The most recent estimate of people living with post-COVID-19 condition (also known as long COVID) globally has surpassed 65 million1 and, without clear diagnostic or treatment options available, this number is steadily increasing. There are more than 200 reported symptoms associated with long COVID,1 affecting virtually every organ system.
Although some long haulers recover, many people have had symptoms since early 2020. The condition is a blanket diagnosis that represents a heterogeneous set of pathophysiological processes. As such, several factors can affect the presentation of long COVID, such as the severity of acute infection, age, sex, pre-existing comorbidities, genetics, socioeconomic factors, and other environmental factors. Long haulers who survived severe acute SARS-CoV-2 infection are most likely to be men older than 50 years with lingering tissue damage and scarring. People with long COVID after a less-severe infection are most likely to be younger women (aged 36–50 years) whose acute infection has triggered adverse physiological responses.
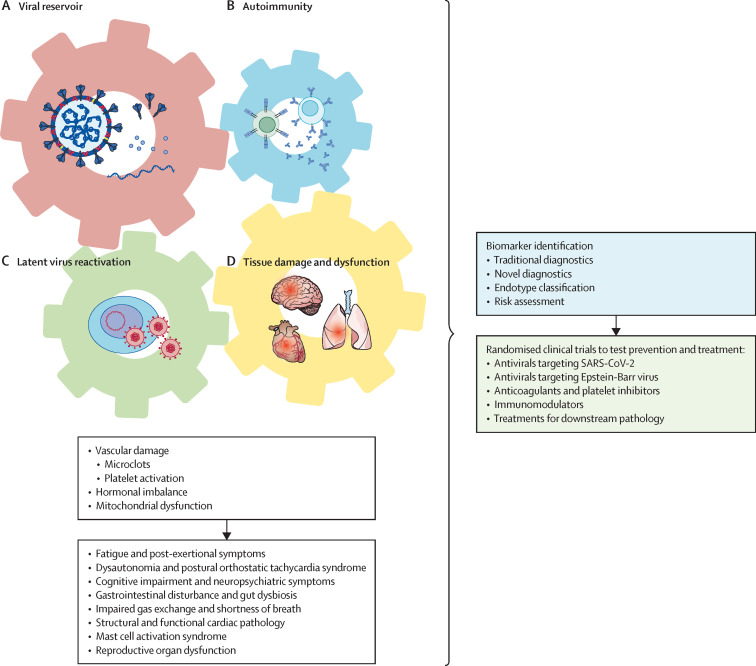
The main disease hypotheses for the root causes of long COVID include viral persistence (infectious virus, viral RNA, or viral proteins), autoimmunity triggered by the infection, reactivation of latent viruses, and inflammation-triggered chronic changes leading to tissue dysfunction and damage (figure ).
Figure.
Causal model of interaction of viral persistence, autoimmunity, latent viral reactivation, and tissue damage and dysfunction as root cause drivers of Long COVID
There is growing and compelling evidence that SARS-CoV-2 infects and produces its RNA and proteins in a wide range of cell types in tissues, including the gastrointestinal, respiratory, cardiovascular, lymphoid, endocrine, urogenital, ocular, skin, muscular, and peripheral nervous system and CNS tissues.2 Circulating spike proteins are observed in 60% of patients with long COVID between 2 and 12 months after infection.3 Although the presence of viral RNA and proteins do not necessarily indicate persistent infection, viral RNA can trigger innate immune responses and viral proteins might cause tissue damage and stimulate persistent activation of lymphocytes, which lead to chronic inflammation.
In people with long COVID, assessment of autoantibodies to human exoproteome using rapid extracellular antigen profiling revealed no significant differences from people without long COVID.4 However, autoantibodies to intracellular antigens5 or autoreactive T cells might have a role in long COVID.
There is also emerging evidence for latent herpesviruses reactivation in people with long COVID. Reactivation of latent Epstein-Barr virus, but not the acute mononucleosis infection, is found in people with long COVID,4, 6 and Epstein-Barr virus viraemia at the time of acute COVID is predictive for long COVID.5
Local inflammatory response to SARS-CoV-2 in one organ can cause lasting alterations in distant tissues and organs. In a mouse model, even mild lung-restricted COVID-19 (in which the infectious virus became undetectable within a week) was found to induce prolonged changes in the CNS, including microglial activation, oligodendrocyte loss, and reduced myelination, for up to 7 weeks after infection.7
Beyond these potential root causes, many secondary pathological changes have been observed in people with long COVID, including the formation of micro-clots and platelet activation,8 reduced cortisol,4, 5 and mitochondrial dysfunction.9
Despite the multifactorial pathogenesis, available data show that long COVID is an organic post-acute infection syndrome (PAIS) with clear physiological dysfunction that is often not consistently apparent using standard medical diagnostic tests. This discrepancy highlights the need for a new generation of more sensitive testing procedures for people with PAIS. Although it is not known whether pre-existing psychological diagnoses might influence the risk of long COVID (eg, by affecting the host endocrine and immune systems), it is neither productive nor clinically or scientifically valid to classify long COVID as a psychosomatic condition.
As avoiding COVID-19 becomes increasingly difficult, we argue that deep biological analyses will identify biomarkers for long COVID and possibly identify distinct endotypes driven by different root causes so that the risk of contracting long COVID is better defined. Biomarker identification will not only be crucial for identifying predisposing factors but also allow us to implement safer, evidence-based policies. Similarly, molecular, cellular, and physiological analyses will inform precision interventions that target the root causes of each long COVID endotype. For example, persistent viral infection could be targeted by antivirals; long COVID driven by autoimmune disease could be treated using monoclonal antibodies that target lymphocytes or drugs that block cytokines and cytokine signalling; and, if the reactivation of herpesviruses contributes to disease, targeting such viruses using antivirals or vaccines could be considered. Diagnostic criteria considering the root causes to prevent and treat long COVID will require large longitudinal studies. If therapeutic targeting of root causes is not feasible, the downstream pathological changes of long COVID could still be treated.
Ideally, potential therapies should be assessed in double-blinded, placebo-controlled, randomised clinical trials. However, such studies are quite costly, are labour intensive, and require substantial government, regulatory, and industry support.
Syndromes like Long COVID are not new. Other PAISs, such as myalgic encephalomyelitis and chronic fatigue syndrome and post-treatment Lyme disease syndrome, have overlapping symptoms with long COVID.10 Thus, the inclusion of individuals with other forms of PAIS as comparison groups in long COVID research is important for broadening overall understanding and widening the impact of this research. Finally, the current knowledge surrounding long COVID would not be possible without the hard work and dedication of patient-led communities striving for answers.
AI is a principal investigator in a decentralised phase 2 efficacy and safety study of nirmatrelvir and ritonavir in adult participants with long COVID (PAXLC; NCT05668091); is a consultant for RIGImmune, Xanadu Bio, Invisishield, and Paratus Bio; and is the vice president of the American Association of Immunologists. DP declares no competing interest.
References
- 1.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023 doi: 10.1038/s41579-022-00846-2. published online Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein SR, Ramelli SC, Grazioli A, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac722. https://doi.org/101.093/cid/ciac722 published online Sept 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein J, Wood J, Jaycox J, et al. Distinguishing features of long COVID identified through immune profiling. medRxiv. 2022 doi: 10.1038/s41586-023-06651-y. https://doi.org/101.101/20220.80.92.2278592 published online Aug 10. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peluso MJ, Deveau TM, Munter SE, et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest. 2023 doi: 10.1172/JCI163669. https://doi.org/10.1172%2FJCI163669 published online Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Castañeda A, Lu P, Geraghty AC, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468.e16. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pretorius E, Venter C, Laubscher GJ, et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/post-acute sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 2022;21:148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guntur VP, Nemkov T, de Boer E, et al. Signatures of mitochondrial dysfunction and impaired fatty acid metabolism in plasma of patients with post-acute sequelae of COVID-19 (PASC) Metabolites. 2022;12 doi: 10.3390/metabo12111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911–923. doi: 10.1038/s41591-022-01810-6. [DOI] [PubMed] [Google Scholar]