Objective
Our objective was to evaluate SARS-CoV-2 excretion in different maternal sites at delivery and during the postpartum period. We also aimed to evaluate the materno-fetal transmission rate according to the WHO classification.
Study design
COVIPREG study is a prospective study conducted in France (The study was approved by the national ethics comity, the CPP SUD MEDITERRANEE (2020-A00924–35) on April 23, 2020, and recorded in the clinical trial registry (NCT04355234) during the first two waves of COVID-19 pandemic (inclusion between 04/28/2020 and 01/13/2021) and before vaccines availability. A written consent was obtained from both parents. In women with positive SARS-CoV-2 nasopharyngeal RT-PCR at any time during pregnancy, several samples were collected at delivery or in the 48H later, for RT-PCR analysis (vagina (VS), rectum (RS), Maternal blood (MB), nasopharynx (NP), Placenta (P), Amniotic fluid (AF) and milk (M)). In the neonate, Umbilical Cord Blood (UCB), NP, gastric fluid (GF), urine (U), and RS were collected to investigate transmission rate. A total of 12 samples could be collected from either the mother or the child. (Methods in Supplemental Data).
Results
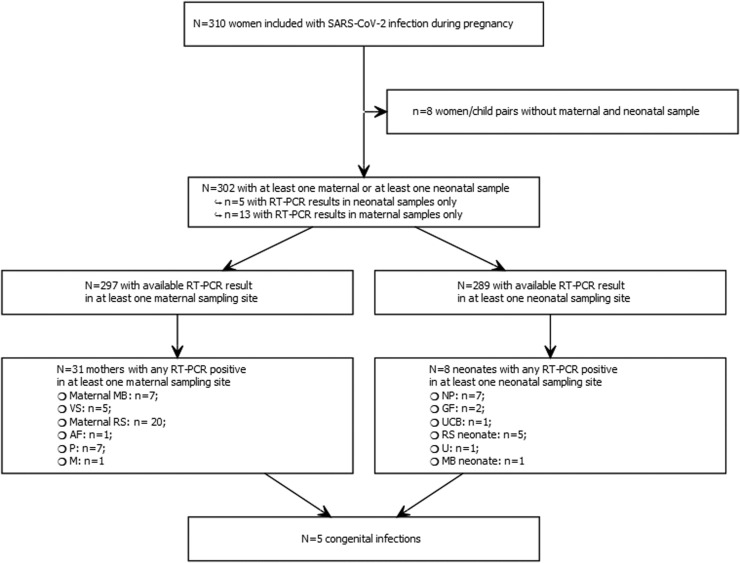
310 women/child pairs were included in the study but unfortunately not all 12 samples were collected for each pair: in 297 pairs at least one maternal and one neonatal sample (excluding blood samples) was available (Fig. 1 ).
Fig. 1.
flowchart of positive RT-PCR for each compartment for the mother and the newborn. vagina (VS), rectum (RS), Maternal blood (MB), nasopharynx (NP), Placenta (P), Amniotic fluid (AF), milk (M), Umbilical Cord Blood (UCB), NP, gastric fluid (GF), urine (U).
In our series, we identified a total of 31 women with at least one positive SARS-CoV-2 RT-PCR in one sample at delivery or in the post-partum period. Among those 7/31 (22.5%) were infected more than 5 weeks before delivery between 11.4 WG and 31.4 WG. The main maternal excretion sites were RS (20/272, 7.4%), followed by VS (5/255, 2.0%) and P (7/262, 2.7%). AF and M were found positive only once in two different mothers (1/176, 0.6%; 1/197, 0.5%). NP RT-PCR at delivery were negative for all mothers. There was no correlation between the different excretion sites.
At birth or during the first 48 h of life, 8/289 (2.8%) neonates had at least one SARS-CoV-2 positive RT-PCR in one sample site, and according to WHO classification, 5/289 (1.7%) cases of proven materno-fetal infection were diagnosed.
Among the 31 women with at least one positive SARS-CoV-2 RT-PCR and the 8 neonates with at least one positive SARS-CoV-2 RT-PCR, 5 women/child pairs both had at least one positive RT-PCR, 26 women had at least one positive RT-PCR without positive RT-PCR in their children and 3 children had at least one positive RT-PCR with an unknown mother's status at delivery. Among the 34 positive pairs (31 mother with a positive sample + 3 neonates with a positive sample with a mother without positive sample). 25positive mothers had symptoms.23/25 symptomatic mothers had a positive sample. 7 neonates had a positive sample (5/7 congenital infections, for 5 positive women/child pairs and 2 positive neonates with an unknown mother status). Ten mothers were hospitalized, 9 had a positive sample and 4 neonates had a positive sample (one positive neonate with an unknown woman status). Therefore, for the five identified neonates with congenital infections all mothers were symptomatic and 2/5 were hospitalized.
Discussion
Excretion in these different sites has been reported in small series in the literature (5 to 56 patients) [1], [2], [3], [4]. Therefore, our series is the larger to date. Furthermore, in these series the delay between maternal SARS-CoV-2 infection and delivery was not stated.
Interestingly, none of the mothers whose neonate had a congenital infection, had positive RT-PCR in blood. However, all had either VS or RS positive RT-PCR. This observation suggests transmission mainly occurs during delivery. One neonate was positive at 48 h of life but was negative at birth, suggesting an immediate postnatal infection. Three neonates were positive at birth but negative in a RT-PCR later, suggesting a superficial exposure to SARS-CoV-2 (one mother had three positive samples (VS, RS and P), and two did not have any RT-PCR positive among samples collected). The rate of transmission we observe (1.7%) is similar to that was reported in the review of Poblete et al. [5].
Conclusion
Our cohort is the largest one before the anti-SARS-CoV-2 vaccination era, and it aimed to evaluate maternal viral excretion at delivery and in the post-partum period. Routes of transmission remain to be confirmed but materno-fetal transmission is a rare event.
The COVIPREG study group:
H. Abdoul, V. Alessandrini, PY Ancel, E. Azria, A.Benachi, C. Borie, V. Buth, C. Cabanne, AG Cordier, C. Couffignal, M. Dommergues, V Alessandrini, S. Dreux, E. Dufour, M. Eloit, J. Guibourdenche, O. Launay, L. Lecomte, F. Goffinet, G. Kayem, M Leruez-Ville, D. Luton, E. Marcault, AA Mariaggi, JF Meritet, B. Parfait, P. Peretout, O. Picone, S. Prieur, J. Rousseau, F. Rozenberg, V. Tsatsaris, Ch Vauloup Fellous, A. Vivanti, Y. Ville.
Funding
Different sources of funding were obtained for the COVIPREG study: a grant from the AP-HP Foundation, a grant from the foundation of the GHU AP-HP centre, a Ferring pharmaceutics grant, a grant from the Université de Paris, a grant from the Groupe de Recherche sur les Infections pendant la Grossesse.
Declaration of Competing Interest
C.V.F. has obtained a grand from Ferring pharmaceutics for the COVIPREG study.
Acknowledgments
The sponsor was Assistance Publique – Hôpitaux de Paris (Délégation à la Recherche Clinique et a l'Innovation)
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jogoh.2023.102547.
Appendix. Supplementary materials
References
- 1.Schwartz A., Yogev Y., Zilberman A., Alpern S., Many A., Yousovich R., Gamzu R. Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vaginal swabs of women with acute SARS-CoV-2 infection: a prospective study. BJOG. 2021;128(1):97–100. doi: 10.1111/1471-0528.16556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenizia C., Saulle I., Di Giminiani M., Vanetti C., Trabattoni D., Parisi F., Biasin M., Savasi V. Unlikely SARS-CoV-2 transmission during vaginal delivery. Reprod Sci. 2021;28(10):2939–2941. doi: 10.1007/s43032-021-00681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinaci S., Ocal D.F., Seven B., Anuk A.T., Besimoglu B., Keven M.C., Goncu Ayhan S., Akin M.S., Tayman C., Keskin H.L., Yapar Eyi E.G., Dinc B., Moraloglu Tekin O., Sahin D. Vertical transmission of SARS-CoV-2: a prospective cross-sectional study from a tertiary center. J Med Virol. 2021;93(10):5864–5872. doi: 10.1002/jmv.27128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y., Liu C., Dong L., Zhang C., Chen Y., Liu J., Zhang C., Duan C., Zhang H., Mol B.W., Dennis C.-.L., Yin T., Yang J., Huang H. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG. 2020;127(9):1109–1115. doi: 10.1111/1471-0528.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poblete C.A., Bancalari A.M. Vertical transmission of Covid - 19. What does the evidence say? Andes Pediatr. 2021;92(5):790–798. doi: 10.32641/andespediatr.v92i5.3488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.