Introduction
Studies providing detailed information on colonoscopy use are important for the interpretation of patterns and trends in colorectal cancer incidence and mortality, but there is a lack of such studies from Germany. To fill this gap, we aimed to describe the 10-year prevalence of colonoscopy use based on German health claims data.
Methods
Using the German Pharmacoepidemiological Research Database (short GePaRD; claims data from ~20% of the German population), we determined the 10-year prevalence of colonoscopy use for the year 2017. We determined this prevalence for any colonoscopy, screening (reimbursable from age 55) and diagnostic colonoscopy, stratified by sex, age, educational level and regional factors (e.g. federal state, physicians density in the district of residence).
Results
In men, the 10-year prevalence of colonoscopy use was as follows (not all age groups reported): 30–34 years: 8%, 40–44 years: 12%, 50–54 years: 21%, 55–59 years: 33% (screening: 10%), 60–64 years: 44% (screening: 23%), 70–74 years: 53% (screening: 23%), 80–84 years: 52% (screening: 15%). In women, the prevalences were similar, with differences mostly less than or equal to 3 percentage points. Also, in analyses stratified by educational level or regional factors, prevalences were mostly similar or varied by less than or equal to 4 percentage points.
Conclusion
In 2017, about 45–50% of men and women in Germany aged 60–84 years had any colonoscopy in the previous 10 years, and about 11–26% had a screening colonoscopy. Our findings suggest no relevant social or regional disparities in the utilization of colonoscopy in Germany.
Keywords: claims data, colonoscopy, colorectal cancer, diagnostic, Germany, prevalence, screening, utilization
Introduction
Colorectal cancer (CRC) is the second most common cancer and the cause of cancer-related death in Europe (Ferlay et al., 2018). Within Europe, there are marked differences regarding current levels as well as recent trends in CRC incidence and mortality. In 2020, estimated age-standardized CRC incidence (per 100.000) was, for example, 21.0 in Austria, 35.8 in Spain and 41.9 in Norway; and age-standardized CRC mortality (per 100.000) was 8.8 in Finland, 13.0 in Portugal and 21.0 in Slovakia (Ferlay et al., 2020). Germany is one of the few countries where not only CRC mortality, but also CRC incidence has started to decline in recent years. Between 2000 and 2016, age-standardized CRC incidence decreased by 22% in men and 26% in women, and age-standardized CRC mortality decreased by 36% in men and 41% in women between 2000 and 2018 (Cardoso et al., 2021). In Germany, screening colonoscopy has been offered since 2002 as part of an opportunistic screening programme to persons aged 55 or older as an alternative to the faecal occult blood test offered from age 50 onwards (Haug, 2018). An organized CRC screening programme has been introduced in 2019 (Gemeinsamer Bundesausschuss, 2020).
While changes in the prevalence of CRC risk factors may also be important, there is a general consensus that colonoscopy use plays an important role regarding trends in CRC incidence and mortality given the convincing evidence on the effectiveness of lower endoscopy in preventing CRC and CRC-related death (Lauby-Secretan et al., 2018). High-quality information on the prevalence of colonoscopy use at the population level is thus important for understanding recent and projecting future trends in CRC incidence and mortality. Such information is also useful to detect potential subgroups of the population with low use of colonoscopy who may be targeted for educational interventions. Ideally, data to determine the prevalence of colonoscopy use would not only capture screening colonoscopies but also –as a separate category – diagnostic colonoscopies given that both are expected to exert a preventive effect (Brenner et al., 2014). Furthermore, longitudinal data with a sufficiently long observation period are required in view of the long-lasting effect of colonoscopy. 10-year prevalence is a reasonable measure and more informative as compared to prevalences determined for shorter time periods. Finally, given that many risk factors of CRC have been reported to be correlated with a low socioeconomic status (SES) (Doubeni et al., 2012), determining the prevalence of colonoscopy use stratified by SES would also provide important insights. Even though there have been reports on colonoscopy use in Germany (Altenhofen; Stock et al., 2010, 2011; Starker and Saß, 2013; Altenhofen, 2016; Chen et al., 2017; Starker et al., 2017; Cardoso et al., 2019; Chen et al., 2019b; Tillmanns et al., 2019; Cardoso et al., 2020; Guo et al., 2020; Steffen et al., 2020), there is – to the best of our knowledge – no study from Germany doing justice to all aspects mentioned above.
To overcome this gap, we aimed to use the potential of the German Pharmacoepidemiological Research Database (GePaRD), a large claims database, to determine 10-year prevalence of colonoscopy use for the year 2017, stratified by screening vs. diagnostic colonoscopy, age, sex, estimates of the SES and regional factors.
Methods
Data source
This study was conducted using GePaRD which is based on claims data from four statutory health insurance providers in Germany and currently includes information on approximately 25 million persons who have been insured with one of the participating providers since 2004 or later. In addition to the demographic data, GePaRD contains information on drug dispensations as well as outpatient (i.e. from general practitioners and specialists) and inpatient services and diagnoses. Per data year, there is information on approximately 20% of the general population and all geographical regions of Germany are represented.
In GePaRD, information on colonoscopy use, including the date of the procedure, is obtained based on codes of the German Uniform Assessment Standard (EBM) and the Operations and Procedures Coding System (OPS). There are different codes for screening and diagnostic colonoscopy, that is, these two types of colonoscopy can be distinguished.
In Germany, the utilization of health insurance data for scientific research is regulated by the Code of Social Law. All involved health insurance providers as well as the German Federal Office for Social Security and the Senator for Health, Women and Consumer Protection in Bremen as their responsible authorities approved the use of GePaRD data for this study. Informed consent for studies based on claims data is required by law unless obtaining consent appears unacceptable and would bias results, which was the case in this study. According to the Ethics Committee of the University of Bremen, studies based on GePaRD are exempt from institutional review board review.
Study design and study population
To determine 10-year prevalence for the year 2017, we included all persons with valid information on sex and age, place of residence in Germany and a continuous insurance period from at least 2006 to 2017 (denominator). Among these, we determined the number of persons who had at least one colonoscopy between 2008 and 2017 (numerator) and calculated the 10-year prevalence of colonoscopy use for the year 2017. If a person had both a screening and a diagnostic colonoscopy in this 10-year time period, we used the first examination to classify persons into screening vs. diagnostic colonoscopy use. We used a pre-observation period of 2 years to assess whether the persons with a colonoscopy already had any codes indicating a prevalent CRC before colonoscopy. For example, for persons with a colonoscopy in 2008, we used the data from 2006 and 2007 as pre-observation period regarding prevalent CRC. For comparison, we also calculated the 5-year prevalence of colonoscopy use for 2017 in an analogous way.
Data analysis
We characterized included men and women (denominator) with respect to the distribution of age, SES (two categories defined by educational level) and region of residence. Similarly, we described 10-year prevalence of colonoscopy use in men and women with respect to these factors, both for colonoscopy overall and stratified by type of the first colonoscopy (screening vs. diagnostic). With respect to regional factors, we considered federal state, geographical region (Northern, Western, Southern or Eastern Germany), urbanization of the district of residence (urban vs. rural) (Bundesinstitut für Bau- Stadt- und Raumforschung, 2020) as well as density of physicians (any physician, gastroenterologists) in the respective district of residence (Statistisches Bundesamt, 2021).
We calculated 95% confidence intervals (CIs) of colonoscopy prevalence using the Wald method. The analyses were carried out with the statistical software SAS 9.4 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
Overall, a total of 7 475 668 persons (55% female) were included. Of these, 2 287 472 had at least one colonoscopy between 2008 and 2017, yielding a 10-year prevalence of colonoscopy use of 31% overall (diagnostic colonoscopy: 21%, screening colonoscopy: 10%).
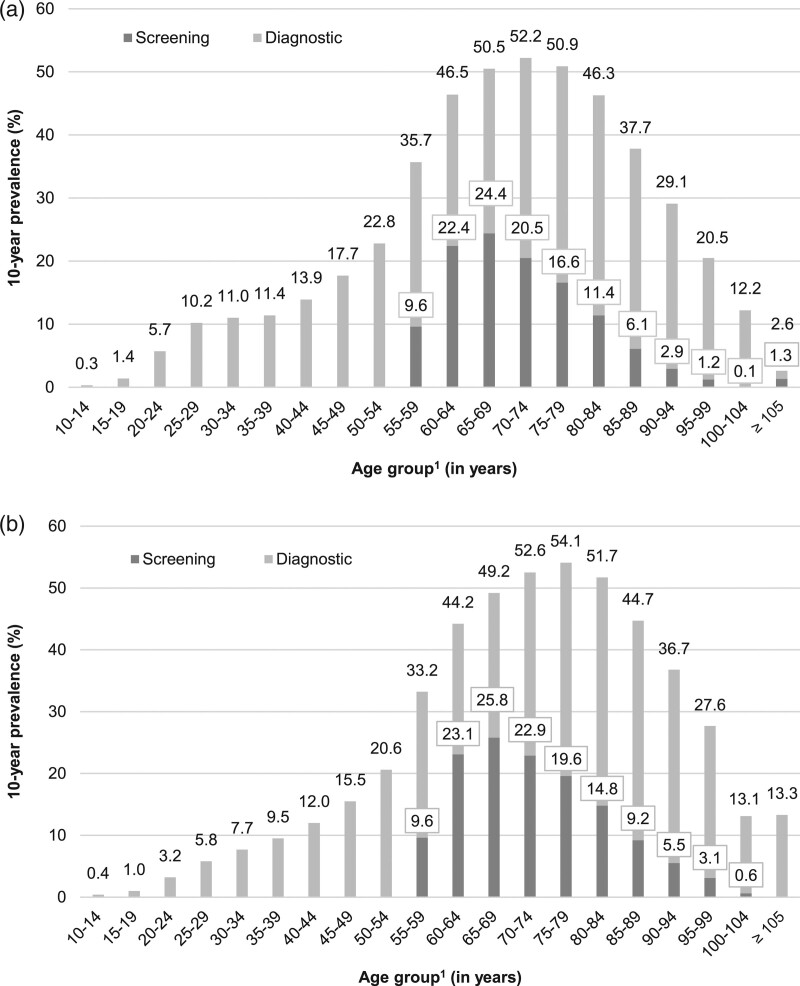
As shown in Fig. 1a (women) and Fig. 1b (men), the 10-year prevalence of colonoscopy varied by age. In women, the overall 10-year prevalence increased from 10 to 14% at age 25–44 to 23% at age 50–54 and was 36% at age 55–59. At age 65–79 it was highest with a 10-year prevalence of 51–52% and it remained above 35% until age 85–89. In men, the age pattern regarding the overall 10-year prevalence of colonoscopy was similar, with some minor differences in younger and older adults: Below age 65, the prevalence was up to 2–4 percentage points lower and from the ages 75–79 to 95–99, the prevalence was 3–8 percentage points higher as compared to women. The 10-year prevalence of screening colonoscopy in men and women was 10% at age 55–59 and 22–26% at age 60–69. It started to decline thereafter: in women from 21% (men: 23%) at age 70–74 to 6% (men: 9%) at age 85–89 (see also Supplemental digital content 1, http://links.lww.com/EJCP/A348, showing sample sizes and CIs). The 5-year prevalence showed patterns similar to the 10-year prevalence. In almost all age and sex groups, it was more than half as high as the 10-year prevalence; at age 55–59, it amounted to 76% of the 10-year prevalence (see Supplemental digital content 2, http://links.lww.com/EJCP/A348).
Fig. 1.
(a) 10-year prevalence of colonoscopy use (screening and diagnostic) among women in 2017 according to age. (b) 10-year prevalence of colonoscopy use (screening and diagnostic) among men in 2017 according to age. 1Age in 2017.
Table 1 shows the 10-year prevalence of screening and diagnostic colonoscopy use in 2017 according to SES in men and women, stratified by age (≤65 vs. >65 years). Regarding SES, the 10-year prevalence of screening colonoscopy was 4 percentage points lower among men above age 65 with a (basic) secondary degree as compared to those with a higher educational degree (24.1 vs. 28.1%). In women less than or equal to 65 years, the 10-year prevalence of colonoscopy overall was 4.6 percentage points higher among those with a (basic) secondary degree as compared to those with a higher educational degree (24.1 vs. 19.5%). In all other subgroups, the difference in the prevalence of screening or diagnostic colonoscopy according to educational level was below 3 percentage points.
Table 1.
10-year prevalence of colonoscopy use (overall, screening and diagnostic) in 2017 according to educational level in women and men aged ≤65 and >65 years
| Age ≤65 yearsa | Age >65 yearsa | |||||
|---|---|---|---|---|---|---|
| Overall | Type of colonoscopy | Overall | Type of colonoscopy | |||
| Screening | Diagnostic | Screening | Diagnostic | |||
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Women | ||||||
| Total | 22.7 (22.6–22.7) | 5.5 (5.5–5.5) | 17.2 (17.1–17. 2) | 48.0 (47.9–48.0) | 16.2 (16.2–16.3) | 31.7 (31.7–31.8) |
| Educational levelb | ||||||
| (Basic) secondary degree | 24.1 (24.1–24.2) | 6.0 (6.0–6.1) | 18.1 (18.1–18.2) | 51.0 (50.8–51.3) | 24.5 (24.3–24.8) | 26.5 (26.3–26.8) |
| Higher education | 19.5 (19.5–19.6) | 4.3 (4.3–4.3) | 15.2 (15.2–15.3) | 52.2 (51.8–52.6) | 26.4 (26.0–26.8) | 25.8 (25.4–26.2) |
| Men | ||||||
| Total | 19.6 (19.6–19.7) | 5.3 (5.3–5.4) | 14.3 (14.2–14.3) | 51.2 (51.1–51.3) | 19.7 (19.6–19.7) | 31.5 (31.5–31.6) |
| Educational levelb | ||||||
| (Basic) secondary degree | 17.2 (17.1–17. 2) | 4.3 (4.3–4.4) | 12.8 (12.8–12.9) | 50.5 (50.3–50.8) | 24.1 (23.8–24.3) | 26.5 (26.2–26.7) |
| Higher education | 20.1 (20.0–20.2) | 5.6 (5.6–5.7) | 14.5 (14.4–14.6) | 53.7 (53.4–53.9) | 28.1 (27.8–28.3) | 25.6 (25.4–25.8) |
CI, confidence interval.
Age in 2017.
Category degree unkown/no formal degree not shown here.
Table 2 shows the 10-year prevalence of colonoscopy use – overall, screening and diagnostic – in 2017 according to federal state and other regional factors, stratified by age (≤65 vs. >65 years). Among persons aged less than or equal to 65 years, the prevalence of overall colonoscopy use ranged from 18% (minimum) in Saxony to 22–23% (maximum) in Hesse, Rhineland-Palatinate, Baden-Württemberg, Bavaria, Saarland and Mecklenburg-Western Pomerania. Among persons aged greater than 65 years, the prevalence of overall colonoscopy use was 46–47% in Bremen, North Rhine-Westphalia, Saarland, Saxony-Anhalt and Thuringia, 48–49% in Saxony and Brandenburg and 50–52% in all other federal states. The 10-year prevalence of screening colonoscopy tended to be higher in federal states with a lower prevalence of diagnostic colonoscopy and vice versa (e.g. Hesse and Brandenburg). When grouping the federal states into Northern, Southern, Eastern and Western Germany, the prevalence of colonoscopy use overall ranged from 19% (Eastern Germany) to 23% (Southern Germany) in persons aged less than or equal to 65 years and from 47% (Eastern Germany) to 51% (Southern Germany) in persons aged greater than 65 years. In persons aged greater than 65 years, the prevalence of screening colonoscopy was higher in the Northern (21%) than in the Western part of Germany (15%) but for diagnostic colonoscopy, it was inverse (Western: 34%; Northern: 29%). Stratification by urbanization of the district of residence (urban vs. rural) did not show any differences in the prevalence of colonoscopy use, neither in persons less than or equal to 65 years nor in persons aged greater than 65 years. Also, the 10-year prevalence did not vary when we distinguished between federal states with a high vs. a low density of physicians (any kind) or gastroenterologists.
Table 2.
10-year prevalence of colonoscopy use (overall, screening and diagnostic) in 2017 according to federal state and other regional factors in persons aged ≤65 and >65 years
| Age ≤65 yearsa | Age >65 yearsa | |||||
|---|---|---|---|---|---|---|
| Overall | Type of colonoscopy | Overall | Type of colonoscopy | |||
| Screening | Diagnostic | Screening | Diagnostic | |||
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Federal state | ||||||
| Schleswig-Holstein | 20.2 (20.1–20.4) | 5.9 (5.8–6.0) | 14.3 (14.2–14.5) | 49.7 (49.4–50.0) | 21.1 (20.9–21.3) | 28.6 (28.3–28.8) |
| Hamburg | 21.0 (20.8–21.2) | 6.0 (5.9–6.1) | 15.0 (14.9–15.2) | 52.3 (51.9–52.6) | 26.1 (25.8–26.4) | 26.2 (25.9–26.5) |
| Lower Saxony | 21.2 (21.1–21.3) | 6.3 (6.2–6.4) | 14.9 (14.8–15.0) | 49.7 (49.5–49.8) | 20.9 (20.8–21.1) | 28.7 (28.6–28.9) |
| Bremen | 20.6 (20.3–20.8) | 5.3 (5.2–5.4) | 15.3 (15.1–15.5) | 47.0 (46.7–47.4) | 17.4 (17.1–17.6) | 29.7 (29.3–30.0) |
| North Rhine-Westphalia | 20.5 (20.5–20.6) | 5.1 (5.0–5.1) | 15.5 (15.4–15.5) | 47.3 (47.2–47.5) | 15.4 (15.3–15.5) | 31.9 (31.8–32.0) |
| Hesse | 21.8 (21.7–21.9) | 3.7 (3.6–3.7) | 18.1 (18.0–18.2) | 49.6 (49.4–49.8) | 12.0 (11.8–12.1) | 37.6 (37.4–37.8) |
| Rhineland-Palatinate | 23.0 (22.8–23.2) | 5.4 (5.3–5.5) | 1 7.6 (17.4–17 . 7) | 49.8 (49.5–50.1) | 16.1 (15.9–16.3) | 33.7 (33.4–34.0) |
| Baden-Wuerttemberg | 22.2 (22.1–22.3) | 5.8 (5.7–5.8) | 16.4 (16.3–16.5) | 50.4 (50.2–50.6) | 17.2 (17.1–17.4) | 33.2 (33.0–33.3) |
| Bavaria | 23.1 (23.0–23.2) | 5.0 (5.0–5.1) | 18.1 (18.0–18.2) | 51.6 (51.4–51.8) | 17.4 (17.2–17.5) | 34.2 (34.1–34.4) |
| Saarland | 22.8 (22.4–23.1) | 5.8 (5.6–6.0) | 17.0 (16.7–17.3) | 47.3 (46.7–47.9) | 17.2 (16.7–17.6) | 30.2 (29.6–30.7) |
| Berlin | 18.9 (18.8–19.0) | 5.6 (5.5–5.7) | 13.3 (13.2–13.4) | 51.5 (51.2–51.8) | 23.1 (22.8–23.3) | 28.5 (28.2–28.8) |
| Brandenburg | 19.8 (19.6–20.0) | 7.0 (6.9–7.1) | 12.8 (12.6–12.9) | 48.8 (48.5–49.1) | 22.1 (21.8–22.3) | 26.8 (26.5–27.1) |
| Mecklenburg-Western Pomerania | 22.0 (21.8–22.3) | 6.6 (6.4–6.7) | 15.4 (15.2–15.7) | 50.8 (50.4–51.2) | 17.3 (17.0–17.6) | 33.6 (33.2–33.9) |
| Saxony | 18.2 (18.0–18.4) | 5.8 (5.7–5.9) | 12.4 (12.2–12.6) | 47.9 (47.5–48.2) | 19.8 (19.5–20.1) | 28.1 (27.8–28.4) |
| Saxony-Anhalt | 19.7 (19.4–20.0) | 6.0 (5.8–6.2) | 13.7 (13.5–13.9) | 46.2 (45.7–46.6) | 17.5 (17.1–17.8) | 28.7 (28.3–29.1) |
| Thuringia | 20.5 (20.2–20.7) | 6.0 (5.8–6.1) | 14.5 (14.2–14.7) | 45.7 (45.3–46.1) | 16.7 (16.4–17.0) | 29.0 (28.6–29.4) |
| Geographical regionb | ||||||
| North | 20.5 (20.4–20.6) | 6.1 (6.1–6.1) | 14.4 (14.3–14.4) | 49.9 (49.8–50.0) | 21.2 (21.1–21.3) | 28.7 (28.6–28.8) |
| South | 22.7 (22.6–22.8) | 5.4 (5.3–5.4) | 17.3 (17.3–17.4) | 51.0 (50.9–51.1) | 17.3 (17.2–17.4) | 33.7 (33.6–33.8) |
| East | 19.3 (19.1–19.4) | 5.9 (5.8–6.0) | 13.4 (13.3–13.5) | 46.8 (46.5–47.0) | 18.2 (18.1–18.4) | 28.5 (28.3–28.7) |
| West | 21.3 (21.2–21.3) | 4.8 (4.8–4.9) | 16.4 (16.4–16.5) | 48.3 (48.1–48.4) | 14.8 (14.7–14.8) | 33.5 (33.4–33.6) |
| Urbanization of district of residence | ||||||
| Urban | 21.2 (21.1–21.2) | 5.3 (5.3–5.3) | 15.9 (15.8–15.9) | 49.7 (49.6–49.8) | 17 .7 (17.6–17.7) | 32.0 (32.0–32.1) |
| Rural | 21.5 (21.4–21.5) | 5.7 (5.7–5.8) | 15.8 (15.7–15.8) | 48.5 (48.4–48.6) | 17 .7 (17.7–17.8) | 30.7 (30.6–30.8) |
| Density of physicians | ||||||
| Lowc | 21.4 (21.4–21.5) | 5.5 (5.5–5.6) | 15.9 (15.8–15.9) | 49.3 (49.3–49.4) | 17.5 (17.4–17.6) | 31.8 (31.8–31.9) |
| Highd | 21.1 (21.1–21.2) | 5.3 (5.3–5.4) | 15.8 (15.7–15.8) | 49.4 (49.3–49.4) | 17.9 (17.8–17.9) | 31.5 (31.4–31.6) |
| Density of gastroenterologists | ||||||
| Lowe | 21.3 (21.3–21.4) | 5.4 (5.4–5.4) | 15.9 (15.9–16.0) | 49.4 (49.3–49.5) | 17 .3 (17. 3–17.4 ) | 32.1 (32.0–32.2) |
| Highf | 21.2 (21.1–21.2) | 5.5 (5.5–5.5) | 15.7 (15.6–15.7) | 49.3 (49.2–49.4) | 18.3 (18.2–18.4) | 31.0 (30.9–31.1) |
CI, confidence interval.
Age in 2017.
North: Schleswig-Holstein, Hamburg, Lower Saxony, Bremen, Berlin, Brandenburg, Mecklenburg-Western Pomerania; South: Baden-Wuerttemberg, Bayern; East: Saxony, Saxony-Anhalt, Thuringia; West: North Rhine-Westphalia, Hesse, Rhineland-Palatinate, Saarland.
Federal states with low density of physicians: Baden-Wuerttemberg, Brandenburg, Hesse, Lower Saxony, Rhineland-Palatinate, Saxony, Saxony-Anhalt, Thuringia.
Federal states with high density of physicians: Bavaria, Berlin, Bremen, Hamburg, Mecklenburg-Western Pomerania, North Rhine-Westphalia, Saarland, Schleswig-Holstein.
Federal states with low density of gastroenterologists: Baden-Wuerttemberg, Bavaria, Berlin, Mecklenburg-Western Pomerania, North Rhine-Westphalia, Saarland, Schleswig-Holstein, Thuringia.
Federal states with high density of gastroenterologists: Brandenburg, Bremen, Hamburg, Hesse, Lower Saxony, Rhineland-Palatinate, Saxony, Saxony-Anhalt.
The proportion of persons with colonoscopy with codes indicating the presence of prevalent CRC in the 2 years before colonoscopy was 3% overall (screening: 2%, diagnostic: 4%). It increased with age, from below 3% in persons aged less than 65 years to 5% in persons aged 75–79 and it was 7% in persons aged greater than or equal to 80 years (see Supplemental digital content 3, http://links.lww.com/EJCP/A348). In Supplemental digital content 4, http://links.lww.com/EJCP/A348, the prevalence of codes for relevant diagnoses, symptoms or other conditions recorded in persons before diagnostic colonoscopy is shown, which may reflect potential reasons for diagnostic colonoscopies.
Discussion
To the best of our knowledge, this is the most comprehensive study investigating the 10-year prevalence of colonoscopy use in Germany based on claims data, with a sample size facilitating a detailed description of colonoscopy use by age, sex, estimates of SES and regional factors. The 10-year prevalence of colonoscopy increased from ~20% at age 50–54, to ~30% at age 55–59 and ~45–50% at age 60–84. Also, in persons aged 85–94, the prevalence remained at levels of ~30–40%. Screening colonoscopy accounted for ~40–50% of the 10-year prevalence in persons aged 60–74, whereas in most other age groups eligible for screening, it had a share of one-third or less. Overall, the patterns in 10-year prevalence by age and type of colonoscopy were rather similar in men and women. Women tended to show a slightly higher prevalence regarding overall colonoscopy use compared to men below the age of 55 (maximum difference 4.4 percentage points) whereas it tended to be lower above the age of 74 (maximum difference 7.6 percentage points). There were no relevant differences in the overall 10-year prevalence according to levels of SES or regional factors such as the density of physicians and gastroenterologists in the district of residence.
The 10-year prevalence of overall and screening colonoscopy use observed in our study is in line with findings presented in a report based on data from 2006 to 2017 from a large statutory health insurance provider not included in our study. Nonetheless, the maximum level of 10-year prevalence mentioned in that report (44% at age 65–75) is somewhat lower than the maximum levels observed in our study (~50%) suggesting minor differences between statutory health insurance providers in Germany. A detailed comparison to our study, however, is not possible as both the methodology and the results in that report were only described roughly and there was no stratification by sex or other factors (Tillmanns et al., 2019). There was one further study based on German claims data describing 10-year prevalence of colonoscopy use but the sample size was small (n = 108 225); the data are old (data from 2000 to 2008) and they did not cover a 10-year period, so extrapolation was needed (Stock et al., 2011). Studies based on survey data from Germany reported 10-year prevalences even higher than our study. For example, two studies using national health survey data reported a 10-year prevalence of 55% among persons greater than or equal to 55 years in 2008–2011 and of 59% in 2014–2015 (Starker and Saß, 2013; Starker et al., 2017). The higher estimates compared to claims data-based findings may be explained by recall bias. In a study by Hoffmeister et al. (2007), comparing medical or physician records to self-reports of endoscopy use showed reasonable agreement (79%) regarding the time point of prior endoscopy only for relatively recent examinations whereas the validity of this information decreased if the endoscopy had been performed more than 4 years before. Also, nonresponder bias might be an issue in health surveys that can often not fully be overcome by weighting factors. In our study using claims data, neither nonresponder nor recall bias played a role.
In comparison with other countries, the results of our study suggest that Germany is one of the countries with the highest 10-year prevalence of overall colonoscopy use in persons aged 55 or older. Only in the US, a higher 10-year prevalence of colonoscopy use in persons aged 50–75 was reported (55–62%) but this information was based on survey data (Chen et al., 2017; Cardoso et al., 2019). Within Europe, an analysis based on data from the European Health Interview Survey suggested that the 10-year prevalence of colonoscopy use in persons aged 50–74 years is highest in Germany, Austria and Luxemburg (~50%), whereas markedly lower prevalences were reported for other countries, for example, for Slovakia (~15%), Finland (~18%) and Norway (~28%) (Cardoso et al., 2020).
Our findings strikingly illustrate that screening colonoscopy provides only a part of the picture of colonoscopy use in Germany, whereas diagnostic colonoscopy plays a major role in most age groups, that is, it is essential to consider both types of colonoscopy when interpreting CRC trends or planning observational studies on CRC screening in Germany. Given that screening colonoscopy is only offered to persons aged 55 or older, the highest levels of (overall) 10-year prevalence were only observed in persons aged 60 or older. According to modelling studies typically proposing a starting age of 50 years or even earlier for CRC screening (Knudsen et al., 2016; Peterse et al., 2018; Chen et al., 2019a), this age pattern seems suboptimal in terms of fully realizing the potential of CRC screening. At the same time, 10-year prevalence of colonoscopy use overall was high even at very high ages, which raises concerns regarding overdiagnosis. Furthermore, complications due to colonoscopy have been reported to occur more frequently among persons with comorbidities, which are particularly common among older persons (Stock et al., 2013; Chukmaitov et al., 2016; Causada-Calo et al., 2020). With respect to the risk–benefit ratio of colonoscopy use, a shift towards uptake at a younger age of overall and screening colonoscopy would thus be favourable. After decades of opportunistic CRC screening in Germany, an organized screening programme has been introduced in 2019. Since then, letters with information on CRC screening have been sent to all persons aged 50, 55, 60 and 65 and screening colonoscopy has been offered to men already at age 50 (Gemeinsamer Bundesausschuss, 2020). It will be important to monitor whether this change will have a favourable effect on age patterns in colonoscopy use.
In our study, there were no relevant differences in colonoscopy use according to estimates of SES defined by educational level. This is consistent with the results of the analyses by Hermann et al. (2015) including 15 014 participants aged 35–60 years of the EPIC study in Germany where no association with educational but a weak positive association with vocational training was observed. In a study by Sieverding et al. (2010), also no clear association was found between education and colonoscopy use in Germany in both sexes. This suggests that there is equal access to colonoscopy in Germany, unlike in other countries, where often positive associations between colonoscopy use and level of income or education have been reported (Chen et al., 2017). Furthermore, the absence of relevant differences in colonoscopy use according to regional factors suggests that there are sufficient colonoscopy resources and broad access to colonoscopy across Germany.
In the interpretation of our study, the following limitations should be considered. First, our database (GePaRD) contains all information on colonoscopies performed in the in- and outpatient setting except for diagnostic colonoscopies performed in the hospital but billed as outpatient procedures because patients were not hospitalized. According to estimates of a large health insurance provider in Germany, this kind of billing applied to about 18% of all colonoscopies in 2014 (Friedrich and Tillmanns, 2016). However, according to reasons mentioned in guidelines justifying their conduct, we assume that most of these colonoscopies are early repeat colonoscopies conducted in specific risk constellations (Pox et al., 2013), that is, they are preceded or followed by additional colonoscopies in the outpatient setting and are thus not relevant regarding the prevalence of any colonoscopy. Also, the fact that the prevalence of colonoscopy observed in our study is well in line or even higher than in the report mentioned above from a health insurance provider with access to data on these specific colonoscopies (Tillmanns et al., 2019) supports that they do not have a relevant impact on prevalence. Unlike previous studies investigating colonoscopy use based on claims data, we were the first to consider an individual estimate of the SES in the analysis. However, it has to be kept in mind that the available information is not optimal: It only takes into account education but no other dimensions such as income, and there was a high proportion of missing values in older age groups (see Supplemental digital content 5, http://links.lww.com/EJCP/A348). We only included persons with a continuous insurance period from at least 2006 to 2017. In some countries, such an inclusion criterion would result in systematically excluding unemployed persons who often have a very low SES. In Germany, however, statutory health insurance coverage is independent of occupational status and regulated by the Code of Social Law; so, we do not think that differences by SES have been underestimated due to this inclusion criterion. This is also supported by the fact that our results regarding the association between SES and colonoscopy use were consistent with other studies from Germany as mentioned before. Finally, we cannot rule out that some physicians billed a colonoscopy conducted because of a positive faecal occult blood test as a screening colonoscopy even though coding guidelines state to code it as a diagnostic colonoscopy.
In conclusion, in 2017, about 45–50% of men and women at age 60–84 had a colonoscopy in the previous 10 years of whom about 11–26% had a screening colonoscopy. Analyses stratified by SES and regional factors suggest no relevant social or regional disparities in the utilization of colonoscopy in Germany.
Acknowledgements
The authors would like to thank all statutory health insurance providers which provided data for this study, namely AOK Bremen/Bremerhaven, DAK-Gesundheit, Die Techniker (TK) and hkk Krankenkasse. The authors would also like to thank Alina Ludewig and Inga Schaffer for statistical programming of the datasets and analyses.
M.H., S.S. and U.H. conceptualized the study and developed the data analysis plan. M.H. and S.S. contributed to data analysis. M.H. drafted the first version of the manuscript. All authors contributed to the interpretation of the results and critically revised the manuscript draft. All authors approved the final version of the manuscript. U.H. supervised the project.
As we are not the owners of the data, we are not legally entitled to grant access to the data of the German Pharmacoepidemiological Research Database. In accordance with German data protection regulations, access to the data is granted only to BIPS employees on the BIPS premises and in the context of approved research projects. Third parties may only access the data in cooperation with BIPS and after signing an agreement for guest researchers at BIPS.
Guarantor of the article: Ulrike Haug.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.eurjcancerprev.com).
References
- Altenhofen L. Projekt. Wissenschaftliche Begleitung von Früherkennungs-Koloskopien in Deutschland. Berichtszeitraum 2012. 10. Jahresbericht. https://www.zi.de/fileadmin/images/content/PDFs_alle/Jahresbericht_2012_Vers_1_2.pdf. [Accessed 01 March 2021].
- Altenhofen L. Projekt. Wissenschaftliche Begleitung von Früherkennungs-Koloskopien in Deutschland Berichtszeitraum 2014. 12. Jahresbericht. https://www.zi.de/fileadmin/user_upload/Jahresbericht_2014_Darmkrebs_Frueherkennung.pdf. Published 2016. [Accessed 01 March 2021].
- Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M. (2014). Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 146:709–717. [DOI] [PubMed] [Google Scholar]
- Bundesinstitut für Bau- Stadt- und Raumforschung. Downloads. Raumbegrenzungen: Referenzdatei und Karten. https://www.bbsr.bund.de/BBSR/DE/forschung/raumbeobachtung/downloads/downloadsReferenz2.html?nn=2544954. Published 2020. [Accessed 22 January 2021].
- Cardoso R, Niedermaier T, Chen C, Hoffmeister M, Brenner H. (2019). Colonoscopy and sigmoidoscopy use among the average-risk population for colorectal cancer: a systematic review and trend analysis. Cancer Prev Res (Phila). 12:617–630. [DOI] [PubMed] [Google Scholar]
- Cardoso R, Guo F, Heisser T, Hoffmeister M, Brenner H. (2020). Utilisation of colorectal cancer screening tests in European countries by type of screening offer: results from the European Health Interview Survey. Cancers (Basel). 12:E1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso R, Zhu A, Guo F, Heisser T, Hoffmeister M, Brenner H. (2021). Incidence and mortality of proximal and distal colorectal cancer in Germany. Dtsch Arztebl Int. 118:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causada-Calo N, Bishay K, Albashir S, Al Mazroui A, Armstrong D. (2020). Association between age and complications after outpatient colonoscopy. JAMA Netw Open. 3:e208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Läcke E, Stock C, Hoffmeister M, Brenner H. (2017). Colonoscopy and sigmoidoscopy use among older adults in different countries: a systematic review. Prev Med. 103:33–42. [DOI] [PubMed] [Google Scholar]
- Chen C, Stock C, Hoffmeister M, Brenner H. (2019a). Optimal age for screening colonoscopy: a modeling study. Gastrointest Endosc. 89:1017–1025.e12. [DOI] [PubMed] [Google Scholar]
- Chen C, Stock C, Jansen L, Chang-Claude J, Hoffmeister M, Brenner H. (2019b). Trends in colonoscopy and fecal occult blood test use after the introduction of dual screening offers in Germany: results from a large population-based study, 2003-2016. Prev Med. 123:333–340. [DOI] [PubMed] [Google Scholar]
- Chukmaitov A, Siangphoe U, Dahman B, Bradley CJ, BouHaidar D. (2016). Patient comorbidity and serious adverse events after outpatient colonoscopy: population-based study from three states, 2006 to 2009. Dis Colon Rectum. 59:677–687. [DOI] [PubMed] [Google Scholar]
- Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, et al. (2012). Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 104:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. (2018). Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. https://gco.iarc.fr/today. Published 2020. [Accessed 27 October 2021].
- Friedrich J, Tillmanns H. (2016). Ambulante Operationen im Krankenhaus. In: Krankenhausreport 2016 Schwerpunkt: Ambulant im Krankenhaus. Klauber J GM, Friedrich J, Wasem J, editors. Schattauer. pp. 127–147. [Google Scholar]
- Gemeinsamer Bundesausschuss. Richtlinie des Gemeinsamen Bundesausschusses für organisierte Krebsfrüherkennungsprogramme - oKFE-Richtlinie/oKFE-RL. https://www.g-ba.de/downloads/62-492-2237/ebd5a8e79221f2c60d6803df7bb36573/oKFE-RL-2020-06-18-iK-2020-08-28.pdf. Published 2020. [Accessed 27 October 2021].
- Guo F, Chen C, Schöttker B, Holleczek B, Hoffmeister M, Brenner H. (2020). Changes in colorectal cancer screening use after introduction of alternative screening offer in Germany: prospective cohort study. Int J Cancer. 146:2423–2432. [DOI] [PubMed] [Google Scholar]
- Haug U. (2018). Current evidence and development of colorectal cancer screening in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 61:1513–1516. [DOI] [PubMed] [Google Scholar]
- Hermann S, Friedrich S, Haug U, Rohrmann S, Becker N, Kaaks R. (2015). Association between socioeconomic and demographic characteristics and utilization of colonoscopy in the EPIC-Heidelberg cohort. Eur J Cancer Prev. 24:81–88. [DOI] [PubMed] [Google Scholar]
- Hoffmeister M, Chang-Claude J, Brenner H. (2007). Validity of self-reported endoscopies of the large bowel and implications for estimates of colorectal cancer risk. Am J Epidemiol. 166:130–136. [DOI] [PubMed] [Google Scholar]
- Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, et al. (2016). Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US preventive services task force. JAMA. 315:2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B, Vilahur N, Bianchini F, Guha N, Straif K; International Agency for Research on Cancer Handbook Working Group (2018). The IARC perspective on colorectal cancer screening. N Engl J Med. 378:1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. (2018). The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 124:2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pox C, Aretz S, Bischoff SC, Graeven U, Hass M, Heußner P, et al. ; Leitlinienprogramm Onkologie der AWMF; Deutschen Krebsgesellschaft e. V; Deutschen Krebshilfe e. V (2013). S3-guideline colorectal cancer version 1.0. Z Gastroenterol. 51:753–854. [DOI] [PubMed] [Google Scholar]
- Sieverding M, Matterne U, Ciccarello L, Haug U. (2010). Colonoscopy use in a country with a long-standing colorectal cancer screening programme: evidence from a large German survey. Z Gastroenterol. 48:1351–1357. [DOI] [PubMed] [Google Scholar]
- Starker A, Saß AC. (2013). Participation in cancer screening in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 56:858–867. [DOI] [PubMed] [Google Scholar]
- Starker A, Buttmann-Schweiger N, Kraywinkel K, Kuhnert R. (2017). The utilization of colonoscopy in Germany. J Health Monit. 2:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistisches Bundesamt. Bei den Ärztekammern registrierte Ärztinnen und Ärzte mit Gebiets- und Facharztbezeichnung (absolut, je 100.000 Einwohner und Einwohner je Arzt). Gliederungsmerkmale: Jahre, Region, Alter, Geschlecht, Gebiets-/Facharztbezeichnung, Tätigkeitsbereich. https://www.gbe-bund.de/gbe/pkg_isgbe5.prc_menu_olap?p_uid=gast&p_aid=47357853&p_sprache=D&p_help=3&p_indnr=656&p_indsp=&p_ityp=H&p_fid=. Published 2021. [Accessed 22 January 2021].
- Steffen A, Holstiege J, Hagen B, Akmatov MK, Bätzing J. (2020). Inanspruchnahme der Darmkrebsfrüherkennung in den Jahren 2009 bis 2018: eine Bestandsaufnahme auf Basis bundesweiter vertragsärztlicher Abrechnungsdaten. Zentralinstitut für die kassenärztliche Versorgung in Deutschland (Zi). https://www.versorgungsatlas.de/fileadmin/ziva_docs/108/VA_Bericht_20-02_Darmkrebsfr%C3%BCherkennung_2020-05-14.pdf. [Accessed 11 March 2021].
- Stock C, Haug U, Brenner H. (2010). Population-based prevalence estimates of history of colonoscopy or sigmoidoscopy: review and analysis of recent trends. Gastrointest Endosc. 71:366–381.e2. [DOI] [PubMed] [Google Scholar]
- Stock C, Ihle P, Schubert I, Brenner H. (2011). Colonoscopy and fecal occult blood test use in Germany: results from a large insurance-based cohort. Endoscopy. 43:771–781. [DOI] [PubMed] [Google Scholar]
- Stock C, Ihle P, Sieg A, Schubert I, Hoffmeister M, Brenner H. (2013) Adverse events requiring hospitalization within 30 days after outpatient screening and nonscreening colonoscopies. Gastrointest Endosc. 77:419–429. [DOI] [PubMed] [Google Scholar]
- Tillmanns H, Schillinger G, Dräther H. (2019). Früherkennung bei Erwachsenen in der gesetzlichen Krankenversicherung: Ergebnisse einer AOK-Sekundärdatenanalyse. In: Versorgungs-Report Früherkennung. Günster C, Klauber J, Robra BP, Schmacke N, Schmuker C, editors. MWV Medizinisch Wissenschaftliche Verlagsgesellschaft. pp. 49–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.