Abstract
To study the extent of heterogeneity of mesothelin overexpression in primary ovarian cancers and their peritoneal and lymph node metastases, a tissue microarray (TMA) was constructed from multiple sites of 220 ovarian cancers and analyzed by immunohistochemistry. One tissue core each was taken from up to 18 different tumor blocks per cancer, resulting in a total of 2460 tissue spots from 423 tumor sites (188 primary cancers, 162 peritoneal carcinosis, and 73 lymph node metastases). Positive mesothelin expression was found in 2041 of the 2342 (87%) arrayed tissue spots and in 372 of the 392 (95%) tumor sites that were interpretable for mesothelin immunohistochemistry. Intratumoral heterogeneity was found in 23% of 168 primary cancer sites interpretable for mesothelin and decreased to 12% in 154 peritoneal carcinosis and to 6% in 71 lymph node metastases (P<0.0001). Heterogeneity between the primary tumor and matched peritoneal carcinosis was found in 16% of 102 cancers with interpretable mesothelin results. In these cancers, the mesothelin status switched from positive in the primary tumor to negative in the peritoneal carcinosis (3 cancers) in or vice versa (2 cancers), or a mixture of positive and negative peritoneal carcinoses was found (11 cancers). No such switch was seen between the mesothelin-interpretable primary tumors and their nodal metastases of 59 cancers, and only 1 mesothelin-positive tumor had a mixture of positive and negative lymph node metastases. In conclusion, mesothelin expression is frequent and highly homogeneous in ovarian cancer.
Key Words: mesothelin, immunohistochemistry, tissue microarray, ovarian cancer, heterogeneity
The human mesothelin (MSLN) gene, located at chromosome 16p13.3, encodes for a membranous precursor glycoprotein that is subsequently cleaved into the soluble 31 kD protein megakaryocyte potentiating factor and the 40 kD membrane-bound protein mesothelin.1–3 Mesothelin was first described as a membrane protein expressed on normal and neoplastic mesothelial cells, but subsequent studies demonstrated a broader expression pattern.1,4–9 The function of mesothelin is not fully understood. Mesothelin is expressed in only few normal tissues but has been found to be overexpressed in various tumor types at a relevant frequency.4–10 Therefore, and because of its membranous location, mesothelin represents an attractive molecule for targeted cancer therapies. Several therapy types, including adaptive immunotherapy (CAR-T cells, TC-210 T cells), monoclonal antibodies (amatuximab/MORAb-009), recombinant immunotoxins (SS1P and LMB-100/RG7787), antibody-drug conjugates (anetumab ravtansine/BAY94-9343, DMOT4039A, BAY2287411, BMS-986148, and h7D9.v3), listeria monocytogene–induced antitumor immune response (CRS-207 and JNJ-64041757), and immunocytokines (IL12-SS1) have provided encouraging data in animal models and/or clinical phase I and II trials.11–24
To identify tumor entities that might benefit most from antimesothelin therapies, it will be necessary to determine their mesothelin expression level in tumor cells. It is a conceptual weakness of biomarker testing in tumor biopsies, however, that a biomarker status is determined on primary tumor tissue removed during initial surgery, whereas the treatment is used to target tumor metastases, which were not analyzed. A change of the mesothelin expression status in metastases could either prevent response to therapy or—in case of a change from mesothelin negative to positive—lead to a situation where a treatable cancer would not be detected by standard diagnostic procedures. Studies analyzing the extent of heterogeneity of biomarker expression in cancer have shown that the level of heterogeneity depends on both the biomarker and the tumor type. For example, heterogeneity of high-level HER2 amplification and overexpression has been found to be minimal in breast cancer 25 and moderate in stomach, bladder, or colorectal cancer.26–28 A high level of heterogeneity was found for ALK rearrangements in lung cancer,29 Phosphatase and Tensin homolog (PTEN) deletion in prostate cancer,30 and BRAF mutation in lung adenocarcinoma.31
To study the heterogeneity of mesothelin expression in ovarian cancer, an “ovarian cancer heterogeneity tissue microarray (TMA)” was constructed and analyzed by immunohistochemistry (IHC). From each of 220 ovarian cancer patients, this TMA contained up to 18 different samples (average 11.2) from different tumor blocks derived from the primary tumor, as well as corresponding peritoneal and/or nodal metastases.
MATERIALS AND METHODS
TMA
The ovarian cancer heterogeneity TMA was constructed from the cancers of 220 patients who underwent surgery at the Department of Gynecology of the University Medical Center Hamburg-Eppendorf between 2000 and 2010. The histologic subtype was serous in 165 (75%) cases, mucinous in 17 (8%), endometrioid in 16 (7%), malignant Mullerian mix tumor in 12 (5%), and clear cell in 11 (5%) cases. Selection criteria included ovarian cancers with multiple archived tumor-containing tissue blocks, which were preferably not only from the primary tumor but also included 1 or several blocks from peritoneal carcinosis and/or lymph node metastasis. In total, 2460 tumor blocks from 220 patients were included in this study. Up to 18 (average 11.2) tumor blocks, including 1 to 9 tumor blocks from the primary tumor, 1 to 9 tumor blocks from peritoneal metastases, and up to 9 blocks from different lymph node metastases, were available from each of the 220 patients. For TMA construction, 1 single 0.6 mm tissue core was taken from each block, resulting in a tissue microarray with a total of 2460 tissue cores. Among the 220 patients, 77 had tissue samples from the primary tumor and the peritoneal carcinosis, 53 from the primary tumor, the peritoneal carcinosis and the lymph node metastases, 45 only from the primary cancer, 25 only from the peritoneal carcinosis, 13 from the primary tumor and from the lymph node metastasis, and 7 patients had tissue samples from the peritoneal carcinosis and the lymph node metastasis. The detailed composition of the TMA is given in Supplemental Table S1, Supplemental Digital Content 1, http://links.lww.com/AIMM/A374. Tissues were fixed in a final concentration of 4% buffered formalin (ie, 10% dilution of 37% formalin stock solution) and then embedded in paraffin. The TMA manufacturing process was described earlier in detail.32,33 In brief, one tissue spot (diameter: 0.6 mm) was transmitted from a cancer containing donor block (≥70% cancer cells) in an empty recipient paraffin block. The paper is exempt from informed consent of the subjects because the use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes, as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
IHC
Freshly prepared TMA sections were immunostained on 1 day in 1 experiment. All immunostaining experiments were performed manually. Slides were deparaffinized with xylol, rehydrated through a graded alcohol series, and exposed to heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 9 DakoTarget Retrieval Solution (Agilent; #S2367). Endogenous peroxidase activity was blocked with Dako Peroxidase Blocking Solution (Agilent; #52023) for 10 minutes. Primary antibody specific against mesothelin protein (mouse monoclonal, MSVA-235M, cat. #2198-235M, MS Validated Antibodies) was applied at 37°C for 60 minutes at a dilution of 1:150. For antibody validation, a second independent antibody (EPR19025-42) was also used for a comparative normal tissue analysis by using an identical protocol but a higher antibody concentration (1:75). Bound antibody was then visualized using the EnVision Kit (Agilent; #K5007), according to the manufacturer’s directions. The sections were counterstained with hemalaun. Staining was usually membranous and often accompanied by less intense cytoplasmic positivity. All detectable membranous and cytoplasmic stainings were considered positive. The percentage of mesothelin-positive tumor cells was estimated in each tissue spot and the staining intensity was semiquantitatively recorded (0, 1+, 2+, 3+). The staining results were categorized into 4 groups as follows: negative: no staining at all, weak staining: staining intensity of 1+ in ≤70% or staining intensity of 2+ in ≤30% of tumor cells, moderate staining: staining intensity of 1+ in >70%, staining intensity of 2+ in >30% but in ≤70% or staining intensity of 3+ in ≤30% of tumor cells, strong staining: staining intensity of 2+ in > 70% or staining intensity of 3+ in >30% of tumor cells. For heterogeneity analysis, tumors were regrouped per tumor localization (ie, primary tumor, peritoneal carcinosis, and lymph node metastasis) into 33 categories including negative (absence of any detectable staining in all belonging tumor spots), homogeneously positive (at least weak staining in all analyzable tumor spots), and heterogeneously positive (at least 1 tumor spot positive and at least 1 tumor spot negative). Only tumor samples with at least 2 interpretable tissue spots were included into the following analyses.
Antibody Validation
A normal tissue array containing 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on 1 slide) was used for validation of IHC staining obtained by MSVA-235M by a second independent antibody (EPR19025-42). All cell types with positive stainings obtained by MSVA-235M (squamous epithelium of tonsil crypts, some colorectal epithelial cell groups, anal transitional, amnion cells of the placenta, some elements of corpuscles of Hassall of the thymus, scattered cells and groups of cells of endocervical mucosa and endometrium, epithelial cells of fallopian tube, some epithelial cells of the stomach, and respiratory epithelium) were confirmed by EPR19025-42 (Supplemental Figure 1, Supplemental Digital Content 2, http://links.lww.com/AIMM/A375).
Large Section Validation
A large section validation of mesothelin-negative and mesothelin-positive tissue spots was performed from 10 heterogeneously positive cancers.
Statistics
Contingency table analysis and χ2 test were used to study associations between mesothelin expression and tumor phenotype.
RESULTS
Technical Results
Mesothelin IHC was interpretable in 2342 of the 2460 (95.2%) of the arrayed tumor samples. The remaining 118 tissue samples were not interpretable because of insufficient numbers of tumor cells in the tissue spot or lack of the entire tissue spot in the TMA section. All raw IHC data are summarized in Supplementary Table 2, Supplemental Digital Content 3, http://links.lww.com/AIMM/A376.
Heterogeneity Within Tumor Sites
Tumor sites with at least 2 interpretable tissue spots included 168 primary cancers, 154 peritoneal carcinoses, and 71 lymph node metastases (Table 1). Positive mesothelin expression was found in 2041 of the 2342 (87%) interpretable tissue spots, and in 372/392 (95%) of the tumor sites. Accordingly, our mesothelin IHC analysis identified only little intratumoral heterogeneity, which gradually decreased from the primary cancers (23% heterogeneously positive tumors) to the peritoneal carcinosis (12%) and the lymph node metastases (6%, P<0.0001). Remarkably, 32 (53%) of the 60 tumor sites with heterogeneous positivity had only tissue spots with negative or weak staining, suggesting low-level expression resulting in borderline IHC findings. The examples of immunostainings with homogeneously positive, heterogeneously positive, and negative findings are shown in Figure 1.
TABLE 1.
Mesothelin Heterogeneity Status in Different Sites of Ovarian Cancer
| Mesothelin IHC | Primary cancers | Peritoneal carcinoses | Lymph node metastasis | P |
|---|---|---|---|---|
| n=168 | n=154 | n=71 | <0.0001 | |
| Negative (%) | 8.9 | 3.2 | 1.4 | — |
| Heterogeneously positive (%) | 22.6 | 11.7 | 5.6 | — |
| Homogeneously positive (%) | 68.5 | 85.1 | 93.0 | — |
IHC indicates immunohistochemistry.
FIGURE 1.
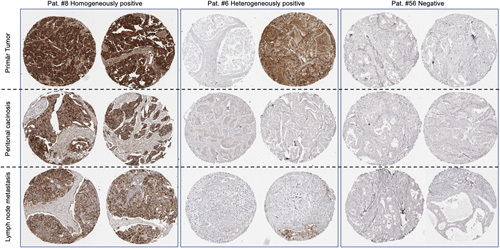
Examples of mesothelin immunostaining results in cases with homogeneous, heterogeneous, and negative findings. Pat. # corresponds to the identifier given in Supplementary Table 2.
Heterogeneity Between Primary Tumors and Metastases
A total of 102 primary tumors with interpretable mesothelin data had matched peritoneal metastases and 59 primary tumors had matched lymph node metastases. The comparison between the primary and metastatic tumor sites is shown in Figure 2 for the peritoneal metastases and in Figure 3 for the lymph node metastases. Again, there was only little heterogeneity: virtually, all primary tumors with homogenous mesothelin expression had homogenously positive peritoneal carcinoses (95% of 75 primary cancers) or lymph node metastases (98% of 52 primary cancers). Also, primary tumors with heterogeneous mesothelin expression showed high rates of positive metastatic sites, including 100% homogeneously positive lymph node metastases and 81% (56% homogeneously and 25% heterogeneously) positive peritoneal carcinoses. Overall, there were 16 of 102 (16%) cancers where the mesothelin status changed between the primary tumor and the peritoneal carcinoses from positive to negative (3 cancers) or from negative to homogeneously positive (2 cancers), or where a mixture of mesothelin-positive and mesothelin-negative peritoneal carcinoses was found (11 cancers). However, the staining differences were often only small. For example, in 4 cases with mesothelin-negative primary cancers but mesothelin-positive peritoneal carcinoses (see ID #157, #177, #199, and #200 in Supplementary Table 2, Supplemental Digital Content 3, http://links.lww.com/AIMM/A376) the positive staining was only weak. No switch of the mesothelin status was seen between the primary cancers and the nodal metastases.
FIGURE 2.
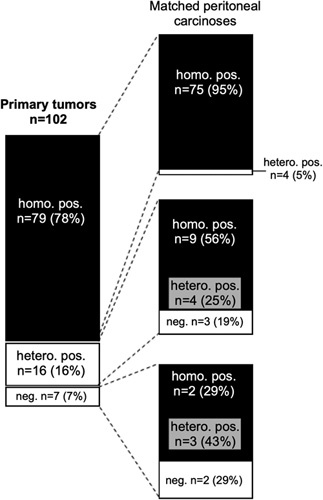
Comparison of mesothelin immunostaining results obtained from the primary tumor and the matched peritoneal carcinoses of 102 ovarian cancers.
FIGURE 3.
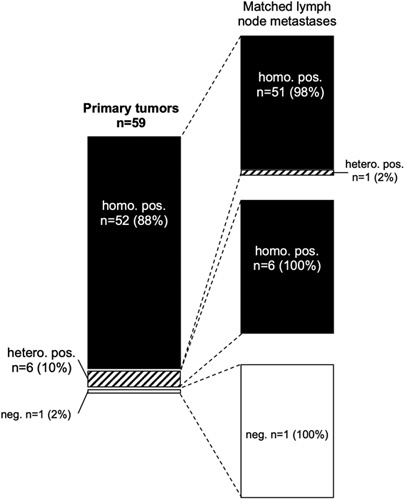
Comparison of mesothelin immunostaining results obtained from the primary tumor and the matched nodal metastases of 102 ovarian cancers.
Large Section Validation
Large section validation of a total of 20 tumors containing tissue blocks from 10 ovarian cancers confirmed a heterogeneous mesothelin staining in these patients (Supplemental Figure 2, Supplemental Digital Content 4, http://links.lww.com/AIMM/A377).
DISCUSSION
The analysis of tumor heterogeneity is challenging, especially in advanced ovarian carcinomas presenting with large intra-abdominal tumor masses. In case of a tumor measuring 7 cm in diameter, the analysis of one 4 µm conventional whole section containing 3×2 cm of cancer tissues would only enable the analysis of 1/21,380 of the entire tumor mass all located in 1 specific area of the tumor. To cost-effectively analyze many different tumor regions, we constructed a heterogeneity TMA containing >11 cancer samples on average per patient. These samples were from different areas (different tumor blocks) including multiple areas from the primary tumor, as well as multiple different peritoneal and lymph node metastases. This enabled a comprehensive heterogeneity analysis of 2460 ovarian cancer patients by staining only 6 TMA sections. The data suggest that mesothelin expression is frequent and highly homogeneous in ovarian cancer. We had earlier used the concept of heterogeneity TMAs to study heterogeneity of ETS Transcription Factor (ERG) fusion,49 PTEN alterations,30 and deletions of chromosomes 3p,68 5q,69 and 6q70 in prostate cancer, amplifications of HER2, EGFR, CCND1, and MYC in gastric cancer,71 HER2 and p53 in colon cancer,28 and CCND1 amplification in breast cancer,72 and EGFR copy number alterations in lung cancer.52 In these studies, the validity of the approach had also been validated by whole section analyses.
The antibody used for this study had been validated according to the criteria of the International Working Group for Antibody Validation, which requires either a comparison of the findings obtained by 2 different independent antibodies or a comparison with expression data obtained by another independent method.34 That all staining obtained by MSVA-235M on 76 different normal tissues were confirmed by a second independent antibody (EPR19025-42) demonstrates that our assay lacks significant cross-reactivities.
The results of this study show a high positivity rate of 82% in our series of 216 interpretable ovarian carcinomas. This is in the upper range of earlier studies describing a positivity rate of 30% to 100% for endometroid8,10,35,36 and of 55% to 100% of serous ovarian cancer.5,9,10,35–41 It is assumed that most of the variability of data in the literature are because of the use of different antibodies, different immunostaining protocols, and divergent criteria to categorize mesothelin immunostaining as positive or negative in these studies. The particularly high rate of positivity in our study may also be because of the excessive tissue sampling. It is well known that the use of multiple samples per tumor on a TMA leads to more positive cases.42,66,67 However, 2041 out of 2342 tumor-containing TMA samples showed a positive staining in our study (87%), indicating that our extensive tissue sampling did not dramatically increase the positivity rate. Moreover, in a recent TMA study, we analyzed immunohistochemical mesothelin expression across >12,600 individual tumors derived from 122 different human tumor types and virtually all normal tissues.43 Using only a single 0.6 mm core per tumor, different subtypes of ovarian cancers ranked among the top 8 indications with most frequent mesothelin expression, including 71% positive mucinous carcinomas, 77% positive endometroid carcinomas, 83% positive clear cell carcinomas, and 97% positive serous ovarian cancers.
The heterogeneity rates found within primary cancers (23%) and between the primary cancers and the peritoneal carcinosis (15%) are not neglectable but consistent with mesothelin representing a suitable therapeutic target. The intratumoral heterogeneity rate is in the range of HER2/neu amplification in breast cancer (1% to 34%)25 and stomach cancer (5% to 75%)44,45 or other drug targets such as EGFR mutation in lung adenocarcinoma or PIK3CA mutation in squamous cell carcinoma of the lung.46 A similarly or even higher rate of heterogeneity was also reported for key molecular alterations of other cancers such as the 8% to 42% heterogeneous cases for TMPRSS2:ERG fusions in prostate cancer47–49 or the 0% to 13% heterogeneous cases of p53 alterations in colorectal cancer.28,50 Using a similar heterogeneity TMA approach as for this study, we had earlier reported HER2/neu heterogeneity between matched primary cancers and metastasis in 16% of breast cancers,51 and found a much higher heterogeneity rate for prognostic alterations occurring later during cancer progression such as the 92% heterogeneous PTEN deletions in prostate cancer30 or the 54% heterogeneous EGFR amplifications in lung cancer.52
Because of inherent technical issues coming along with IHC on formalin fixed tissues, we believe, that the fraction of mesothelin heterogeneous cases was rather overestimated than underestimated in this study. Some false-negative immunostaining results always occur in TMAs because not all tissues are properly fixed in all areas.53 Unequal fixation across a tissue results in an inhomogeneous immunostaining that leads to an immunostaining gradient across a large section and will result in false-negative immunostainings, if TMA cores are taken from areas with poor reactivity.54 Taking multiple samples per tumor, especially if this is from different tumor blocks as in this project, increases the likelihood for both detecting true heterogeneity and sampling nonimmunoreactive tissues. That heterogeneity was confirmed by large section analysis in 10 of 10 validated cases argues for a high rate of truly heterogeneous cases identified in our study, however. It seems possible that a higher degree of heterogeneity occurs in cancers with low-level mesothelin expression.
That the heterogeneity rate was higher in primary tumors than in peritoneal and nodal metastases and that many primary tumors with heterogeneous mesothelin expression developed homogeneously positive metastatic lesions would be consistent with a higher likelihood of mesothelin-positive ovarian cancer cells for peritoneal or nodal tumor spread. A higher aggressiveness of mesothelin-positive cancers would indeed be supported by functional in vitro and in vivo studies suggesting a role of mesothelin in several cancer-related cellular processes, including the PI3K/AkT and MAPK/ERK pathway.55,56 Furthermore, increased mesothelin expression has been shown to promote cell death resistance, increased cell proliferation, invasive and metastatic properties, and angiogenesis.55–60 In colorectal cancer, 4 studies have shown associations between high mesothelin expression and unfavorable tumor phenotype or poor prognosis.61–64 In ovarian cancer, only 4 studies have estimated the clinical relevance of mesothelin expression. Two of them have found a strong relationship between high mesothelin expression and shorter progression-free survival 65 and overall survival.40 One has shown a significant association between high mesothelin expression and prolonged overall survival 39 and one found no association between mesothelin expression and overall or progression-free survival.41
CONCLUSIONS
Our data demonstrate frequent high level and homogeneous mesothelin expression in ovarian cancer. If antimesothelin therapies should prove efficient in the future, ovarian cancer will be an ideal cancer type for such treatments. Small biopsies are likely to be sufficiently representative for determining the mesothelin expression status of these tumors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Melanie Witt, Inge Brandt, Maren Eisenberg, Laura Behm, and Sünje Seekamp for excellent technical assistance.
Footnotes
S.W., R.S., G.S., and P.L.: contributed to conception, design, data collection, data analysis, and manuscript writing. N.G., C.B., T.S.C., F.J., K.J., B.S., L.W., P.P., E.B., U.H., R.U., S.S., T.K., A.M., and E.B.: conception and design, collection of samples. C.F., C.H.M., M.L., and R.S.: collection and data analysis. P.L., W.W., and G.S.: study supervision.
The mesothelin antibody clone MSVA-235 was provided from MS Validated Antibodies GmbH (owned by a family member of G.S.).
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.appliedimmunohist.com.
Contributor Information
Sören Weidemann, Email: s.weidemann@uke.de.
Natalia Gorbokon, Email: n.gorbokon@uke.de.
Maximilian Lennartz, Email: m.lennartz@uke.de.
Claudia Hube-Magg, Email: c.hube@uke.de.
Christoph Fraune, Email: c.fraune@uke.de.
Christian Bernreuther, Email: c.bernreuther@uke.de.
Till S. Clauditz, Email: t.clauditz@uke.de.
Frank Jacobsen, Email: f.jacobsen@uke.de.
Kristina Jansen, Email: k.jansen@uke.de.
Barbara Schmalfeldt, Email: b.schmalfeldt@uke.de.
Linn Wölber, Email: lwoelber@uke.de.
Peter Paluchowski, Email: peter.paluchowski@sana.de.
Enikö Berkes, Email: ronald_simon_de@yahoo.de.
Uwe Heilenkötter, Email: u.heilenkoetter@kh-itzehoe.de.
Guido Sauter, Email: g.sauter@uke.de.
Ria Uhlig, Email: r.uhlig@uke.de.
Waldemar Wilczak, Email: w.wilczak@uke.de.
Stefan Steurer, Email: s.steurer@uke.de.
Ronald Simon, Email: r.simon@uke.de.
Till Krech, Email: t.krech@uke.de.
Andreas Marx, Email: cl.hube@gmail.com.
Eike Burandt, Email: e.burandt@uke.de.
Patrick Lebok, Email: p.lebok@uke.de.
REFERENCES
- 1. Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamaguchi N, Hattori K, Oh-eda M, et al. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y5. J Biol Chem. 1994;269:805–808. [PubMed] [Google Scholar]
- 3. Urwin D, Lake RA. Structure of the Mesothelin/MPF gene and characterization of its promoter. Mol Cell Biol Res Commun. 2000;3:26–32. [DOI] [PubMed] [Google Scholar]
- 4. Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–381. [DOI] [PubMed] [Google Scholar]
- 5. Inaguma S, Wang Z, Lasota J, et al. Comprehensive immunohistochemical study of mesothelin (MSLN) using different monoclonal antibodies 5B2 and MN-1 in 1562 tumors with evaluation of its prognostic value in malignant pleural mesothelioma. Oncotarget. 2017;8:26744–26754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alvarez H, Rojas PL, Yong KT, et al. Mesothelin is a specific biomarker of invasive cancer in the Barrett-associated adenocarcinoma progression model: translational implications for diagnosis and therapy. Nanomedicine. 2008;4:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. [DOI] [PubMed] [Google Scholar]
- 8. Frierson HF, Jr, Moskaluk CA, Powell SM, et al. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 2003;34:605–609. [DOI] [PubMed] [Google Scholar]
- 9. Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766–3772. [DOI] [PubMed] [Google Scholar]
- 10. Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. [DOI] [PubMed] [Google Scholar]
- 11. Bauss F, Lechmann M, Krippendorff BF, et al. Characterization of a re-engineered, mesothelin-targeted Pseudomonas exotoxin fusion protein for lung cancer therapy. Mol Oncol. 2016;10:1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haas AR, Tanyi JL, O’Hara MH, et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther. 2019;27:1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hassan R, Alley E, Kindler H, et al. Clinical response of live-attenuated, listeria monocytogenes expressing mesothelin (CRS-207) with chemotherapy in patients with malignant pleural mesothelioma. Clin Cancer Res. 2019;25:5787–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassan R, Blumenschein GR, Jr, Moore KN, et al. First-in-human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol. 2020;38:1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res. 2014;20:5927–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang Q, Ghafoor A, Mian I, et al. Enhanced efficacy of mesothelin-targeted immunotoxin LMB-100 and anti-PD-1 antibody in patients with mesothelioma and mouse tumor models. Sci Transl Med. 2020;12:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim H, Gao W, Ho M. Novel immunocytokine IL12-SS1 (Fv) inhibits mesothelioma tumor growth in nude mice. PLoS One. 2013;8:e81919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lanitis E, Poussin M, Hagemann IS, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazzerini L, Johrens K, Sehouli J, et al. Favorable therapeutic response after anti-Mesothelin antibody-drug conjugate treatment requires high expression of mesothelin in tumor cells. Arch Gynecol Obstet. 2020;302:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizukami T, Kamachi H, Fujii Y, et al. The anti-mesothelin monoclonal antibody amatuximab enhances the anti-tumor effect of gemcitabine against mesothelin-high expressing pancreatic cancer cells in a peritoneal metastasis mouse model. Oncotarget. 2018;9:33844–33852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scales SJ, Gupta N, Pacheco G, et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol Cancer Ther. 2014;13:2630–2640. [DOI] [PubMed] [Google Scholar]
- 22. Terwisscha van Scheltinga AG, Ogasawara A, Pacheco G, et al. Preclinical efficacy of an antibody-drug conjugate targeting mesothelin correlates with quantitative 89Zr-immunoPET. Mol Cancer Ther. 2017;16:134–142. [DOI] [PubMed] [Google Scholar]
- 23. Weekes CD, Lamberts LE, Borad MJ, et al. Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol Cancer Ther. 2016;15:439–447. [DOI] [PubMed] [Google Scholar]
- 24. Yu L, Feng M, Kim H, et al. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J Cancer. 2010;1:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marchio C, Annaratone L, Marques A, et al. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol. 2021;72:123–135. [DOI] [PubMed] [Google Scholar]
- 26. Zhang H, Wang Y, Wang Y, et al. Intratumoral and intertumoral heterogeneity of HER2 immunohistochemical expression in gastric cancer. Pathol Res Pract. 2020;216:153229. [DOI] [PubMed] [Google Scholar]
- 27. Moktefi A, Pouessel D, Liu J, et al. Reappraisal of HER2 status in the spectrum of advanced urothelial carcinoma: a need of guidelines for treatment eligibility. Mod Pathol. 2018;31:1270–1281. [DOI] [PubMed] [Google Scholar]
- 28. Stahl PR, Schnellert J, Koop C, et al. Determination of tumor heterogeneity in colorectal cancers using heterogeneity tissue microarrays. Pathol Oncol Res. 2015;21:1183–1189. [DOI] [PubMed] [Google Scholar]
- 29. Zito Marino F, Liguori G, Aquino G, et al. Intratumor heterogeneity of ALK-rearrangements and homogeneity of EGFR-mutations in mixed lung adenocarcinoma. PLoS One. 2015;10:e0139264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krohn A, Freudenthaler F, Harasimowicz S, et al. Heterogeneity and chronology of PTEN deletion and ERG fusion in prostate cancer. Mod Pathol. 2014;27:1612–1620. [DOI] [PubMed] [Google Scholar]
- 31. Tatematsu T, Sasaki H, Shimizu S, et al. Intra-tumor heterogeneity of BRAF V600E mutation in lung adenocarcinomas. Exp Ther Med. 2015;9:1719–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dancau AM, Simon R, Mirlacher M, et al. Tissue microarrays. Methods Mol Biol. 2016;1381:53–65. [DOI] [PubMed] [Google Scholar]
- 33. Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. [DOI] [PubMed] [Google Scholar]
- 34. Uhlen M, Bandrowski A, Carr S, et al. A proposal for validation of antibodies. Nat Methods. 2016;13:823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hassan R, Kreitman RJ, Pastan I, et al. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. [DOI] [PubMed] [Google Scholar]
- 36. Drapkin R, Crum CP, Hecht JL. Expression of candidate tumor markers in ovarian carcinoma and benign ovary: evidence for a link between epithelial phenotype and neoplasia. Hum Pathol. 2004;35:1014–1021. [DOI] [PubMed] [Google Scholar]
- 37. Illei PB, Alewine C, Zahurak M, et al. Mesothelin expression in advanced gastroesophageal cancer represents a novel target for immunotherapy. Appl Immunohistochem Mol Morphol. 2016;24:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanner WA, Galgano MT, Stoler MH, et al. Distinguishing breast carcinoma from Mullerian serous carcinoma with mammaglobin and mesothelin. Int J Gynecol Pathol. 2008;27:491–495. [DOI] [PubMed] [Google Scholar]
- 39. Yen MJ, Hsu CY, Mao TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12(3 Pt 1):827–831. [DOI] [PubMed] [Google Scholar]
- 40. Yildiz Y, Kabadayi G, Yigit S, et al. High expression of mesothelin in advanced serous ovarian cancer is associated with poor prognosis. J BUON. 2019;24:1549–1554. [PubMed] [Google Scholar]
- 41. Magalhaes I, Fernebro J, Abd Own S, et al. Mesothelin expression in patients with high-grade serous ovarian cancer does not predict clinical outcome but correlates with CD11c(+) expression in tumor. Adv Ther. 2020;37:5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tennstedt P, Koster P, Bruchmann A, et al. The impact of the number of cores on tissue microarray studies investigating prostate cancer biomarkers. Int J Oncol. 2012;40:261–268. [DOI] [PubMed] [Google Scholar]
- 43. Weidemann S, Gagelmann P, Gorbokon N, et al. Mesothelin expression in human tumors: a tissue microarray study on 12,679 tumors. Biomedicines. 2021;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grillo F, Fassan M, Sarocchi F, et al. HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J Gastroenterol. 2016;22:5879–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurokawa Y, Matsuura N, Kimura Y, et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18:691–697. [DOI] [PubMed] [Google Scholar]
- 46. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376:2109–2121. [DOI] [PubMed] [Google Scholar]
- 47. Nie L, Pan X, Zhang M, et al. The expression profile and heterogeneity analysis of ERG in 633 consecutive prostate cancers from a single center. Prostate. 2019;79:819–825. [DOI] [PubMed] [Google Scholar]
- 48. Suh JH, Park JH, Lee C, et al. Intrafocal heterogeneity of ERG protein expression and gene fusion pattern in prostate cancer. Prostate. 2017;77:1438–1445. [DOI] [PubMed] [Google Scholar]
- 49. Minner S, Gartner M, Freudenthaler F, et al. Marked heterogeneity of ERG expression in large primary prostate cancers. Mod Pathol. 2013;26:106–116. [DOI] [PubMed] [Google Scholar]
- 50. Scott N, Sagar P, Stewart J, et al. p53 in colorectal cancer: clinicopathological correlation and prognostic significance. Br J Cancer. 1991;63:317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simon R, Nocito A, Hubscher T, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93:1141–1146. [DOI] [PubMed] [Google Scholar]
- 52. Grob TJ, Hoenig T, Clauditz TS, et al. Frequent intratumoral heterogeneity of EGFR gene copy gain in non-small cell lung cancer. Lung Cancer. 2013;79:221–227. [DOI] [PubMed] [Google Scholar]
- 53. Tapia C, Schraml P, Simon R, et al. HER2 analysis in breast cancer: reduced immunoreactivity in FISH non-informative cancer biopsies. Int J Oncol. 2004;25:1551–1557. [PubMed] [Google Scholar]
- 54. Fraune C, Simon R, Hube-Magg C, et al. MMR deficiency in urothelial carcinoma of the bladder presents with temporal and spatial homogeneity throughout the tumor mass. Urol Oncol. 2020;38:488–495. [DOI] [PubMed] [Google Scholar]
- 55. Bharadwaj U, Marin-Muller C, Li M, et al. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis. 2011;32:1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chang MC, Chen CA, Chen PJ, et al. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem J. 2012;442:293–302. [DOI] [PubMed] [Google Scholar]
- 57. Servais EL, Colovos C, Rodriguez L, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res. 2012;18:2478–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Uehara N, Matsuoka Y, Tsubura A. Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol Cancer Res. 2008;6:186–193. [DOI] [PubMed] [Google Scholar]
- 59. Wang Y, Wang L, Li D, et al. Mesothelin promotes invasion and metastasis in breast cancer cells. J Int Med Res. 2012;40:2109–2116. [DOI] [PubMed] [Google Scholar]
- 60. Zheng C, Jia W, Tang Y, et al. Mesothelin regulates growth and apoptosis in pancreatic cancer cells through p53-dependent and -independent signal pathway. J Exp Clin Cancer Res. 2012;31:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Inoue S, Tsunoda T, Riku M, et al. Diffuse mesothelin expression leads to worse prognosis through enhanced cellular proliferation in colorectal cancer. Oncol Lett. 2020;19:1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shiraishi T, Shinto E, Nearchou IP, et al. Prognostic significance of mesothelin expression in colorectal cancer disclosed by area-specific four-point tissue microarrays. Virchows Arch. 2020;477:409–420. [DOI] [PubMed] [Google Scholar]
- 63. Shiraishi T, Shinto E, Mochizuki S, et al. Mesothelin expression has prognostic value in stage IotaIota/IotaIotaIota colorectal cancer. Virchows Arch. 2019;474:297–307. [DOI] [PubMed] [Google Scholar]
- 64. Kawamata F, Homma S, Kamachi H, et al. C-ERC/mesothelin provokes lymphatic invasion of colorectal adenocarcinoma. J Gastroenterol. 2014;49:81–92. [DOI] [PubMed] [Google Scholar]
- 65. Hanaoka T, Hasegawa K, Kato T, et al. Correlation between tumor mesothelin expression and serum mesothelin in patients with epithelial ovarian carcinoma: a potential noninvasive biomarker for esothelin-targeted Therapy. Mol Diagn Ther. 2017;21:187–198. [DOI] [PubMed] [Google Scholar]
- 66. Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80:1943–1949. [DOI] [PubMed] [Google Scholar]
- 67. Rubin MA, Dunn R, Strawderman M, et al. Tissue microarray sampling strategy for prostate cancer biomarker analysis. Am J Surg Pathol. 2002;26:312–319. [DOI] [PubMed] [Google Scholar]
- 68. Kluth M, Volta H, Hussein M, et al. Deletion of 3p13 is a late event linked to progression of TMPRSS2:ERG fusion prostate cancer. Cancer Manag Res. 2018;10:5909–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kluth M, Al Kilani Z, Ozden C, et al. 5q21 deletion is often heterogeneous in prostate cancer. Genes Chromosomes Cancer. 2019;58:509–515. [DOI] [PubMed] [Google Scholar]
- 70. Kluth M, Meyer D, Krohn A, et al. Heterogeneity and chronology of 6q15 deletion and ERG-fusion in prostate cancer. Oncotarget. 2016;7:3897–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stahl P, Seeschaaf C, Lebok P, et al. Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol. 2015;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Burandt E, Grunert M, Lebeau A, et al. Cyclin D1 gene amplification is highly homogeneous in breast cancer. Breast Cancer. 2016;23:111–119. [DOI] [PubMed] [Google Scholar]


