrene | 4.2 | 3.2 | 850 | 560 | (50) |
| CdSe:Au NC/9-ACA/DPA | 12 | 200 | 495 | 425 | (55) |
| CsPbX3 NC/AEDPA/DPA | 0.65 | 25 | ∼610 | 425 | (56) |
| CuInS2/ZnS NC/9-ACA/DPA | 9.3 | 4700 | 505 | 425 | (57) |
| InP/ZnSe/ZnS NC/9-ACA/DPA | 5.0 | 0.57 | 540 | 425 | (58) |
| ZnSe/ZnS NC/BCA/DTBN | 3.1 | 2.4 | 400 | 335 | (59) |
| Si NC/9-EA/DPA | 7.5 | N/A | N/A (488 nm ex) | 425 | (60) |
| CdSe NC/9-ACA/DPA | 7.9 | N/A | 511 | 425 | (61) |
| CdTe NR/9-ACA/DPA | 1.92 | 93 | 612 | 425 | (62) |
| CdSe NPL/9-ACA/DPA | 2.52 | 237 | 545 | 425 | (63) |
| PbS-ZnS NC/rubrene | 0.14 | N/A | 983 | 560 | (64) |
| PbS NC/5-CT/rubrene | 5.9 | 53 400 | 836 | 560 | (65) |
| CdSe NC/9-ACA/DPA | 5.9 | N/A | 504 | 425 | (66) |
| CdSe NC/1-ACA/DPA | 1.9 | N/A | 504 | 425 | (66) |
| CdSe NC/1-ADTC/DPA | 1.5 | N/A | 504 | 425 | (66) |
| CsPbBr3 NC/2-ACA/DPA | 6.5 | 6900 | 450 | 425 | (67) |
| CdSe NC/ADP/DPA | 8.5 | 163 | 500 | 425 | (68) |
| PbS NC/TES-ADT | 0.145 | 66 000 | 864 | 580 | (69) |
| CdSe NC/DPA | 3.47 | N/A | 535 | 425 | (70) |
| solid-state devices | |||||
| PbS NC/rubrene/DBP | 3.5a | N/A | 790 | 610 | (71) |
| PbS NC/rubrene/DBP/AlQ3 | 0.8a | 11 000 | 960 | 560 | (72) |
| PbS NC/rubrene/DBP (microcavity) | 0.05 | 13 | 960 | 560 | (73) |
| CdSe NC/9-ACA/DPA | 1.5 | N/A | ∼520 | 425 | (74) |
| CdSe/CdS NC/anthracene MOF | 0.00045 | 1000 | ∼620 | ∼440 | (75) |
Internal UC QYs normalized to the QY of rubrene/DBP.
The NC composition determines the Bohr exciton diameter and the spectrum of possible band gap energies and thus, some compositions will be better suited for certain applications. The previously introduced work by Huang et al. highlights this idea, where matching compatible NC compositions with annihilator molecules results in different apparent anti-Stokes shifts.37 The electronic properties of the NC can also be tuned by doping, which can be used to adjust the energetic alignment between NC and annihilator. For example, Ronchi et al. doped CdSe NC sensitizers with gold in order to mitigate alternative nonradiative decay pathways, such as hole transfer, thus allowing the excitonic energy transfer from NC to transmitter ligand (denoted ET1) to be a more favorable process.55
In addition to IV–VI and II–IV semiconductor NCs, perovskite nanocrystals (PeNCs) have become a popular triplet sensitizer since the initial report in 2017, where green-to-blue UC was achieved using CsPbBr3 PeNCs.56,76 While IV–VI, II–IV, and PeNC compositions are among the most widely used for TTA-UC, there is apprehension on account of their toxic Cd- and Pb-based compositions. This is particularly relevant when considering the possibility of implementing these systems into in vivo applications such as bioimaging or optogenetics.6,77 Thus, the development of more benign NCs for TTA-UC has been pursued.57
Wu and co-workers were the first to report nontoxic NC-sensitized TTA-UC by using shelled ternary CuInS2 NCs, 9-ACA transmitter ligands, and DPA annihilators (see section 2.2.4 for more about NC passivation and shelling).57 Furthermore, InP NCs have been pursued to replace the more toxic II–VI NCs,58 but the low native PL QYs of InP NCs indicates that surface passivation is required for use in optoelectronic applications.78 For ultraviolet (UV)-to-visible UC, Wu and co-workers have recently reported TTA-UC sensitized by ZnSe/ZnS NCs, representing an important first step in UV light generation using less toxic NCs.59 Si NCs have also been utilized as triplet sensitizers in NIR-to-visible TTA-UC systems that can maintain UC performance under ambient conditions for longer time periods than the more typical CdSe NC-based systems due to polymerization of the passivating ligands on the NCs.60 The enhanced oxygen resistance of the Si system was significant given that spin-triplet states are quenched by oxygen. The oxygen sensitivity of TTA-UC systems is an important hurdle to overcome, especially for in vivo systems. In all, the development of nontoxic NC sensitizers is vital for the pursuit of TTA-UC in vivo.
2.2.2. Size Dependence
One of the most notable features of NCs is their size-dependent band gap.79 Small NCs, relative to the Bohr exciton diameter, will have a higher band gap due to a larger degree of quantum confinement. NC sizes can be varied by the synthetic conditions, such as temperature or growth time. For triplet sensitization, it is vital that the NC size be tuned such that sufficient driving force for energy transfer to a triplet acceptor is obtained.80 In line with the Marcus normal regime,81 an increase in energetic driving force from NC sensitizer to triplet transmitter ligand (ET1 in Figure 1b and c) generally leads to a higher energy transfer rate and efficiency, which can in turn contribute to a higher UC efficiency.61,82−84 Indeed, this trend has been demonstrated in both CdSe NCs and Pb-based NC-sensitized systems.61,82,83 Since increasing the driving force results in greater energetic losses during ET1, the increased UC efficiencies must be weighed against the cost of reduction of the apparent anti-Stokes shift.85
Utilization of smaller NCs has also been observed to correlate to longer excited triplet lifetimes of the bound transmitter ligand, measured by transient absorption spectroscopy.61 Such elongated transmitter ligand lifetimes allow more time for diffusion-mediated energy transfer from the transmitter ligand to the annihilator (ET2 in Figure 1b) to occur.86 While the exact reasoning for this observation is not yet fully understood, this is yet another way in which smaller (more confined) NCs have been shown to have superior sensitization performance.
Wu and co-workers have argued that the wave function amplitude on NC surfaces, rather than the energetic driving force, is the primary source of improved ET1 outcomes and UC efficiencies for smaller sized NCs.67,84,87 Given that NCs with a greater degree of quantum confinement feature relatively higher exciton wave function leakage beyond the NC surface19,88 and ET1 is a Dexter-type process that requires wave function overlap between donor and acceptor,45 it should follow that smaller, more quantum confined NCs are more effective sensitizers.45 Indeed, a linear relationship between the rate of ET1 and the carrier probability density at the NC surface was observed in CsPbBr3 NC-sensitized systems.84 This theory may also explain the observation that PbSe NCs, with a Bohr exciton diameter of 92 nm, outperform comparable PbS NCs, with a Bohr exciton diameter of 32 nm.37,83,89 Additionally, it explains why CdSe NCs with a Bohr exciton diameter of 10.2 nm must be functionalized with transmitter ligands in order to produce relatively efficient UC, as shown in the seminal work on NC-sensitized UC.37,83,89
2.2.3. Variation of Morphology
Most commonly, sphere- or cube-shaped quantum NCs which are confined in all three dimensions, i.e.., zero dimensional (0D) NCs or quantum dots (QDs), have been used as triplet sensitizers. Quantum confinement effects can be relaxed in one or two dimensions, resulting in one-dimensional (1D) NCs, such as nanorods (NRs)90 or nanowires,91 or two-dimensional (2D) NCs, such as nanoplatelets (NPLs),92 or nanosheets.93 The elongation of these materials leads to unique anisotropic properties which could be leveraged in UC schemes. For example, 1D NRs and 2D NPLs can be readily self-assembled into ordered macrostructures with oriented dipole moments,94−96 which could be especially useful for solid-state NC sensitization. Additionally, CdSe NPLs have received attention97 on account of their higher relative PL QYs than CdSe QD counterparts,98 quantized emission dependent upon their thickness (by monolayer),99 and large absorption cross sections.100 Recently, both NRs and NPLs have been used as triplet sensitizers by Nienhaus and co-workers.62,63
While different NC morphologies can be manipulated to harness their unique electronic and optical responses, doing so also presents a unique set of challenges. Specifically, direct comparison of 5.5 monolayer CdSe NPL to CdSe QD sensitized ACA/DPA UC systems resulted in UC efficiencies that were three times higher for the QD-sensitized systems compared to the NPL-sensitized system.63 Interestingly, the power threshold Ith for the NPL-sensitized system was less than half of that of the QD-sensitized system (237 mW/cm2 compared to 574 mW/cm2), indicating the strong promise of the NPL-based system, despite the lower efficiency. The discrepancy in NPL versus QD sensitized TTA-UC efficiency was partially attributed to NPL stacking in solution,101 which limits the overall efficiency of the energy transfer processes due to the possibility of buried transmitter ligands (ET2 inefficient) and inaccessible binding sites (ET1 inefficient). In addition, the high oscillator strength of the NPLs, especially compared to the QDs, results in a higher probability of back transfer to the NPL sensitizer, which is the likely root cause of the observed discrepancies in efficiency.
CdTe NRs have also been used as triplet sensitizers in an 9-ACA/DPA UC system showing red-to-blue upconversion with a large apparent anti-Stokes shift.62 Again, low Ith values were observed, (93 mW/cm2) and UC efficiencies comparable to the NPL-sensitized system were obtained, even with a nearly 0.4 eV lower energetic driving force for ET1. The NR-sensitized system could benefit from further optimization, where more uniformly shaped NRs could potentially yield more efficient triplet sensitization due to better and more uniform coupling between the NR and the transmitter ligand. Further, the ordered self-assembly of NRs, especially in the solid-state,94 could be useful in increasing the UC QY of solid-state devices. In addition, the combination of ordered NRs with an ordered annihilator film may enable directed TTA-UC, as well as polarization-dependent UC for optical switching.102
2.2.4. Passivation and Heterostructures
Historically, long-chain organic ligands have been used to passivate the surface of NCs and minimize trap emission and nonradiative decay processes.103,104 Thus, care must be taken to properly passivate surface defects such as dangling bonds to maximize the PL QY through the addition of ligands or other agents that passivate the surface, since it has been demonstrated that higher native PL QYs can lead to higher UC efficiencies.82 Additionally, the long-chain organic ligands impart colloidal stability and allow for dispersion in nonpolar solvents such as toluene or hexanes.103
The growth of an epitaxial shell on the surface of a NC is a common approach for achieving NC surface passivation,105,106 and different configurations of core/shell NCs are found depending on the energetic alignment between core and shell valence and conduction bands.107 Type I band alignment, where the core bands are sandwiched between the shell bands, is most commonly employed in triplet sensitization schemes.46,50,85,108−110 Here, the shell has two roles: the wide band-gap shell passivates the core NC surface and spatially confines the exciton to the core. However, as a result, the shell becomes a tunneling barrier for ET1. Therefore, a marked interplay between the core–shell native PL QY and the UC efficiency exists as the shell thickness changes.50 A thin shell will typically cause an increase in both the native PL QY of the NC and UC efficiency, since the shell passivates the surface ions of the core, resulting in the suppression of parasitic nonradiative decay pathways, i.e., charge trapping at defect sites. A thicker shell, on the other hand, results in a decrease in the rate of ET1, since the tunneling barrier is wider and the wave function overlap between the donor and acceptor is reduced.50 Thus, depending on the degree of surface passivation by the shell, the shell thickness for optimal UC efficiency will vary.50,108 Additionally, the energetic difference between the core and shell can impact ET1, where minimizing the core/shell energetic difference decreases the damping coefficient for Dexter energy transfer, thereby increasing the probability of tunneling, thus facilitating more efficient ET1.50 Care must also be taken to choose the core and shell composition such that interfacial lattice strain is minimized, which can also be alleviated by the addition of an intermediate shell.107
In a deviation from a typical type I core/shell NCs for TTA-UC, Zamkov and co-workers have touched on TTA-UC sensitized by CdS/CdSe core/nanoshell structures.111 This structure is made up of a bulk-sized core and a shell quantum confined in one dimension.111 This results in a reverse type I band alignment such that the exciton is localized in the shell.112 It has been proposed that localization of excitons in the quantum-confined shell might result in more wave function overlap between NC triplet donor and transmitter ligand triplet acceptor.111
NC surfaces can also be passivated through surface treatments such as ligand exchange113,114 or addition of adsorbing ions (e.g., metal salts)115,116 or alternatively through synthetic precursor adjustments.117 Passivation by these methods may be favored over shelling, as the incorporation of a tunneling barrier for ET1 is avoided. Different NC compositions may require different passivation techniques because of the unique NC surface and crystal structure where one passivation technique may have an advantage over the other. Specifically, the ligand binding energies are facet dependent, and can vary depending on whether a CdSe NC, for example, is wurtzite or zinc blende.118,119 Computational studies investigating the crystal structures of various NC compositions can provide insight into trap sites and how to mitigate them on a case-by-case basis.120
For CdSe NC systems, surface trap sites have been theorized to result from a lone pair on Se or an empty orbital on Cd.115,121 A density functional theory (DFT) study predicted that empty sites resulting from undercoordinated surface anions along the NC surface were the sole source of traps in both II–VI and III–V semiconductor NCs.122 Experimental evidence supporting this prediction was observed in CdTe NCs where the addition of two electron-accepting Z-type ligands led to significantly improved PL QYs exceeding 90%.122 The exact mechanism for II–VI and III–V NC surface passivation is not yet fully understood, especially since treatment with L-type Lewis base ligands can also be used to improve PL QYs of CdSe NCs, albeit to a lesser degree than the Z-type ligands, presumably by filling the empty orbitals on Cd.121 While Z-type ligand treatment of II–VI NC sensitizers in UC schemes has not yet been demonstrated, L-type ligand treatment of NC sensitizers has led to an increase in the rate of ET1,47 likely due to the fact that trap-induced nonradiative recombination processes in the NC were circumvented.
The addition of adsorbing cations, or sub-monolayer shells, has been used for passivation of PbS NC triplet sensitizers.64 Treating PbS NCs with Cd2+ (or Zn2+) prior to the addition of the rubrene annihilator solution led to an increase in the UC QY of several orders of magnitude compared to untreated NC systems. The authors speculated that adsorbate addition leads to both passivation and generation of surface defects, with the latter yielding an energetic state within the band gap that promotes direct ET to rubrene. Further study is warranted in order to verify these claims spectroscopically, as it is unclear whether the passivation in this manner allows for differences in the ET, or, simply causes a change in the ligand shell through the addition of Z-type ligand metal salts.115
The overall quality of a NC surface can also be influenced by adjustment of the synthetic precursors. In a study by Tang and co-workers, PbS NCs made with two different sulfur precursors, bis(trimethylsilyl) sulfide and thiourea, were compared, with the resultant NCs denoted PbS-S and PbS-T, respectively.65 While the native PL QY of PbS-T was no more than 5% greater than that of PbS-S, the efficiency of ET1 from PbS-T was over 10% higher than that of its counterpart. Improved ET1 efficiencies resulted from the elongation of NC lifetimes resulting from a higher-quality NC surface. With the better passivated surface, the systems with PbS-T NCs had a UC QY of 5.9% compared to only 2.3% for the PbS-S NC system. This demonstrates how precursor choice affects not only PLQYs but can fundamentally alter the NC surface on an atomic scale, which in turn affects energy transfer capabilities.
2.3. Triplet Mediation via Triplet Transmitter Ligands
As previously mentioned, triplet transmitter ligands are often used in NC-sensitized TTA-UC schemes to enable or improve the ET1 efficiency. Transmitter ligands are triplet acceptors that directly bind to the NC surface, resulting in enhanced wave function overlap between triplet donor and acceptor, and thus more efficient Dexter-type ET.37 Transmitter ligands are especially important considering that UC efficiencies have been shown to be primarily dependent on the efficiency of ET1.86 Common motifs for transmitter ligands involve anthracene or tetracene based backbones with carboxylic acid functionalization to bind to the NC surface as an X-type ligand.37,49,50,108 The anthracene or tetracene moieties of the transmitter ligands resemble the backbones of common annihilators, such as DPA and rubrene, respectively. The triplet energy level of the transmitter ligand must be conducive to its role as both a triplet donor and acceptor, enabling downhill ET1 and ET2 processes while minimizing reverse ET.48
The widespread use of the 9-ACA transmitter ligand for CdSe NCs is, in part, inspired by a 2017 study by Xia et al. comparing the performance of anthracene isomers functionalized with either carboxylic acid (ACA) or dithiocarbamate (ADTC) binding groups as transmitter ligands for CdSe NCs and freely diffusing DPA as the annihilator.66 In this comparative analysis, systems utilizing 9-ACA transmitter ligands demonstrated the highest UC efficiencies and the fastest rates of ET1. While 9-ACA and 9-ADTC exhibited similar rates for ET1, the UC QY for the 9-ADTC-sensitized system was significantly lower than that sensitized by 9-ACA (0.05% versus 5.95%).66 This comparison demonstrates the importance of considering the entire TTA-UC system when optimizing a single component. Here, the 9-ADTC transmitter ligand was able to effectively quench the NC but could not efficiently perform ET2 to the annihilator, likely due to the presence of other quenching pathways, including hole transfer.
The aforementioned study also compared the 1-, 2-, and 9- locations of the binding group on the transmitter ligands.66 The variation in UC efficiency for each position was a result of how well the orientation of the anthracene moiety enabled Dexter-type ET with the NC. Thus, it was no coincidence that the 9-functionalized isomers had the highest rate of ET1 for both carboxylic acid and dithiocarbamate binding groups. The ET1 rates for the 2-functionalized isomers were the lowest, so it can be interpreted that the ligand orientation created by the 2-functionalization is the least conducive to Dexter-type ET. He et al. later demonstrated that 2-ACA is actually a better transmitter ligand than 9-ACA for weakly quantum confined NCs.67 This result was attributed to a difference in ET1 mechanism depending on the degree of quantum confinement. Specifically, in weakly confined NCs, ET1 occurs more efficiently via a through-bond coupling, enabled by the resonance effect in 2-ACA, while in strongly confined NCs ET1 occurs most efficiently through space by a direct wave function overlap. This is reflected in rapid ET1 in strongly confined NCs to 9-ACA, where the anthracene moiety is physically closer in space to the NC than it would be for 2-ACA, resulting in efficient ET1 enabled by the high degree of wave function leakage in strongly confined NCs. Thus, transmitter ligands should be selected based on the optimal ET1 mechanism based on the extent of quantum confinement in the NC.
While monodentate transmitter ligands like 9-ACA are most often employed, bidentate transmitter ligands have also been reported.68,123 Just as with monodentate ligands, the geometry of bidentate ligands with respect to the NC surface can be controlled in order to maximize wave function overlap.123 The UC QY obtained in a study using bidentate anthracene diphosphate (ADP) ligands was 8.5%.68 In comparison with an analogous 9-ACA transmitter ligand system, the ET1 rate was roughly one order of magnitude higher for the ADP ligand system. It is worth noting that the diphosphate groups have a much stronger binding affinity to the NC surface than the carboxylic acids of 9-ACA do, and therefore it was supposed that the ligands were much more strongly coordinated in the case of ADP.
Transmitter ligand binding can be a dynamic process (see section 3.2). The solubility of the transmitter ligand in the solvent medium will affect the number of ligands bound to each NC.82 Using solvents in which the transmitter ligand is weakly soluble is helpful in order to preferentially promote ligand binding to the NC and results in higher UC efficiencies. Addition of the annihilator solution can also alter the transmitter ligand solubility, and should be taken into consideration.124
In lieu of distinct transmitter ligands and annihilator molecules, directly attached 5,11-bis(triethylsilylethynyl)anthradithiophene annihilators on PbS NCs have been used to obtain NIR-to-visible TTA-UC.125 It was theorized that the annihilator binding might be a dynamic process, with annihilators detaching from the NC surface after triplet sensitization, but this theory warrants further study. While the UC QYs for this system were below 0.05%, they were later optimized to 0.145% by Tripathi et al. by ligand exchanging native ligands for shortened passivating ligands prior to addition of attached annihilators.69
The replacement of native passivating ligands with shorter passivating ligands has also been used in absence of triplet transmitter ligands. In doing so, direct Dexter-type TET to the annihilator is enhanced or enabled. This was demonstrated by Nienhaus et al. in a solid-state PbS NC rubrene annihilator thin film where the passivating ligand on the PbS NC was varied by carbon chain length, from the 18-C native oleic acid ligand down to a 4-C ligand.71 6-C ligands enabled high TET rates and sufficient surface passivation, yielding internal UC QYs of 3.5%, compared to 0.6% for oleic acid.
Recently, Miyashita et al. reported that replacing native oleic acid ligands on CdSe NCs with 8-C octanoic acid ligands enabled direct sensitization from the NCs to the freely diffusing DPA annihilator, resulting in a UC QY up to 3.47%.70 This is especially significant considering that transmitter ligands had previously been required for CdSe NCs to be used as sensitizers.37
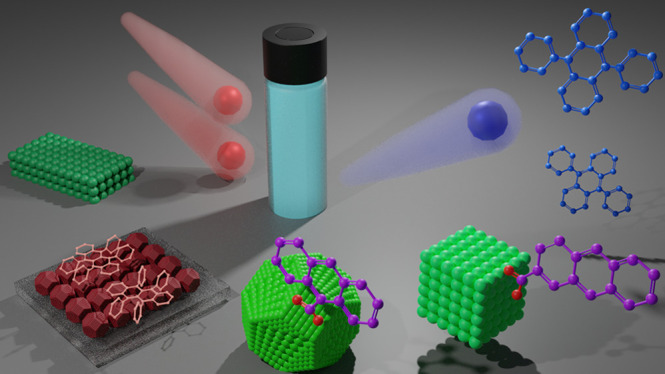
Optimization of transmitter ligands has been a major driver of improvements for UC QYs in NC-sensitized TTA-UC systems since their inception, summarized in Figure 3. When native, long-chain ligands are employed, like 18-C oleic acid, direct sensitization is difficult, demonstrated in Figure 3a. While shortening the passivating ligands can be used to achieve direct TET to annihilators and obtain UC emission (Figure 3b), incorporating transmitter ligands tends to yield much higher efficiencies (Figure 3c).
Figure 3.
Schematics of energy transfer in various UC systems. (a) ET from NCs to freely diffusing annihilators impeded by presence of long, 18-C oleic acid passivating ligands. (b) Direct ET from NCs to freely diffusing annihilators occurring when 8-C octanoic acid passivating ligands are used. (c) ET1 from NC sensitizer to 9-ACA transmitter ligand followed by ET2 from 9-ACA to DPA annihilator. The 2-ACA transmitter ligand is shown on the left of the NC; there is less wave function overlap between the 2-ACA and the NC as compared to the 9-ACA and the NC.
2.4. Initialization and Development of Solid-State Semiconductor NC-Sensitized TTA-UC Devices
NC-sensitized TTA-UC is typically studied in colloidal solutions, but solution-based UC is not ideal for photovoltaic or detection device integration.38 It is challenging to implement efficient TTA-UC systems into the solid state. This is due to several factors, including the absence of free diffusion of triplet acceptors, the short exciton diffusion lengths in sensitizer films, partially due to long-chain ligands, which reduce the electronic coupling between adjacent NCs, and a decrease in the PL QY in solid-state films of the organic annihilators due to intermolecular interactions. While a solid media is not well-suited to facilitate annihilator diffusion, solid-state integration of NC-sensitized TTA-UC has been achieved with some initial success.
The Baldo and Bawendi groups have pioneered solid-state NIR-to-visible UC sensitized by PbS NCs. To prepare the thin films, a layer of PbS NCs is spin-coated onto a substrate, followed by thermal evaporation of a doped rubrene layer.38 The rubrene annihilator is doped with dibenzotetraphenylperiflanthene (DBP) emitter molecules to harvest the high energy singlet produced by TTA in rubrene via Förster resonance energy transfer (FRET).126 DBP increase the QY of the UC process, as FRET outcompetes triplet pair separation in rubrene films.127 Recall from section 2.3 that the passivating ligands on the NC can be optimized to a length of six carbons in order to enhance TET to rubrene.71
For these systems, only one monolayer of PbS NCs can be added before TET to rubrene becomes inefficient, demonstrated in the schematic of Figure 4. The excitons are unable to diffuse through layers of NCs to the annihilator layer of the film.38 Additionally, thick NC layers increase the likelihood of reabsorption of UC emission. Thin layers of NCs, however, are only able to absorb a fraction of a percent of incident light.38 Photonic management by the addition of a silver back-reflector and optical spacer redirects any incident NIR light that is transmitted through the film, reflecting the light back into the absorbing NC layer.72 These additions led to an increase in the percentage of light absorbed by the NC up to 1.5% and internal UC QYs up to 0.8%, compared to 0.255% without photonic management.38,72
Figure 4.
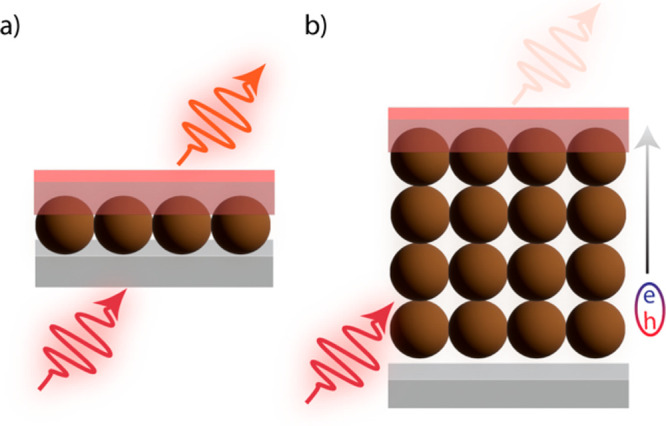
Schematic detailing the difficulty of solid-state NC-sensitized TTA-UC, where single-monolayers of nanocrystals (a) outperform thicker sensitizer films (b) as exciton diffusion cannot occur through thicker films.
Other optimizations to PbS/rubrene UC systems include the incorporation of a Fabry-Pérot microcavity with a Bragg reflector.73 NC absorption was maximized to approximately 48% at the resonant wavelength (980 nm) of the device, a nearly 74-fold increase in the PbS NC absorption. This setup enabled lower Ith values than those in the group’s previous studies, and enough UC emission was obtained such that an external quantum efficiency (EQE) of 0.05% was measured.
CdSe NC-sensitized upconverting thin films have also been demonstrated.46,74 Initially, “blended” films consisted of a spin-casted layer of a solution containing both sensitizer and annihilator were fabricated, rather than layering two separate sensitizer and annihilator solutions.46 Transmitter ligands typical of colloidal systems were utilized for CdSe NC-based films, but Rigsby et al. theorized that the inefficiency of triplet annihilation is the main culprit for the low UC efficiencies.74 They suggest that rather than simply spin-casting an optimized colloidal solution, it might be necessary to tailor the system for the solid-state by enabling different energy transfer mechanisms, such as exciton hopping or even direct sensitization of the annihilator without transmitter ligands.71,74,128
Beyond solution deposition onto thin films, TTA-UC can be incorporated into the solid-state by utilizing metal–organic frameworks (MOFs). MOFs are a type of molecular assembly structure which can be synthesized in solution and dried into powders.129 There have been numerous reports of TTA-UC obtained by doping molecular triplet sensitizers into annihilator MOFs.130,131 Amemori et al. reported a similar system wherein semiconductor NC sensitizers were encapsulated within an annihilator MOF.75 Specifically, they reported TTA-UC using core/shell CdSe/CdS NCs and PbS NCs as triplet sensitizers doped into anthracene and tetracene-based MOFs, respectively. While the reported UC efficiencies for the systems were low, NC-doped molecular assemblies are an alternative setup for TTA-UC systems.
3. Toward Device Relevance: Improvements and Mechanistic Insights
As discussed briefly in the Introduction, TTA-UC could be leveraged by many applications, effectively increasing the range of light absorption in applications such as photocatalysis or in PV, for example, by upconverting light that could otherwise not be absorbed. In such device-driven applications, an upconverting component must be in the solid-state, necessitating large-scale improvements to existing solid-state NC-sensitized TTA-UC systems for meaningful device improvements. In the deposition of NC sensitizers onto films, any defects or issues at the NC-ligand interface are magnified. Additionally, the long-chain organic ligands necessary for surface passivation and colloidal stability in solution are problematic in the solid-state, limiting coupling between NCs and at the NC/annihilator interface.
A more mechanistic understanding and finer control of NC-ligand interactions could benefit both solution-phase and solid-state TTA-UC systems. For example, even with the rich, yet recent history of literature into NC-sensitized TTA-UC systems,18 the exact mechanism underpinning energy transfer from NC to transmitter ligand is unknown in most cases. Further insights gleaned from a complete understanding of this mechanism for all systems under study could yield further breakthroughs in the efficiency of both energy transfer pathways, in addition to increases in the efficiency.
3.1. Solid-State TTA-UC Device Approaches
As discussed in section 2.4, the development of solid-state NC-sensitized TTA-UC systems has mainly utilized NIR PbS NC sensitizers, with progress mainly capped by the limited exciton diffusion through the NC ensemble film, necessitating very thin sensitizer films.132 Thus, NC sensitizer layers with improved excitonic diffusion through the layer could yield more efficient devices. Here, the field could take advantage of the wealth of work done in colloidal NC devices, where solid-state ligand exchange and deposition techniques have been developed in order to improve electronic coupling.133
Additionally, the self-assembly of NCs has shown the ability to endow unique properties, which could allow for more efficient NC-sensitized TTA-UC devices. For example, CdSe NPLs are able to rapidly self-assemble into ordered stacks in both solution96 and in solid state.134 CdSe NPLs have previously been used as triplet sensitizers, but stacking in solution decreased the UC efficiency of the system. In the solid state, though, ordered arrays of CdSe NPLs feature rapid energy transfer through the stack of NPLs.135 Further, novel deposition techniques have been recently employed, which allow for the deposition of NPLs into well-ordered layered stacks.136 Therefore, controlled and ordered stacks of CdSe NPLs could prove to be superior solid-state NC sensitizers than those previously studied, as exciton diffusion would no longer be a bottleneck in the UC process.18,137
Self-assembly could be leveraged using 0D NCs as well. Recently, the self-assembly of PeNCsNCs has resulted in the formation of superlattices capable of superfluorescence.138 While this in and of itself is not directly applicable to triplet sensitization, it suggests that the degree of coupling necessary for superfluorescence can be leveraged to direct energy transfer in a solid-state TTA-UC device. More recently, superlattices of multiple packing motifs, including a perovskite superlattice, have been demonstrated,139 which could enable superior electronic coupling between PeNCsNCs, allowing for further improvements to PeNCs–which already feature promising exciton diffusion lengths.140
3.2. Semiconductor Nanocrystal/Ligand Control
Precise control of the NC/ligand surface is paramount for the advancement of TTA-UC systems, since either the native ligands must be made short enough to enable direct triplet transfer to a triplet annihilator or novel ligands must be exchanged which can mediate TET from the NC to the annihilator. Interestingly, the specific methods of ligand exchange vary when transmitter ligands are employed. For example, Tang and co-workers37 commonly employ a mixing procedure of the NCs and transmitter ligands before repurifying/crashing the NC/ligand complex, whereas Nienhaus and co-workers62,63 and Albinsson and co-workers141 do not repurify the NC/ligand complex postexchange, instead employing a “direct-mixing” method. Other groups employ sonication in their ligand exchange techniques, upon which the NC/ligand complex is either repurified142 or simply syringe filtered postexchange.67,84 The resulting ligand concentration can be estimated through either UV–vis absorption spectroscopy, NMR, or a combination of the two. The effect of exact ligand exchange method on the TET mechanism between NC and transmitter ligand, and the concomitant UC process remains unclear.
Specifically, the exact effect of excess transmitter ligand is currently unknown, even though there is a marked decrease in the UC QY of a NC-sensitized system above a threshold transmitter ligand concentration.49,61,62,68 This phenomenon is further complicated by dynamic NC/ligand environments which have been previously reported in CdSe NCs,143 NPLs,98 and PeNCs.144 In these dynamic systems, an excess of ligand can cause rapid desorption and rebinding when the native ligand and the transmitter ligand have the same binding motif, i.e.. carboxylate-based transmitter ligands in the case of native oleic acid ligation. Applying this to the TTA-UC systems described in this Perspective, there are many phenomena that are yet unstudied. Wilson and co-workers have begun to elucidate some of these dynamics in PbS systems, as they studied the dynamics of 9-ACA for oleic acid ligand exchange using NMR.145 Interestingly, they found that, upon exchange, ACA primarily segregates to the facet edge and does not easily exchange with native oleic acid after adsorbing to the NC facet edge. However, it is still unknown whether ACA can then easily exchange with other ACA molecules at these weaker binding sites or what occurs when ACA is in excess in solution. Additionally, it is unclear whether the aforementioned passivation methods affect the ligand binding sites. For example, the addition of surface amines which bind to the surface, or Z-type metal salts that may bind differently than the most common X-type carboxylates results in a more limited binding environment for the transmitter ligands.
Therefore, we propose multiple possible phenomena which could be occurring in solution with NCs, native ligands, and photoactive transmitter ligands. First, the standard model, described first by Tang and co-workers,37 where the transmitter ligand displaces the native ligand and simply acts as a triplet mediator is likely to occur. However, there are two other possibilities which can occur, especially with free transmitter ligand present, or in NC systems which exhibit rapid and dynamic ligand exchange. The transmitter ligand can act as a free transmitter in solution, allowing the free ligand to accept triplets from bound transmitter ligands and also act as a donor to the free annihilator in solution. Additionally, the long triplet lifetime of triplet transmitter ligands could enable a scenario in which the transmitter ligand triplet state is populated after excitation of the NC via ET1 and then desorbs, likely after exchange with another ligand molecule. The now freely diffusing transmitter ligand could then either undergo TTA with another transmitter molecule, perform hetero-TTA with the annihilator, or perform ET2, populating the triplet state of the annihilator.
Regardless, detailed studies of the transmitter ligand and native ligand interactions, in the style of Wilson and co-workers,145 which uses advanced NMR techniques146 to probe NC/ligand interactions, should be further employed in an attempt to elucidate the dynamics occurring between NC, the native ligand, and the transmitter ligand. The successful illumination of these dynamics will allow for further optimizations and improvements of these NC-sensitized TTA-UC systems. For example, one possible improvement may come because of transmitter ligand/annihilator diffusion away from the NC after or just before TTA, since this would decrease the probability for singlet back transfer. This would be furthered by the two latter scenarios described above and could be a further source of improvement for these systems.
Recently, a novel triplet sensitization mechanism has been elucidated by Wu and co-workers using the same NC/transmitter ligand system described above.147 Here, a PeNC/rhodamine B (RhB) hybrid system was investigated, where the triplet state of RhB can potentially be populated by the NC itself, or through a spin mixing process mediated by the NC, which can occur after excitation of the RhB molecule. This triplet state can then be harvested for TTA-UC or can be used for photochemical transformations. While the end result remains the same here, i.e., triplet sensitization, this preliminary work bears further study.
Additionally, there are novel configurations of the “standard” NC/transmitter ligand/annihilator system that could be employed that could potentially lead to further increases in UC efficiency. Novel annihilators composed of dimers capable of TTA in the same molecule have recently been reported,148 potentially allowing for a transmitter ligand that can be directly bound and act as an annihilator. This is along the same vein as the multilayer TTA-UC/solar cell device reported by Beery et al.(149) Interestingly, their oriented multilayer film composed of CdSe QDs and a phosphonic acid anthracene derivative which binds to both the QD and TiO2 resulted in Ith values below the solar flux, which is especially notable, as CdSe NC-sensitized systems are typically orders of magnitude higher.37,63,68 Another useful strategy may be to find a transmitter ligand composition capable of acting as the native ligand, in other words being able to maintain the colloidal stability of the NC while passivating the surface to the degree of common native ligands, such as oleic acid. This could prove difficult, as shorter/smaller ligands can vastly change the NC growth and nucleation when used as compared to longer-chain ligands, such as oleic acid.114 Novel long-chain conjugated ligands could still be useful, however, as Tang and co-workers have recently reported the observation of an energy transfer mechanism which is mediated by hopping, allowing for triplet energy transfer to occur at distances longer than 1 nm.128
4. Conclusions/Outlook
In summary, the past decade of research into NC-sensitized TTA-UC has yielded more efficient UC because of NC improvements, novel and/or optimization of ligands, or a combination thereof. Furthermore, NC-sensitized TTA-UC in the solid state has become more efficient through various optimizations and through novel device structures aimed at improving NC absorption. In short, there is still plenty of room for improvement. A schematic of potential improvements is shown in Figure 5, outlined with potential approaches outlined in section 3. In all cases, interest should be turned toward mechanistic insights of both energy transfer processes, which can be leveraged toward further improvements. Especially in the case of solid-state sensitization, the wealth of literature for NC self-assembly and colloidal NC optoelectronic devices, which both aim at making well-ordered NC substrates with some degree of electronic coupling, should be leveraged to fabricate NC layers with maximal excitonic diffusion.
Figure 5.
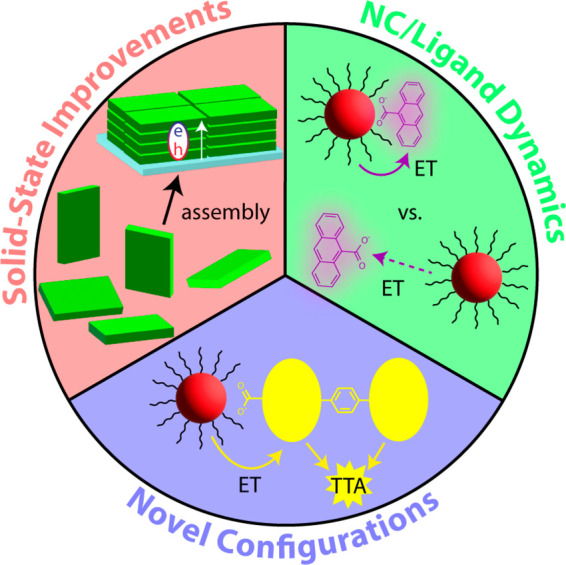
Schematic detailing potential improvements of NC-sensitized TTA-UC systems.
Acknowledgments
The authors acknowledge Florida State University for funding. Z.A.V. acknowledges Dr. Joseph Manion for Blender tutorials and assets which assisted in the generation of the Table of Contents graphic.
Author Contributions
† R.W. and Z.A.V: Equal contribution. CRediT: Rachel Weiss writing-original draft (equal), writing-review & editing (equal); Zachary A VanOrman conceptualization (equal), writing-original draft (equal), writing-review & editing (equal); Colette M Sullivan writing-original draft (equal); Lea Nienhaus conceptualization (equal), supervision (lead), writing-original draft (equal), writing-review & editing (equal).
The authors declare no competing financial interest.
References
- Trupke T.; Green M. A.; Würfel P. Improving Solar Cell Efficiencies by Up-Conversion of Sub-Band-Gap Light. J. Appl. Phys. 2002, 92 (7), 4117–4122. 10.1063/1.1505677. [DOI] [Google Scholar]
- Schulze T. F.; Schmidt T. W. Photochemical Upconversion: Present Status and Prospects for Its Application to Solar Energy Conversion. Energy Environ. Sci. 2015, 8 (1), 103–125. 10.1039/C4EE02481H. [DOI] [Google Scholar]
- Shockley W.; Queisser H. J. Detailed Balance Limit of Efficiency of P-n Junction Solar Cells. J. Appl. Phys. 1961, 32 (3), 510–519. 10.1063/1.1736034. [DOI] [Google Scholar]
- Zhou J.; Liu Q.; Feng W.; Sun Y.; Li F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115 (1), 395–465. 10.1021/cr400478f. [DOI] [PubMed] [Google Scholar]
- Chatterjee D.; Rufaihah A.; Zhang Y. Upconversion Fluorescence Imaging of Cells and Small Animals Using Lanthanide Doped Nanocrystals. Biomaterials 2008, 29 (7), 937–943. 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]
- Chen S.; Weitemier A. Z.; Zeng X.; He L.; Wang X.; Tao Y.; Huang A. J. Y.; Hashimotodani Y.; Kano M.; Iwasaki H.; Parajuli L. K.; Okabe S.; Teh D. B. L.; All A. H.; Tsutsui-Kimura I.; Tanaka K. F.; Liu X.; McHugh T. J. Near-Infrared Deep Brain Stimulation via Upconversion Nanoparticle–Mediated Optogenetics. Science 2018, 359 (6376), 679–684. 10.1126/science.aaq1144. [DOI] [PubMed] [Google Scholar]
- Ansari M. I. H.; Qurashi A.; Nazeeruddin M. K. Frontiers, Opportunities, and Challenges in Perovskite Solar Cells: A Critical Review. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2018, 35, 1–24. 10.1016/j.jphotochemrev.2017.11.002. [DOI] [Google Scholar]
- Singh-Rachford T. N.; Castellano F. N. Photon Upconversion Based on Sensitized Triplet–Triplet Annihilation. Coord. Chem. Rev. 2010, 254 (21–22), 2560–2573. 10.1016/j.ccr.2010.01.003. [DOI] [Google Scholar]
- Joubert M.-F. Photon Avalanche Upconversion in Rare Earth Laser Materials. Opt. Mater. 1999, 11 (2), 181–203. 10.1016/S0925-3467(98)00043-3. [DOI] [Google Scholar]
- Parker C. A.; Hatchard C. G.; Bowen E. J. Delayed Fluorescence from Solutions of Anthracene and Phenanthrene. Proc. R. Soc. London, Ser. A 1962, 269 (1339), 574–584. 10.1098/rspa.1962.0197. [DOI] [Google Scholar]
- Kozlov D. V.; Castellano F. N. Anti-Stokes Delayed Fluorescence from Metal–Organic Bichromophores. Chem. Commun. 2004, (24), 2860–2861. 10.1039/B412681E. [DOI] [PubMed] [Google Scholar]
- Baluschev S.; Miteva T.; Yakutkin V.; Nelles G.; Yasuda A.; Wegner G. Up-Conversion Fluorescence: Noncoherent Excitation by Sunlight. Phys. Rev. Lett. 2006, 97 (14), 143903. 10.1103/PhysRevLett.97.143903. [DOI] [PubMed] [Google Scholar]
- Amemori S.; Sasaki Y.; Yanai N.; Kimizuka N. Near-Infrared-to-Visible Photon Upconversion Sensitized by a Metal Complex with Spin-Forbidden yet Strong S0-T1 Absorption. J. Am. Chem. Soc. 2016, 138 (28), 8702–8705. 10.1021/jacs.6b04692. [DOI] [PubMed] [Google Scholar]
- VanOrman Z. A.; Nienhaus L. Bulk Metal Halide Perovskites as Triplet Sensitizers: Taking Charge of Upconversion. ACS Energy Lett. 2021, 6 (10), 3686–3694. 10.1021/acsenergylett.1c01794. [DOI] [Google Scholar]
- VanOrman Z. A.; Drozdick H. K.; Wieghold S.; Nienhaus L. Bulk Halide Perovskites as Triplet Sensitizers: Progress and Prospects in Photon Upconversion. J. Mater. Chem. C 2021, 9 (8), 2685–2694. 10.1039/D1TC00245G. [DOI] [Google Scholar]
- Younts R.; Duan H.-S.; Gautam B.; Saparov B.; Liu J.; Mongin C.; Castellano F. N.; Mitzi D. B.; Gundogdu K. Efficient Generation of Long-Lived Triplet Excitons in 2D Hybrid Perovskite. Adv. Mater. 2017, 29 (9), 1604278. 10.1002/adma.201604278. [DOI] [PubMed] [Google Scholar]
- Maiti S.; Poonia D.; Schiettecatte P.; Hens Z.; Geiregat P.; Kinge S.; Siebbeles L. D. A. Generating Triplets in Organic Semiconductor Tetracene upon Photoexcitation of Transition Metal Dichalcogenide ReS2. J. Phys. Chem. Lett. 2021, 12 (22), 5256–5260. 10.1021/acs.jpclett.1c01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.; He S.; Wu K. Molecular Triplet Sensitization and Photon Upconversion Using Colloidal Semiconductor Nanocrystals. ACS Energy Lett. 2021, 6, 3151–3166. 10.1021/acsenergylett.1c01348. [DOI] [Google Scholar]
- Brus L. E. Electron–Electron and Electron-hole Interactions in Small Semiconductor Crystallites: The Size Dependence of the Lowest Excited Electronic State. J. Chem. Phys. 1984, 80 (9), 4403–4409. 10.1063/1.447218. [DOI] [Google Scholar]
- Efros A. L.; Brus L. E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15 (4), 6192–6210. 10.1021/acsnano.1c01399. [DOI] [PubMed] [Google Scholar]
- Saha A.; Chellappan K. V.; Narayan K. S.; Ghatak J.; Datta R.; Viswanatha R. Near-Unity Quantum Yield in Semiconducting Nanostructures: Structural Understanding Leading to Energy Efficient Applications. J. Phys. Chem. Lett. 2013, 4 (20), 3544–3549. 10.1021/jz401958u. [DOI] [Google Scholar]
- Zhou J.; Zhu M.; Meng R.; Qin H.; Peng X. Ideal CdSe/CdS Core/Shell Nanocrystals Enabled by Entropic Ligands and Their Core Size-, Shell Thickness-, and Ligand-Dependent Photoluminescence Properties. J. Am. Chem. Soc. 2017, 139 (46), 16556–16567. 10.1021/jacs.7b07434. [DOI] [PubMed] [Google Scholar]
- Murray C. B.; Norris D. J.; Bawendi M. G. Synthesis and Characterization of Nearly Monodisperse CdE (E = Sulfur, Selenium, Tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115 (19), 8706–8715. 10.1021/ja00072a025. [DOI] [Google Scholar]
- Medintz I. L.; Uyeda H. T.; Goldman E. R.; Mattoussi H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4 (6), 435–446. 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Wood V.; Bulović V. Colloidal Quantum Dot Light-Emitting Devices. Nano Rev. 2010, 1 (1), 5202. 10.3402/nano.v1i0.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath R.; Bakr O.; Sargent E. H. Solution-Processed Colloidal Quantum Dot Photovoltaics: A Perspective. Energy Environ. Sci. 2011, 4 (12), 4870–4881. 10.1039/c1ee02279b. [DOI] [Google Scholar]
- Drozdick H. K.; Weiss R.; Sullivan C. M.; Wieghold S.; Nienhaus L. Widespread Opportunities for Materials Engineering of Nanocrystals: Synthetically Tailorable Effects and Methodologies. Matter 2022, 5 (6), 1645–1669. 10.1016/j.matt.2022.04.023. [DOI] [Google Scholar]
- Lu Z.; Yoon T. P. Visible Light Photocatalysis of [2 + 2] Styrene Cycloadditions by Energy Transfer. Angew. Chem., Int. Ed. 2012, 51 (41), 10329–10332. 10.1002/anie.201204835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113 (7), 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Wang C.; Rogers C. R.; Kodaimati M. S.; Weiss E. A. Regio- and Diastereoselective Intermolecular [2 + 2] Cycloadditions Photocatalysed by Quantum Dots. Nat. Chem. 2019, 11 (11), 1034–1040. 10.1038/s41557-019-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.; Xia P.; Zhao G.; Nie C.; Gao K.; He S.; Wang L.; Wu K. Energy-Transfer Photocatalysis Using Lead Halide Perovskite Nanocrystals: Sensitizing Molecular Isomerization and Cycloaddition. Angew. Chem., Int. Ed. Engl. 2022, e202208241. 10.1002/anie.202208241. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Weiss E. A. Colloidal Quantum Dots as Photocatalysts for Triplet Excited State Reactions of Organic Molecules. J. Am. Chem. Soc. 2020, 142 (36), 15219–15229. 10.1021/jacs.0c07421. [DOI] [PubMed] [Google Scholar]
- Turro N. J.Modern Molecular Photochemistry; University Science Books, 1991. [Google Scholar]
- Amemori S.; Yanai N.; Kimizuka N. Metallonaphthalocyanines as Triplet Sensitizers for Near-Infrared Photon Upconversion beyond 850 Nm. Phys. Chem. Chem. Phys. 2015, 17 (35), 22557–22560. 10.1039/C5CP02733K. [DOI] [PubMed] [Google Scholar]
- Wang X.; Del Guerzo A.; Schmehl R. H. Photophysical Behavior of Transition Metal Complexes Having Interacting Ligand Localized and Metal-to-Ligand Charge Transfer States. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2004, 5 (1), 55–77. 10.1016/j.jphotochemrev.2004.01.002. [DOI] [Google Scholar]
- Tabachnyk M.; Ehrler B.; Gélinas S.; Böhm M. L.; Walker B. J.; Musselman K. P.; Greenham N. C.; Friend R. H.; Rao A. Resonant Energy Transfer of Triplet Excitons from Pentacene to PbSe Nanocrystals. Nat. Mater. 2014, 13 (11), 1033–1038. 10.1038/nmat4093. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Li X.; Mahboub M.; Hanson K. M.; Nichols V. M.; Le H.; Tang M. L.; Bardeen C. J. Hybrid Molecule–Nanocrystal Photon Upconversion Across the Visible and Near-Infrared. Nano Lett. 2015, 15 (8), 5552–5557. 10.1021/acs.nanolett.5b02130. [DOI] [PubMed] [Google Scholar]
- Wu M.; Congreve D. N.; Wilson M. W. B.; Jean J.; Geva N.; Welborn M.; Van Voorhis T.; Bulović V.; Bawendi M. G.; Baldo M. A. Solid-State Infrared-to-Visible Upconversion Sensitized by Colloidal Nanocrystals. Nature Photon 2016, 10 (1), 31–34. 10.1038/nphoton.2015.226. [DOI] [Google Scholar]
- Efros Al. L.; Rosen M.; Kuno M.; Nirmal M.; Norris D. J.; Bawendi M. Band-Edge Exciton in Quantum Dots of Semiconductors with a Degenerate Valence Band: Dark and Bright Exciton States. Phys. Rev. B 1996, 54 (7), 4843–4856. 10.1103/PhysRevB.54.4843. [DOI] [PubMed] [Google Scholar]
- Scholes G. D.; Rumbles G. Excitons in Nanoscale Systems. Nat. Mater. 2006, 5 (9), 683. 10.1038/nmat1710. [DOI] [PubMed] [Google Scholar]
- Nirmal M.; Brus L. Luminescence Photophysics in Semiconductor Nanocrystals. Acc. Chem. Res. 1999, 32 (5), 407–414. 10.1021/ar9700320. [DOI] [Google Scholar]
- Nirmal M.; Norris D. J.; Kuno M.; Bawendi M. G.; Efros Al. L.; Rosen M. Observation of the “Dark Exciton” in CdSe Quantum Dots. Phys. Rev. Lett. 1995, 75 (20), 3728–3731. 10.1103/PhysRevLett.75.3728. [DOI] [PubMed] [Google Scholar]
- Furis M.; Hollingsworth J. A.; Klimov V. I.; Crooker S. A. Time- and Polarization-Resolved Optical Spectroscopy of Colloidal CdSe Nanocrystal Quantum Dots in High Magnetic Fields. J. Phys. Chem. B 2005, 109 (32), 15332–15338. 10.1021/jp051738b. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Chiga Y.; Kouno H.; Yanai N.; Kimizuka N.; Teranishi T.; Sakamoto M. Exciton Recycling in Triplet Energy Transfer from a Defect-Rich Quantum Dot to an Organic Molecule. J. Phys. Chem. C 2022, 126 (28), 11674–11679. 10.1021/acs.jpcc.2c03107. [DOI] [Google Scholar]
- Dexter D. L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21 (5), 836–850. 10.1063/1.1699044. [DOI] [Google Scholar]
- Okumura K.; Mase K.; Yanai N.; Kimizuka N. Employing Core-Shell Quantum Dots as Triplet Sensitizers for Photon Upconversion. Chem.—Eur. J. 2016, 22 (23), 7721–7726. 10.1002/chem.201600998. [DOI] [PubMed] [Google Scholar]
- Rigsby E. M.; Lee K.; Sun J.; Fishman D. A.; Tang M. L. Primary Amines Enhance Triplet Energy Transfer from Both the Band Edge and Trap State from CdSe Nanocrystals. J. Chem. Phys. 2019, 151 (17), 174701. 10.1063/1.5125021. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Tang M. L. Designing Transmitter Ligands That Mediate Energy Transfer between Semiconductor Nanocrystals and Molecules. J. Am. Chem. Soc. 2017, 139 (28), 9412–9418. 10.1021/jacs.6b08783. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Simpson D. E.; Mahboub M.; Li X.; Tang M. L. Ligand Enhanced Upconversion of Near-Infrared Photons with Nanocrystal Light Absorbers. Chem. Sci. 2016, 7 (7), 4101–4104. 10.1039/C6SC00257A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboub M.; Huang Z.; Tang M. L. Efficient Infrared-to-Visible Upconversion with Subsolar Irradiance. Nano Lett. 2016, 16 (11), 7169–7175. 10.1021/acs.nanolett.6b03503. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Castellano F. N.; Schmidt T. W.; Hanson K. On the Quantum Yield of Photon Upconversion via Triplet–Triplet Annihilation. ACS Energy Lett. 2020, 5 (7), 2322–2326. 10.1021/acsenergylett.0c01150. [DOI] [Google Scholar]
- Monguzzi A.; Mezyk J.; Scotognella F.; Tubino R.; Meinardi F. Upconversion-Induced Fluorescence in Multicomponent Systems: Steady-State Excitation Power Threshold. Phys. Rev. B 2008, 78 (19), 195112. 10.1103/PhysRevB.78.195112. [DOI] [Google Scholar]
- Ronchi A.; Brazzo P.; Sassi M.; Beverina L.; Pedrini J.; Meinardi F.; Monguzzi A. Triplet-Triplet Annihilation Based Photon up-Conversion in Hybrid Molecule–semiconductor Nanocrystal Systems. Phys. Chem. Chem. Phys. 2019, 21, 12353–12359. 10.1039/C9CP01692A. [DOI] [PubMed] [Google Scholar]
- Cheng Y. Y.; Fückel B.; Khoury T.; Clady R. G. C. R.; Ekins-Daukes N. J.; Crossley M. J.; Schmidt T. W. Entropically Driven Photochemical Upconversion. J. Phys. Chem. A 2011, 115 (6), 1047–1053. 10.1021/jp108839g. [DOI] [PubMed] [Google Scholar]
- Ronchi A.; Capitani C.; Pinchetti V.; Gariano G.; Zaffalon M. L.; Meinardi F.; Brovelli S.; Monguzzi A. High Photon Upconversion Efficiency with Hybrid Triplet Sensitizers by Ultrafast Hole-Routing in Electronic-Doped Nanocrystals. Adv. Mater. 2020, 32 (37), 2002953. 10.1002/adma.202002953. [DOI] [PubMed] [Google Scholar]
- Mase K.; Okumura K.; Yanai N.; Kimizuka N. Triplet Sensitization by Perovskite Nanocrystals for Photon Upconversion. Chem. Commun. 2017, 53 (59), 8261–8264. 10.1039/C7CC03087H. [DOI] [PubMed] [Google Scholar]
- Han Y.; He S.; Luo X.; Li Y.; Chen Z.; Kang W.; Wang X.; Wu K. Triplet Sensitization by “Self-Trapped” Excitons of Nontoxic CuInS2 Nanocrystals for Efficient Photon Upconversion. J. Am. Chem. Soc. 2019, 141 (33), 13033–13037. 10.1021/jacs.9b07033. [DOI] [PubMed] [Google Scholar]
- Lai R.; Sang Y.; Zhao Y.; Wu K. Triplet Sensitization and Photon Upconversion Using InP-Based Quantum Dots. J. Am. Chem. Soc. 2020, 142 (47), 19825–19829. 10.1021/jacs.0c09547. [DOI] [PubMed] [Google Scholar]
- Lin X.; Chen Z.; Han Y.; Nie C.; Xia P.; He S.; Li J.; Wu K. ZnSe/ZnS Core/Shell Quantum Dots as Triplet Sensitizers toward Visible-to-Ultraviolet B Photon Upconversion. ACS Energy Lett. 2022, 7 (3), 914–919. 10.1021/acsenergylett.2c00017. [DOI] [Google Scholar]
- Xia P.; Schwan J.; Dugger T. W.; Mangolini L.; Tang M. L. Air-Stable Silicon Nanocrystal-Based Photon Upconversion. Advanced Optical Materials 2021, 9 (17), 2100453. 10.1002/adom.202100453. [DOI] [Google Scholar]
- Rigsby E. M.; Miyashita T.; Jaimes P.; Fishman D. A.; Tang M. L. On the Size-Dependence of CdSe Nanocrystals for Photon Upconversion with Anthracene. J. Chem. Phys. 2020, 153 (11), 114702. 10.1063/5.0017585. [DOI] [PubMed] [Google Scholar]
- VanOrman Z. A.; Conti C. R.; Strouse G. F.; Nienhaus L. Red-to-Blue Photon Upconversion Enabled by One-Dimensional CdTe Nanorods. Chem. Mater. 2021, 33 (1), 452–458. 10.1021/acs.chemmater.0c04468. [DOI] [Google Scholar]
- VanOrman Z. A.; Bieber A. S.; Wieghold S.; Nienhaus L. Green-to-Blue Triplet Fusion Upconversion Sensitized by Anisotropic CdSe Nanoplatelets. Chem. Mater. 2020, 32 (11), 4734–4742. 10.1021/acs.chemmater.0c01354. [DOI] [Google Scholar]
- Mahboub M.; Xia P.; Van Baren J.; Li X.; Lui C. H.; Tang M. L. Midgap States in PbS Quantum Dots Induced by Cd and Zn Enhance Photon Upconversion. ACS Energy Lett. 2018, 3 (4), 767–772. 10.1021/acsenergylett.8b00122. [DOI] [Google Scholar]
- Huang Z.; Xu Z.; Mahboub M.; Liang Z.; Jaimes P.; Xia P.; Graham K. R.; Tang M. L.; Lian T. Enhanced Near-Infrared-to-Visible Upconversion by Synthetic Control of PbS Nanocrystal Triplet Photosensitizers. J. Am. Chem. Soc. 2019, 141 (25), 9769–9772. 10.1021/jacs.9b03385. [DOI] [PubMed] [Google Scholar]
- Xia P.; Huang Z.; Li X.; Romero J. J.; Vullev V. I.; Pau G. S. H.; Tang M. L. On the Efficacy of Anthracene Isomers for Triplet Transmission from CdSe Nanocrystals. Chem. Commun. 2017, 53 (7), 1241–1244. 10.1039/C6CC08229G. [DOI] [PubMed] [Google Scholar]
- He S.; Lai R.; Jiang Q.; Han Y.; Luo X.; Tian Y.; Liu X.; Wu K. Engineering Sensitized Photon Upconversion Efficiency via Nanocrystal Wavefunction and Molecular Geometry. Angew. Chem., Int. Ed. 2020, 59 (40), 17726–17731. 10.1002/anie.202009066. [DOI] [PubMed] [Google Scholar]
- De Roo J.; Huang Z.; Schuster N. J.; Hamachi L. S.; Congreve D. N.; Xu Z.; Xia P.; Fishman D. A.; Lian T.; Owen J. S.; Tang M. L. Anthracene Diphosphate Ligands for CdSe Quantum Dots; Molecular Design for Efficient Upconversion. Chem. Mater. 2020, 32 (4), 1461–1466. 10.1021/acs.chemmater.9b04294. [DOI] [Google Scholar]
- Tripathi N.; Ando M.; Akai T.; Kamada K. Efficient NIR-to-Visible Upconversion of Surface-Modified PbS Quantum Dots for Photovoltaic Devices. ACS Appl. Nano Mater. 2021, 4 (9), 9680–9688. 10.1021/acsanm.1c02059. [DOI] [Google Scholar]
- Miyashita T.; Jaimes P.; Lian T.; Tang M. L.; Xu Z. Quantifying the Ligand-Induced Triplet Energy Transfer Barrier in a Quantum Dot-Based Upconversion System. J. Phys. Chem. Lett. 2022, 13 (13), 3002–3007. 10.1021/acs.jpclett.2c00514. [DOI] [PubMed] [Google Scholar]
- Nienhaus L.; Wu M.; Geva N.; Shepherd J. J.; Wilson M. W. B.; Bulović V.; Van Voorhis T.; Baldo M. A.; Bawendi M. G. Speed Limit for Triplet-Exciton Transfer in Solid-State PbS Nanocrystal-Sensitized Photon Upconversion. ACS Nano 2017, 11 (8), 7848–7857. 10.1021/acsnano.7b02024. [DOI] [PubMed] [Google Scholar]
- Wu M.; Jean J.; Bulović V.; Baldo M. A. Interference-Enhanced Infrared-to-Visible Upconversion in Solid-State Thin Films Sensitized by Colloidal Nanocrystals. Appl. Phys. Lett. 2017, 110 (21), 211101. 10.1063/1.4984136. [DOI] [Google Scholar]
- Wu M.; Lin T.-A.; Tiepelt J. O.; Bulović V.; Baldo M. A. Nanocrystal-Sensitized Infrared-to-Visible Upconversion in a Microcavity under Subsolar Flux. Nano Lett. 2021, 21 (2), 1011–1016. 10.1021/acs.nanolett.0c04060. [DOI] [PubMed] [Google Scholar]
- Rigsby E. M.; Miyashita T.; Fishman D. A.; Roberts S. T.; Tang M. L. CdSe Nanocrystal Sensitized Photon Upconverting Film. RSC Adv. 2021, 11 (49), 31042–31046. 10.1039/D1RA06562A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemori S.; Gupta R. K.; Böhm M. L.; Xiao J.; Huynh U.; Oyama T.; Kaneko K.; Rao A.; Yanai N.; Kimizuka N. Hybridizing Semiconductor Nanocrystals with Metal–Organic Frameworks for Visible and near-Infrared Photon Upconversion. Dalton Trans. 2018, 47 (26), 8590–8594. 10.1039/C7DT04794K. [DOI] [PubMed] [Google Scholar]
- Okumura K.; Yanai N.; Kimizuka N. Visible-to-UV Photon Upconversion Sensitized by Lead Halide Perovskite Nanocrystals. Chem. Lett. 2019, 48 (11), 1347–1350. 10.1246/cl.190473. [DOI] [Google Scholar]
- Sasaki Y.; Oshikawa M.; Bharmoria P.; Kouno H.; Hayashi-Takagi A.; Sato M.; Ajioka I.; Yanai N.; Kimizuka N. Near-Infrared Optogenetic Genome Engineering Based on Photon-Upconversion Hydrogels. Angew. Chem., Int. Ed. Engl. 2019, 58 (49), 17827–17833. 10.1002/anie.201911025. [DOI] [PubMed] [Google Scholar]
- Li Y.; Hou X.; Dai X.; Yao Z.; Lv L.; Jin Y.; Peng X. Stoichiometry-Controlled InP-Based Quantum Dots: Synthesis, Photoluminescence, and Electroluminescence. J. Am. Chem. Soc. 2019, 141 (16), 6448–6452. 10.1021/jacs.8b12908. [DOI] [PubMed] [Google Scholar]
- Rossetti R.; Ellison J. L.; Gibson J. M.; Brus L. E. Size Effects in the Excited Electronic States of Small Colloidal CdS Crystallites. J. Chem. Phys. 1984, 80 (9), 4464–4469. 10.1063/1.447228. [DOI] [Google Scholar]
- Zhang J.; Kouno H.; Yanai N.; Eguchi D.; Nakagawa T.; Kimizuka N.; Teranishi T.; Sakamoto M. Number of Surface-Attached Acceptors on a Quantum Dot Impacts Energy Transfer and Photon Upconversion Efficiencies. ACS Photonics 2020, 7 (7), 1876–1884. 10.1021/acsphotonics.0c00771. [DOI] [Google Scholar]
- Marcus R. A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. I. J. Chem. Phys. 1956, 24 (5), 966–978. 10.1063/1.1742723. [DOI] [Google Scholar]
- Huang Z.; Li X.; Yip B. D.; Rubalcava J. M.; Bardeen C. J.; Tang M. L. Nanocrystal Size and Quantum Yield in the Upconversion of Green to Violet Light with CdSe and Anthracene Derivatives. Chem. Mater. 2015, 27 (21), 7503–7507. 10.1021/acs.chemmater.5b03731. [DOI] [Google Scholar]
- Mahboub M.; Maghsoudiganjeh H.; Pham A. M.; Huang Z.; Tang M. L. Triplet Energy Transfer from PbS(Se) Nanocrystals to Rubrene: The Relationship between the Upconversion Quantum Yield and Size. Adv. Funct. Mater. 2016, 26 (33), 6091–6097. 10.1002/adfm.201505623. [DOI] [Google Scholar]
- Luo X.; Lai R.; Li Y.; Han Y.; Liang G.; Liu X.; Ding T.; Wang J.; Wu K. Triplet Energy Transfer from CsPbBr3 Nanocrystals Enabled by Quantum Confinement. J. Am. Chem. Soc. 2019, 141 (10), 4186–4190. 10.1021/jacs.8b13180. [DOI] [PubMed] [Google Scholar]
- Lai R.; Wu K. Red-to-Blue Photon Upconversion Based on a Triplet Energy Transfer Process Not Retarded but Enabled by Shell-Coated Quantum Dots. J. Chem. Phys. 2020, 153 (11), 114701. 10.1063/5.0023052. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Huang Z.; Li C.; Huang T.; Evangelista F. A.; Tang M. L.; Lian T. Tuning the Quantum Dot (QD)/Mediator Interface for Optimal Efficiency of QD-Sensitized Near-Infrared-to-Visible Photon Upconversion Systems. ACS Appl. Mater. Interfaces 2020, 12 (32), 36558–36567. 10.1021/acsami.0c10269. [DOI] [PubMed] [Google Scholar]
- Han Y.; Luo X.; Lai R.; Li Y.; Liang G.; Wu K. Visible-Light-Driven Sensitization of Naphthalene Triplets Using Quantum-Confined CsPbBr3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10 (7), 1457–1463. 10.1021/acs.jpclett.9b00597. [DOI] [PubMed] [Google Scholar]
- Brus L. E. A Simple Model for the Ionization Potential, Electron Affinity, and Aqueous Redox Potentials of Small Semiconductor Crystallites. J. Chem. Phys. 1983, 79 (11), 5566–5571. 10.1063/1.445676. [DOI] [Google Scholar]
- Ekimov A. I.; Hache F.; Schanne-Klein M. C.; Ricard D.; Flytzanis C.; Kudryavtsev I. A.; Yazeva T. V.; Rodina A. V.; Efros A. L. Absorption and Intensity-Dependent Photoluminescence Measurements on CdSe Quantum Dots: Assignment of the First Electronic Transitions. J. Opt. Soc. Am. B, JOSAB 1993, 10 (1), 100–107. 10.1364/JOSAB.10.000100. [DOI] [Google Scholar]
- Peng X.; Manna L.; Yang W.; Wickham J.; Scher E.; Kadavanich A.; Alivisatos A. Shape Control of CdSe Nanocrystals. Nature 2000, 404 (6773), 59–61. 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- Yan R.; Gargas D.; Yang P. Nanowire Photonics. Nat. Photonics 2009, 3 (10), 569–576. 10.1038/nphoton.2009.184. [DOI] [Google Scholar]
- Ithurria S.; Dubertret B. Quasi 2D Colloidal CdSe Platelets with Thicknesses Controlled at the Atomic Level. J. Am. Chem. Soc. 2008, 130 (49), 16504–16505. 10.1021/ja807724e. [DOI] [PubMed] [Google Scholar]
- Shamsi J.; Dang Z.; Bianchini P.; Canale C.; Di Stasio F.; Brescia R.; Prato M.; Manna L. Colloidal Synthesis of Quantum Confined Single Crystal CsPbBr3 Nanosheets with Lateral Size Control up to the Micrometer Range. J. Am. Chem. Soc. 2016, 138 (23), 7240–7243. 10.1021/jacs.6b03166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talapin D. V.; Shevchenko E. V.; Murray C. B.; Kornowski A.; Förster S.; Weller H. CdSe and CdSe/CdS Nanorod Solids. J. Am. Chem. Soc. 2004, 126 (40), 12984–12988. 10.1021/ja046727v. [DOI] [PubMed] [Google Scholar]
- Abécassis B.; Tessier M. D.; Davidson P.; Dubertret B. Self-Assembly of CdSe Nanoplatelets into Giant Micrometer-Scale Needles Emitting Polarized Light. Nano Lett. 2014, 14 (2), 710–715. 10.1021/nl4039746. [DOI] [PubMed] [Google Scholar]
- Jana S.; Davidson P.; Abécassis B. CdSe Nanoplatelets: Living Polymers. Angew. Chem., Int. Ed. 2016, 55 (32), 9371–9374. 10.1002/anie.201603880. [DOI] [PubMed] [Google Scholar]
- Yu J.; Chen R. Optical Properties and Applications of Two-Dimensional CdSe Nanoplatelets. InfoMat 2020, 2 (5), 905–927. 10.1002/inf2.12106. [DOI] [Google Scholar]
- Singh S.; Tomar R.; ten Brinck S.; De Roo J.; Geiregat P.; Martins J. C.; Infante I.; Hens Z. Colloidal CdSe Nanoplatelets, A Model for Surface Chemistry/Optoelectronic Property Relations in Semiconductor Nanocrystals. J. Am. Chem. Soc. 2018, 140 (41), 13292–13300. 10.1021/jacs.8b07566. [DOI] [PubMed] [Google Scholar]
- Ithurria S.; Tessier M. D.; Mahler B.; Lobo R.; Dubertret B.; Efros A. L. Colloidal Nanoplatelets with Two-Dimensional Electronic Structure. Nat. Mater. 2011, 10 (12), 936–941. 10.1038/nmat3145. [DOI] [PubMed] [Google Scholar]
- Yeltik A.; Delikanli S.; Olutas M.; Kelestemur Y.; Guzelturk B.; Demir H. V. Experimental Determination of the Absorption Cross-Section and Molar Extinction Coefficient of Colloidal CdSe Nanoplatelets. J. Phys. Chem. C 2015, 119 (47), 26768–26775. 10.1021/acs.jpcc.5b09275. [DOI] [Google Scholar]
- Guillemeney L.; Lermusiaux L.; Landaburu G.; Wagnon B.; Abécassis B. Curvature and Self-Assembly of Semi-Conducting Nanoplatelets. Commun. Chem. 2022, 5 (1), 1–11. 10.1038/s42004-021-00621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börjesson K.; Rudquist P.; Gray V.; Moth-Poulsen K. Photon Upconversion with Directed Emission. Nat. Commun. 2016, 7 (1), 12689. 10.1038/ncomms12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M.; Lee J. K.; Dabbousi B. O.; Mikulec F. V.; Bawendi M. G. The Band Edge Luminescence of Surface Modified CdSe Nanocrystallites: Probing the Luminescing State. J. Chem. Phys. 1997, 106 (23), 9869–9882. 10.1063/1.473875. [DOI] [Google Scholar]
- Heuer-Jungemann A.; Feliu N.; Bakaimi I.; Hamaly M.; Alkilany A.; Chakraborty I.; Masood A.; Casula M. F.; Kostopoulou A.; Oh E.; Susumu K.; Stewart M. H.; Medintz I. L.; Stratakis E.; Parak W. J.; Kanaras A. G. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119 (8), 4819–4880. 10.1021/acs.chemrev.8b00733. [DOI] [PubMed] [Google Scholar]
- Dabbousi B. O.; Rodriguez-Viejo J.; Mikulec F. V.; Heine J. R.; Mattoussi H.; Ober R.; Jensen K. F.; Bawendi M. G. CdSe)ZnS Core–Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B 1997, 101 (46), 9463–9475. 10.1021/jp971091y. [DOI] [Google Scholar]
- Vasudevan D.; Gaddam R. R.; Trinchi A.; Cole I. Core–Shell Quantum Dots: Properties and Applications. J. Alloys Compd. 2015, 636, 395–404. 10.1016/j.jallcom.2015.02.102. [DOI] [Google Scholar]
- Reiss P.; Protière M.; Li L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5 (2), 154–168. 10.1002/smll.200800841. [DOI] [PubMed] [Google Scholar]
- Gray V.; Xia P.; Huang Z.; Moses E.; Fast A.; Fishman D. A.; Vullev V. I.; Abrahamsson M.; Moth-Poulsen K.; Lee Tang M. CdS/ZnS Core–Shell Nanocrystal Photosensitizers for Visible to UV Upconversion. Chem. Sci. 2017, 8 (8), 5488–5496. 10.1039/C7SC01610G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.; Xia P.; Megerdich N.; Fishman D. A.; Vullev V. I.; Tang M. L. ZnS Shells Enhance Triplet Energy Transfer from CdSe Nanocrystals for Photon Upconversion. ACS Photonics 2018, 5 (8), 3089–3096. 10.1021/acsphotonics.8b00338. [DOI] [Google Scholar]
- Huang Z.; Xu Z.; Mahboub M.; Li X.; Taylor J. W.; Harman W. H.; Lian T.; Tang M. L. PbS/CdS Core–Shell Quantum Dots Suppress Charge Transfer and Enhance Triplet Transfer. Angew. Chem., Int. Ed. 2017, 56 (52), 16583–16587. 10.1002/anie.201710224. [DOI] [PubMed] [Google Scholar]
- Cassidy J.; Zamkov M. Nanoshell Quantum Dots: Quantum Confinement beyond the Exciton Bohr Radius. J. Chem. Phys. 2020, 152 (11), 110902. 10.1063/1.5126423. [DOI] [PubMed] [Google Scholar]
- Razgoniaeva N.; Moroz P.; Yang M.; Budkina D. S.; Eckard H.; Augspurger M.; Khon D.; Tarnovsky A. N.; Zamkov M. One-Dimensional Carrier Confinement in “Giant” CdS/CdSe Excitonic Nanoshells. J. Am. Chem. Soc. 2017, 139 (23), 7815–7822. 10.1021/jacs.7b02054. [DOI] [PubMed] [Google Scholar]
- Wang X.-S.; Dykstra T. E.; Salvador M. R.; Manners I.; Scholes G. D.; Winnik M. A. Surface Passivation of Luminescent Colloidal Quantum Dots with Poly(Dimethylaminoethyl Methacrylate) through a Ligand Exchange Process. J. Am. Chem. Soc. 2004, 126 (25), 7784–7785. 10.1021/ja0489339. [DOI] [PubMed] [Google Scholar]
- Calvin J. J.; Brewer A. S.; Alivisatos A. P. The Role of Organic Ligand Shell Structures in Colloidal Nanocrystal Synthesis. Nat. Synth 2022, 1 (2), 127–137. 10.1038/s44160-022-00025-4. [DOI] [Google Scholar]
- Kirkwood N.; Monchen J. O. V.; Crisp R. W.; Grimaldi G.; Bergstein H. A. C.; du Fossé I.; van der Stam W.; Infante I.; Houtepen A. J. Finding and Fixing Traps in II–VI and III–V Colloidal Quantum Dots: The Importance of Z-Type Ligand Passivation. J. Am. Chem. Soc. 2018, 140 (46), 15712–15723. 10.1021/jacs.8b07783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscher B. A.; Swabeck J. K.; Bronstein N. D.; Alivisatos A. P. Essentially Trap-Free CsPbBr3 Colloidal Nanocrystals by Postsynthetic Thiocyanate Surface Treatment. J. Am. Chem. Soc. 2017, 139 (19), 6566–6569. 10.1021/jacs.7b02817. [DOI] [PubMed] [Google Scholar]
- Hendricks M. P.; Campos M. P.; Cleveland G. T.; Jen-La Plante I.; Owen J. S. A Tunable Library of Substituted Thiourea Precursors to Metal Sulfide Nanocrystals. Science 2015, 348 (6240), 1226–1230. 10.1126/science.aaa2951. [DOI] [PubMed] [Google Scholar]
- Manna L.; Wang; Cingolani R.; Alivisatos A. P. First-Principles Modeling of Unpassivated and Surfactant-Passivated Bulk Facets of Wurtzite CdSe: A Model System for Studying the Anisotropic Growth of CdSe Nanocrystals. J. Phys. Chem. B 2005, 109 (13), 6183–6192. 10.1021/jp0445573. [DOI] [PubMed] [Google Scholar]
- Yin Y.; Alivisatos A. P. Colloidal Nanocrystal Synthesis and the Organic–Inorganic Interface. Nature 2005, 437 (7059), 664–670. 10.1038/nature04165. [DOI] [PubMed] [Google Scholar]
- Giansante C.; Infante I. Surface Traps in Colloidal Quantum Dots: A Combined Experimental and Theoretical Perspective. J. Phys. Chem. Lett. 2017, 8 (20), 5209–5215. 10.1021/acs.jpclett.7b02193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasieniak J.; Mulvaney P. From Cd-Rich to Se-Rich – the Manipulation of CdSe Nanocrystal Surface Stoichiometry. J. Am. Chem. Soc. 2007, 129 (10), 2841–2848. 10.1021/ja066205a. [DOI] [PubMed] [Google Scholar]
- Houtepen A. J.; Hens Z.; Owen J. S.; Infante I. On the Origin of Surface Traps in Colloidal II–VI Semiconductor Nanocrystals. Chem. Mater. 2017, 29 (2), 752–761. 10.1021/acs.chemmater.6b04648. [DOI] [Google Scholar]
- Li X.; Fast A.; Huang Z.; Fishman D. A.; Tang M. L. Complementary Lock-and-Key Ligand Binding of a Triplet Transmitter to a Nanocrystal Photosensitizer. Angew. Chem., Int. Ed. 2017, 56 (20), 5598–5602. 10.1002/anie.201701929. [DOI] [PubMed] [Google Scholar]
- Piland G. B.; Huang Z.; Lee Tang M.; Bardeen C. J. Dynamics of Energy Transfer from CdSe Nanocrystals to Triplet States of Anthracene Ligand Molecules. J. Phys. Chem. C 2016, 120 (11), 5883–5889. 10.1021/acs.jpcc.5b12021. [DOI] [Google Scholar]
- Nishimura N.; Allardice J. R.; Xiao J.; Gu Q.; Gray V.; Rao A. Photon Upconversion Utilizing Energy beyond the Band Gap of Crystalline Silicon with a Hybrid TES-ADT/PbS Quantum Dots System. Chem. Sci. 2019, 10 (18), 4750–4760. 10.1039/C9SC00821G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster Th Zwischenmolekulare Energiewanderung und Fluoreszenz. Annalen der Physik 1948, 437 (1–2), 55–75. 10.1002/andp.19484370105. [DOI] [Google Scholar]
- Bossanyi D. G.; Sasaki Y.; Wang S.; Chekulaev D.; Kimizuka N.; Yanai N.; Clark J. In Optimized Rubrene-Based Nanoparticle Blends for Photon Upconversion, Singlet Energy Collection Outcompetes Triplet-Pair Separation, Not Singlet Fission. J. Mater. Chem. C 2022, 10 (12), 4684–4696. 10.1039/D1TC02955J. [DOI] [Google Scholar]
- Huang Z.; Xu Z.; Huang T.; Gray V.; Moth-Poulsen K.; Lian T.; Tang M. L. Evolution from Tunneling to Hopping Mediated Triplet Energy Transfer from Quantum Dots to Molecules. J. Am. Chem. Soc. 2020, 142 (41), 17581–17588. 10.1021/jacs.0c07727. [DOI] [PubMed] [Google Scholar]
- Yanai N.; Kimizuka N. Recent Emergence of Photon Upconversion Based on Triplet Energy Migration in Molecular Assemblies. Chem. Commun. 2016, 52 (31), 5354–5370. 10.1039/C6CC00089D. [DOI] [PubMed] [Google Scholar]
- Meinardi F.; Ballabio M.; Yanai N.; Kimizuka N.; Bianchi A.; Mauri M.; Simonutti R.; Ronchi A.; Campione M.; Monguzzi A. Quasi-Thresholdless Photon Upconversion in Metal–Organic Framework Nanocrystals. Nano Lett. 2019, 19 (3), 2169–2177. 10.1021/acs.nanolett.9b00543. [DOI] [PubMed] [Google Scholar]
- Rowe J. M.; Zhu J.; Soderstrom E. M.; Xu W.; Yakovenko A.; Morris A. J. Sensitized Photon Upconversion in Anthracene-Based Zirconium Metal–Organic Frameworks. Chem. Commun. 2018, 54 (56), 7798–7801. 10.1039/C8CC01893F. [DOI] [PubMed] [Google Scholar]
- Geva N.; Nienhaus L.; Wu M.; Bulovic V.; Baldo M. A.; Van Voorhis T.; Bawendi M. G. A Heterogeneous Kinetics Model for Triplet Exciton Transfer in Solid-State Upconversion. J. Phys. Chem. Lett. 2019, 10 (11), 3147–3152. 10.1021/acs.jpclett.9b01058. [DOI] [PubMed] [Google Scholar]
- Kagan C. R.; Murray C. B. Charge Transport in Strongly Coupled Quantum Dot Solids. Nat. Nanotechnol. 2015, 10 (12), 1013–1026. 10.1038/nnano.2015.247. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Weidman M. C.; Tisdale W. A. CdSe Nanoplatelet Films with Controlled Orientation of Their Transition Dipole Moment. Nano Lett. 2017, 17 (6), 3837–3843. 10.1021/acs.nanolett.7b01237. [DOI] [PubMed] [Google Scholar]
- Rowland C. E.; Fedin I.; Zhang H.; Gray S. K.; Govorov A. O.; Talapin D. V.; Schaller R. D. Picosecond Energy Transfer and Multiexciton Transfer Outpaces Auger Recombination in Binary CdSe Nanoplatelet Solids. Nat. Mater. 2015, 14 (5), 484–489. 10.1038/nmat4231. [DOI] [PubMed] [Google Scholar]
- Erdem O.; Foroutan S.; Gheshlaghi N.; Guzelturk B.; Altintas Y.; Demir H. V. Thickness-Tunable Self-Assembled Colloidal Nanoplatelet Films Enable Ultrathin Optical Gain Media. Nano Lett. 2020, 20 (9), 6459–6465. 10.1021/acs.nanolett.0c02153. [DOI] [PubMed] [Google Scholar]
- Nienhaus L.; Wu M.; Bulovic V.; Baldo M. A.; Bawendi M. G. Using Lead Chalcogenide Nanocrystals as Spin Mixers: A Perspective on near-Infrared-to-Visible Upconversion. Dalton Trans. 2018, 47 (26), 8509–8516. 10.1039/C8DT00419F. [DOI] [PubMed] [Google Scholar]
- Rainò G.; Becker M. A.; Bodnarchuk M. I.; Mahrt R. F.; Kovalenko M. V.; Stöferle T. Superfluorescence from Lead Halide Perovskite Quantum Dot Superlattices. Nature 2018, 563 (7733), 671–675. 10.1038/s41586-018-0683-0. [DOI] [PubMed] [Google Scholar]
- Cherniukh I.; Rainò G.; Stöferle T.; Burian M.; Travesset A.; Naumenko D.; Amenitsch H.; Erni R.; Mahrt R. F.; Bodnarchuk M. I.; Kovalenko M. V. Perovskite-Type Superlattices from Lead Halide Perovskite Nanocubes. Nature 2021, 593 (7860), 535–542. 10.1038/s41586-021-03492-5. [DOI] [PubMed] [Google Scholar]
- Yang M.; Moroz P.; Miller E.; Porotnikov D.; Cassidy J.; Ellison C.; Medvedeva X.; Klinkova A.; Zamkov M. Energy Transport in CsPbBr3 Perovskite Nanocrystal Solids. ACS Photonics 2020, 7 (1), 154–164. 10.1021/acsphotonics.9b01316. [DOI] [Google Scholar]
- Hou L.; Olesund A.; Thurakkal S.; Zhang X.; Albinsson B. Efficient Visible-to-UV Photon Upconversion Systems Based on CdS Nanocrystals Modified with Triplet Energy Mediators. Adv. Funct. Mater. 2021, 31 (47), 2106198. 10.1002/adfm.202106198. [DOI] [Google Scholar]
- Mongin C.; Garakyaraghi S.; Razgoniaeva N.; Zamkov M.; Castellano F. N. Direct Observation of Triplet Energy Transfer from Semiconductor Nanocrystals. Science 2016, 351 (6271), 369–372. 10.1126/science.aad6378. [DOI] [PubMed] [Google Scholar]
- Fritzinger B.; Capek R. K.; Lambert K.; Martins J. C.; Hens Z. Utilizing Self-Exchange To Address the Binding of Carboxylic Acid Ligands to CdSe Quantum Dots. J. Am. Chem. Soc. 2010, 132 (29), 10195–10201. 10.1021/ja104351q. [DOI] [PubMed] [Google Scholar]
- De Roo J.; Ibáñez M.; Geiregat P.; Nedelcu G.; Walravens W.; Maes J.; Martins J. C.; Van Driessche I.; Kovalenko M. V.; Hens Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10 (2), 2071–2081. 10.1021/acsnano.5b06295. [DOI] [PubMed] [Google Scholar]
- Green P. B.; Yarur Villanueva F.; Imperiale C. J.; Hasham M.; Demmans K. Z.; Burns D. C.; Wilson M. W. B. Directed Ligand Exchange on the Surface of PbS Nanocrystals: Implications for Incoherent Photon Conversion. ACS Appl. Nano Mater. 2021, 4 (6), 5655–5664. 10.1021/acsanm.1c00853. [DOI] [Google Scholar]
- Hens Z.; Martins J. C. A Solution NMR Toolbox for Characterizing the Surface Chemistry of Colloidal Nanocrystals. Chem. Mater. 2013, 25 (8), 1211–1221. 10.1021/cm303361s. [DOI] [Google Scholar]
- Liu M.; Wang J.; Liang G.; Luo X.; Zhao G.; He S.; Wang L.; Liang W.; Li J.; Wu K. Spin-Enabled Photochemistry Using Nanocrystal-Molecule Hybrids. Chem. 2022, 8 (6), 1720–1733. 10.1016/j.chempr.2022.03.003. [DOI] [Google Scholar]
- Olesund A.; Gray V.; Mårtensson J.; Albinsson B. Diphenylanthracene Dimers for Triplet–Triplet Annihilation Photon Upconversion: Mechanistic Insights for Intramolecular Pathways and the Importance of Molecular Geometry. J. Am. Chem. Soc. 2021, 143 (15), 5745–5754. 10.1021/jacs.1c00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery D.; Wheeler J. P.; Arcidiacono A.; Hanson K. CdSe Quantum Dot Sensitized Molecular Photon Upconversion Solar Cells. ACS Appl. Energy Mater. 2020, 3 (1), 29–37. 10.1021/acsaem.9b01765. [DOI] [Google Scholar]