Abstract
Background
Anti-interleukin (IL)-17 biological agents (BAs) have significant efficacy in the treatment of psoriasis and psoriatic arthritis; however, adverse events (AEs) are common, and their safety has not been systematically evaluated.
Objectives
The purpose of this systematic review and meta-analysis was to summarize the number and corresponding rates of AEs caused by anti-IL-17 BAs in patients with psoriasis and psoriatic arthritis to improve clinical decision-making regarding their use.
Methods
PubMed, Embase, Cochrane Library, and Web of Science databases were independently searched by three authors for articles on the treatment of psoriasis with anti-IL-17 BAs that were published before March 1, 2022, and included at least one AE. Dichotomous variables and 95% confidence intervals (CI) were analyzed using R software (version 4.1.3) and the Meta and Metafor software packages. Funnel plots and meta-regression were used to test for the risk of bias, I2 was used to assess the magnitude of heterogeneity, and subgroup analysis was used to reduce heterogeneity.
Results
A total of 57 studies involving 28,424 patients with psoriasis treated with anti-IL-17 BAs were included in the meta-analysis. Subgroup analysis showed that anti-IL-17A (73.48%) and anti-IL-17A/F (73.12%) BAs were more likely to cause AEs than anti-IL-17R BAs (65.66%). The incidence of AEs was as high as 72.70% with treatment durations longer than one year, and long-term use of medication had the potential to lead to mental disorders. Infection (33.16%), nasopharyngitis (13.74%), and injection site reactions (8.28%) were the most common AEs. Anti-IL-17 BAs were most likely to cause type α (33.52%) AEs. Type δ AEs (1.01%) were rarely observed.
Conclusions
Anti-IL-17 BAs used for the treatment of psoriasis and psoriatic arthritis caused a series of AEs, but the symptoms were generally mild.
Keywords: anti-IL-17, biological agents, adverse events, meta-analysis, systematic review
1. Introduction
Psoriasis has long been a significant research topic in dermatology. Psoriasis usually affects the head, trunk, and elbows. Thimble nails and wispy hair are idiopathic symptoms. Psoriasis is known as an “undying disease” due to recurring outbreaks that are difficult to cure and can lead to depression and even suicidal tendencies in severe cases (1). Unfortunately, the incidence of psoriasis has increased annually. Psoriasis can occur with a variety of comorbidities such as cardiovascular disease, chronic obstructive pulmonary disease, metabolic disorders, and psoriatic arthritis (PSA); approximately 30% of patients with psoriasis develop PSA (2). There is no one-stop solution for the treatment of moderate-to-severe psoriasis. Dermatologists commonly use glucocorticoids, vitamin D analogs, calcium-regulated phosphatase inhibitors, and phototherapy to control the course of psoriasis, but the results are unsatisfactory.
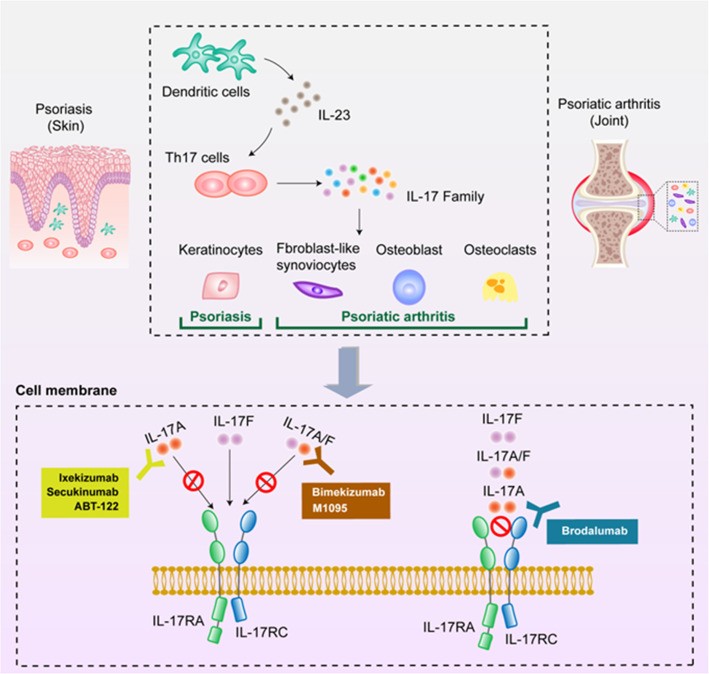
The IL-23/Th17 immune axis is thought to play a central role in the development of psoriasis. Th17-related cytokines, such as interleukin (IL)-17A and IL-17F, are significantly elevated in psoriasis. Previous studies have shown that the expression levels of IL-17A and IL-17F are up to eight times higher in psoriatic skin lesions than those in healthy patients. IL-17 causes increased proliferation of keratinocytes and inflammation by stimulating pro-inflammatory cytokines, pro-proliferative cytokines, antimicrobial peptides, and chemokines. IL-17 can also upregulate inflammatory factors such as IL-6 and intracellular adhesion molecule-1 in endothelial cells, further enhancing the inflammatory response. Similarly, IL-17A plays a central role in PSA by enhancing the inflammatory response and promoting joint damage. IL-17A targets osteoblasts, osteoclast precursors, and synovial-like joint fibroblasts (3) ( Figure 1 ). Moreover, clinical studies have shown that the discontinuation rate of traditional systemic therapy is as high as 66% owing to its low efficacy and side effects (4).
Figure 1.
Schematic diagram of the mechanism of action of anti-interleukin-(IL)-17 drugs. The mechanism of anti-IL-17 monoclonal antibodies in psoriasis and psoriatic arthritis. The pathogenesis of both psoriasis and psoriatic arthritis is closely related to the IL-23/IL-17 axis. First, dendritic cells secrete IL-23 to activate Th17 cells and then the activated Th17 cells secrete large amounts of IL-17, thereby triggering an inflammatory cascade. Next, the IL-17 family induces excessive proliferation of keratinocytes, resulting in localized papules, erythema, and silvery-white plaques on the skin. In addition, the IL-17 family act on the joint to activate synovial fibroblasts, osteoblasts, and osteoclasts, triggering local tissue inflammation. This study included three types of monoclonal antibodies that alleviate psoriasis and psoriatic arthritis by blocking IL-17A, IL-17R, and IL-17A/F, thereby interrupting the inflammatory cascade.
BAs are highly active immunologic agents that exert their effects by blocking specific inflammatory factors produced by the immune system. BAs are commonly used in the treatment of autoimmune diseases such as rheumatoid arthritis, PSA, systemic lupus erythematosus, and ankylosing spondylitis (5). In 2004, the European Medicines Agency (EMA) approved the first BA, etanercept; adalimumab and ustekinumab have since emerged (6). A meta-analysis of 28 studies involving IL-12, IL-23, and IL-17 inhibitors showed these biologics to be more effective than placebos in the short term, although they increased the risk of adverse events (AEs) (7). The primary reason that BAs cause AEs is that they are highly immunogenic proteins, such as monoclonal antibodies, fusion proteins, and cytokines, that directly act on the immune system. Monoclonal antibodies targeting IL-17, IL-17A/F, and IL-17R have been developed and put into clinical use, which has benefited patients with moderate-to-severe psoriasis. Compared to traditional therapies, BAs have a high curative effect, are simple and convenient to use, and are favored by the majority of patients (8). Secukinumab, a humanized IgG1 monoclonal antibody against IL-17A, was first approved by the FDA in 2015 for the treatment of moderate-to-severe plaque psoriasis, and in 2016 for the treatment of PSA (9). Subsequently, ixekizumab, a humanized IgG4 monoclonal antibody against IL-17A, was developed. The primary mechanism by which IL-17 antibodies attach to cytokines and prevent them from interacting with receptors to block the inflammatory cascade (10). A real-world study monitored 645 psoriasis patients taking ixekizumab and 1152 taking secukinumab for more than a year and showed lower discontinuation rates and higher adherence to ixekizumab (11). Bimekizumab is a humanized IgG1 monoclonal antibody that acts on the dual targets IL-17A and IL-17F. Due to its dual inhibitory effect, bimekizumab more strongly downregulates inflammatory factors, and its clinical efficacy is also considered to be better than that of the anti-IL-17A monoclonal antibody (12). Brodalumab acts on IL-17 receptors on the surface of keratinocytes.
Currently, there is no summary of the safety of IL-17 BAs. To improve their clinical use, it is necessary to comprehensively summarize and analyze the safety of IL-17 BAs. Therefore, the purpose of this systematic review and meta-analysis was to evaluate the incidence of AEs caused by IL-17 BAs to provide data that contribute to better decision-making regarding their clinical application, rational drug use, and reducing the occurrence of AEs.
2. Methods
2.1. Literature search strategy
We used a combination of subject terms and free words to retrieve relevant articles published before March 1, 2022 from four major databases: PubMed, Embase, Cochrane, and Web of Science. The search terms included “psoriasis;” the IL-17A-related items “ixekizumab,” “ABT-122,” and “secukinumab;” the IL-17A/F related BAs “bimekizumab” and “M1095;” and the IL-17R related biologic “brodalumab.” Search terms for biologics also included trade names. The keywords “safety,” “adverse reactions,” “adverse events,” and “side effects” were also included. Additionally, our search covered anti-IL-17 agents for both psoriatic arthritis and psoriasis. This systematic review was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions ( Supplementary Table 1 ) (13).
2.2. Study selection
The inclusion criteria were as follows: (1) randomized controlled trials using anti-IL-17 biologics and (2) studies containing more than one AE. The exclusion criteria were as follows: (1) non-anti-IL-17 drugs, (2) non-randomized controlled trials, (3) preclinical studies, (4) non-experimental articles such as case reports, reviews, meta-analyses, and conferences, (5) articles with incomplete basic information or that provided the abstract only. Two authors (JW and LL) independently screened the articles, and any difference of opinions was resolved by a third-party (XL) intervention.
2.3. Data extraction
Three authors (JW, CW, and LL) independently extracted the following information: (1) first author and publication year; (2) research stage; (3) disease diagnosis; (4) BA type and target, trade name, and constituent; (5) total number of individuals included in the study; (6) experimental dose of monoclonal antibody; (7) mode of administration; and (8) study registration number. AEs-related data were also included. AEs are divided into five categories according to their target and biological consequences: α, β, γ, δ, and ϵ. Type α is often a systemic inflammatory response caused by the release of high levels of pro-inflammatory factors such as IL-6, IL-8, and INF. Type β is often considered to be an immediate or delayed hypersensitivity reaction. Type γ is associated with immune imbalance. Type δ is related to cross-reactions. Type ϵ typically represents psychiatric diseases and neurological symptoms ( Supplementary Table 2 ) (14).
2.4. Quality assessment
The risk of bias in the included randomized controlled trials (RCTs) was categorized as high, medium, or low, and was assessed using the Cochrane risk of bias tool, which comprises seven parameters. The specific parameters are presented in Supplementary Table 3 . Independent evaluation was performed by three authors (JW, LL, and CW), and disagreements were resolved by a fourth author (XL).
2.5. Statistical analysis
Dichotomous variables and 95% confidence intervals (CI) were analyzed using R software (version 4.1.3) and the Meta and Metafor software packages. The original rates were transformed using the Freeman–Tukey double arcsine transformation and then tested for normality. Metaprop was used to calculate the rates and 95% confidence intervals for each independent study, and the Q test and I2 value were used to analyze the heterogeneity among groups. An I2 >50% indicated that the heterogeneity was high; in these cases, a subgroup analysis was required, and a random effects model was used.
3. Results
3.1. Characteristics of the included studies
The final analysis included 57 RCTs; the process used to screen articles is shown in Supplementary Figure 1 . Adverse reactions caused by at least one monoclonal antibody were recorded from each study; if a study included multiple dose cohorts, each dose was analyzed independently. The characteristics of adverse events caused by anti-IL-17 agents are summarized in Table 1 .
Table 1.
Characteristics of the 57 included studies.
| Study | Design (Phase) | Diagnosis | Drug/Target | Total number of patients (M/F) | Dose (mg/kg) | mode of administration | Identifier |
|---|---|---|---|---|---|---|---|
| Blauvelt A 2021 (15) | III | PSO | Ixekizumab/IL-17A | 1346 (N/A) | 80 | i.h. | NCT01646177 |
| Reich K 2019 (16) | III | PSO | Secukinumab/IL-17A | 514 (342/172) | 300 | i.h. | NCT03090100 |
| Mease PJ 2020 (17) | IIIb/IV | PSA | Ixekizumab/IL-17A | 566 (312/254) | 80 | i.h. | NCT03151551 |
| Smolen JS 2020 (18) | IIIb/IV | PSA | Ixekizumab/IL-17A | 566 (312/254) | 80 | i.h. | NCT03151551 |
| Mrowietz U 2019 (19) | IIIb | PSO | Secukinumab/IL-17A | 237 (51/186) | 300; 150 | i.h. | NCT02008890 |
| Chandran V 2020 (20) | III | PSA | Ixekizumab/IL-17A | 386 (N/A) | 80 | N/A | NCT01695239 |
| Gelfand JM 2020 (21) | III | PSO | Secukinumab/IL-17A | 91 (61/30) | 300 | i.h. | NCT02690701 |
| Mease PJ 2017 (22) | III | PSA | Ixekizumab/IL-17A | 417 (192/225) | 80 | i.h. | NCT01695239 |
| McInnes IB 2017 (23) | N/A | PSA | Secukinumab/IL-17A | 299 (N/A) | 300; 150; 75 | N/A | NCT01752634 |
| Bagel J 2017 (24) | IIIb | PSO | Secukinumab/IL-17A | 102 (N/A) | 300 | i.h. | NCT02267135 |
| van der Heijde D 2018 (25) | III | PSA | Ixekizumab/IL-17A | 191 (80/111) | 80 | i.h. | NCT01695239 |
| Okubo Y 2019 (26) | N/A | PSO | Secukinumab/IL-17A | 13 (9/4) | 300 | N/A | NCT01406938 |
| Imafuku S 2017 (27) | III | PSO | Ixekizumab/IL-17A | 1296 (N/A) | 80 | N/A | NCT01474512 |
| Paul C 2015 (28) | III | PSO | Secukinumab/IL-17A | 182 (125/57) | 300; 150 | i.h. | NCT01636687 |
| Blauvelt A 2017 (29) | III | PSO | Ixekizumab/IL-17A | 771 (512/259) | 80 | N/A | NCT01646177 |
| Reich K 2020 (30) | IIIb | PSO | Ixekizumab/IL-17A | 54 (42/12) | 80 | i.h. | NCT02634801 |
| Wu NL 2017 (31) | III | PSO | Secukinumab/IL-17A | 51 (41/10) | 300; 150 | i.h. | N/A |
| Gottlieb A 2017 (32) | IIIb | PSO | Secukinumab/IL-17A | 205 (112/93) | 300; 150 | i.h. | NCT01806597 |
| Paul C 2019 (33) | IIIb | PSO | Ixekizumab/IL-17A | 136 (90/46) | 80 | i.h. | NCT02561806 |
| Ohtsuki M 2014 (34) | III | PSO | Secukinumab/IL-17A | 58 (49/9) | 300; 150 | i.h. | NCT01365455 |
| Valenzuela F 2017 (35) | III | PSO | Ixekizumab/IL-17A | 1604 (1061/543) | 80 | N/A | NCT01646177 |
| LeoNardi C 2018 (36) | III | PSO | Ixekizumab/IL-17A | 1346 (N/A) | 80 | i.h. | NCT01646177 |
| Reich K 2017 (37) | IIIb | PSO | Ixekizumab/IL-17A | 302 (202/100) | 80 | i.h. | NCT02561806 |
| Sticherling M 2017 (38) | N/A | PSO | Secukinumab/IL-17A | 200 (124/76) | 150 | i.h. | NCT02474082 |
| Blauvelt A 2017 (39) | III | PSO | Ixekizumab/IL-17A | 1226 (N/A) | 80 | i.h. | NCT01474512; NCT01597245 |
| Warren RB 2020 (40) | III | PSO | Secukinumab/IL-17A | 327 (213/114) | 300 | i.h. | NCT03478787 |
| Mease PJ 2018 (41) | II | PSA | ABT-122/IL-17A | 240 (121/119) | 240; 120 | i.h. | NCT02349451 |
| Genovese MC 2018 (42) | III | PSA | Ixekizumab/IL-17A | 310 (146/164) | 80 | i.h. | NCT02349295 |
| Körber A 2018 (43) | III | PSO | Secukinumab/IL-17A | 906 (407/498) | 300 | i.h. | NCT01365455; NCT01358578; NCT02074982 |
| Griffiths CE 2015 (44) | III | PSO | Ixekizumab/IL-17A | 2562 (1739/823) | 80 | i.h. | NCT01597245; NCT01646177 |
| Richard G 2014 (45) | III | PSO | Secukinumab/IL-17A | 2044 (1438/606) | 300; 150 | i.h. | NCT01365455; NCT01358578 |
| Kenneth B 2014 (46) | II | PSO | Ixekizumab/IL-17A | 129 (N/A) | 10; 25; 75; 150 | i.h. | NCT01107457 |
| Blauvelt A 2021 (47) | IV | PSO | Ixekizumab/IL-17A | 1025 (N/A) | 80 | i.h. | NCT03573323 |
| Rich P 2013 (48) | II | PSO | Secukinumab/IL-17A | 404 (306/98) | 150 | i.h. | NCT00941031 |
| Gordon KB 2016 (49) | III | PSO | Ixekizumab/IL-17A | 3866 (2622/1244) | 80 | i.h. | NCT01474512; NCT01597245; NCT01646177 |
| Bagel J 2021 (50) | IIIb | PSO | Secukinumab/IL-17A | 1102 (N/A) | 300 | i.h. | NCT02826603 |
| Lebwohl MG 2020 (51) | N/A | PSO | Ixekizumab/IL-17A | 1274 (N/A) | 80 | N/A | NCT01646177 |
| Stebut EV 2019 (52) | N/A | PSO | Secukinumab/IL-17A | 151 (102/49) | 300; 150 | N/A | N/A |
| Leonardi C 2020 (53) | III | PSO | Ixekizumab/IL-17A | 206 (140/66) | 80 | N/A | NCT01597245 |
| Thaci D 2015 (54) | IIIb | PSO | Secukinumab/IL-17A | 676 (481/195) | 300 | i.h. | NCT02074982 |
| Nash P 2017 (55) | III | PSA | Ixekizumab/IL-17A | 363 (169/194) | 80 | i.h. | NCT02349295 |
| Mease P 2017 (56) | III | PSA | Secukinumab/IL-17A | 996 (500/496) | 300; 150 | i.h. | NCT02404350 |
| D’Agostino MA 2021 (57) | III | PSA | Secukinumab/IL-17A | 166 (75/91) | 300; 150 | i.h. | NCT02662985 |
| McInnes IB 2020 (58) | III | PSA | Secukinumab/IL-17A | 397 (N/A) | 150 | i.h. | NCT01752634 |
| Mease PJ 2020 (59) | III | PSA | Brodalumab/IL-17R | 962 (483/479) | 140 | i.h. | NCT02029495; NCT02024646 |
| Seo SJ 2020 (60) | III | PSO | Brodalumab/IL-17R | 62 (38/24) | 210 | i.h. | NCT02982005 |
| Nakagawa H 2015 (61) | II | PSO | Brodalumab/IL-17R | 151 (120/31) | 70; 140; 210 | i.h. | N/A |
| Papp KA 2012 (62) | II | PSO | Brodalumab/IL-17R | 198 (127/71) | 70; 140; 210; 280 | i.h. | NCT00975637 |
| Pinter A 2021 (63) | IV | PSO | Brodalumab/IL-17R | 210 (145/65) | 210 | i.h. | NCT03331835 |
| Reich K 2021 (64) | III | PSO | Bimekizumab/IL-17A/F | 567 (406/161) | 320 | i.h. | NCT03370133 |
| Gordon KB 2021 (65) | III | PSO | Bimekizumab/IL-17A/F | 435 (313/122) | 320 | i.h. | NCT03410992 |
| Papp KA 2018 (66) | IIb | PSO | Bimekizumab/IL-17A/F | 250 (163/87) | 64; 160; 320; 480 | i.h. | NCT02905006 |
| Glatt S 2018 (67) | Ib | PSA | Bimekizumab/IL-17A/F | 53 (26/27) | 40; 80; 160; 320; 560 | i.v. | NCT02141763 |
| Blauvelt A 2020 (68) | IIb | PSA | Bimekizumab/IL-17A/F | 170 (N/A) | 64; 160; 320 | N/A | NCT03010527 |
| Glatt S 2018 (69) | I | PSO | Bimekizumab/IL-17A/F | 39 (30/9) | 8; 40; 160; 480; 640 | i.v. | NCT02529956 |
| Svecova D 2019 (70) | I | PSO | M1095/IL-17A/F | 41 (35/6) | 30; 60; 120; 240 | i.h. | NCT02156466 |
PSO, psoriasis; PSA, psoriatic arthritis; UC, ulcerative colitis; N/A, not available; M/F, Male/Female; i.v., intravenous injection; i.h., hypodermic injection.
3.2. Study quality
The SYRCLE risk of bias tool ( Supplementary Table 3 ) was used to assess the risk of bias in each study; 90% of the research was of high quality. Four studies were included as high risk because their methods of data collection and results were potentially biased due to open labeling, unblinded researchers, or inconsistent patient administration, which may have affected the outcome assessment. The risk of bias was also assessed by a funnel plot constructed using the R language package. As shown in Supplementary Figure 2 , the studies were concentrated and symmetrical, indicating that they had a small risk of bias. There was no evidence of publication bias according to Egger’s test (P=0.8927>0.05). A meta-regression analysis was used to re-examine the included studies without risk of bias ( Supplementary Figure 3 ). A radial plot showed that the study is heterogeneous and therefore required subgroup analysis ( Supplementary Figure 4 ). Sensitivity analyses showed that the overall incidence did not change significantly after removing any of the items individually; therefore, the model was considered stable ( Supplementary Figure 5 ).
3.3. Outcomes
3.3.1. Incidence of AEs with Anti-IL-17 BAs
The meta-analysis showed that 28,424 patients from 57 RCTs received anti-IL-17 drugs, and the incidence of AEs was 72.70% (95% CI 70.14–75.20., I2 = 95%) ( Table 2 , Supplementary Figure 6 ). The incidence of AEs in patients with psoriasis (73.22% [95% CI 70.25–76.10, I2 = 96%]) was comparable to that in patients with PSA (71.22% [95% CI 66.02–76.17, I2 = 93%]) ( Table 2 , Supplementary Figure 7 ).
Table 2.
Estimates of adverse events in the 57 included studies.
| Trials | Any Grade Adverse Events | |||||
|---|---|---|---|---|---|---|
| Studies | Patients of AEs | Total | Incidence/95% CI | I2 | P Value | |
| 1. Diagnosis | ||||||
| Psoriasis (PSO) | 93 | 16,762 | 23,763 | 0.732 [0.702, 0.761] | 96 | <0.0001 |
| Psoriasis arthritis (PSA) | 33 | 3,291 | 4,661 | 0.712 [0.660, 0.762] | 93 | <0.01 |
| 2. Dose Adjustment | ||||||
| < 80mg | ||||||
| Secukinumab | 1 | 84 | 99 | 0.848 [0.762, 0.913] | N/A | N/A |
| Brodalumab | 2 | 48 | 77 | 0.626 [0.452, 0.785] | 58 | 0.12 |
| Bimekizumab | 4 | 42 | 62 | 0.702 [0.567, 0.824] | 44 | 0.15 |
| 80mg | ||||||
| Ixekizumab | 49 | 12674 | 18105 | 0.720 [0.680, 0.758] | 97 | <0.01 |
| 120-160mg | ||||||
| Secukinumab | 15 | 1793 | 2399 | 0.778 [0.701, 0.847] | 94 | <0.01 |
| Brodalumab | 4 | 338 | 613 | 0.565 [0.504, 0.625] | 43 | 0.15 |
| Bimekizumab | 4 | 151 | 200 | 0.724 [0.523, 0.890] | 88 | <0.01 |
| ABT-122 | 1 | 33 | 71 | 0.464 [0.345, 0.587] | N/A | N/A |
| 210-300mg | ||||||
| Brodalumab | 7 | 425 | 641 | 0.733 [0.621, 0.832] | 90 | <0.01 |
| ABT-122 | 1 | 31 | 73 | 0.425 [0.310, 0.546] | N/A | N/A |
| Secukinumab | 19 | 3092 | 4102 | 0.770 [0.713, 0.822] | 93 | <0.01 |
| > 300mg | ||||||
| Bimekizumab | 10 | 1011 | 1460 | 0.733 [0.645, 0.814] | 90 | <0.01 |
| 3. Courses of Medication | ||||||
| 12W | 37 | 4,709 | 8,035 | 0.587 [0.576, 0.598] | 43 | <0.01 |
| IL-17A | 20 | 3,917 | 6,721 | 0.584 [0.572, 0.596] | 48 | <0.01 |
| IL-17R | 10 | 464 | 752 | 0.642 [0.581, 0.702] | 55 | 0.02 |
| IL-17A/F | 7 | 328 | 562 | 0.585 [0.543, 0.626] | 0 | 0.82 |
| 16W | 6 | 851 | 1,460 | 0.586 [0.540, 0.631] | 66 | 0.01 |
| IL-17A | 3 | 299 | 472 | 0.634 [0.590, 0.677] | 0 | 0.6 |
| IL-17R | 2 | 339 | 639 | 0.531 [0.492, 0.569] | 0 | 0.46 |
| IL-17A/F | 1 | 213 | 349 | 0.610 [0.557, 0.662] | N/A | N/A |
| 20W | 9 | 48 | 64 | 0.827 [0.621, 0.973] | 59 | 0.01 |
| IL-17A/F | 9 | 48 | 64 | 0.827 [0.621, 0.973] | 59 | 0.01 |
| 24W | 13 | 1,404 | 2,086 | 0.707 [0.654, 0.757] | 82 | <0.01 |
| IL-17A | 12 | 1,313 | 1,982 | 0.689 [0.644, 0.733] | 73 | <0.01 |
| IL-17R | 1 | 91 | 104 | 0.875 [0.796, 0.932] | N/A | N/A |
| 48W | 7 | 1,342 | 2,381 | 0.592 [0.452, 0.725] | 98 | <0.01 |
| IL-17A | 7 | 1,342 | 2,381 | 0.592 [0.452, 0.725] | 98 | <0.01 |
| 52W | 21 | 3,566 | 4,597 | 0.795 [0.756, 0.831] | 86 | <0.01 |
| IL-17A | 20 | 3,243 | 4,202 | 0.794 [0.752, 0.832] | 86 | <0.01 |
| IL-17A/F | 1 | 323 | 395 | 0.818 [0.776, 0.855] | N/A | N/A |
| >1Y | 51 | 6,360 | 9,128 | 0.794 [0.745, 0.840] | 97 | <0.01 |
| IL-17A | 29 | 5,898 | 8,565 | 0.802 [0.746, 0.852] | 97 | <0.01 |
| IL-17R | 1 | 91 | 104 | 0.525 [0.361, 0.685] | N/A | N/A |
| IL-17A/F | 5 | 371 | 459 | 0.801 [0.731, 0.864] | 63 | 0.03 |
| 4. Frequency of Administration | ||||||
| Q1wk | ||||||
| ABT-122 | 2 | 64 | 144 | 0.444 [0.363, 0.527] | 0 | 0.63 |
| Q2wk | ||||||
| lxekizumab | 11 | 2139 | 3503 | 0.658 [0.590, 0.724] | 91 | <0.01 |
| Secukinumab | 1 | 357 | 387 | 0.922 [0.891, 0.947] | N/A | N/A |
| Brodalumab | 13 | 909 | 1513 | 0.652 [0.578, 0.721] | 85 | <0.01 |
| M1095 | 1 | 21 | 33 | 0.636 [0.451, 0.796] | N/A | N/A |
| Q4wk | ||||||
| Secukinumab | 36 | 4743 | 6433 | 0.758 [0.716, 0.798] | 91 | <0.01 |
| lxekizumab | 26 | 6798 | 10668 | 0.741 [0.698, 0.781] | 96 | <0.01 |
| Brodalumab | 1 | 30 | 41 | 0.731 [0.571, 0.858] | N/A | N/A |
| Bimekizumab | 16 | 1131 | 1634 | 0.670 [0.627, 0.768] | 87 | <0.01 |
| Q8wk | ||||||
| Bimekizumab | 1 | 77 | 100 | 0.770 [0.675, 0.848] | N/A | N/A |
| Q12wk | ||||||
| lxekizuma | 1 | 168 | 227 | 0.740 [0.678, 0.796] | N/A | N/A |
| 5. Drugs | ||||||
| IL-17A | 88 | 17,841 | 25,051 | 0.735 [0.705, 0.764] | 96 | <0.0001 |
| Ixekizumab | 50 | 12,768 | 18,225 | 0.721 [0.682, 0.759] | 97 | <0.01 |
| Secukinumab | 36 | 5,009 | 6,682 | 0.768 [0.724, 0.809] | 93 | <0.01 |
| ABT-122 | 2 | 64 | 144 | 0.444 [0.363, 0.527] | 0 | 0.63 |
| IL-17R | 14 | 939 | 1,554 | 0.657 [0.588, 0.723] | 84 | <0.01 |
| Brodalumab | 14 | 939 | 1,554 | 0.657 [0.588, 0.723] | 84 | <0.01 |
| IL-17A/F | 24 | 1,273 | 1,819 | 0.731 [0.667, 0.791] | 85 | <0.01 |
| Bimekizumab | 23 | 1,252 | 1,786 | 0.736 [0.669, 0.799] | 85 | <0.01 |
| M1095 | 1 | 21 | 33 | 0.636 [0.451, 0.796] | N/A | N/A |
| 6. Types of AEs | ||||||
| Type-α | 106 | 8,363 | 25,577 | 0.334 [0.285, 0.385] | 98 | <0.0001 |
| Type-β | 108 | 6,113 | 25,568 | 0.223 [0.191, 0.257] | 96 | <0.0001 |
| Type-γ | 69 | 733 | 19,753 | 0.047 [0.036, 0.059] | 93 | <0.01 |
| Type-δ | 35 | 153 | 14,744 | 0.010 [0.007, 0.013] | 66 | <0.01 |
| Type-ϵ | 32 | 117 | 7,092 | 0.016 [0.010, 0.023] | 69 | <0.01 |
| 6.1 Type α | ||||||
| Viral URI | 8 | 325 | 1,876 | 0.155 [0.088, 0.235] | 95 | <0.01 |
| URI | 82 | 1,828 | 22,861 | 0.078 [0.066, 0.092] | 89 | <0.01 |
| Diarrhea | 32 | 336 | 7,189 | 0.040 [0.030, 0.052] | 82 | <0.01 |
| Headache | 69 | 1,397 | 19,722 | 0.060 [0.052, 0.068] | 77 | <0.01 |
| Back pain | 25 | 331 | 6,897 | 0.042 [0.034, 0.052] | 66 | <0.01 |
| Urinary tract infection | 24 | 132 | 3,895 | 0.033 [0.022, 0.045] | 73 | <0.01 |
| Candida infections | 46 | 533 | 14,253 | 0.035 [0.026, 0.046] | 86 | <0.01 |
| Cough | 19 | 184 | 4,028 | 0.041 [0.030, 0.054] | 59 | <0.01 |
| Arthralgia | 41 | 753 | 15,268 | 0.046 [0.037, 0.056] | 79 | <0.01 |
| Nausea | 17 | 97 | 3,034 | 0.033 [0.020, 0.049] | 65 | <0.01 |
| Oropharyngeal pain | 13 | 156 | 3,462 | 0.032 [0.020, 0.045] | 62 | <0.01 |
| Infections | 55 | 5,008 | 18,842 | 0.332 [0.276, 0.390] | 99 | <0.0001 |
| Serious infections | 26 | 91 | 7,029 | 0.011 [0.008, 0.015] | 27 | 0.1 |
| Fatigue | 4 | 26 | 538 | 0.045 [0.028, 0.065] | 0 | 0.56 |
| Influenza | 3 | 68 | 1,145 | 0.051 [0.029, 0.078] | 50 | 0.14 |
| Pain in extremity | 5 | 20 | 257 | 0.074 [0.043, 0.111] | 0 | 0.49 |
| Dizziness | 3 | 3 | 72 | 0.022 [0.000, 0.085] | 9 | 0.33 |
| Vomiting | 4 | 20 | 426 | 0.046 [0.027, 0.069] | 0 | 0.98 |
| Gastrointestinal disorders | 5 | 12 | 92 | 0.105 [0.039, 0.189] | 0 | 0.52 |
| Abdominal pain | 2 | 3 | 12 | 0.246 [0.027, 0.550] | 0 | 0.56 |
| 6.2 Type β | ||||||
| Injection-site reaction | 59 | 1,866 | 19,736 | 0.083 [0.063, 0.105] | 95 | <0.01 |
| Bronchitis | 25 | 333 | 5,964 | 0.051 [0.041, 0.063] | 68 | <0.01 |
| Injection-site erythema | 10 | 125 | 2,773 | 0.053 [0.032, 0.078] | 83 | <0.01 |
| Allergic reaction/hypersensitivity events | 24 | 228 | 5,253 | 0.042 [0.030, 0.057] | 83 | <0.01 |
| Nasopharyngitis | 97 | 3,715 | 23,662 | 0.137 [0.118, 0.159] | 92 | <0.01 |
| Sinusitis | 15 | 157 | 3,390 | 0.046 [0.035, 0.058] | 48 | 0.02 |
| Pruritus | 19 | 209 | 5,102 | 0.061 [0.033, 0.095] | 83 | <0.01 |
| Decreased neutrophils | 10 | 94 | 2,378 | 0.025 [0.008, 0.049] | 92 | <0.01 |
| Eczema | 9 | 67 | 1,230 | 0.080 [0.042, 0.126] | 69 | <0.01 |
| Urticaria | 6 | 20 | 232 | 0.082 [0.048, 0.124] | 0 | 0.66 |
| Neutropenia | 9 | 17 | 998 | 0.019 [0.000, 0.066] | 68 | <0.01 |
| 6.3 Type γ | ||||||
| Hepatic event | 27 | 219 | 6,668 | 0.034 [0.025, 0.046] | 79 | <0.01 |
| Hypertension | 31 | 308 | 6,689 | 0.040 [0.033, 0.048] | 51 | <0.01 |
| Cerebro‐cardiovascular events | 36 | 225 | 14,588 | 0.015 [0.011, 0.020] | 80 | <0.01 |
| Cytopenia | 4 | 5 | 239 | 0.017 [0.002, 0.041] | 0 | 0.49 |
| PSA/PSO | 12 | 42 | 1,207 | 0.021 [0.011, 0.032] | 26 | 0.19 |
| Crohn’s disease | 13 | 20 | 9,570 | 0.002 [0.001, 0.004] | 14 | 0.3 |
| Ulcerative colitis | 4 | 5 | 1,329 | 0.004 [0.001, 0.008] | 0 | 0.94 |
| Inflammatory bowel disease | 14 | 21 | 8,807 | 0.002 [0.001, 0.003] | 0 | 0.94 |
| 6.4 Type Σ | ||||||
| Depression | 22 | 73 | 4,930 | 0.014 [0.010, 0.018] | 26 | 0.13 |
| Nervous system disorders | 5 | 19 | 92 | 0.188 [0.104, 0.285] | 0 | 0.81 |
| 7. Severity of AE | ||||||
| TEAE | 44 | 7485 | 11532 | 0.713 [0.662, 0.761] | 98 | <0.0001 |
| SAE | 1516 | 24749 | 24794 | 0.045 [0.031, 0.060] | 91 | <0.01 |
AEs, adverse events; URI, Upper Respiratory Tract Infection; PSA, psoriatic arthritis; PSO, psoriasis; TEAE, Treatment-emergent adverse events; SAE, Serious adverse events.
3.3.2. Effect of doses of anti-IL-17 BAs
A subgroup analysis showed that doses less than 80 mg resulted in the lowest incidence of AEs for brodalumab at 62.6% (95% CI 0.452– 0.785, I2 = 58%), and doses ranging from 120–160 mg were associated with the lowest incidence of AEs for ABT-122 at 46.4% (95% CI 0.345–0.587) and the highest incidence of AEs for secukinumab at 77.8% (95% CI 0.701–0.847, I2 = 94%). At doses ranging from 210–300 mg, the lowest incidence of AEs due to ABT-122 was 42.5% (95% CI 0.310–0.546) ( Table 2 , Supplementary Figure 8, 8.1–8.7 ).
3.3.3. Effect of duration of anti-IL-17 treatment
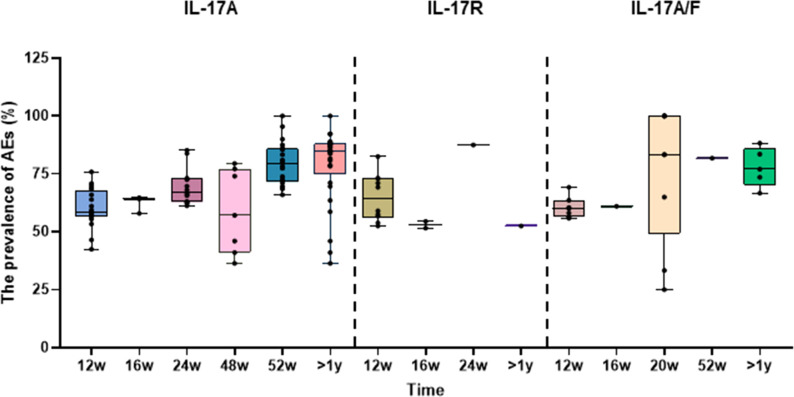
A subgroup analysis of the treatment duration in 57 studies showed that anti-IL-17A and anti-IL-17A/F BAs were relatively safe when administered for less than 48 weeks, and the incidence of AEs was approximately 58–69%. However, the incidence of AEs reached 80% for treatment durations longer than 52 weeks. The 20-week data were not included because the number of patients was inadequate. In addition, due to the small sample size in the clinical trials, the AEs of anti-IL-17R BAs were insufficient to determine the effect of treatment duration ( Table 2 , Supplementary Figure 9 , and Supplementary Figure 9.1–9.7 ). The boxplot clearly and intuitively reflects the effect of treatment duration on the safety of each anti-IL-17 BA ( Figure 2 ).
Figure 2.
Box diagram of subgroup analysis. Box diagram of the incidence of changes in adverse events among different medication courses.
3.3.4. Effect of dosing interval of anti-IL-17 BAs
A subgroup analysis of dosing intervals showed that Secukinumab had a 92.2% (95% CI 0.891–0.947) incidence of AEs when administered at a mean interval of 2 weeks, and the incidence of AEs decreased to 75.8% (95% 0.716–0.798, I2 = 91%) when the interval was prolonged to 4 weeks. The incidence of AEs did not differ significantly when the dosing intervals of other BAs were changed within the safe limits ( Table 2 , Supplementary Figure 10 , 10.1-10.5 ).
3.3.5. Targeted subunits and commercial drugs
The subgroup analyses showed that the incidence of AEs associated with anti-IL-17A and anti-IL-17A/F BAs was similar, both of which were higher than those associated with anti-IL-17R BAs. Further subgroup analysis showed that the incidence of AEs was 73.48% (95% CI 70.48–76.38, I2 = 96%) for the anti-IL-17A BAs and 73.12% (95% CI 66.71–79.14, I2 = 83%) for the anti-IL-17A/F BAs, which were not significantly different. These were both higher than the incidence of AEs for BAs against IL-17R, which was 65.66% (95% CI 58.75–72.26, I2 = 84%) ( Table 2 , Supplementary Figure 11 ). Further subgroup analyses were performed to assess BAs with different targets, for which the incidence of AEs were: 72.10% (95% CI 68.18–75.88, I2 = 97%) for ixekizumab, 76.79% (95% CI 72.44–80.88, I2 = 93%) for secukinumab, 44.44% (95% CI 36.35–52.68, I2 = 0%) for ABT-122, 65.66% (95% CI 58.75–72.26, I2 = 84%) for brodalumab, 73.61% (95% CI 69.92–79.87, I2 = 84%) for bimekizumab, and 63.64% (95% CI 45.13–79.60) for M1095 ( Table 2 , Supplementary Figure 12 ).
3.3.6. Incidence of the five types of AEs
A subgroup analysis of five types of AEs showed that type α was the most common (33.4%), followed by types β (22.3%), γ (4.7%), δ(1.0%), and ϵ (1.6%). Detailed values are listed in Table 2 and Supplementary Figure 13 .
3.3.6.1. Type α AEs
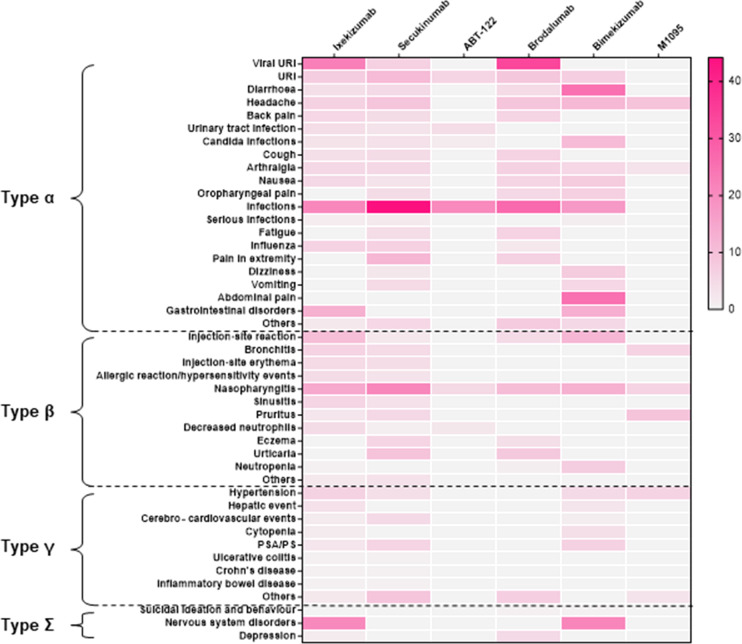
According to the subgroup analyses, anti-IL-17 BAs were the most likely to cause type α AEs and had the highest probability of causing infection, with an incidence rate of 26.98%. Biologics against IL-17A/F most frequently induced diarrhea and abdominal pain, both occurring in 25% of the patients. The most frequent AE induced by IL-17R BAs was viral upper respiratory tract infection, with an incidence as high as 33.65%, followed by infection (26.76%). Detailed values are listed in Table 2 , Supplementary Table 4 and Supplementary Figure 14 . The dark areas of the heat map shown in Figure 3 represent a high incidence of AEs and vice versa.
Figure 3.
Heat map of different types of symptoms caused by different drugs. Red areas indicate greater relative probability of occurrence and lighter-colored areas indicate a slight or null relative probability of occurrence.
3.3.6.2. Type β AEs
The incidence of type β AEs was second only to that of type α. Biologics targeting IL-17A, IL-17A/F, and IL-17R were the most common causes of nasopharyngitis, with incidence rates of 16.05%, 12.62%, and 10.27%, respectively. The second most common type β AEs caused by anti-IL-17A and anti-IL-17A/F BAs were injection site reactions with an incidence of 9.52% and 11.54%. Anti-IL-17R was likely to induce urticaria, with an incidence of 8.08%. Patients treated with anti-IL-17A/F BAs were also prone to pruritus, with an incidence of 9.09%. The incidence of other type β AEs, including sinusitis and neutropenia, was less than 3–6% ( Table 2 , Supplementary Table 4 , Supplementary Figure 14 ).
3.3.6.3. Type γ AEs
Anti-IL-17A and anti-IL-17A/F BAs were more likely to induce type γ AEs than anti-IL-17R BAs. Anti-IL-17A BAs were most likely to induce hypertension (4.59%) and hepatic events (3.41%), and anti-IL-17A/F BAs were most likely to induce psoriasis and PSA (6.11%), followed by hypertension (4.82%) ( Table 2 , Supplementary Table 4 , Supplementary Figure 14 ).
3.3.6.4. Type ϵ AEs
Anti-IL-17A and anti-IL-17A/F BAs were also more likely to induce type ϵ adverse reactions than anti-IL-17R BAs. The most common were neurological diseases, with these two different biologics causing these AEs with incidence rates of 20.37% and 21.05% ( Table 2 , Supplementary Table 4 , Supplementary Figure 14 ).
3.3.7. Severity of AE
According to their severity, AEs are classified as treatment-emergent AEs (TEAEs) or serious AEs (SAEs). An SAE is frequently described as an adverse reaction that necessitates the discontinuation of therapy. This study revealed that when anti-IL-17 BAs were used to treat moderate to severe psoriasis, the incidence of TEAEs was 71.32%, whereas that of SAEs was 4.46% ( Supplementary Figures 15 , 16 ).
4. Discussion
Psoriasis is one of the most common chronic inflammatory skin diseases worldwide. Repeated disease recurrence is a major bottleneck in current treatments. A survey study showed that the top treatment needs of patients with psoriasis in the Asia-Pacific region were long-term remission and reduced recurrence. In this region, 68% of patients with psoriasis believe that the rapid clearance of skin lesions is the key to psoriasis treatment options (71). Similarly, the 2018 edition of the Chinese Psoriasis Diagnosis and Treatment Guidelines considers the prevention of recurrence as an important goal of psoriasis treatment and emphasizes that reducing disease progression and recurrence and improving quality of life are crucial for patients (72). However, traditional methods of treating psoriasis can no longer meet the needs of patients with moderate-to-severe psoriasis. The public has become aware of BAs due to their efficacy, but the safety of these treatments is a problem that cannot be ignored. The main adverse reactions to almost all anti-IL-17 BAs are infection, diarrhea, headache, and back pain. Brodalumab, which was co-developed by AstraZeneca and Amgen, caused occasional suicidal ideation or behavioral events in patients during 2015, but a recent study showed no link between brodalumab and suicide (73). However, a meta-analysis of 163 RCTs and 46 extension studies showed that biologics are associated with significantly higher rates of adverse events Compared to the control group (74). In addition, psoriasis often presents with comorbidities such as cardiovascular disease, abnormal lipid metabolism, chronic obstructive pulmonary disease, and inflammatory bowel disease. Therefore, the safety of BAs must be considered not only for psoriasis but also with regard to its comorbidities.
To our knowledge, this is the first systematic review and meta-analysis to comprehensively evaluate AEs caused by IL-17 BAs in the treatment of psoriasis. We included all relevant RCTs to date, which lends credibility to the data. Table 2 lists all the AE-related information retrieved from the 57 studies included in the analysis. Our study found a higher incidence of AEs associated with anti-IL-17A (73.48%) and anti-IL-17A/F (73.12%) than with anti-IL-17R (65.66%). With treatment durations longer than one year, the incidence of adverse reactions was as high as 79%, and long-term use of medication had the potential to lead to mental disorders. The most common AEs caused by BAs were type α, with an incidence of 33.52%, of which infection was the most common. This was followed by type β adverse reactions with an incidence rate of 22.29%, of which nasopharyngitis was the most common. The incidence rates of type γ, δ, and ϵ AEs were 4.72%, 1.01%, and 1.66%, respectively. Hepatic events were the most common among type γ AEs, which occurred in 3.44% of patients. However, a longitudinal cohort study of 1061 patients with PSA showed liver abnormalities in these patients; therefore, biologics are not the sole contributors to liver events (75). Depression was the most common type ϵ adverse reaction induced by BAs, with an incidence of 1.37%. This may be explained by studies that have shown that mental disorders are comorbid with psoriasis, inflammation and depression share certain molecular mechanisms, and inflammatory factors such as IL-6 are significantly increased in patients with depression (1). However, long-term medication use may also lead to mental disorders. Therefore, regardless of whether BAs are used, doctors should pay more attention to the psychological state of patients and provide psychological treatment when necessary to prevent the deterioration of the condition. The lowest incidence of AEs was type δ (1.01%), which includes adverse reactions such as malignant tumors and acne. Two studies (76, 77) systematically reviewed biologics in the treatment of rheumatoid arthritis and malignancies, and evidence suggests that biologics not only do not cause malignancy but may also reduce the incidence of tumors due to their inhibitory effect on inflammation. However, there is currently no relevant evidence in patients with psoriasis, and we believe that BAs are safe and effective and that the occurrence of malignant tumors is likely unrelated to BAs. Our study found that only 4.46% of patients treated with anti-IL-17 BAs experienced SAEs, at which point the treatment had to be discontinued and the rest of the adverse events could be managed during the remission phase.
This systematic review and meta-analysis evaluated the safety of IL-17-related biologics in the treatment of psoriasis and PSA by summarizing all relevant RCTs to date to facilitate better clinical decision-making. The quality of the included studies was high. Additionally, this study included more than 28,000 patients. The large sample size and use of high-quality studies suggest that our data analysis is convincing and the evidence sufficient and reliable. Each author independently screened the literature and extracted article data, which ensured that it was reliable and reduced the occurrence of errors. Finally, we analyzed and compared multiple subgroups with adverse reactions and fully mined the data. However, this study has some limitations. First, the included studies did not exclude the underlying diseases of the patients and could not fully determine whether some AEs were caused by BAs. Second, individual articles did not clearly define SAEs, which are divided into severe and serious AEs, which may have produced slight errors in the data analysis. Third, some articles were unclear about the definition of certain AEs, such as skin system diseases and musculoskeletal diseases. To ensure accuracy, we discarded these data. Fourth, there are too few IL-17R-related studies, and the evidence is not sufficient. Therefore, it is recommended to conduct large-scale, multi-center clinical trials to confirm the safety of anti-IL-17R BAs in the future. Biologics will likely become the focus of psoriasis treatment in the future; however, shortcomings remain. In the future, it is necessary to further clarify the mechanism of common adverse reactions caused by related monoclonal antibodies and their impact on the comorbidities of psoriasis to reduce their occurrence and benefit the majority of patients.
4.1. Conclusion
Among the adverse events caused by the use of anti-IL-17 agents, cytokine release syndrome and allergic reactions were the most common. Given that immunologic agents are increasingly used in the clinical setting, their AEs should receive more attention.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
XL and BL proposed and designed the study; XL obtained funding; JW and LL retrieved and selected the data; JW, LL, and CW extracted the data; SH and YR assessed the quality of all studies; NL, MZ, and XS performed the statistical analyses of all the data; JW and CW drafted the manuscript; and XL revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was sponsored by the National Natural Science Foundation of China (Nos. 81874470, 82074427, 81904200).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.993057/full#supplementary-material
References
- 1. Koo J, Marangell LB, Nakamura M, Armstrong A, Jeon C, Bhutani T, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. (2017) 31(12):1999–2009. doi: 10.1111/jdv.14460 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. Jama (2020) 323(19):1945–60. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 3. Akiyama S, Sakuraba A. Distinct roles of interleukin-17 and T helper 17 cells among autoimmune diseases. J Transl Autoimmun (2021) 4:100104. doi: 10.1016/j.jtauto.2021.100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tveit KS, Duvetorp A, Østergaard M, Skov L, Danielsen K, Iversen L, et al. Treatment use and satisfaction among patients with psoriasis and psoriatic arthritis: results from the NORdic PAtient survey of psoriasis and psoriatic arthritis (NORPAPP). J Eur Acad Dermatol Venereol. (2019) 33(2):340–54. doi: 10.1111/jdv.15252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ru Y, Ding X, Luo Y, Li H, Sun X, Zhou M, et al. Adverse events associated with anti-IL-23 agents: Clinical evidence and possible mechanisms. Front Immunol (2021) 12:670398. doi: 10.3389/fimmu.2021.670398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rencz F, Kemény L, Gajdácsi JZ, Owczarek W, Arenberger P, Tiplica GS, et al. Use of biologics for psoriasis in central and Eastern European countries. J Eur Acad Dermatol Venereol. (2015) 29(11):2222–30. doi: 10.1111/jdv.13222 [DOI] [PubMed] [Google Scholar]
- 7. Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: A systematic review and network meta-analysis of randomized controlled trials. J Immunol Res (2019) 2019:2546161. doi: 10.1155/2019/2546161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichiyama S, Ito M, Funasaka Y, Abe M, Nishida E, Muramatsu S, et al. Assessment of medication adherence and treatment satisfaction in Japanese patients with psoriasis of various severities. J Dermatol (2018) 45(6):727–31. doi: 10.1111/1346-8138.14225 [DOI] [PubMed] [Google Scholar]
- 9. Apremilast (Otezla) [Internet]. (2017). Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; [PubMed] [Google Scholar]
- 10. Markham A. Ixekizumab: First global approval. Drugs (2016) 76(8):901–5. doi: 10.1007/s40265-016-0579-y [DOI] [PubMed] [Google Scholar]
- 11. Blauvelt A, Shi N, Burge R, Malatestinic WN, Lin CY, Lew CR, et al. Comparison of real-world treatment patterns among patients with psoriasis prescribed ixekizumab or secukinumab. J Am Acad Dermatol (2020) 82(4):927–35. doi: 10.1016/j.jaad.2019.11.015 [DOI] [PubMed] [Google Scholar]
- 12. Oliveira DG, Faria R, Torres T. An overview of bimekizumab for the treatment of psoriatic arthritis: The evidence so far. Drug Des Devel Ther (2021) 15:1045–53. doi: 10.2147/DDDT.S267405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aubin F, Carbonnel F, Wendling D. The complexity of adverse side-effects to biological agents. J Crohns Colitis (2013) 7(4):257–62. doi: 10.1016/j.crohns.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 15. Blauvelt A, Lebwohl MG, Mabuchi T, Leung A, Garrelts A, Crane H, et al. Long-term efficacy and safety of ixekizumab: a 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol (2021) 85(2):360–8. doi: 10.1016/j.jaad.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 16. Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet (2019) 394(10201):831–9. doi: 10.1016/S0140-6736(19)31773-8 [DOI] [PubMed] [Google Scholar]
- 17. Mease PJ, Smolen JS, Behrens F, Nash P, Liu Leage S, Li L, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis (2020) 79(1):123–31. doi: 10.1136/annrheumdis-2019-215386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smolen JS, Mease P, Tahir H, Schulze-Koops H, de la Torre I, Li L, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheumatic Dis. (2020) 79(10):1310–9. doi: 10.1136/annrheumdis-2020-217372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mrowietz U, Bachelez H, Burden AD, Rissler M, Sieder C, Orsenigo R, et al. Secukinumab for moderate-to-severe palmoplantar pustular psoriasis: Results of the 2PRECISE study. J Am Acad Dermatol (2019) 80(5):1344–52. doi: 10.1016/j.jaad.2019.01.066 [DOI] [PubMed] [Google Scholar]
- 20. Chandran V, van der Heijde D, Fleischmann RM, Lespessailles E, Helliwell PS, Kameda H, et al. Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1). Rheumatol (Oxford) (2020) 59(10):2774–84. doi: 10.1093/rheumatology/kez684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-Severe plaque psoriasis (VIP-s). J Invest Dermatol (2020) 140(9):1784–1793.e1782. doi: 10.1016/j.jid.2020.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis (2017) 76(1):79–87. doi: 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatol (Oxford) (2017) 56(11):1993–2003. doi: 10.1093/rheumatology/kex301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bagel J, Duffin KC, Moore A, Ferris LK, Siu K, Steadman J, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol (2017) 77(4):667–74. doi: 10.1016/j.jaad.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 25. van der Heijde D, Gladman DD, Kishimoto M, Okada M, Rathmann SS, Moriarty SR, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol (2018) 45(3):367–77. doi: 10.3899/jrheum.170429 [DOI] [PubMed] [Google Scholar]
- 26. Okubo Y, Ohtsuki M, Morita A, Yamaguchi M, Shima T, Tani Y, et al. Long-term efficacy and safety of secukinumab in Japanese patients with moderate to severe plaque psoriasis: 3-year results of a double-blind extension study. J Dermatol (2019) 46(3):186–92. doi: 10.1111/1346-8138.14761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imafuku S, Torisu-Itakura H, Nishikawa A, Zhao F, Cameron GS, Japanese UNCOVER-1 Study Group . Efficacy and safety of ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis: Subgroup analysis of a placebo-controlled, phase 3 study (UNCOVER-1). J Dermatol (2017) 44(11):1285–90. doi: 10.1111/1346-8138.13927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. (2015) 29(6):1082–90. doi: 10.1111/jdv.12751 [DOI] [PubMed] [Google Scholar]
- 29. Blauvelt A, Gooderham M, Iversen L, Ball S, Zhang L, Agada NO, et al. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3). J Am Acad Dermatol (2017) 77(5):855–62. doi: 10.1016/j.jaad.2017.06.153 [DOI] [PubMed] [Google Scholar]
- 30. Reich K, Augustin M, Thaçi D, Pinter A, Leutz A, Henneges C, et al. A 24-week multicentre, randomized, open-label, parallel-group study comparing the efficacy and safety of ixekizumab vs. fumaric acid esters and methotrexate in patients with moderate-to-severe plaque psoriasis naive to systemic treatment. Br J Dermatol (2020) 182(4):869–79. doi: 10.1111/bjd.18384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu NL, Hsu CJ, Sun FJ, Tsai TF. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: Subanalysis from ERASURE phase III study. J Dermatol (2017) 44(10):1129–37. doi: 10.1111/1346-8138.13900 [DOI] [PubMed] [Google Scholar]
- 32. Gottlieb A, Sullivan J, van Doorn M, Kubanov A, You R, Parneix A, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: Results from GESTURE, a randomized controlled trial. J Am Acad Dermatol (2017) 76(1):70–80. doi: 10.1016/j.jaad.2016.07.058 [DOI] [PubMed] [Google Scholar]
- 33. Paul C, Griffiths CEM, van de Kerkhof PCM, Puig L, Dutronc Y, Henneges C, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-s, a phase 3 study. J Am Acad Dermatol (2019) 80(1):70–79.e73. doi: 10.1016/j.jaad.2018.06.039 [DOI] [PubMed] [Google Scholar]
- 34. Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol (2014) 41(12):1039–46. doi: 10.1111/1346-8138.12668 [DOI] [PubMed] [Google Scholar]
- 35. Valenzuela F, de la Cruz Fernandez C, Galimberti RL, Gürbüz S, McKean-Matthews M, Goncalves L, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis: Subgroup analysis of Latin American patients in the phase 3 randomized UNCOVER-3 study. Actas Dermosifiliogr. (2017) 108(6):550–63. doi: 10.1016/j.ad.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 36. Leonardi C, Maari C, Philipp S, Goldblum O, Zhang L, Burkhardt N, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol (2018) 79(5):824–830.e2. doi: 10.1016/j.jaad.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 37. Reich K, Pinter A, Lacour JP, Ferrandiz C, Micali G, French LE, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. (2017) 177(4):1014–23. doi: 10.1111/bjd.15666. [DOI] [PubMed] [Google Scholar]
- 38. Sticherling M, Mrowietz U, Augustin M, Thaçi D, Melzer N, Hentschke C, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. (2017) 177(4):1024–32. doi: 10.1111/bjd.15707. [DOI] [PubMed] [Google Scholar]
- 39. Blauvelt A, Papp KA, Sofen H, Augustin M, Yosipovitch G, Katoh N, et al. Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. J Eur Acad Dermatol Venereol. (2017) 31(6):1004–13. doi: 10.1111/jdv.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warren RB, Blauvelt A, Poulin Y, Beeck S, Kelly M, Wu T, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol (2021) 184(1):50–9. doi: 10.1111/bjd.19341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mease PJ, Genovese MC, Weinblatt ME, Peloso PM, Chen K, Othman AA, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-Targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol (2018) 70(11):1778–89. doi: 10.1002/art.40579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Genovese MC, Combe B, Kremer JM, Tsai TF, Behrens F, Adams DH, et al. Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: Week 52 results from SPIRIT-P2. Rheumatol (United Kingdom) (2018) 57(11):2001–11. doi: 10.1093/rheumatology/key182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Körber A, Papavassilis C, Bhosekar V, Reinhardt M. Efficacy and safety of secukinumab in elderly subjects with moderate to severe plaque psoriasis: A pooled analysis of phase III studies. Drugs Aging. (2018) 35(2):135–44. doi: 10.1007/s40266-018-0520-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet (2015) 386(9993):541–51. doi: 10.1016/S0140-6736(15)60125-8 [DOI] [PubMed] [Google Scholar]
- 45. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. New Engl J Med (2014) 371(4):326–38. doi: 10.1056/NEJMoa1314258 [DOI] [PubMed] [Google Scholar]
- 46. Gordon KB, Leonardi CL, Lebwohl M, Blauvelt A, Cameron GS, Braun D, et al. A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. J Am Acad Dermatol (2014) 71(6):1176–82. doi: 10.1016/j.jaad.2014.07.048 [DOI] [PubMed] [Google Scholar]
- 47. Blauvelt A, Leonardi C, Elewski B, Crowley JJ, Guenther LC, Gooderham M, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol (2021) 184(6):1047–58. doi: 10.1111/bjd.19509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol (2013) 168(2):402–11. doi: 10.1111/bjd.12070 [DOI] [PubMed] [Google Scholar]
- 49. Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-Severe plaque psoriasis. N. Engl J Med (2016) 375(4):345–56. doi: 10.1056/NEJMoa1512711 [DOI] [PubMed] [Google Scholar]
- 50. Bagel J, Blauvelt A, Nia J, Hashim P, Patekar M, de Vera A, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). J Eur Acad Dermatol Venereol. JEADV. (2021) 35(1):135–42. doi: 10.1111/jdv.16558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lebwohl MG, Gordon KB, Gallo G, Zhang L, Paul C. Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER-3 study. J Eur Acad Dermatol Venereol. (2020) 34(2):301–9. doi: 10.1111/jdv.15921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. von Stebut E, Reich K, Thaci D, Koenig W, Pinter A, Körber A, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol (2019) 139(5):1054–62. doi: 10.1016/j.jid.2018.10.042 [DOI] [PubMed] [Google Scholar]
- 53. Leonardi C, Reich K, Foley P, Torii H, Gerdes S, Guenther L, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-Severe psoriasis: Long-term results from the UNCOVER-1 and UNCOVER-2 phase-3 randomized controlled trials. Dermatol Ther (2020) 10(3):431–47. doi: 10.1007/s13555-020-00367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol (2015) 73(3):400–9. doi: 10.1016/j.jaad.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 55. Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet (2017) 389(10086):2317–27. doi: 10.1016/S0140-6736(17)31429-0 [DOI] [PubMed] [Google Scholar]
- 56. Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheumatic Dis. (2018) 77(6):890–7. doi: 10.1136/annrheumdis-2017-212687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. D'Agostino MA, Schett G, Lopez-Rdz A, Šenolt L, Fazekas K, Burgos-Vargas R, et al. Response to secukinumab on synovitis using power Doppler ultrasound in psoriatic arthritis: 12-week results from a phase III study, ULTIMATE. Rheumatol (Oxford England) (2021) 61(5):1867–76. doi: 10.1093/rheumatology/keab628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McInnes IB, Mease PJ, Kivitz AJ, Nash P, Rahman P, Rech J, et al. Long-term efficacy and safety of secukinumab in patients with psoriatic arthritis: 5-year (end-of-study) results from the phase 3 FUTURE 2 study. Lancet Rheumatol (2020) 2(4):e227–35. doi: 10.1016/S2665-9913(20)30036-9 [DOI] [PubMed] [Google Scholar]
- 59. Mease PJ, Helliwell PS, Hjuler KF, Raymond K, McInnes I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis (2021) 80(2):185–93. doi: 10.1136/annrheumdis-2019-216835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Seo SJ, Shin BS, Lee JH, Jeong H. Efficacy and safety of brodalumab in the Korean population for the treatment of moderate to severe plaque psoriasis: A randomized, phase III, double-blind, placebo-controlled study. J Dermatol (2021) 48(6):807–17. doi: 10.1111/1346-8138.15733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci (2016) 81(1):44–52. doi: 10.1016/j.jdermsci.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 62. Nakagawa H, Niiro H, Ootaki K, Japanese brodalumab study group . Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci (2016) 82(1):44–52. doi: 10.1016/j.jdermsci.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 63. Pinter A, Hoffmann M, Reich K, Augustin M, Kaplan K, Gudjónsdóttir SD, et al. A phase 4, randomized, head-to-head trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate-to-severe plaque psoriasis (CHANGE). J Eur Acad Dermatol Venereol. (2021) 35(3):701–11. doi: 10.1111/jdv.16932 [DOI] [PubMed] [Google Scholar]
- 64. Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus secukinumab in plaque psoriasis. N. Engl J Med (2021) 385(2):142–52. doi: 10.1056/NEJMoa2102383 [DOI] [PubMed] [Google Scholar]
- 65. Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet (2021) 397(10273):475–86. doi: 10.1016/S0140-6736(21)00126-4 [DOI] [PubMed] [Google Scholar]
- 66. Papp KA, Merola JF, Gottlieb AB, Griffiths CEM, Cross N, Peterson L, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: Results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol (2018) 79(2):277–286.e210. doi: 10.1016/j.jaad.2018.03.037 [DOI] [PubMed] [Google Scholar]
- 67. Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson ADG, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheumatic Dis. (2018) 77(4):523–32. doi: 10.1136/annrheumdis-2017-212127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, et al. Bimekizumab for patients with moderate to severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled, phase 2b extension study. J Am Acad Dermatol (2020) 83(5):1367–74. doi: 10.1016/j.jaad.2020.05.105 [DOI] [PubMed] [Google Scholar]
- 69. Glatt S, Helmer E, Haier B, Strimenopoulou F, Price G, Vajjah P, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol (2017) 83(5):991–1001. doi: 10.1111/bcp.13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Svecova D, Lubell MW, Casset-Semanaz F, Mackenzie H, Grenningloh R, Krueger JG. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin 17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol (2019) 81(1):196–203. doi: 10.1016/j.jaad.2019.03.056 [DOI] [PubMed] [Google Scholar]
- 71. Tada Y, Jo SJ, Huang YH, Wahking B, Lee BY, Gowindah R, et al. Uncovering the unmet needs among psoriasis patients in the Asia-pacific region. J Dermatol (2021) 48(11):1665–74. doi: 10.1111/1346-8138.16072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Committee CMADBPP . Guideline for the diagnosis and treatment of psoriasis in China (2018 complete edition). Chin J Dermatol (2019) 52(10). doi: 10.35541/cjd.20190847 [DOI] [Google Scholar]
- 73. Lebwohl M, Leonardi C, Wu JJ, Armstrong A, Rawnsley N, Merchant M, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb) (2021) 11(1):173–80. doi: 10.1007/s13555-020-00472-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and cochrane overview. Cochrane Database Syst Rev (2011) 2011(2):Cd008794. doi: 10.1002/14651858.CD008794.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pakchotanon R, Ye JY, Cook RJ, Chandran V, Gladman DD. Liver abnormalities in patients with psoriatic arthritis. J Rheumatol (2020) 47(6):847–53. doi: 10.3899/jrheum.181312 [DOI] [PubMed] [Google Scholar]
- 76. Cho SK, Lee J, Han M, Bae SC, Sung YK. The risk of malignancy and its incidence in early rheumatoid arthritis patients treated with biologic DMARDs. Arthritis Res Ther (2017) 19(1):277. doi: 10.1186/s13075-017-1482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yamamoto K, Goto H, Hirao K, Nakajima A, Origasa H, Tanaka K, et al. Longterm safety of tocilizumab: Results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol (2015) 42(8):1368–75. doi: 10.3899/jrheum.141210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.