Abstract
Rationale and Objective:
APOL1 risk alleles are associated with increased cardiovascular and chronic kidney disease (CKD) risk. It is unknown whether knowledge of APOL1 risk status motivates patients and providers to attain recommended blood pressure (BP) targets to reduce cardiovascular disease.
Study Design:
Multicenter, pragmatic, randomized controlled clinical trial
Setting and Participants:
6650 individuals with African ancestry and hypertension from 13 health systems.
Intervention:
APOL1 genotyping with clinical decision support (CDS) results are returned to participants and providers immediately (intervention) or at 6 months (control). A subset of participants are re-randomized to pharmacogenomic testing for relevant antihypertensive medications (pharmacogenomic sub-study). CDS alerts encourage appropriate CKD screening and antihypertensive agent use.
Outcomes:
Blood pressure and surveys are assessed at baseline, 3 and 6 months. The primary outcome is change in systolic BP from enrollment to 3 months in individuals with two APOL1 risk alleles. Secondary outcomes include new diagnoses of CKD, systolic blood pressure at 6 months, diastolic BP, and survey results. The pharmacogenomic sub-study will evaluate the relationship of pharmacogenomic genotype and change in systolic BP between baseline and 3 months.
Results:
To date, the trial has enrolled 3423 participants.
Conclusions:
The effect of patient and provider knowledge of APOL1 genotype on systolic blood pressure has not been well-studied. GUARDD-US addresses whether blood pressure improves when patients and providers have this information. GUARDD-US provides a CDS framework for primary care and specialty clinics to incorporate APOL1 genetic risk and pharmacogenomic prescribing in the electronic health record.
Trial registration:
Keywords: pharmacogenomics, blood pressure, genotype, chronic kidney disease
Introduction
Background and rationale:
Approximately 150 million Americans have hypertension including 57% of all African Americans1. Only half achieve desired control of blood pressure1, 2. Hypertension and chronic kidney disease (CKD) are inextricably linked as the prevalence of hypertension increases with CKD severity3. In turn, CKD increases the cardiovascular mortality of individuals with uncontrolled hypertension4, 5.
One in seven individuals with African ancestry are homozygous for risk alleles of the Apolipoprotein L1 (APOL1) locus. Homozygosity for the G1 and G2 alleles of APOL1 confers a high-risk genotype (APOL1-HR) for CKD progression. These high risk genotypes are nearly absent in populations without African ancestry6 and explain up to 70% of the excess prevalence of CKD and end-stage kidney disease (ESKD) in hypertensive individuals of African ancestry7–10. APOL1 risk alleles do not significantly increase the incident risk of CKD in hypertensive diabetics; however, among those with prevalent CKD, APOL1 risk alleles exacerbate progression to ESKD regardless of the presence of diabetes mellitus8. CKD and ESKD are both key drivers of cardiovascular disease4. Strict blood pressure control is associated with improved cardiovascular mortality in African Americans with APOL1-HR genotypes11.
GUARDD-US builds upon a prior pilot study conducted in New York city at 2 sites12. The pilot study randomized 2050 adults of African ancestry with hypertension but without CKD (eGFR>60 ml/min and no albuminuria) to undergo immediate (intervention) or delayed (waiting list control group) APOL1 testing with return of results by trained staff in a 7:1 ratio. The co-primary outcomes were change in the 3-month systolic blood pressure and 12-month microalbumin screening between intervention patients with APOL1-HR genotypes and low-risk APOL1 genotypes (APOL1-LR). Secondary outcomes compared these outcomes between intervention APOL1-HR patients and controls. The blood pressure change from baseline at 3 months was significantly higher in APOL1-HR (6 mm of Hg) vs. APOL1-LR (3 mm of Hg) or controls (3 mm of Hg), however, this change was not sustained at 12 months. At 12 months there was a non-significant 12% increase in urine microalbumin testing among patients with APOL1–HR, 6% with APOL1-LR, and 7% in controls. Patients with APOL1-HR reported more changes in lifestyle and more frequent antihypertensive medication use.
GUARDD-US expands upon this prior study by including individuals with and without CKD in a multi-institutional environment. The primary outcome of the pilot study compared systolic blood pressure in APOL1-HR individuals to APOL1-LR individuals. In the multicenter study, systolic blood pressure is compared between APOL1-HR individuals with immediate or delayed genotyping. Hypertension management requires coordinated efforts between patients and providers (physicians, nurse practitioners, and other health care personnel who manage antihypertensive medications). Patients control their dietary salt intake, exercise regimen, and adherence to prescribed medications. Trust between patients and providers is particularly important in individuals of African ancestry because racial disparities in CKD have been associated with perceived racial discrimination, social determinants, and poor blood pressure control13–23. In the prior pilot study24, patients felt that knowledge of their APOL1 related-risk of CKD helped to counter stereotypes of non-adherence or low-literacy and provided the impetus for themselves and their providers to better achieve hypertension goals12, 25.
Objectives:
The clinical indications warranting APOL1 testing are evolving26, 27. It is not yet known whether disclosure of APOL1 risk will impact clinical care, patient behaviors, or patient outcomes. The Genetic Testing to Understand and Address Renal Disease Disparities Across the United States (GUARDD-US) tests the hypothesis that knowledge of APOL1 genotype will lead to improved blood pressure control in individuals with two risk alleles. The study is a part of the National Human Genome Research Institute (NHGRI)’s Implementing Genomics in Practice (IGNITE) network which seeks to enhance the use of genomic medicine in clinical care and explore methods for effective implementation, diffusion and sustainability in diverse clinical environments28. By December 2021, the trial had enrolled 3,423 individuals with a goal of 6,650 by June 2023.
Methods
Trial design:
GUARDD-US is a multicenter, unblinded, dual-arm, pragmatic randomized controlled trial that will randomize 6650 or more individuals with hypertension and African ancestry to immediate or delayed APOL1 testing (Figure 1). The primary aim is to determine the effect of participant and provider knowledge of the APOL1-HR genotype on a change in systolic blood pressure from baseline to 3 months. Secondary aims include an appropriate CKD diagnosis entered into the electronic health record (EHR), urine microalbumin/ creatinine testing, appropriate use of angiotensin converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs) in subjects with CKD, as well as cost effectiveness, mediators, moderators, and psycho-behavioral impacts of result disclosure. A subset of individuals with the APOL1-LR genotype are re-randomized to a pharmacogenomic sub-study as described below. The study significantly expands upon a prior single center GUARDD pilot study because it is powered to compare individuals with high-risk genotypes in the intervention group to those with high-risk genotypes in a control group and includes individuals with or without CKD24.
Figure 1:
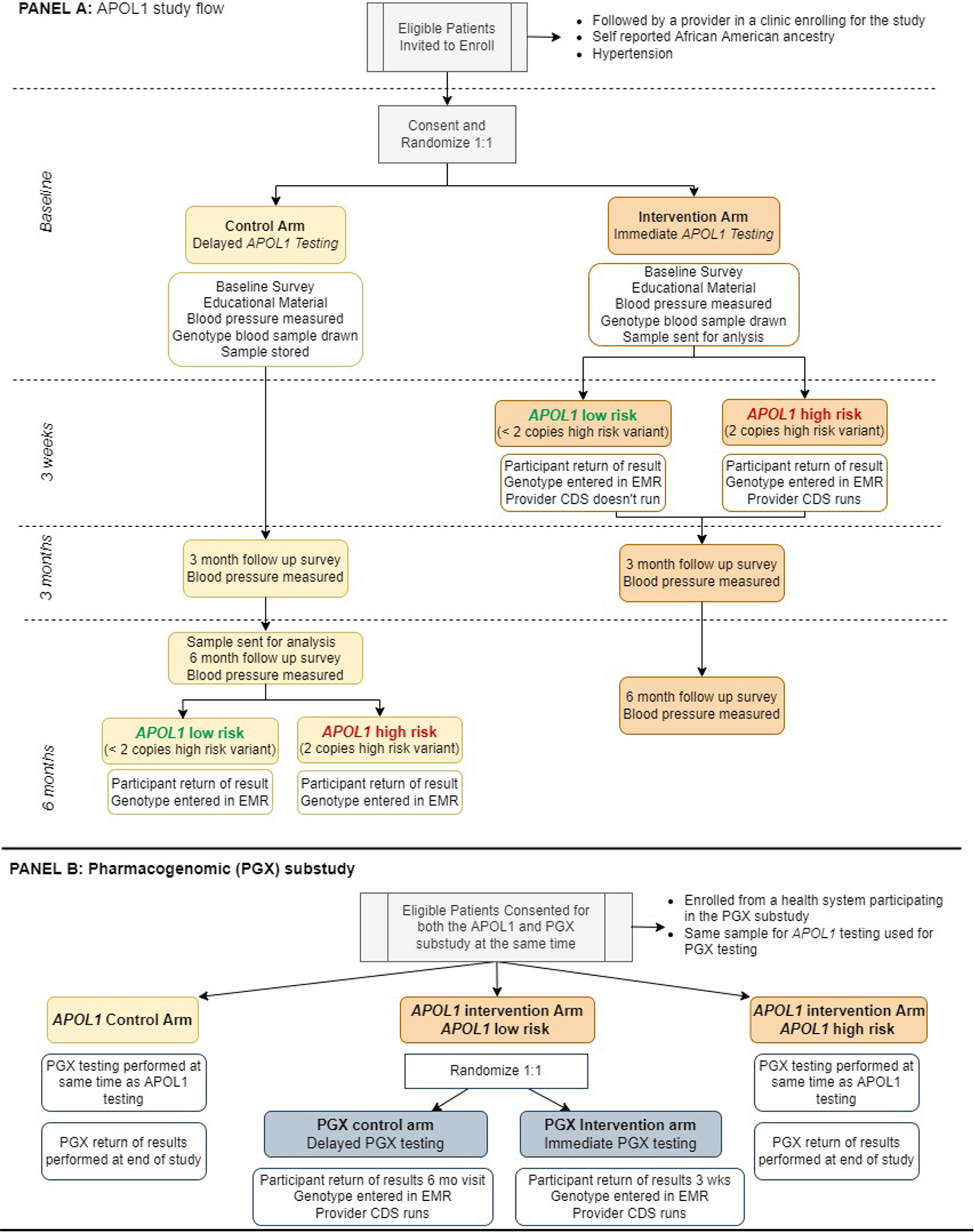
GUARDD-US study design. After obtaining written consent, eligible subjects are randomized 1:1 in the control or intervention arms. In the control arm, subjects receive delayed genetic testing with return of results after completion of the study at 6 months. In the intervention arm, subjects and their providers receive immediate disclosure of their APOL1 test results. The primary outcome is change in systolic blood pressure at 3 months between the control and intervention subjects with a high-risk APOL1 genotype. Subjects with a low-risk APOL1 genotype are not included in the primary outcome measure. A subset of the APOL1-LR subjects are re-randomized to test the efficacy of immediate and delayed antihypertensive pharmacogenomic testing. All subjects receive their APOL1 and pharmacogenomic test results at the conclusion of the study.
The study innovates in several respects. First, stakeholder and community engagement are paramount as described below. Second, the intervention is patient oriented as clinical research coordinators (CRCs) are trained to return results directly to participants in addition to providers. Finally, actionable clinical decision support (CDS) and best practice advisories (BPAs) were developed to aid primary care providers, nephrologists, and other specialists managing hypertension29. These BPAs include recommendations for CKD screening in individuals with high-risk variants or to provide disease modifying therapies such as ACE-Is/ARBs to those with early CKD.
Intervention:
The intervention for GUARDD-US is a combination of the return of results to participants and the CDS to providers. These two components are designed to enhance awareness of disease risk as it relates to the APOL1 genotype and prompt clinicians to achieve recommended blood pressure goals to reduce risk in those with APOL1-HR genotypes.
Setting:
Subjects are recruited from diverse primary care, nephrology, and cardiology clinics in academic, community, and safety-net health systems in urban and rural areas across the US. The IGNITE Network members include Duke University as the coordinating center (CC), and 13 health systems spanning 10 distinct regions (Figure 2).
Figure 2:
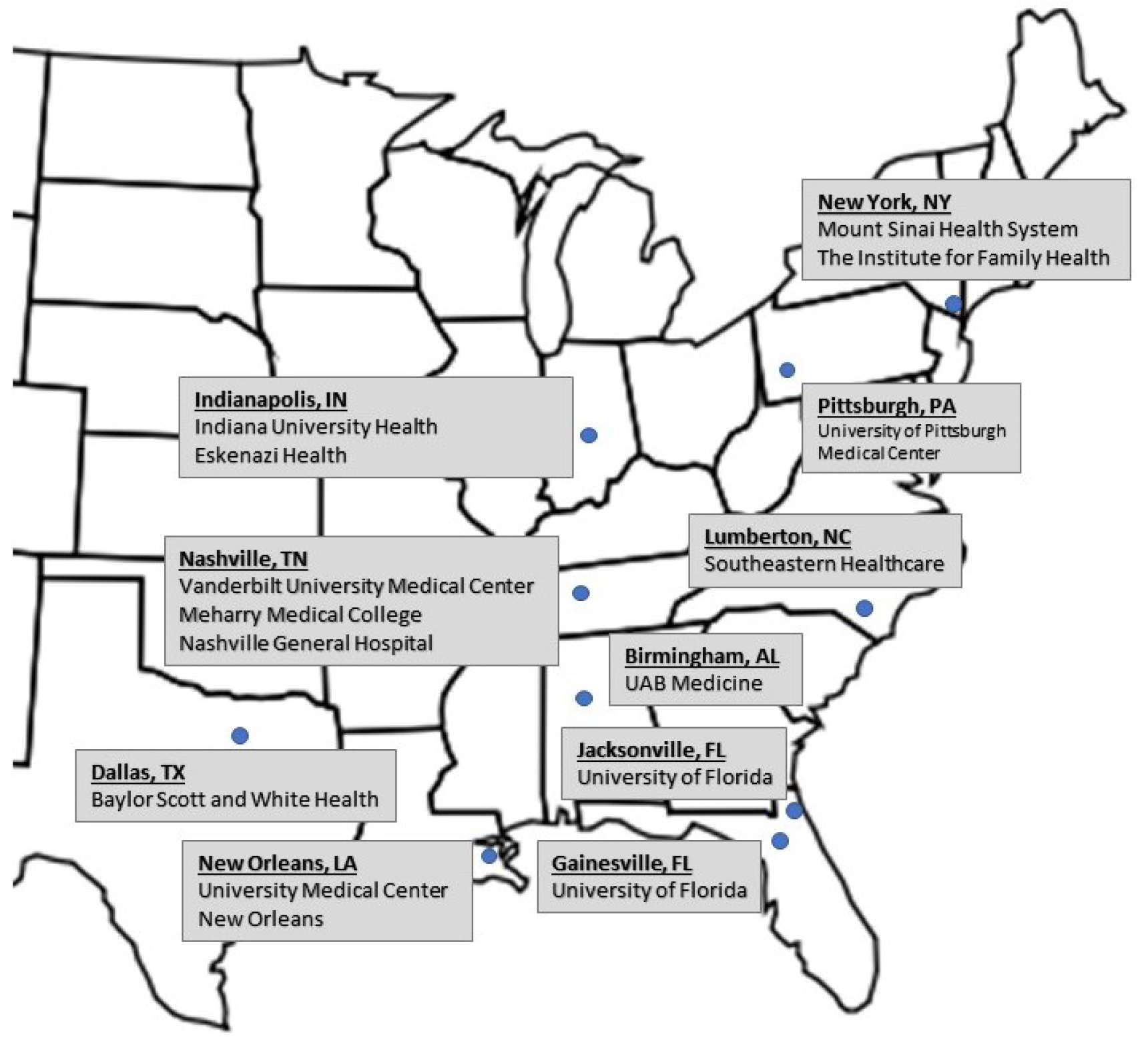
Recruitment from 13 health systems is distributed across 10 geographic regions in the United States.
Eligibility and informed consent:
Inclusion criteria include: (1) self-identified African American/Black/Afro-Caribbean/Afro-Latino or having any African Ancestry; (2) English speaking; (3) age 18–70 years; (4) diagnosed hypertension (ICD 10 codes or on problem list, and/or on antihypertensive therapy, and/or 2 systolic BP readings >140); (5) seen at least once in the past year at a participating primary care or specialty practice. Exclusion includes: (1) diabetes without CKD (eGFR < 60 ml/min per 1.73 m2 or microalbumin/proteinuria level >30 mg/g for 3 or more months; (2) ESKD (on dialysis or eGFR<15 ml/min); (3) terminally ill (metastatic cancer, palliative care or hospice); (4) declared pregnancy; (5) liver, kidney, or bone marrow transplant; (6) too cognitively impaired, ill or institutionalized to provide informed consent and/or complete study protocol; (7) plan to move out of the area within 6 months of enrollment; (8) not receiving care for their hypertension at a participating site; and (9) previous APOL1 testing. CRCs review all criteria and obtain informed consent.
Overall Study Flow:
The overall study flow is depicted in Figure 1. Participants are recruited from eligible clinics at one of the 13 health systems. After consent, participants are randomized to the intervention or control arms. All participants then undergo three study visits over 6 months. At baseline, all subjects have blood pressure measured, complete a baseline survey, and receive an educational booklet with information about hypertension, kidney disease, and APOL1 testing. All participants are advised to speak with their provider if their BP is greater than 140/90 mm Hg. Subjects with a systolic blood pressure above 180 or diastolic BP above 100 are contacted by a study investigator with clinical experience to direct the subject to receive additional care if medically indicated. All subjects provide a sample for APOL1 genotyping. Both arms return at 3 and 6 months to undergo follow-up surveys and blood pressure measurements. Samples are analyzed immediately in the intervention arm and at 6 months in the control arm; thus, all subjects ultimately receive their APOL1 genotype status. Once analyzed, CRCs review genotype results and their health implications with participants immediately after the genotype is available in the intervention arm and at the 6-month follow-up visit in the control arm.
Clinical measures:
At baseline, 3- and 6-month follow-ups study visits, CRCs measure BP and pulse digitally, three times each 2 minutes apart30. BP is obtained from participants in an upright seated position, legs uncrossed, with back straight and supported, feet flat on the floor, and the arm supported on a surface at the level of the heart with palm facing up. Most study participants have their blood pressure measured with a study-specific Omron HEM-907XL monitor with one of four cuff sizes. The cuff size used is determined by the patient’s upper arm circumference31. Participants are asked to remove long shirt sleeves (not roll them), remove jewelry or other implements, and select the preferred arm for measurement. The same arm and cuff size are used throughout the study. BP measurement is avoided on arms with a contraindication, such as an arteriovenous fistula or prior major surgery. For the day of the study visit, participants are instructed to take their blood pressure medications as normally prescribed. In certain circumstances a wrist cuff (A&D UB-543) is used, but only in the event that a subject’s arm is too big to fit the extra-large HEM-907XL cuff or if an error is repeatedly obtained using the Omron monitor32. All cuffs undergo validation with standards set by the Association for the Advancement of Medical Instrumentation, British Hypertension Society, and European Society of Hypertension-International Protocol33.
CRCs were trained on measuring BPs by video, PowerPoint slides, and one-on-one supervision to learn how to obtain BPs according to the study’s manual of operations. The first training session was performed at an IGNITE kickoff meeting. Newer CRCs review the content from this meeting and are observed and tested for competency by senior study team members before they conduct study visits. Of the 3 BP and pulse readings, the first measurement is discarded, while the second and third are recorded in the database and averaged to generate the official study blood pressure and pulse measurement. Measures of renal function are obtained from the EHR. APOL1 testing (from a blood, saliva or buccal swab sample) is performed at baseline in the intervention arm. Specimens are transferred to a Clinical Laboratory Improvement Amendments (CLIA) certified laboratory to interrogate for APOL1 G1 (rs73885319 and rs609101) and G2 (rs71785313) variants. Those homozygous or compound heterozygous for G1 and/or G2 are defined as “high-risk” of CKD (APOL1-HR); those heterozygous G1 or G2 carriers or homozygous wild type are defined as “low risk” of CKD result (APOL1-LR).
Surveys:
The Genomic Medicine Integrative Research (GMIR) framework served as the foundation of the surveys.34 For the patient survey at baseline, 3 and 6 months, we ask about general health35, comorbidities, family history of hypertension and kidney disease36, beliefs surrounding hypertension, kidney disease37, medications38, medication adherence39, and the patient–provider relationship40. We ask about contextual factors: impacts of the COVID-19 pandemic41, perceived racism42, patient activation43, knowledge about genetics44, 45, depression46, general anxiety47, perceived stress48, social support49, health literacy50, access to health care51, diet and physical activity52, health care utilization53, and demographics52. Participants are asked about knowledge, attitudes, and life-style changes surrounding genetic testing, APOL1 testing, and anticipated reactions to testing and results54–56. For a medication inventory, participants are asked to bring all prescription medications or a list of these. The provider baseline survey focuses on experience with pharmacogenetic testing, genetic testing for chronic conditions, knowledge of genetic testing results, and impacts on the patient-provider relationship57–61.
Main study endpoints and sample size:
The primary outcome compares changes in systolic BP in APOL1-HR participants in the control arm with APOL1-HR participants in the intervention arm at 3-months post enrollment. Secondary outcomes include diastolic BP and BP measured 6-months post enrollment (3 and 6-month visits may occur up to 42 days after the projected dates).
Using data from the pilot GUARDD study and 2 other studies8, 62, a 14% prevalence of APOL1-HR individuals was assumed. The approximately 6650 participants are 1:1 randomized (3325 in each arm), with an expected 931 being APOL1-HR (approximately 466 in each arm). Using a two tailed t-test with a 5% level of significance, 10% lost to follow-up, one interim analysis (adjusted using Lan-Demets spending function approximating O’Brein-Fleming boundaries), and a standard deviation of 18.1 mm of Hg for SBP (from pilot GUARDD12), a sample size of 6650 with 931 APOL1-HR participants will detect a 3.5 mm Hg difference in SBP from baseline to the 3-month follow-up between the APOL1 intervention and control arms with at least 80% power.
An additional secondary outcome is the inclusion of an appropriate ICD10 diagnosis of CKD entered into the EHR which corresponds with estimated glomerular filtration rate or proteinuria. Providers do not include CKD on the problem list of 40% of patients with laboratory evidence of CKD, and there is widespread patient unawareness of CKD. Urine protein tests, essential for early diagnosis and prognosis of CKD, are missing in up to 30% of participants with CKD. Utilization of urine microalbumin testing is a secondary outcome as well. Disclosure of APOL1 results could increase CKD diagnosis and treatment, and impact hypertension treatment through increased clinician action or changes in patients’ behaviors.
Statistical methods:
To determine the effect of knowledge of APOL1-HR status on SBP, the change in SBP from baseline to 3 months in the Intervention APOL1-HR group, will be compared to the change in SBP from baseline to 3 months of the Control APOL1-HR group using a two-sided t-test with a two-sided type I error of 0.025. Participants that are APOL1-LR in both the Intervention and Control groups will not be included in the primary analysis. The primary analysis will be conducted according to the principle of intention-to-treat (ITT) with participants analyzed and endpoints attributed according to the treatment arm to which the participants were randomized, regardless of subsequent crossover or post-randomization medical care within the pre-specified subset of APOL1-HR participants.
Participant Return of Results:
The goal of participant return of results is to ensure the participant is aware of their APOL1 genotype, its associated phenotype, and what actions they can take to reduce their risk (if they have a high-risk genotype). Once genotype results are available, CRCs generate a personalized patient-oriented educational document about their result and review the result and its clinical implications with the participant over the phone. To ensure comprehension, CRCs employ the participant teach back method, asking patients to explain the results, and provide clarification when needed. The personalized results document is shared via mail and/or email. In addition, participants are given the option to speak with a genetic counselor or physician with relevant expertise about their results at no charge.
Provider Clinical decision support (CDS):
The goal of CDS is to ensure that providers are aware of the participant’s APOL1 genotype (i.e. no high risk variants, 1 high risk variant, or 2 high risk variants), the clinical implications of their phenotype, and risk management recommendations based on their medical conditions, blood pressure readings, and current medications. The CDS was designed by a CDS committee comprised of study personnel with expertise in informatics, hypertension, and chronic kidney disease to optimize both process and content. Given that this multi-site study engaged a wide variety of health systems with different capabilities, EHRs, and expertise, the CDS core features were defined by the committee with example implementations; but each site ultimately determined how to implement. The CDS core features included 1) actions to recommend based on genotype, CKD status, blood pressure, and microalbumin testing, 2) wording of clinical decision support for each actionable event, and 3) timing of the alert. Examples of CDS algorithms with actions are show in Table 1 for those with a high-risk genotype. An overview of CDS design and development has been published29. Ultimately, sites chose one of two primary implementation pathways: fully electronic using best practice alerts or manual using clinical notes with provider messaging. There were, of course, nuanced differences between health systems even within one pathway (such as electronic) but these were small compared to the larger infrastructure differences between the electronic and manual pathways. Both pathways started with a discrete genotype result. In the electronic pathway, the genotype result prompts analysis of other EHR variables (blood pressure, microalbumin, medical conditions) needed to specify the appropriate best practice alert. The alert is then automatically presented the next time the provider opens the participant’s chart (interruptive alert). In the manual pathway the genotype result is received by the study team from the laboratory and manually entered as a discrete laboratory value. At the same time the study team reviews the chart, follows the algorithm, and determines which CDS content should be presented to the provider. The content is then uploaded in the participant’s chart as a clinical note and the provider alerted to review the note. The published CDS algorithms for the pharmacogenomics sub-study mirrors that of the primary study29.
Table 1:
CDS Algorithms for subjects with a high-risk APOL1 genotype
| Alert Type | Microalbumin/Creat. Ratio | BP | RAAS Antagonist | Alert recommendation |
|---|---|---|---|---|
| No alert | Normal | <140/90 | N/A | No alert fires in this circumstance |
| 1 | Not on file | any | N/A | Microalbumin/creatinine ratio order suggested |
| 2 | Normal (<30 mg/g) | >140/90 | N/A | General BP recommendations given |
| 3 | Elevated (>30 mg/g) | >140/90 | Not prescribed | Add RAAS antagonist for BP control |
| 4 | Elevated (>30 mg/g) | <140/90 | Not prescribed | Add RAAS antagonist for CKD prevention |
| 5 | Elevated (>30 mg/g) | >130/80 | Prescribed | CKD monitoring or titrate RAAS blockade |
The effectiveness of the study’s intervention depends on health professionals viewing and acting on CDS. Orders such as microalbumin screening and antihypertensive agent initiation are embedded within the CDS alerts so that providers can order these from the alert itself. Providers are educated at the beginning of the study, but all prescriptions and orders are made at their behest. To understand CDS fidelity, the consortium has an EHR workgroup which will measure prescribing and ordering behavior of practitioners at the conclusion of the study. Such information will facilitate assessment of the CDS implementation efficacy.
Clinician recruitment:
Providers (physicians, nurse practitioners, physician assistants) from participating sites receive a brief presentation, information sheet, or email with study background and details. Providers are then emailed a link to complete a baseline survey.
Participant recruitment:
A computable phenotype-based search identifies patients that meet specific EHR criteria and CRCs verify these through screening questions and EHR review. According to local requirements, CRCs may mail potentially eligible participants a recruitment letter on behalf of their provider and/or practice. If they do not decline participation, CRCs attempt to contact these individuals by phone. In some clinical sites, mailing participants is not required. Using a Research Electronic Data Capture (REDCap) database63, upcoming clinic appointments for potentially eligible participants are added to the calendar so CRCs can meet them at a clinic visit (i.e., clinic intercept). CRCs distribute personalized study flyers and business cards and use recruitment scripts which have been vetted by study stakeholder partners. Providers are encouraged to refer their eligible patients. Regardless of the method of initial contact, CRCs then discuss the study and screen them for enrollment. If interested and eligible, CRCs review and obtain informed consent.
Recruiter Training, Recruitment and Retention:
CRCs are extensively trained in recruitment, consent, survey administration, clinical measures, specimen collection for genetic testing, return of results, and REDCap data entry all of which are documented in a study manual of procedures64. Clinical groups target recruitment of coordinators with African ancestry, from the communities where patients are recruited or experienced in working with diverse populations. As part of their training, CRCs learn procedures proven effective in recruiting diverse patients and interview mock patients and/or are observed by experienced study personnel during the initial training period to receive feedback. Across the Network and within sites, CRC meetings are held to discuss recruitment progress, highlight successful techniques, and brainstorm ways to handle difficult situations that arise.
To best retain participants, CRCs personalize all their interactions with participants, attempt intercepts at clinical visits, and reach out to alternative contacts when necessary. Participants receive a $40 gift card (or cash) after each visit and some sites provide transportation. Evening and weekend visits are available. Sites use weekly data reports to track enrollments, declines, ineligibles, patients who have been contacted at least once, patients who have been scheduled for future enrollment, and patients who have not showed to study visits, stratified by enrolling site and by research coordinator. This allows teams to highlight and learn from successes, address individual, site and other challenges, and engage stakeholders including community members, clinicians and the broader IGNITE membership to strategize solutions. For example, teams revised recruitment scripts and approaches during the COVID-19 pandemic to address participant-identified concerns about safety of study visits and the focus on African ancestry individuals.
Randomization and Blinding:
Eligible patients are randomized in a 1:1 allocation to the APOL1 genotyping intervention or control arm with stratification according to clinical site, and block randomization within each site. Participants, providers, and study personnel are not blinded to randomization assignments since genotype results are deposited in EHRs, and the primary aim is BP control following the knowledge of risk results. To minimize bias in the primary outcome, participants are not provided their randomization assignments until after the baseline survey and BP readings are completed. Participants that are APOL1-LR in the intervention arm are re-randomized to a pharmacogenomic sub-study if their site is participating in the sub-study, as described below.
The randomization scheme was generated in SAS 9.4 and stratified by clinical site with a random block size within each stratum. The scheme was uploaded to the randomization module of the study database in REDCap. During the creation and upload of the randomization scheme, only the Data Safety and Monitoring Board (DSMB, discussed below) statistician and the database programmer had access to the scheme. Only the data coordinating center has access to the full study database, and the DCC operational team provides operational reporting to the investigators. To prevent bias, the operational reporting does not include any reports by treatment. By treatment analyses are only generated by the DSMB statistician and reported solely to the DSMB in a closed session.
Data Collection and management:
A recruitment database of potential participants is uploaded to a local database that includes demographics, upcoming clinical visits, contact data, contact attempts, screening questions, inclusion/exclusion criteria, and eligibility. Those providing informed consent are assigned a unique patient identifier in a network-wide central REDCap database. After all baseline data is completed, participants are randomized, samples are sent to designated laboratories, and results are transmitted to the EHR and coordinating center at the appropriate time(s). BP measurements must be taken in person, surveys can be completed by phone. If BP measurements are missing, any available BP during the follow-up window in EHRs are entered (and distinguished from those obtained by CRCs).
Pharmacogenomic sub-study:
Pharmacogenetic (PGx) testing may help guide initial or add-on antihypertensive therapy management for patients with hypertension. However, data are limited regarding the impact of PGx testing in hypertension, especially among African Americans. Therefore, we implemented a PGx sub-study within GUARDD-US to determine the effect of knowledge of genetic test results that predict efficacy of various antihypertensive medications on change in systolic blood pressure from baseline to 3 months in individuals who are APOL1-LR (Figure 1). Based on existing literature65, the PGx sub-study analyzes the relationship between two gene-drug pairs: YEATS4 (rs7297610) and thiazide diuretics66, and NAT2 and hydralazine67 (a 4 SNP-model of rs1799931, rs1801279, rs1799930, rs1801280). Variability in YEATS4 can impact the effectiveness of thiazide diuretics. Approximately 50% of the GUARDD population are expected to be carriers of the T allele in rs7297610 which is associated with reduced whole blood expression of YEATS4 and a reduced response to a thiazide diuretic (up to a 5 mm Hg less improvement in systolic BP as compared to those with a C/C genotype). Variability in NAT2 can impact both efficacy and toxicity of hydralazine. Approximately 50% of GUARDD participants are predicted to be NAT2 normal or intermediate metabolizers and thus are predicted to have reduced hydralazine efficacy, whereas NAT2 poor metabolizers (50% of participants) may demonstrate increased hydralazine efficacy and/or higher likelihood of adverse events.
Participants in the APOL1 intervention arm who were APOL1-LR and from a site participating in the sub-study (Florida, New York, and Indiana), are re-randomized in a 1:1 allocation to the sub-study intervention (immediate PGx testing and return of results) or control (delayed PGx testing and return of results) group. Meanwhile, participants in the APOL1 intervention arm that are APOL1-HR are excluded from the sub-study and receive delayed PGx gene testing and return of results at the 6-month visit. The expected enrollment from Florida, New York, and Indiana is 4,400 individuals, of which 2,200 will be randomized to the immediate genotyping arm and 1,892 are expected to be APOL1-LR. Thus, approximately 1,892 individuals are expected to participate in the PGx sub-study. Clinical decision support was established for the YEATS4 and NAT2 genes which allowed return of results to occur using the same methods described for the APOL1 return of results29. In contrast to the main GUARDD-US study, the sub-study’s CDS core component ‘actions to recommend’ are tailored to the class of anti-hypertensive medications the participant is taking and the CDS core component ‘wording’ reflects recommendations for starting or stopping specific anti-hypertensive classes. Subjects are not required to be on a thiazide or hydralazine for enrollment; however, providers are alerted to the efficacy of these medications with the return of the pharmacogenomic test results and with CDS when these medications are prescribed. For YEATS4, Subjects with a C/T genotype are expected to have reduced expression of YEATS4 and reduced response to thiazides and should be treated with a calcium channel blocker as first line therapy. For NAT2, subjects with a *4/*4 genotype are rapid acetylators and would be expected to have reduced response to hydralazine.
Stakeholder engagement
This translational research study engages transdisciplinary stakeholders locally and nationally. Building on the successes of the GUARDD pilot study24, we leveraged the broad expertise of NHGRI’s Implementing Genomics in Practice Pragmatic Trials Network (IGNITE) consortium whose Patient, Provider, and Payer Advisory Board helped establish the study framework, eligibility criteria, and build information to assist payers in making coverage decisions. Mount Sinai’s pre-existing Genomics Stakeholder Board co-built educational and recruitment materials and ensured diverse input, including from patients and community leaders with African ancestry.
GUARDD-US’s recruitment materials and surveys were designed through collaborations with the community leaders, patients, advocates, and clinicians to build interest and trust and to engage diverse individuals from varied educational and socioeconomic backgrounds in the study. It is an opportunity to utilize precision medicine for everyday practice in the context of chronic illness and empowers patients to reflect upon their own ancestry and genomic risks. Remaining clear that race is a social construct, but ancestry has some genetic underpinnings allows the study team to have difficult, but critical discussions about the intersectionality between race and ancestry. Our diverse teams strive to work with an anti-racist research lens. GUARDD-US helps inform the technical, economic, and social aspects of tailoring clinical interventions. By focusing on and partnering with individuals from Black communities- those most disproportionately and unjustly impacted by chronic illnesses- we also are building a roadmap to address health disparities and widen the diversity in genomic studies.
Operations
Principal investigators, project managers, and administrators meet regularly and collaborate to ensure all enrolling institutions are coordinating effectively. Study principal investigators, project managers, and coordinating center staff meet weekly during Protocol Implementation Team (PIT) calls to discuss protocol questions and challenges. CRCs from across the network meet biweekly to discuss recruitment and retention successes and challenges. To foster candid dialogue, the CRC meetings are not attended by PIs. Specific issues that require decisions or input from study leadership are relayed back to the coordinating center for discussion during the next PIT meeting. An EHR workgroup oversees the necessary extraction of data from the medical record. IGNITE’s DSMB provides safety oversight, and monitors for adverse events secondary to study interventions. Because GUARDD-US is an observational study of behavior following clinical decision support guidance based on APOL1 genotype, adverse event reporting is minimal. Distress from return of results and events of interest are monitored for oversight by the DSMB. Adverse events suspected to be related to study interventions, like a genotyping blood draw, are reported to the reviewing IRB under local policies. Reportable events including unanticipated study related deaths are collected in the study database.
Results:
Baseline Characteristics
The GUARDD-US study enrolled its first patient in July 2020 and will continue enrolling through June 2023, with an additional 6 months of follow-up planned until December 2023. As of December 2021, the study had enrolled 3423 participants with baseline study visits completed. The baseline characteristics of these participants are included in Table 2.
Table 2.
Baseline Demographics Characteristics
| Baseline Demographics Characteristics | All Participants N=3423 |
|---|---|
| Age at randomization (years) | |
| Mean (SD) | 55.7 (10.14) |
| Median (Q1, Q3) | 58.0 (50.0, 63.0) |
| Min, Max | 19, 71 |
| Sex at birth | |
| Female | 2156/3423 (63.0%) |
| Male | 1267/3423 (37.0%) |
| Gender | |
| Female | 2152/3423 (62.9%) |
| Male | 1268/3423 (37.0%) |
| Transgender | 1/3423 (0.0%) |
| Do not identify as female, male, or transgender | 2/3423 (0.1%) |
| Race/Ethnicity | |
| American Indian, Native American or Alaska Native | 18/3423 (0.5%) |
| Asian | 1/3423 (0.0%) |
| Black or African American | 3027/3423 (88.4%) |
| Native Hawaiian or Other Pacific Islander | 1/3423 (0.0%) |
| White or European American | 1/3423 (0.0%) |
| Middle Eastern or North African/Mediterranean | 2/3423 (0.1%) |
| Latino(a) | 29/3423 (0.8%) |
| Other | 46/3423 (1.3%) |
| Prefer not to answer/unknown | 32/3423 (0.9%) |
| Multiple | 266/3423 (7.8%) |
| Insurance | |
| No insurance | 204/3423 (6.0%) |
| Medicaid | 1154/3423 (33.7%) |
| Medicare | 541/3423 (15.8%) |
| Private health insurance | 1099/3423 (32.1%) |
| Other insurance | 397/3423 (11.6%) |
| Don’t know/Refused | 18/3423 (0.9%) |
| Education | |
| Some high school or less | 512/3423 (15.2%) |
| High school graduate | 836/3423 (24.4%) |
| Some post high school training or Associate degree | 1204/3423 (36.1%) |
| Bachelor’s degree | 507/3423 (14.8%) |
| Graduate or professional degree | 308/3423 (9.0%) |
| Don’t know or Refused | 16/3423 (0.5%) |
| Household’s total family income | |
| Less than $20,000 | 946/3423 (27.6%) |
| $20,000 to $39,999 | 629/3423 (18.4%) |
| $40,000 to $59,999 | 412/3423 (12.0%) |
| $60,000 to $99,999 | 404/3423 (11.8%) |
| $100,000 or more | 261/3423 (7.6%) |
| Don’t know/Refused | 771/3423 22.5%) |
Note: Percentages are based on the total number of patients with non-missing response per question.
Interim analysis
No public interim analysis will be released. An interim analysis was completed in December 2021 to assess efficacy and futility and the results were reviewed in a closed session by the IGNITE II DSMB. Study investigators are blinded to specific results, but overall the analysis determined that the study did not fulfill criteria for either efficacy or futility and will continue enrolling through June 2023.
Challenges
GUARDD-US was initially projected to complete enrollment in June 2022 with 5435 participants. The study has suffered 2 significant challenges. First, enrollment at most sites was depressed below expectations due to the COVID-19 pandemic. Due to the pandemic, the trial initiation was delayed from March 2020 to July 2020, access to clinics was reduced after the trial started, and some subjects were reticent to enroll during surges of the pandemic. Site specific issues included one site closing for months after hurricane Ida and another closing for months during an EHR ransom attack.
An additional challenge has been the gradual uptake of clinical APOL1 testing in nephrology clinics, outside of GUARDD-US. This shift in practice patterns has reduced the ability to recruit from nephrology clinics, because prior APOL1 testing is an exclusion criterion. As a result, a higher than expected proportion of subjects have been recruited from primary care clinics which resulted in a lower than expected APOL1-HR rate (14.5% vs the predicted 17%). Since APOL1-HR rate is a key driver of power in GUARDD-US, the DSMB recommended increasing the study enrollment targets. The interim analysis determined that to maintain 80% power enrollment should expand from 5,435 to 6,650. All statistics and methods described in this manuscript reflect the updated recruitment goals since December 2021. To help address the need for additional participants, GUARDD-US added two new recruiting clinical sites: the University of Alabama Birmingham and University of Pittsburgh. Despite these challenges, study teams have successfully 3423 subjects over 18 months during the pandemic and have maintained a 5.96% loss to follow-up for the primary endpoint.
Conclusions
GUARDD-US is the first large multi-site precision medicine trial designed to address uncontrolled hypertension and chronic kidney disease outcomes in African ancestry populations through APOL1 testing with clinical decision support. This study will provide essential data to inform future innovations to optimize large-scale dissemination of personalized genomic data to providers and patients by understanding how this information can influence knowledge, attitudes, and treatment of hypertension and chronic kidney disease.
The IGNITE consortium is committed to key stakeholder representation and involvement at all stages of research design, execution, interpretation and dissemination28. The GUARDD-US pragmatic trial design employed formative research, directed input from community, academic, clinical and patient stakeholders, and an inclusive approach to engage African ancestry adults of varying ages, socioeconomic backgrounds and urban, suburban, and rural communities24. This engagement approach ensures that this trial is aligned with stakeholder priorities and will increase the likelihood of adoption of the successful strategies identified by this trial.
Patient perspective:
“The GUARDD-US study has positively changed my outlook on my hypertension diagnosis. I have had hypertension for a long time but learned so much from being in the study! My study coordinator was an absolute pleasure to work with and explained everything to me perfectly so that I understood the study and [my] results.”
Acknowledgements
Research reported in this publication was supported by grants from the National Institutes of Health (U01 HG007269, U01 HG010232, U01 HG010248, U01 HG010231, U01 HG010245, U01 HG010225, and by the NIH IGNITE Network (https://gmkb.org/ignite/). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to thank the GUARDD-US team of academic, community, clinical partners, study coordinators, and staff at study sites, and their partners in the IGNITE Network, a consortium of genomic medicine pilot demonstration projects funded and guided by the NHGRI, for their valuable contributions to this project. We would also like to thank the IGNITE Patient, Provider, and Payer Advisory Board and stakeholders at the individual sites, for their devoted time and effort.
Footnotes
Declaration of competing interest:
The authors have nothing to disclose.
References:
- 1.Ostchega YF CD; Nwankwo T; Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. In: SERVICES USDOHAH, ed: National Center for Health Statistics; 2020:1–8. [PubMed] [Google Scholar]
- 2.Ayanian JZ, Landon BE, Newhouse JP, Zaslavsky AM. Racial and ethnic disparities among enrollees in Medicare Advantage plans. N Engl J Med 2014;371(24): 2288–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tedla FM, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens. 2011;2011: 132405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74(10): 1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung AK, Chang TI, Cushman WC, et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3): 559–569. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf AA, Govender MA, Brandenburg JT, Winkler CA. Kidney disease and APOL1. Hum Mol Genet 2021;30(R1): R129–R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 2013;24(9): 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 2013;369(23): 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22(11): 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Fang X, Zhang H, et al. Genetic susceptibility of hypertension-induced kidney disease. Physiol Rep 2021;9(1): e14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku E, Lipkowitz MS, Appel LJ, et al. Strict blood pressure control associates with decreased mortality risk by APOL1 genotype. Kidney Int. 2017;91(2): 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadkarni GN, Fei K, Ramos MA, et al. Effects of Testing and Disclosing Ancestry-Specific Genetic Risk for Kidney Failure on Patients and Health Care Professionals: A Randomized Clinical Trial. JAMA Netw Open. 2022;5(3): e221048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargas RB, Norris KC. Kidney disease progression and screening cost-effectiveness among African Americans. J Am Soc Nephrol 2012;23(12): 1915–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powe NR. To have and have not: Health and health care disparities in chronic kidney disease. Kidney Int 2003;64(2): 763–772. [DOI] [PubMed] [Google Scholar]
- 15.Peralta CA, Katz R, DeBoer I, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol 2011;22(7): 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int 2005;68(3): 914–924. [DOI] [PubMed] [Google Scholar]
- 17.Keith D, Ashby VB, Port FK, Leichtman AB. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol 2008;3(2): 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns TS, Estrella MM, Crews DC, et al. Neighborhood socioeconomic status, race, and mortality in young adult dialysis patients. J Am Soc Nephrol 2014;25(11): 2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 2003;14(11): 2902–2907. [DOI] [PubMed] [Google Scholar]
- 20.Gao SW, Oliver DK, Das N, et al. Assessment of racial disparities in chronic kidney disease stage 3 and 4 care in the department of defense health system. Clin J Am Soc Nephrol 2008;3(2): 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller M, Purnell TS, Mensah GA, Cooper LA. Reducing racial and ethnic disparities in hypertension prevention and control: what will it take to translate research into practice and policy? Am J Hypertens 2015;28(6): 699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolezsar CM, McGrath JJ, Herzig AJM, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol 2014;33(1): 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sims M, Diez-Roux AV, Dudley A, et al. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am J Public Health 2012;102 Suppl 2: S258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horowitz CR, Abul-Husn NS, Ellis S, et al. Determining the effects and challenges of incorporating genetic testing into primary care management of hypertensive patients with African ancestry. Contemp Clin Trials. 2016;47: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horowitz CR, Ferryman K, Negron R, et al. Race, Genomics and Chronic Disease: What Patients with African Ancestry Have to Say. J Health Care Poor Underserved. 2017;28(1): 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman DJ, Pollak MR. APOL1 Nephropathy: From Genetics to Clinical Applications. Clin J Am Soc Nephrol 2021;16(2): 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp JB, Winkler CA. Genetic Testing for APOL1 Genetic Variants in Clinical Practice: Finally Starting to Arrive. Clin J Am Soc Nephrol 2020;15(1): 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginsburg GS, Cavallari LH, Chakraborty H, et al. Establishing the value of genomics in medicine: the IGNITE Pragmatic Trials Network. Genetics in medicine : official journal of the American College of Medical Genetics. 2021;23(7): 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider TM, Eadon MT, Cooper-DeHoff RM, et al. Multi-Institutional Implementation of Clinical Decision Support for APOL1, NAT2, and YEATS4 Genotyping in Antihypertensive Management. J Pers Med 2021;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1): 142–161. [DOI] [PubMed] [Google Scholar]
- 31.Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Press Monit 2007;12(4): 233–242. [DOI] [PubMed] [Google Scholar]
- 32.Fania C, Benetti E, Palatini P. Validation of the A&D BP UB-543 wrist device for home blood pressure measurement according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit 2015;20(4): 237–240. [DOI] [PubMed] [Google Scholar]
- 33.Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens 2018;36(3): 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horowitz CR, Orlando LA, Slavotinek AM, et al. The Genomic Medicine Integrative Research Framework: A Conceptual Framework for Conducting Genomic Medicine Research. Am J Hum Genet 2019;104(6): 1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart AL, Hays RD, Ware JE Jr.,The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26(7): 724–735. [DOI] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- 37.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60(6): 631–637. [DOI] [PubMed] [Google Scholar]
- 38.Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns 1999;37(2): 113–124. [DOI] [PubMed] [Google Scholar]
- 39.Voils CI, Maciejewski ML, Hoyle RH, et al. Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Med Care. 2012;50(12): 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5): 728–739. [DOI] [PubMed] [Google Scholar]
- 41.Joel Stoddard EKR, Ruth Paris, Simone Haller, Sara Johnson, Jodi Zik, Eliza Elliotte, Mihoko Maru, Allison Jaffe, Ajitha Mallidi, Ashley Smith, Raquel G. Hernandez, Heather E. Volk, Melissa A. Brotman, Joan Kaufman The Coronavirus Impact Scale: Construction, Validation, and Comparisons in Diverse Clinical Samples. PsyArXiv Preprints. 2020;2021(May 24th). [Google Scholar]
- 42.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev 2000;57 Suppl 1: 146–161. [DOI] [PubMed] [Google Scholar]
- 43.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39(4 Pt 1): 1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henneman L, Timmermans DR, van der Wal G. Public experiences, knowledge and expectations about medical genetics and the use of genetic information. Community Genet 2004;7(1): 33–43. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson SC, Linderman MD, Suckiel SA, et al. Motivations, concerns and preferences of personal genome sequencing research participants: Baseline findings from the HealthSeq project. Eur J Hum Genet 2016;24(1): 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114(1–3): 163–173. [DOI] [PubMed] [Google Scholar]
- 47.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146(5): 317–325. [DOI] [PubMed] [Google Scholar]
- 48.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24(4): 385–396. [PubMed] [Google Scholar]
- 49.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32(6): 705–714. [DOI] [PubMed] [Google Scholar]
- 50.Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med 2006;21(8): 874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. Jama. 1995;274(4): 305–311. [PubMed] [Google Scholar]
- 52.CfDCaP. CfDCC. National Health and Nutrition Examination Survey Questionnaire.. Vol 2021: Centers for Disease Control; 2017. [Google Scholar]
- 53.Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev 2006;63(2): 217–235. [DOI] [PubMed] [Google Scholar]
- 54.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4): 281–292. [DOI] [PubMed] [Google Scholar]
- 55.Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol 2002;21(6): 564–572. [PubMed] [Google Scholar]
- 56.Holmes-Rovner M, Kroll J, Schmitt N, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making. 1996;16(1): 58–64. [DOI] [PubMed] [Google Scholar]
- 57.Bernhardt BA, Zayac C, Gordon ES, Wawak L, Pyeritz RE, Gollust SE. Incorporating direct-to-consumer genomic information into patient care: attitudes and experiences of primary care physicians. Per Med 2012;9(7): 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonham VL, Sellers SL, Woolford S. Physicians’ knowledge, beliefs, and use of race and human genetic variation: new measures and insights. BMC Health Serv Res 2014;14: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Overby CL, Erwin AL, Abul-Husn NS, et al. Physician Attitudes toward Adopting Genome-Guided Prescribing through Clinical Decision Support. J Pers Med 2014;4(1): 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomarkers. 2013;17(3): 219–225. [DOI] [PubMed] [Google Scholar]
- 61.van Langen IM, Birnie E, Leschot NJ, Bonsel GJ, Wilde AA. Genetic knowledge and counselling skills of Dutch cardiologists: sufficient for the genomics era? Eur Heart J 2003;24(6): 560–566. [DOI] [PubMed] [Google Scholar]
- 62.Spiech KM, Tripathy PR, Woodcock AM, et al. Implementation of a Renal Precision Medicine Program: Clinician Attitudes and Acceptance. Life (Basel). 2020;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horowitz CR, Sabin T, Ramos M, et al. Successful recruitment and retention of diverse participants in a genomics clinical trial: a good invitation to a great party. Genetics in medicine : official journal of the American College of Medical Genetics. 2019;21(10): 2364–2370. [DOI] [PubMed] [Google Scholar]
- 65.Eadon MT, Maddatu J, Moe SM, et al. Pharmacogenomics of Hypertension in CKD: The CKD-PGX Study. Kidney360. 2022;3(2): 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duarte JD, Turner ST, Tran B, et al. Association of chromosome 12 locus with antihypertensive response to hydrochlorothiazide may involve differential YEATS4 expression. Pharmacogenomics J 2013;13(3): 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins KS, Raviele ALJ, Elchynski AL, et al. Genotype-Guided Hydralazine Therapy. Am J Nephrol 2020;51(10): 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]


