Abstract
Background –
Myeloid cells form an important element of the response to ischemia-reperfusion injury (IRI). While the mononuclear phagocyte (MP) system is complex and difficult to study, our knowledge of the cells involved and their impacts has been steadily increasing. However, there is still need to rigorously define and separate the functions of discreet myeloid populations in the kidney. The relatively recent distinction between resident macrophages and infiltrating monocytes in the kidney is an important advance that will enhance our understanding of the various roles of distinct myeloid populations, but specific tools are needed to rigorously define the contributions of each to injury, repair, and the transition to chronic disease.
Summary –
Resident macrophages in the kidney form a network with various supportive roles during development and homeostasis. While the classification of these cells has been frequently convoluted in the literature, evidence for their roles during injury and repair are starting to accumulate. Current indications suggest they may have a minimal role during injury processes but may be important during the recovery phase. However, their involvement may also be dependent on their activation state in response to environmental cues. Investigations of the M1/M2 phenotype of myeloid cells has shed some light on the phenotypes that contribute the manifestation of injury and/or recovery, but it is still difficult to form detailed conclusions. Here we will discuss the potential involvement of resident cells in these processes and the use of the M1/M2 system for defining the myeloid response following IRI.
Key Messages –
There is a need for additional specific analysis of the contribution of resident versus recruited myeloid cells to injury, recovery, and chronic disease in the kidney. In addition, the contribution of myeloid activation states that extend beyond simple M1/M2 classification is an important area that needs close attention. Our ability to assess resident cells is growing and awareness of the shortcoming of the M1/M2 system is also increasing. These are promising developments which bode well for the future of kidney injury and disease research.
Keywords: Resident macrophages, inflammation, acute renal failure, CKD, AKI
Introduction
The innate response to kidney injury is a complex reaction that involves the triggering of inflammatory pathways generally associated with pathogen infection. It is possible and likely that unchecked activation of these pathways in the absence of infection leads to a cascade of inflammatory injury that can culminate in chronic disease, renal failure, and death, rather than repair and recovery. The complexity of this process arises from the variety of cells that are involved in the response; the phenotypic diversity, plasticity, and overlap of myeloid cells; and how these cells respond to changes to the tissue environment during injury. Here we will explore the involvement of resident macrophages in events that manifest downstream of ischemic kidney damage, the leading cause of acute kidney injury (AKI).
Resident Macrophages in IRI and CKD
Resident macrophages are the most abundant mononuclear phagocyte (MP) population in the healthy kidney [1]. During tissue injury, resident macrophages are able to cloak sites of damage to prevent excessive immune cell recruitment and inflammatory cascades [2]. Thus, these cells form a first line of defense against sterile tissue damage and it is possible that AKI only proceeds once they have been overwhelmed.
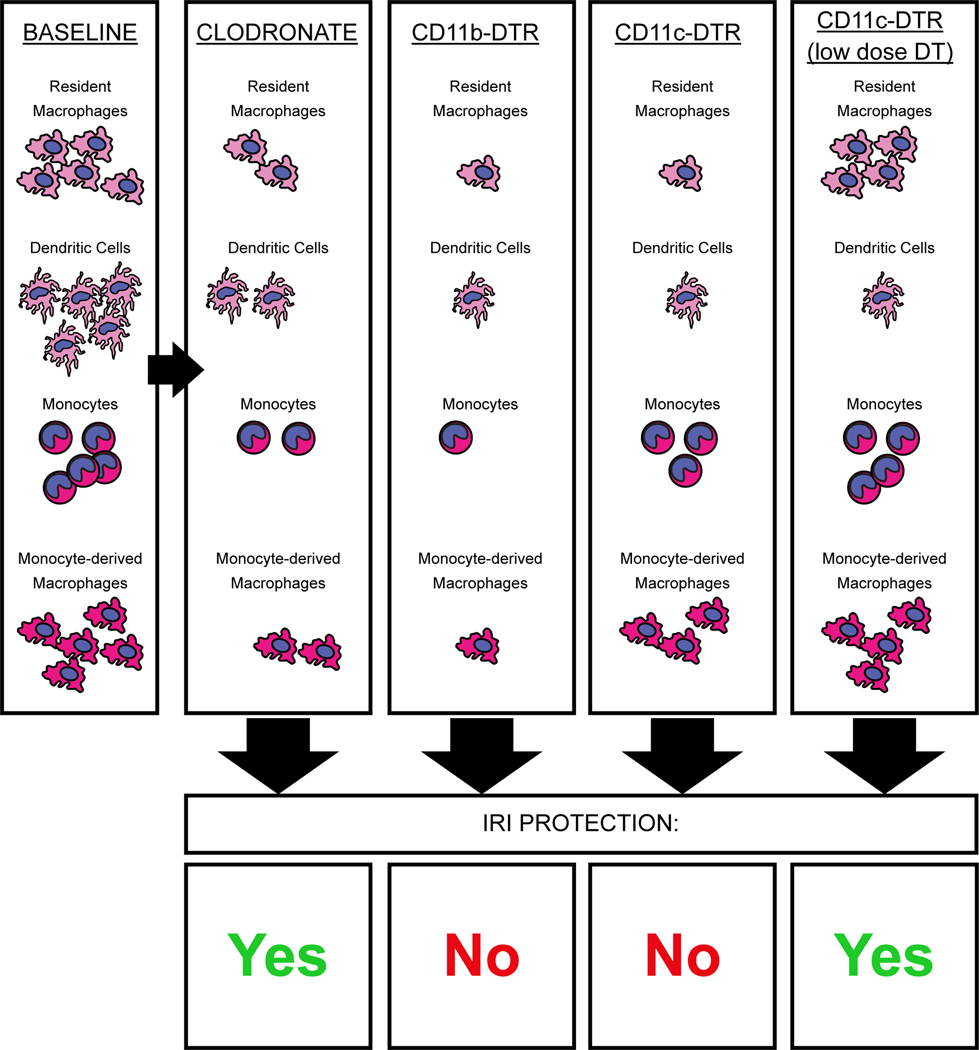
Resident macrophages are F4/80hi CD11bmid and are distinct from their F4/80mid CD11bhi bone marrow-derived monocytic counterparts, as well as dendritic cell (DC) populations [3–6]. Depletion models are useful for determining the roles of specific cell populations, but there are few studies that have achieved selective resident cell depletion without off-target effects. Furthermore, evidence from clodronate- and diphtheria toxin receptor (DTR)-mediated depletion models has painted a complex picture. Clodronate-mediated depletion of phagocytic cells ameliorates IRI [7–9], but this is difficult to reconcile with the fact that depletion in CD11b-DTR and CD11c-DTR mice does not protect from IRI [7,9]. Furthermore, the effect of DTR-mediated depletion is dominant. When clodronate and diphtheria toxin are administered to the same mice, the protection afforded by clodronate treatment is removed [9]. However, the extent of cellular depletion is more complete in DTR models, which raises the possibility that a less phagocytic cell type that is spared by clodronate and expresses CD11b and CD11c has a protective effect during IRI. Interestingly, DC may play a role in IRI since low-dose DT treatment, which should deplete DC but not macrophages, prevented the increase in plasma creatinine [10], further indicating ischemic injury is not mediated by resident macrophages (Fig. 1).
Fig. 1. Impact of some myeloid depletion strategies on IRI.
Clodronate and diphtheria toxin depletion models have varying relative impacts on myeloid cell populations. The removal of cells with clodronate liposomes leads to a function-based removal of phagocytic cells, independent of gene and protein marker expression. The CD11b-DTR and CD11c-DTR models, however, specifically delete cells that express CD11b or CD11c, independent of the cells’ functions. These molecules are expressed to varying degrees on many immune cell populations and depletion efficiency will depend on the level of expression, since this is what drives the production and display of DTR on the cell surface. Thus, CD11c-DTR will deplete CD11chi DC at low doses of DT administration and other CD11c+ cells at higher doses of DT. In general, current evidence indicates that more thorough myeloid depletion is not necessarily IRI-protective. CD11b/CD11c-DTR models remove more cells overall than clodronate and low-dose DT CD11c-DTR, but do not manifest protection. Thus, there is likely functional heterogeneity in these myeloid populations that must be balanced to achieve protection.
Since preventing the influx of neutrophils and other CD11bhi cells often impairs the development of kidney injury and dysfunction, it is possible that the resident cells do not promote injury in the absence of additional inflammatory cells [3,11]. It is also notable that administration of diphtheria toxin to CD11c-DTR mice does not protect from ischemic AKI [7]. This DTR model depletes resident macrophages and dendritic cells in the kidney, but should leave the neutrophil and monocytic compartments largely intact due to low/absent CD11c expression [6,12]. The implication is that resident macrophages and DC are not required for the establishment of ischemic AKI. Additional evidence that resident macrophages do not contribute to early injury has been provided by Park et al. [13]. In this study, they exploited the differential repopulation kinetics of resident macrophages and circulating cells. Clodronate administered 2 weeks prior to injury resulted in a lack of resident macrophages but normal monocytic cell distribution at the time of IRI. Upon IRI, creatinine and blood urea nitrogen (BUN) elevation was unchanged in the absence of resident cells [13].
The potential for these resident cells to promote kidney development is interesting when viewed in the context of repair following AKI [14–16]. Interestingly, although clodronate depletion prior to IRI is protective, clodronate treatment after injury or a specific lack of resident macrophages may lead to impaired recovery in the form of sustained damage, delayed recovery of function, and impaired angiogenesis [13,17,18]. Colony stimulating factor-1 (CSF-1) is required for the development of resident macrophages and the differentiation of monocytes into macrophages [19], but mice treated with this cytokine also exhibit reduced fibrosis after ischemic injury [20]. While CSF-1 is not required for kidney development, lack of CSF-1 signaling results in an absence of macrophages and developmental differences as kidney formation progresses [15,16,19]. Thus, the subtle impacts of macrophages during development may be relevant during the recovery phase following IRI. Indeed, after AKI, resident macrophages take on a phenotype more related to that seen during kidney development, which may be an important element in the repair process [21].
Environmental Cues and Macrophage Activation States
There is a fair amount of focus on the M1/M2 classification system in the kidney field. While there is value in this classification system, it is still problematic due to its inherent oversimplification. This classification is dependent upon the M1/M2 system as created by Mills et al and based off of the Th1/Th2 system [22]. In short, M1 macrophages are considered “classically” activated by IFN-γ, released by type 1 cells and M2 macrophages are considered “alternatively” activated by IL-4, released by type 2 cells [23].
The largest limitation of the M1/M2 system is that, in vitro, the stimuli and states are easily separated, but these results do not translate well in vivo. In vitro, the activation signatures determined for M1 and M2 lack the ability to fully capture or describe the activity of macrophages in their natural environment. Analysis of these activation states should include consideration of factors such as cell source, maturation/differentiation state of the cells, adhesion and extracellular matrix composition, chemo-attractants, and the general tissue milieu – all of which influence the activation state and functional potential of macrophages.
While the M1/M2 system is oversimplified, it is possible to take this into account and design experimental set ups to answer defined questions. For example, one study on macrophage phenotype post IRI assessed whether F4/80+ cells in the kidney were more M1-like or M2-like over 7 days post-IRI [24]. While the cell types were not separated into resident or monocytic, the F4/80+ pool progressively increased expression of arginase-1 (Arg-1) and decreased expression of inducible nitric oxide synthase 2 (iNOS), an M2 and M1 marker respectively. This indicated injury was able to modulate macrophage gene expression, potentially toward a less inflammatory phenotype. They did not test if this was due to alterations in macrophages or the accumulation of infiltrating monocytes with greater Arg-1 expression, but did determine if the kidney environment was capable of switching M1-like cells toward an M2-like state by transferring labeled macrophages derived from the bone marrow. The cells were polarized to M1 in vitro and transferred into mice on IRI day 0. Day 1 post-IRI, the labeled cells were mostly iNOS+, but when the labeled cells were recovered on day 5 post-IRI, they were mostly mannose receptor+ (another M2 marker) rather than iNOS+ [24]. Additionally, transfer of M1 polarized cells immediately after IRI was able to abrogate the protection provided by clodronate treatment 24 hours after injury while transfer of M2 polarized cells maintained the protection [24]. These data nicely show that monocytes/macrophages dynamically alter their state in response to changes in the kidney environment and raises the question if M2 polarization in situ occurs too late to effectively protect kidneys. This study nicely utilizes transfer models to answer specific questions about the ability of cells to respond to the injury environment and the contribution of putative polarization states to injury. However, further analysis of macrophage states and function in vivo will require much more detailed analysis since M1/M2 markers do not necessarily correlate with inflammatory/anti-inflammatory functions in vivo [23,25]. A nice resource has recently been published by Jablonski et al [26]. They performed an extensive and detailed transcriptional analysis of M1 and M2 signatures to identify markers that may better correspond to macrophage functional states.
It must also be kept in mind that macrophages can switch between these states and even display a mixed M1/M2 phenotype at times. Studies have demonstrated that mixed M1/M2 phenotypes exist in diseases such as cancer, inflammatory and autoimmune disorders, and chronic infections [27]. This mixed phenotype is related to a fundamental discrepancy in the M1/M2 system. M1 macrophages are not equivalent to in vivo “classically” activated macrophages and M2 macrophages are not equivalent to “alternatively” activated macrophages. This conclusion is supported by a study comparing in vivo and in vitro activated macrophage transcriptomes, which indicated that genes included in the M1 or M2 signatures can actually be regulated in opposite or apparently unrelated ways during in vivo activation [25]. It is evident that the M1/M2 system for classifying macrophages can be used in vitro, but further studies are necessary to develop a more appropriate system for in vivo macrophage classification. Single cell sequencing technology is aiding this endeavor and recent papers have begun nicely exploring myeloid heterogeneity in the healthy, injured, and repairing kidney with this platform [28,29]. The information gathered from these studies will be invaluable as the field of renal immunology advances and we look toward developing therapies for AKI.
Conclusion
While the picture painted here is complex, it also highlights that progress is underway. We have come about as far as the tools of the past would allow, and now the new knowledge we have gathered regarding the shortcomings of those systems will drive the next generation of experimental design, resource generation, and innovation. Tools that have been recently developed, such as single cell sequencing, high-dimensional flow cytometry, and specific depletion models, will enable the exploration of very specific, directed questions about myeloid cells in the kidney, their contributions to IRI, and their involvement in the recovery vs AKI-to-CKD transition.
Funding Sources
NIH R01 DK062324 and NIH R01 DK056223.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Nash WT, Okusa MD. Chess Not Checkers: Complexities Within the Myeloid Response to the Acute Kidney Injury Syndrome. Front Med. 2021. [cited 2022 Apr 25]. ;8. Available from: 10.3389/fmed.2021.676688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN. Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell. 2019. Apr;177(3):541–555.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Huang L, Sung S-SJ, Vergis AL, Rosin DL, Rose CE, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia–reperfusion injury. Kidney Int. 2008. Dec;74(12):1526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu F, Dai S, Feng D, Qin Z, Peng X, Sakamuri SSVP, et al. Distinct fate, dynamics and niches of renal macrophages of bone marrow or embryonic origins. Nat Commun. 2020. May;11(1):2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salei N, Rambichler S, Salvermoser J, Papaioannou NE, Schuchert R, Pakalniškytė D, et al. The Kidney Contains Ontogenetically Distinct Dendritic Cell and Macrophage Subtypes throughout Development That Differ in Their Inflammatory Properties. J Am Soc Nephrol. 2020. Feb;31(2):257–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami T, Lichtnekert J, Thompson LJ, Karna P, Bouabe H, Hohl TM, et al. Resident Renal Mononuclear Phagocytes Comprise Five Discrete Populations with Distinct Phenotypes and Functions. J Immunol. 2013. Sep;191(6):3358–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Faubel S, He Z, Hernando AA, Jani A, Kedl R, et al. Depletion of Macrophages and Dendritic Cells in Ischemic Acute Kidney Injury. Am J Nephrol. 2012;35(2):181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day Y-J, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal Ischemia-Reperfusion Injury and Adenosine 2A Receptor-Mediated Tissue Protection: The Role of CD4+ T Cells and IFN-γ. J Immunol. 2006. Mar;176(5):3108–14. [DOI] [PubMed] [Google Scholar]
- 9.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TMJ, Marson LP, Kluth DC, et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012. Oct;82(8):928–33. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Okusa MD. Macrophages, Dendritic Cells, and Kidney Ischemia-Reperfusion Injury. Semin Nephrol. 2010. May;30(3):268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Huang L, Sung SJ, Lobo PI, Brown MG, Gregg RK, et al. NKT Cell Activation Mediates Neutrophil IFN-γ Production and Renal Ischemia-Reperfusion Injury. J Immunol. 2007. May;178(9):5899–911. [DOI] [PubMed] [Google Scholar]
- 12.Tittel AP, Heuser C, Ohliger C, Knolle PA, Engel DR, Kurts C. Kidney Dendritic Cells Induce Innate Immunity against Bacterial Pyelonephritis. J Am Soc Nephrol. 2011. Aug;22(8):1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J-G, Lee C-R, Kim M-G, Kim G, Shin HM, Jeon Y-H, et al. Kidney residency of VISTA-positive macrophages accelerates repair from ischemic injury. Kidney Int. 2020. May;97(5):980–94. [DOI] [PubMed] [Google Scholar]
- 14.Wen Y, Yan H-R, Wang B, Liu B-C. Macrophage Heterogeneity in Kidney Injury and Fibrosis. Front Immunol. 2021. [cited 2022 Apr 26]. ;12. Available from: 10.3389/fimmu.2021.681748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r–EGFP transgene reporter. Dev Biol. 2007. Aug;308(1):232–46. [DOI] [PubMed] [Google Scholar]
- 16.Munro DA, Wineberg Y, Tarnick J, Vink CS, Li Z, Pridans C, et al. Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. eLife. 2019. Feb;8:e43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M-G, Su Boo C, Sook Ko Y, Young Lee H, Yong Cho W, Kyu Kim H, et al. Depletion of kidney CD11c+ F4/80+ cells impairs the recovery process in ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010. Sep;25(9):2908–21. [DOI] [PubMed] [Google Scholar]
- 18.Puranik AS, Leaf IA, Jensen MA, Hedayat AF, Saad A, Kim K-W, et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci Rep. 2018. Sep;8(1):13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X-M, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002. Jan;99(1):111–20. [DOI] [PubMed] [Google Scholar]
- 20.Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, et al. Colony-Stimulating Factor-1 Promotes Kidney Growth and Repair via Alteration of Macrophage Responses. Am J Pathol. 2011. Sep;179(3):1243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lever JM, Hull TD, Boddu R, Pepin ME, Black LM, Adedoyin OO, et al. Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight. 2019. Jan;4(2). DOI: 10.1172/jci.insight.125503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J Immunol. 2000. Jun;164(12):6166–73. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014. Mar;6(13). DOI: 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi B-S, et al. Distinct Macrophage Phenotypes Contribute to Kidney Injury and Repair. J Am Soc Nephrol. 2011. Feb;22(2):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS−) vs. Alternatively Activated Macrophages. Front Immunol. 2019;10. DOI: 10.3389/fimmu.2019.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado J de D, Popovich PG, Partida-Sanchez S, et al. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLOS ONE. 2015. Dec;10(12):e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raggi F, Pelassa S, Pierobon D, Penco F, Gattorno M, Novelli F, et al. Regulation of Human Macrophage M1–M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front Immunol. 2017. [cited 2022 Apr 28]. ;8. Available from: 10.3389/fimmu.2017.01097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conway BR, O’Sullivan ED, Cairns C, O’Sullivan J, Simpson DJ, Salzano A, et al. Kidney Single-Cell Atlas Reveals Myeloid Heterogeneity in Progression and Regression of Kidney Disease. J Am Soc Nephrol. 2020. Dec;31(12):2833–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman KA, Bentley MR, Lever JM, Li Z, Crossman DK, Song CJ, et al. Single-Cell RNA Sequencing Identifies Candidate Renal Resident Macrophage Gene Expression Signatures across Species. J Am Soc Nephrol. 2019. May;30(5):767–81. [DOI] [PMC free article] [PubMed] [Google Scholar]