PURPOSE
Fear of cancer recurrence (FCR) is a common distressing condition. We investigated the efficacy of smartphone problem-solving therapy and behavioral activation applications in breast cancer survivors.
METHODS
This was a decentralized randomized trial. Participants were disease-free breast cancer survivors age 20-49 years who were randomly assigned to the smartphone-based intervention or waitlist control. Both groups received treatment as usual. The control group could access the smartphone apps during weeks 8-24. The intervention comprised smartphone problem-solving therapy and behavioral activation apps. The primary end point was the Concerns About Recurrence Scale at week 8. Secondary outcomes included the Fear of Cancer Recurrence Inventory-Short Form (FCRI-SF), the Hospital Anxiety and Depression Scale (HADS), the Short-form Supportive Care Needs Survey (SCNS-SF34), and the Posttraumatic Growth Inventory at weeks 8 and 24 (trial registration: UMIN-CTR: UMIN000031140).
RESULTS
The intervention group included 223 participants, and the control group included 224 participants. Primary outcome data were obtained for 444 participants, and 213 participants in the intervention arm completed the week 24 assessment. The intervention group had statistically greater improvements than controls at week 8 on the Concerns About Recurrence Scale (difference –1.39; 95% CI, –1.93 to –0.85; P < .001), FCRI-SF (difference –1.65; 95% CI, –2.41 to –0.89; P < .001), HADS depression (difference –0.49; 95% CI, –0.98 to 0; P < .05), and SCNS-SF34 psychological domain (difference –1.49; 95% CI, –2.67 to –0.32; P < .05). These scores at week 24 were not statistically significant compared with week 8 although the HADS depression score at week 24 was significantly reduced (P = .03).
CONCLUSION
Novel smartphone psychotherapy offers a promising way to reduce FCR given the large number of survivors and a limited number of therapists to competently conduct psychotherapy.
INTRODUCTION
Advances in early detection and individualized medical treatment have improved the survival rate of patients with breast cancer. The current 10-year survival rate for this population is more than 90%, meaning that the number of breast cancer survivors is increasing.1
CONTEXT
Key Objective
Many breast cancer survivors suffer from fear of cancer recurrence (FCR), and previous studies demonstrated that several interventions, especially cognitive behavioral therapy, reduce FCR among breast cancer survivors. These interventions may be promising; however, a problem with these kinds of interventions is the low participation rate because of time and distance issues, and in addition, the number of therapists who can provide such specialized care may be limited, which presents a serious problem in many countries. The purpose of the present randomized study was to examine the efficacy of smartphone-based problem-solving therapy and behavioral activation interventions in reducing FCR in breast cancer survivors in a decentralized randomized controlled trial.
Knowledge Generated
To our knowledge, the present study is the first to demonstrate the efficacy of smartphone-based psychological therapies in reducing FCR among breast cancer survivors.
Relevance
Novel smartphone psychotherapy offers a promising way to reduce FCR given the large number of cancer survivors and a limited number of therapists to competently conduct appropriate psychotherapy.
It has been suggested that many breast cancer survivors suffer from uncertainty/anxiety and fear of recurrence.2-4 Our previous study found that the most common unmet needs experienced by ambulatory patients with breast cancer were psychological, especially fear of cancer recurrence (FCR); more than half of the participating patients complained of such issues.5 Among patients with breast cancer, FCR is highly prevalent6 and is also associated with poor quality of life.2,5,7-9
Previous studies demonstrated that the several interventions improved FCR among breast cancer survivors, including mindfulness stress reduction (six weekly group sessions), cognitive behavioral therapy (CBT, five individual face-to-face sessions and three e-consultations over a 3-month period), and the novel theoretically based ConquerFear intervention (five individual face-to-face sessions over 10 weeks).10-13 A recent meta-analysis demonstrated that psychological interventions for FCR had a small but significant effect.14 These interventions may be promising; however, a problem with these kinds of interventions is the low participation rate because of time and distance issues (eg, more than 60% of potentially eligible participants decline participation).10,12,13,15,16 In addition, the number of therapists who can provide such specialized care may be limited.
Our past experience and some previous studies indicated that CBT, including problem-solving therapy (PST) and behavioral activation (BA), demonstrated potential effectiveness for reducing FCR.17-20 We previously demonstrated that patients' problem-solving skills were significantly associated with FCR.17 PST and BA are straightforward interventions that can be administered by less experienced therapists, including nurses.21 However, patients willing to undergo PST or BA are rarely able to do so because a typical course of PST or BA comprises 8-12 face-to-face sessions led by trained therapists.22-24
Despite such programs being promising, they appear to suffer similar limitations.10-13 Given the growing number of women diagnosed with breast cancer annually, a completely novel approach to therapy is required. Recent studies have demonstrated the effectiveness of computerized or Internet CBT (iCBT) for the treatment of depression, which can be as effective as face-to face individual, group, or other CBT delivery formats of similar lengths.25-27 CBT delivered via smartphones may be a better treatment option for FCR—in terms of accessibility and portability—than computer-based delivery,28 and these two components have been suggested to be useful for iCBT.29 We recently developed a smartphone CBT app that teaches BA and cognitive restructuring skills and demonstrated its efficacy for antidepressant-resistant major depression in a randomized controlled trial.30 We also developed a PST program as a smartphone app and demonstrated the acceptability and efficacy of smartphone-based PST in a single-arm pilot study with breast cancer survivors.31 The purpose of the present randomized study was to examine the efficacy of smartphone-based PST and BA interventions in reducing FCR among breast cancer survivors.
METHODS
Study Design
We previously described details of the study methodology in a published protocol.32 This fully decentralized individually randomized, parallel-group multicenter trial enrolled participants without any in-person contact.33,34 Some collaborating breast oncologists were asked to hand out study leaflets to their patients, but this did not involve any in-person intervention. Participants were randomly assigned to a smartphone-based intervention plus treatment as a usual group or a waitlist control group receiving treatment as usual.
Participants
The inclusion criteria for participants are as follows: (1) diagnosis of breast cancer, (2) age 20-49 years, (3) 1 year after breast surgery, (4) currently disease-free, and (5) able to complete an electronic patient-reported outcome measure using an iPhone or iPad. We limited participants' age to 20-49 years because another study and our previous investigation demonstrated that individuals in that age group were at high risk for FCR and that more than 50% of this age group have smartphones.9,35,36
The exclusion criteria for participants are as follows: (1) having active, serious physical disease and a current or past history of cancer other than breast cancer; (2) inability to understand Japanese; (3) currently undergoing follow-up and treatment by a psychiatrist or other mental health professional; (4) having previously received structured PST, BA therapy, or CBT; and (5) those judged inappropriate for participation by the researchers (eg, identity theft and duplicate entry).
The study Protocol (online only) was approved by the Institutional Review Board of Nagoya City University on January 15, 2018 (ID: 60-00-1171).
Interventions: Smartphone-Based PST (Kaiketsu-App) and BA (Genki-App)
PST provides patients with a structured, five-step strategy for solving their problems.37 The smartphone-based PST program, called Kaiketsu-App (kaiketsu means solution in Japanese; Life2Bits Inc, Japan) for iPhones and iPads (Apple Inc, Cupertino, CA), was developed for this study. The app development was based on our empirically supported PST manual.20 The Kaiketsu-App comprised nine sessions. Each session took approximately 10 minutes to complete. We also developed the smartphone-based BA program called Genki-App (genki means energy or vitality in Japanese; Life2Bits Inc, Japan).38 BA encourages patients to increase pleasurable and meaningful behaviors. The Genki-App comprised six sessions, with approximately 10 minutes needed to complete each session. Over the 8-week program period, participants were encouraged to complete the sessions and homework through automated e-mail reminders once a week. Both applications were developed for general use and were not specifically tailored to the experiences of breast cancer survivors.
Procedures
Study information was disseminated through several social networking systems, posters displayed in core cancer hospitals, and leaflets introducing the study that collaborating breast oncologists gave to their patients. The study website39 also provided information about this study. After providing electronic informed consent (e-consent) at week 0 and completing the baseline investigation (electric patient-reported outcome), participants were randomly allocated to either the smartphone-based PST and BA group or the waitlist control group at a 1:1 ratio using an electronic data-capturing web program at the data management center. Participants were offered rewards (ie, Amazon gift certificates) of up to 5,000 yen depending on provision of their outcome measurements. The study period was from April 2, 2018, to July 13, 2020. The follow-up was completed on January 15, 2021.
Assessment Measures
Participants were assessed every 2 weeks during the study period (weeks 0-8), and the follow-up assessment was conducted at 24 weeks via smartphone.
The primary outcome was FCR evaluated by the overall fear score on the Japanese version of the Concerns About Recurrence Scale (CARS-J).35,40 The range of possible scores for overall fear was 4-24; a higher score indicated greater fear of recurrence. This scale can be used to screen for high FCR.41 Secondary outcome measures included the Fear of Cancer Recurrence Inventory-Short Form (FCRI-SF), the Hospital Anxiety and Depression Scale (HADS),42 the Short-form Supportive Care Needs Survey questionnaire (SCNS-SF34),43 the Japanese version of the Posttraumatic Growth Inventory (PTGI-J),44 and satisfaction with the intervention (score of 0-100, with 100 indicating complete satisfaction). The CARS-J and HADS were evaluated at weeks 0, 2, 4, 8, and 24. The FCRI-SF, SCNS-SF34, and PTGI-J were administered at weeks 0, 8, and 24. Satisfaction with the intervention was assessed, and a qualitative evaluation of the intervention was conducted at week 8. Satisfaction aimed to include all aspects of the intervention (ie, smartphone application and automated encouragement e-mails), and the qualitative evaluation considered positive and negative aspects and any harms of the intervention. The intervention comprised multiple complex components; therefore, simple structured telephone interviews were conducted at 8 weeks for 30 participants to evaluate the perceived usability, merit, and harms of the intervention.
Sample Size Estimation
Our previous phase II study revealed that the mean CARS-J scores were 12.8 at preintervention (baseline), 12.4 at week 4, and 11.2 at week 8.31 We therefore assumed that the mean CARS-J score at 2 weeks would be 12.6; the overall CARS-J scores in the control arm would not change (12.8 at 0, 2, 4, and 8 weeks); the variance of the score would be 30 at all times; and the intraclass correlation would be 0.82 (ie, a compound symmetry working covariance structure). Therefore, for a sample size on the basis of the power of 0.8 to detect a significant difference at P = .05 (two-sided), 211 participants would be required for each arm. Assuming that 5% of the initial entries would drop out, we needed to recruit 444 participants for this trial. As there were no data regarding the minimal clinically important difference in CARS-J scores and we wanted to conduct this trial as an efficacy trial, we set the sample size on the basis of our previous study.
Statistical Analyses
To examine the treatment effect parameters of all randomly assigned patients in the primary analysis set according to the intention-to-treat principle, we analyzed the primary outcome using a generalized linear model with unstructured covariance and robust SEs. The fixed effects were CARS-J score at baseline, treatment allocation, time, and treatment-by-time interaction. The primary outcome of interest was the difference in CARS-J scores between the two groups at week 8. Effect sizes (ESs) were also calculated on the basis of Cohen's d. Detailed analysis methods, including the secondary end points, are described in the Statistical Analysis Plan (Data Supplement, online only).
RESULTS
Participants' Characteristics
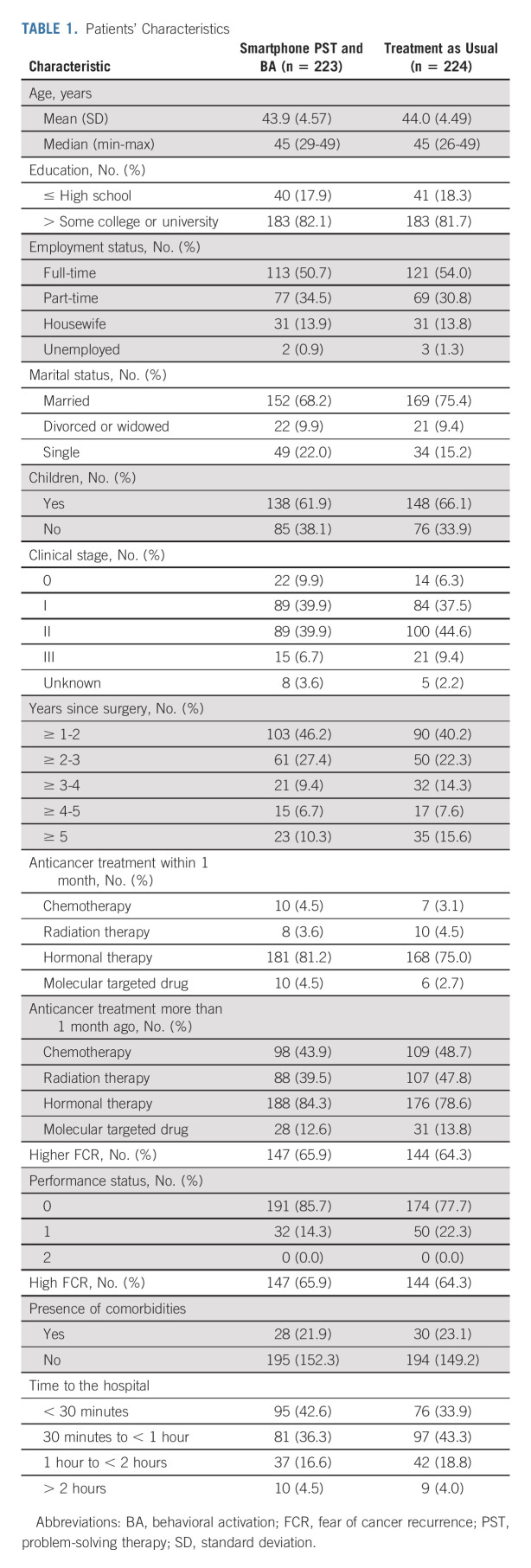
Figure 1 shows the flow of participants through this study. In total, 447 patients provided informed consent and were randomly assigned; 223 were allocated to the intervention group, and 224 to the control group. Primary outcome data at 8 weeks were obtained for 444 randomly assigned participants (99.7% of the total 447 participants). Among the 223 participants who received the intervention, 213 (95.5%) completed the follow-up assessment at week 24. Table 1 shows the baseline demographic and clinical characteristics for each group, which were well balanced. Typically, patients had full-time or part-time employment and were married. Approximately half of the patients received adjuvant chemotherapy, and 65% had high FCR, as estimated in our previous study (ie, scores ≥ 3 [5-point Likert scale: 1 = I never worry about it, 5 = I worry about it all the time] for the fear concerning the consistency item in the CARS-J).41
FIG 1.
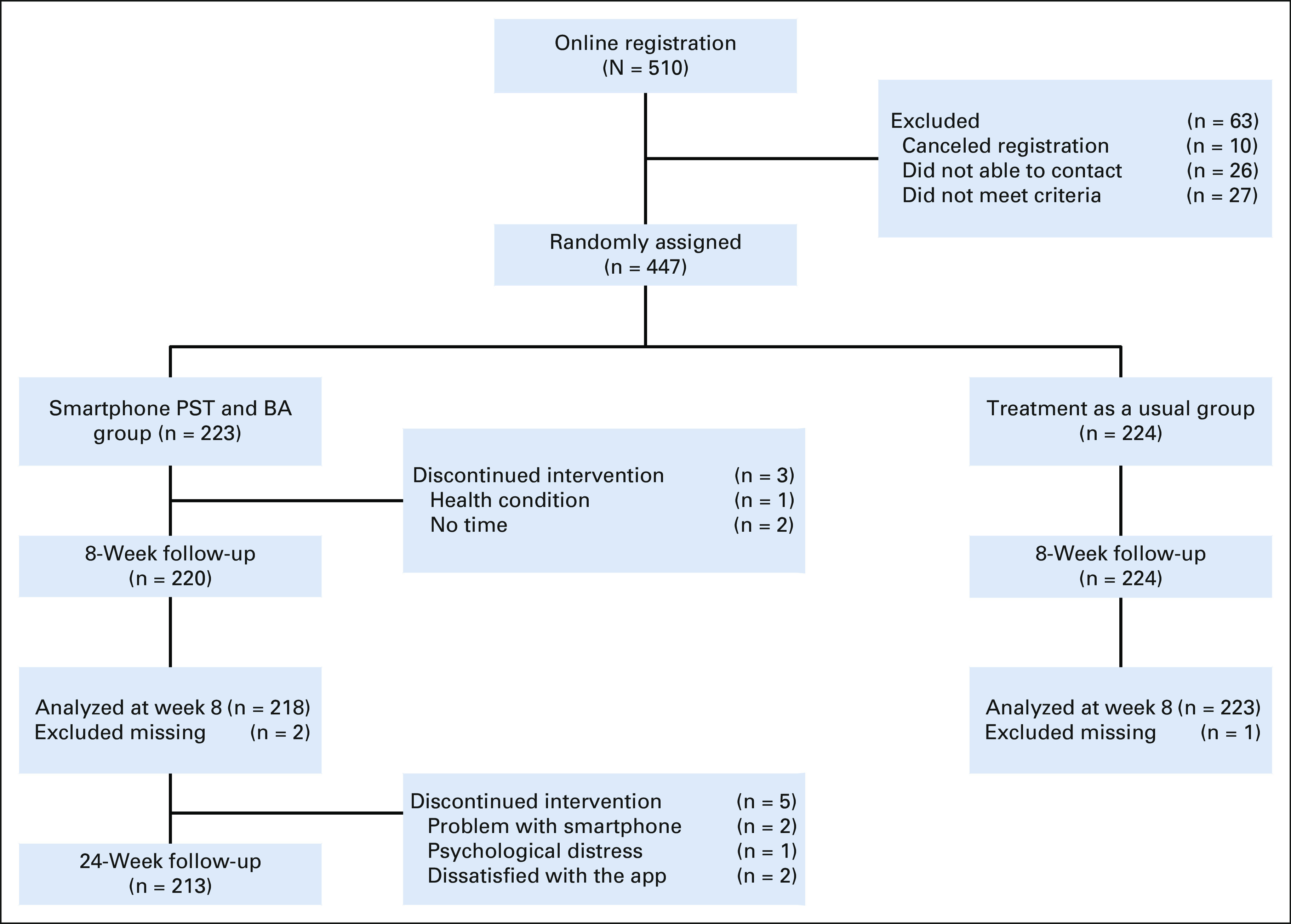
CONSORT diagram. The intervention group, smartphone PST, and BA were evaluated at week 24 in addition to week 8 (primary end point), whereas the treatment as the usual group was evaluated at week 8. BA, behavioral activation; PST, problem-solving therapy.
TABLE 1.
Patients' Characteristics
Treatment Received
For the smartphone apps, most participants in the intervention group completed at least one session of PST (89.2%) and BA (82.1%). The mean, median, and range of completed sessions of the PST app were 6.7 ± 3.3, 9, and 0-9, respectively, and those for the BA app were 4.7 ± 1.9, 6, and 0-6, respectively.
Outcomes
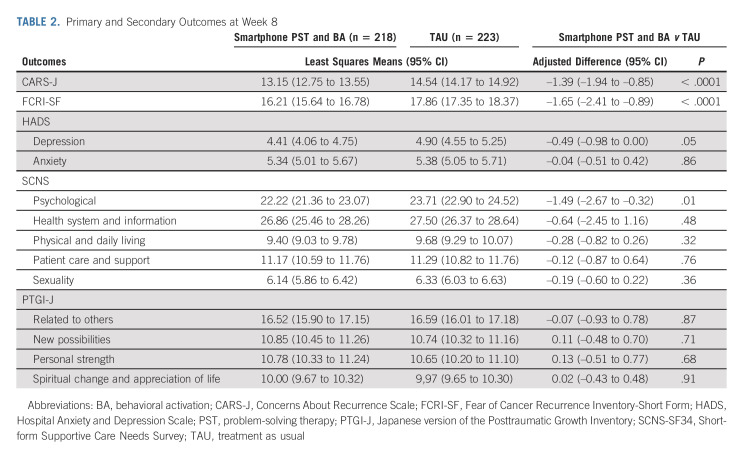
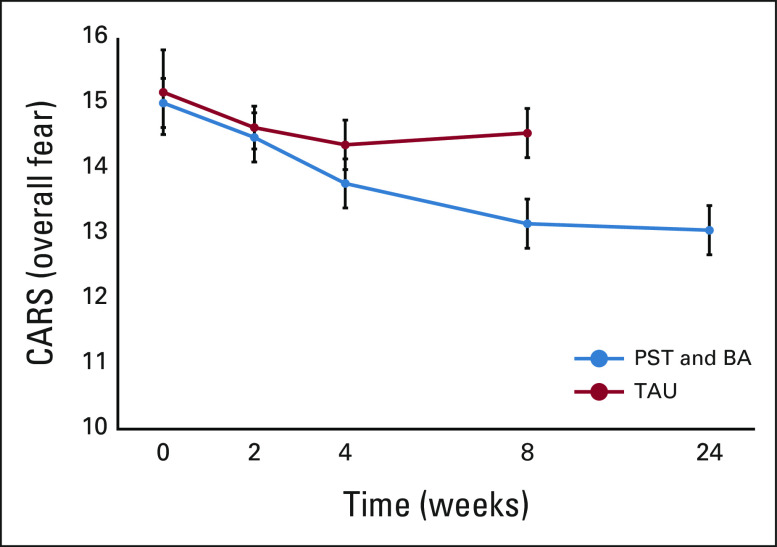
Table 2 shows the outcomes for each group. Participants in the intervention group (n = 223) demonstrated a statistically significant improvement in CARS-J scores at week 8 compared with the control group (difference –1.39, 95% CI, –1.94 to –0.85, P < .001; ES = 0.32). Changes in the overall fear scores from the baseline are shown in Figure 2. In addition, the intervention group showed statistically significant improvement at week 8 in FCRI-SF scores (difference –1.65; 95% CI, –2.41 to –0.89; P < .001; ES = 0.25), HADS depression score (difference –0.49; 95% CI, –0.98 to 0; P < .05; ES = 0.19), and the SCNS-SF34 psychological domain score (difference –1.49; 95% CI, –2.67 to –0.32; P < .05; ES = 0.16). There were no significant differences in scores for other scales between the two groups. The overall satisfaction with treatment in the intervention and control groups was rated at 73.4 (standard deviation = 17.3) and 73.9 (standard deviation = 17.7), respectively, and there was no significant difference (P = .26).
TABLE 2.
Primary and Secondary Outcomes at Week 8
FIG 2.
Changes from baseline in the overall fear scores on the CARS. This shows the mean change from baseline in the overall fear scores. The range of possible scores for overall fear was 4-24; a higher score indicates greater fear of recurrence. I bars indicate SEs. BA, behavioral activation; CARS, Concerns About Recurrence Scale; PST, problem-solving therapy; TAU, treatment as usual.
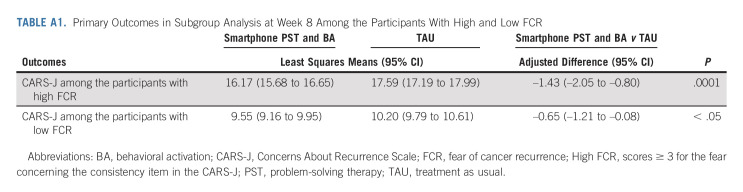
We conducted an additional analysis to determine if the intervention was more effective for those with high FCR (scores ≥ 3 for the CARS-J fear concerning the consistency item). We stratified participants into two groups by their baseline FCR score. Although significant improvement was observed in both groups (week 0 to week 8), greater improvement was observed among those with high FCR (difference –1.43; 95% CI, –2.05 to –0.80; P < .001) compared with those with low FCR (difference –0.65; 95% CI, –1.21 to –0.08; P < .05; subgroup interaction P = .0047; Appendix Table A1, online only). We also divided participants into two groups on the basis of adherence to each of the apps as evaluated by completion of > 80% of sessions. Low adherence to the Kaiketsu-App was defined as completion of seven or fewer sessions, and high adherence as completion of eight or more sessions. Low adherence to the Genki-App was defined as completion of five or fewer sessions, and high adherence as completion of six sessions. We then analyzed the change in study outcomes (week 0 to week 8) in the two groups to investigate the potential influence of adherence to the intervention on the outcome. The degree of engagement with the apps (adherence) was not significantly associated with the outcome (Kaiketsu-App: P = .35; Genki-App: P = .54).
The qualitative evaluation of the interventions showed that the usability of the Kaiketsu-App and Genki-App was positively reported by 43% and 50% of the participants, respectively. Commonly perceived advantages of the Kaiketsu-App included the structured, stepped approach to problem-solving, the sense of receiving a supportive push, and so on. Commonly perceived strengths of the Genki-App included the fun gaming features, encouragement to start small activities, and so on. There were no harms reported although some participants reported negative experiences in that the apps were sometimes complex and difficult to use.
Follow-Up at Week 24
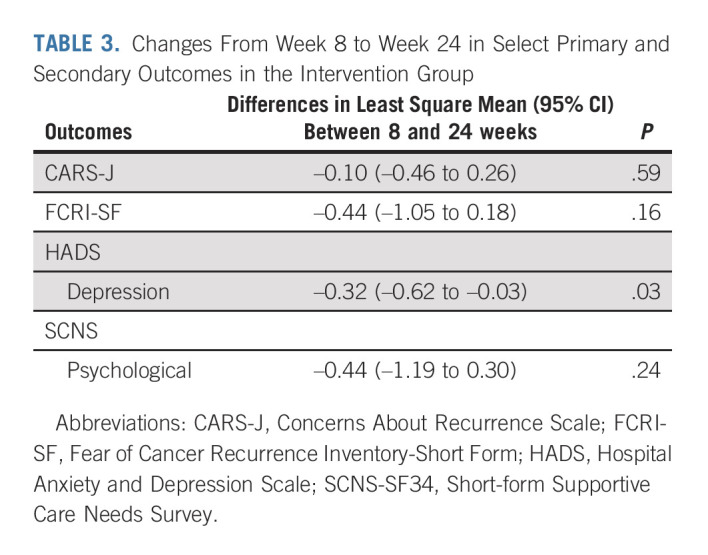
There were no significant differences in the intervention group outcomes at weeks 8 and 24 in terms of CARS-J (Fig 2), FCRI-SF, and SCNS-SF34 psychological domain scores. HADS depression scores at week 24 were significantly further reduced compared with week 8 (P < .05; Table 3).
TABLE 3.
Changes From Week 8 to Week 24 in Select Primary and Secondary Outcomes in the Intervention Group
DISCUSSION
To our knowledge, the present study is the first to demonstrate the efficacy of smartphone-based psychological therapies for FCR among breast cancer survivors. We calculated the ES of the intervention group as a clinical indicator and found that the between-group ES was 0.32 at 8 weeks. This suggested that the smartphone intervention had a small to medium effect on reducing FCR. Although the ES was not large, we believe that the statistically significant difference at 8 weeks reflected a clinically relevant and meaningful change, especially as a meta-analysis investigating the effect of a psychological intervention on FCR demonstrated similar effects (g: 0.24-0.42) for face‐to‐face CBT.14 In addition, our finding that the intervention was more effective for those with high FCR suggests that the current ESs might have underestimated the true intervention effects for survivors with high FCR. With regard to our finding of no significant association between the degree of engagement with the apps (adherence) and the outcome, a further study is needed to address the most effective strategies to provide apps to participants, as we cannot judge causality because adherence was a postrandomization variable (ie, we cannot tell if participants stopped using the app because they improved, or if they did not improve because they had stopped using the app).
Wagner et al45 recently reported that a targeted eHealth intervention that included three cognitive behavioral skills (relaxation, cognitive restricting, and worry practice) failed to show efficacy for FCR among breast cancer survivors with moderate to high FCR. However, there were some differences between our study and that study, including participants' age and the intervention methods and components. Our participants were on average 10 years younger than their participants, and our intervention was delivered via smartphone with PST and BA components. By contrast, Wagner et al delivered their intervention via websites and their components included different cognitive behavioral skills. Our findings showed that smartphone-based PST and BA contributed to reducing FCR among young breast cancer survivors. As most breast cancer survivors need to cope with FCR along with busy daily lives (eg, working) and there are a large number of patients worldwide, smartphone-based psychological therapy, which has benefits such as accessibility, portability, brevity, and cost-effectiveness, may be a promising therapeutic intervention for FCR. Further tasks include dissemination and implementation of this kind of novel intervention.
A recent component network meta-analysis that investigated the efficacy of different cognitive and behavioral skills on depression demonstrated that BA was the most beneficial, followed by behavioral therapy for insomnia and problem-solving.29 A meta-analysis focused on CBT for FCR found that contemporary approaches focused on the process of cognition showed greater efficacy than traditional CBT that focused on the contents of cognition.14 Therefore, different cognitive and behavioral skills have potentially different effects on psychological distress. Further research is needed to clarify which components and combinations of CBT elements are most appropriate for reducing FCR among patients with cancer.
The present study also demonstrated that the intervention had potential efficacy on depression and psychological needs among breast cancer survivors. As depression and unmet psychological needs are common causes of psychological distress among patients with cancer, further research is needed to investigate the efficacy of smartphone-based psychological therapies on other types of distress.
In addition, as smartphone psychotherapy is scalable and has high potential for social implementation, it may be promising to expand the target population to include people living in remote areas and those who have difficulty with face-to-face consultations. Further research should also be conducted in collaboration with local governments and health insurance providers and in health technology and other fields peripheral to medicine.
Finally, we would like to emphasize the potential benefits of our novel strategy of conducting a clinical trial or a decentralized clinical trial although this was not the main focus of the present study. Decentralized clinical trials are characterized by less dependence on traditional research facilities or specialist intermediaries for data collection.33 We experienced less burden on physicians and patients when participating in the clinical trial, and once enrolled, the follow-up rates were excellent (eg, more than 99% completion of the primary outcome, see Fig 1), which suggested that a decentralized clinical trial may be a viable method for further clinical trials.
The present study had some methodological limitations. First, not all patients who were interested in and willing to use the Kaiketsu-App and the Genki-App possessed a smartphone. The present study focused on younger breast cancer survivors who were iPhone users, rather than those with elevated FCR. This may reduce the applicability and generalizability of the results from this trial to all breast cancer survivors with FCR. In particular, the results may not be applicable to patients in developing countries and to those with poor information and communication technology literacy. However, we believe that we targeted a clinically important group of patients, as our eligibility criteria were set to select potential participants who had a strong fear of recurrence even 1 year after surgery and survivors who were assumed to have a need for this service. It must also be noted that smartphone use has rapidly expanded in modern societies and broader circles of people are using these devices on a daily basis. However, further studies are needed to determine the effect of the smartphone apps on older patients and the combined effect on patients receiving psychological or psychiatric treatments. Second, we used a waitlist control group as the comparator because of feasibility and ethical considerations. Different control conditions are used to address different factors, and a meta-analysis found that the odds of response were statistically significantly greater for no treatment compared with a waitlist group.46,47 Third, we used two types of psychotherapies (BA and PST) as interventions. Therefore, we cannot determine which intervention was most efficacious or beneficial in managing FCR. Finally, as the CARS-J comprised four factors in addition to overall worries, we did not evaluate these factors because the factor structure of the CARS-J differed from the original version, meaning that we cannot determine the effect of the intervention on specific domains of FCR.
In conclusion, this study demonstrates the efficacy of PST and BA smartphone apps in reducing FCR at week 8 and the efficacy appears to be maintained at week 24. Given the large number of cancer survivors and the limited number of therapists to competently conduct appropriate psychotherapy, novel smartphone psychotherapy may be a promising way to reduce FCR.
ACKNOWLEDGMENT
We thank Ms Y. Yanase, Ms A. Nomura, Ms K. Tojima, Ms I. Sakakima, and Ms K. Kobori for their support of this study. We also thank Audrey Holmes, MA, from Edanz, for editing a draft of this manuscript.
APPENDIX
TABLE A1.
Primary Outcomes in Subgroup Analysis at Week 8 Among the Participants With High and Low FCR
Tatsuo Akechi
Honoraria: lectures fees from Chugai, Daiichi-Sankyo, Dainippon-Sumitomo, Eizai, Janssen, Kyowa, Lilly, MSD, Meiji-Seika Pharma, Mochida, Otsuka, Takeda, and Viatris, and royalties from Igaku-Shoin
Patents, Royalties, Other Intellectual Property: The inventor of the pending patents (2020-135195) (Inst)
Takuhiro Yamaguchi
Stock and Other Ownership Interests: STATCOM
Consulting or Advisory Role: Ono Pharmaceutical, Kowa, Japan Tobacco Inc, Chugai Pharma, DAIICHI SANKYO COMPANY, LIMITED, Eisai, EP Croit Co., Ltd, 3H Clinical Trial, SONIRE Therapeutics Inc, SEIKAGAKU CORPORATION, Otsuka, Incyte
Speakers' Bureau: Daiichi Sankyo, Takeda, Mebix, AstraZeneca
Research Funding: AC Medical (Inst), A2 Healthcare (Inst), Facet Biotech (Inst), Japan Tobacco Inc (Inst), Japan Media Corporation (Inst), Medidata Solutions, Inc (Inst), Ono Pharmaceutical (Inst), intellim (Inst), Welby (Inst), 3H Medi Solution (Inst), NIPRO CORPORATION (Inst), Hemp Kitchen (Inst), Nobori Ltd (Inst), Puravida Technologies (Inst), Medrio (Inst), 3H Clinical Trial (Inst), Senju Pharmaceutical (Inst), Otsuka (Inst), Eisai (Inst), ClinChoice (Inst), Kyowa Kirin Co., Ltd
Tempei Miyaji
Honoraria: Pfizer, Takeda, Merck, AYUMI, Welby
Research Funding: AC Medical (Inst), A2 Healthcare (Inst), CAC Croit Corporation (Inst), Japan Tobacco Inc (Inst), Japan Media Corporation (Inst), Luminary Medical (Inst), Medidata Solutions, Inc (Inst), Ono Pharmaceutical (Inst), FMD K&L Japan (Inst), intellim (Inst), Welby (Inst), 3H Medi Solution (Inst), Nipro Corporation (Inst), New Age Trading (Inst), NOBORI Ltd (Inst), Puravida Technologies (Inst), Medrio (Inst)
Toshi A. Furukawa
Honoraria: Mitsubishi Tanabe Pharma, SONY, Kyoto University Original, Shionogi
Research Funding: Mitsubishi Tanabe Pharma (Inst), Shionogi (Inst)
Patents, Royalties, Other Intellectual Property: Patent 2018-177688 pending (Inst), Patent 2022-082495 pending (Inst), Intellectual properties for Kokoro-app (Inst)
Akiyo Yoshimura
Honoraria: Chugai Pharma, AstraZeneca, Lilly, Pfizer, ACTmed
Shinji Ohno
Speakers' Bureau: Chugai/Roche, Lilly Japan, AstraZeneca, AstraZeneca, Eisai, Kyowa Kirin International, Lilly Japan, Nihonkayaku, Pfizer, Daiichi Sankyo/UCB Japan, Taiho Pharmaceutical, MSD
Research Funding: Taiho Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Eisai (Inst), Eisai (Inst)
Hiroji Iwata
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo, Lilly Japan, Kyowa Hakko Kirin, Taiho Pharmaceutical, MSD
Consulting or Advisory Role: Chugai Pharma, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly Japan, Kyowa Hakko Kirin, Novartis, MSD, Sanofi
Research Funding: MSD (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nihonkayaku (Inst), Lilly Japan (Inst), Novartis (Inst), Bayer (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Sanofi (Inst), Amgen (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The funders/sponsors had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. T.Y., T. Mashiko, and T. Miyaji had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Data are available from T.A. and U.M. upon reasonable request.
SUPPORT
Supported by a Grant-in-Aid for Japan Agency for Medical Research and Development (No. JP17ck0106324h). This study was also partly supported by a Grant-in-Aid for Scientific Research (No. 25285194) and Young scientists (No. 17k13942) from the Japanese Ministry of Education, Culture, Science, and Technology; a grant from Nagoya City University Foundation for Promotion of Cancer Research in Japan; and The National Cancer Center Research and Development Fund (No. 27-A-3 and 30-A-11).
CLINICAL TRIAL INFORMATION
UMIN-CTR: UMIN000031140
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.00699.
AUTHOR CONTRIBUTIONS
Conception and design: Tatsuo Akechi, Takuhiro Yamaguchi, Fuminobu Imai, Kanae Momino, Fujika Katsuki, Naomi Sakurai, Tempei Miyaji, Toshi A. Furukawa, Yosuke Uchitomi, Hiroji Iwata
Financial support: Tatsuo Akechi
Administrative support: Tatsuo Akechi, Megumi Uchida, Fujika Katsuki, Shinji Ohno, Kenji Higaki, Yosuke Uchitomi
Provision of study materials or patients: Tatsuo Akechi, Naomi Sakurai, Masaru Horikoshi, Toshi A. Furukawa, Akiyo Yoshimura, Shinji Ohno, Natsue Uehiro, Kenji Higaki, Yoshie Hasegawa, Kazuhisa Akahane, Hiroji Iwata
Collection and assembly of data: Tatsuo Akechi, Naomi Sakurai, Tempei Miyaji, Tomoe Mashiko, Akiyo Yoshimura, Shinji Ohno, Natsue Uehiro, Kenji Higaki, Yoshie Hasegawa, Kazuhisa Akahane, Hiroji Iwata
Data analysis and interpretation: Tatsuo Akechi, Takuhiro Yamaguchi, Megumi Uchida, Fuminobu Imai, Tempei Miyaji, Tomoe Mashiko, Masaru Horikoshi, Toshi A. Furukawa, Yosuke Uchitomi, Hiroji Iwata
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Smartphone Psychotherapy Reduces Fear of Cancer Recurrence Among Breast Cancer Survivors: a Fully Decentralized Randomized Controlled Clinical Trial (J-SUPPORT 1703 Study)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tatsuo Akechi
Honoraria: lectures fees from Chugai, Daiichi-Sankyo, Dainippon-Sumitomo, Eizai, Janssen, Kyowa, Lilly, MSD, Meiji-Seika Pharma, Mochida, Otsuka, Takeda, and Viatris, and royalties from Igaku-Shoin
Patents, Royalties, Other Intellectual Property: The inventor of the pending patents (2020-135195) (Inst)
Takuhiro Yamaguchi
Stock and Other Ownership Interests: STATCOM
Consulting or Advisory Role: Ono Pharmaceutical, Kowa, Japan Tobacco Inc, Chugai Pharma, DAIICHI SANKYO COMPANY, LIMITED, Eisai, EP Croit Co., Ltd, 3H Clinical Trial, SONIRE Therapeutics Inc, SEIKAGAKU CORPORATION, Otsuka, Incyte
Speakers' Bureau: Daiichi Sankyo, Takeda, Mebix, AstraZeneca
Research Funding: AC Medical (Inst), A2 Healthcare (Inst), Facet Biotech (Inst), Japan Tobacco Inc (Inst), Japan Media Corporation (Inst), Medidata Solutions, Inc (Inst), Ono Pharmaceutical (Inst), intellim (Inst), Welby (Inst), 3H Medi Solution (Inst), NIPRO CORPORATION (Inst), Hemp Kitchen (Inst), Nobori Ltd (Inst), Puravida Technologies (Inst), Medrio (Inst), 3H Clinical Trial (Inst), Senju Pharmaceutical (Inst), Otsuka (Inst), Eisai (Inst), ClinChoice (Inst), Kyowa Kirin Co., Ltd
Tempei Miyaji
Honoraria: Pfizer, Takeda, Merck, AYUMI, Welby
Research Funding: AC Medical (Inst), A2 Healthcare (Inst), CAC Croit Corporation (Inst), Japan Tobacco Inc (Inst), Japan Media Corporation (Inst), Luminary Medical (Inst), Medidata Solutions, Inc (Inst), Ono Pharmaceutical (Inst), FMD K&L Japan (Inst), intellim (Inst), Welby (Inst), 3H Medi Solution (Inst), Nipro Corporation (Inst), New Age Trading (Inst), NOBORI Ltd (Inst), Puravida Technologies (Inst), Medrio (Inst)
Toshi A. Furukawa
Honoraria: Mitsubishi Tanabe Pharma, SONY, Kyoto University Original, Shionogi
Research Funding: Mitsubishi Tanabe Pharma (Inst), Shionogi (Inst)
Patents, Royalties, Other Intellectual Property: Patent 2018-177688 pending (Inst), Patent 2022-082495 pending (Inst), Intellectual properties for Kokoro-app (Inst)
Akiyo Yoshimura
Honoraria: Chugai Pharma, AstraZeneca, Lilly, Pfizer, ACTmed
Shinji Ohno
Speakers' Bureau: Chugai/Roche, Lilly Japan, AstraZeneca, AstraZeneca, Eisai, Kyowa Kirin International, Lilly Japan, Nihonkayaku, Pfizer, Daiichi Sankyo/UCB Japan, Taiho Pharmaceutical, MSD
Research Funding: Taiho Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Eisai (Inst), Eisai (Inst)
Hiroji Iwata
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo, Lilly Japan, Kyowa Hakko Kirin, Taiho Pharmaceutical, MSD
Consulting or Advisory Role: Chugai Pharma, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly Japan, Kyowa Hakko Kirin, Novartis, MSD, Sanofi
Research Funding: MSD (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Chugai Pharma (Inst), Nihonkayaku (Inst), Lilly Japan (Inst), Novartis (Inst), Bayer (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Sanofi (Inst), Amgen (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Ito Y, Miyashiro I, Ito H, et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 2014;105:1480–1486. doi: 10.1111/cas.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodgkinson K, Butow P, Hunt GE, et al. Breast cancer survivors' supportive care needs 2-10 years after diagnosis. Support Care Cancer. 2007;15:515–523. doi: 10.1007/s00520-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 3. Hartl K, Schennach R, Muller M, et al. Quality of life, anxiety, and oncological factors: A follow-up study of breast cancer patients. Psychosomatics. 2010;51:112–123. doi: 10.1176/appi.psy.51.2.112. [DOI] [PubMed] [Google Scholar]
- 4. Gordon NH, Siminoff LA. Measuring quality of life of long-term breast cancer survivors: The Long Term Quality of Life-Breast Cancer (LTQOL-BC) scale. J Psychosoc Oncol. 2010;28:589–609. doi: 10.1080/07347332.2010.516806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akechi T, Okuyama T, Endo C, et al. Patient's perceived need and psychological distress and/or quality of life in ambulatory breast cancer patients in Japan. Psychooncology. 2011;20:497–505. doi: 10.1002/pon.1757. [DOI] [PubMed] [Google Scholar]
- 6. Luigjes-Huizer YL, Tauber NM, Humphris G, et al. What is the prevalence of fear of cancer recurrence in cancer survivors and patients? A systematic review and individual participant data meta-analysis. Psychooncology. 2022;31:879–892. doi: 10.1002/pon.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrison JD, Young JM, Price MA, et al. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117–1128. doi: 10.1007/s00520-009-0615-5. [DOI] [PubMed] [Google Scholar]
- 8. Koch L, Jansen L, Brenner H, et al. Fear of recurrence and disease progression in long-term (>/= 5 years) cancer survivors—A systematic review of quantitative studies. Psychooncology. 2013;22:1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- 9. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 10. Lengacher CA, Reich RR, Paterson CL, et al. Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: A randomized controlled trial. J Clin Oncol. 2016;34:2827–2834. doi: 10.1200/JCO.2015.65.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18:1261–1272. doi: 10.1002/pon.1529. [DOI] [PubMed] [Google Scholar]
- 12. Butow PN, Turner J, Gilchrist J, et al. Randomized trial of ConquerFear: A novel, theoretically based psychosocial intervention for fear of cancer recurrence. J Clin Oncol. 2017;35:4066–4077. doi: 10.1200/JCO.2017.73.1257. [DOI] [PubMed] [Google Scholar]
- 13. van de Wal M, Thewes B, Gielissen M, et al. Efficacy of blended cognitive behavior therapy for high fear of recurrence in breast, prostate, and colorectal cancer survivors: The SWORD study, a randomized controlled trial. J Clin Oncol. 2017;35:2173–2183. doi: 10.1200/JCO.2016.70.5301. [DOI] [PubMed] [Google Scholar]
- 14. Tauber NM, O'Toole MS, Dinkel A, et al. Effect of psychological intervention on fear of cancer recurrence: A systematic review and meta-analysis. J Clin Oncol. 2019;37:2899–2915. doi: 10.1200/JCO.19.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukui S, Kugaya A, Okamura H, et al. A psychosocial group intervention for Japanese women with primary breast carcinoma. Cancer. 2000;89:1026–1036. doi: 10.1002/1097-0142(20000901)89:5<1026::aid-cncr12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16. Fukui S, Kugaya A, Kamiya M, et al. Participation in psychosocial group intervention among Japanese women with primary breast cancer and its associated factors. Psychooncology. 2001;10:419–427. doi: 10.1002/pon.534. [DOI] [PubMed] [Google Scholar]
- 17. Akechi T, Momino K, Yamashita T, et al. Contribution of problem-solving skills to fear of recurrence in breast cancer survivors. Breast Cancer Res Treat. 2014;145:205–210. doi: 10.1007/s10549-014-2929-3. [DOI] [PubMed] [Google Scholar]
- 18. Akechi T, Hirai K, Motooka H, et al. Problem-solving therapy for psychological distress in Japanese cancer patients: Preliminary clinical experience from psychiatric consultations. Jpn J Clin Oncol. 2008;38:867–870. doi: 10.1093/jjco/hyn115. [DOI] [PubMed] [Google Scholar]
- 19. Momino K, Miyashita M, Yamashita H, et al. Feasibility study of collaborative care intervention for the perceived care needs among breast cancer women undergoing adjuvant therapy after surgery. Jpn J Clin Oncol. 2017;47:213–220. doi: 10.1093/jjco/hyw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirai K, Motooka H, Ito N, et al. Problem-solving therapy for psychological distress in Japanese early-stage breast cancer patients. Jpn J Clin Oncol. 2012;42:1168–1174. doi: 10.1093/jjco/hys158. [DOI] [PubMed] [Google Scholar]
- 21. Patel V, Weobong B, Weiss HA, et al. The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: A randomised controlled trial. Lancet. 2017;389:176–185. doi: 10.1016/S0140-6736(16)31589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nezu AM, Nezu CM, Felgoise SH, et al. Project genesis: Assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol. 2003;71:1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 23. Dimidjian S, Goodman SH, Sherwood NE, et al. A pragmatic randomized clinical trial of behavioral activation for depressed pregnant women. J Consult Clin Psychol. 2017;85:26–36. doi: 10.1037/ccp0000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hopko DR, Armento ME, Robertson SM, et al. Brief behavioral activation and problem-solving therapy for depressed breast cancer patients: Randomized trial. J Consult Clin Psychol. 2011;79:834–849. doi: 10.1037/a0025450. [DOI] [PubMed] [Google Scholar]
- 25. Cuijpers P, Noma H, Karyotaki E, et al. Effectiveness and acceptability of cognitive behavior therapy delivery formats in adults with depression: A network meta-analysis. JAMA Psychiatry. 2019;76:700–707. doi: 10.1001/jamapsychiatry.2019.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wagner B, Horn AB, Maercker A. Internet-based versus face-to-face cognitive-behavioral intervention for depression: A randomized controlled non-inferiority trial. J Affect Disord. 2014;152-154:113–121. doi: 10.1016/j.jad.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 27. Karyotaki E, Ebert DD, Donkin L, et al. Do guided internet-based interventions result in clinically relevant changes for patients with depression? An individual participant data meta-analysis. Clin Psychol Rev. 2018;63:80–92. doi: 10.1016/j.cpr.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 28. Watts S, Mackenzie A, Thomas C, et al. CBT for depression: A pilot RCT comparing mobile phone vs. computer. BMC Psychiatry. 2013;13:49. doi: 10.1186/1471-244X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Furukawa TA, Suganuma A, Ostinelli EG, et al. Dismantling, optimising, and personalising internet cognitive behavioural therapy for depression: A systematic review and component network meta-analysis using individual participant data. Lancet Psychiatry. 2021;8:500–511. doi: 10.1016/S2215-0366(21)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mantani A, Kato T, Furukawa TA, et al. Smartphone cognitive behavioral therapy as an adjunct to pharmacotherapy for refractory depression: Randomized controlled trial. J Med Internet Res. 2017;19:e373. doi: 10.2196/jmir.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imai F, Momino K, Katsuki F, et al. Smartphone problem-solving therapy to reduce fear of cancer recurrence among breast cancer survivors: An open single-arm pilot study. Jpn J Clin Oncol. 2019;49:537–544. doi: 10.1093/jjco/hyz005. [DOI] [PubMed] [Google Scholar]
- 32. Akechi T, Yamaguchi T, Uchida M, et al. Smartphone problem-solving and behavioural activation therapy to reduce fear of recurrence among patients with breast cancer (SMartphone Intervention to LEssen fear of cancer recurrence: SMILE project): Protocol for a randomised controlled trial. BMJ Open. 2018;8:e024794. doi: 10.1136/bmjopen-2018-024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Norman GA. Decentralized clinical trials: The future of medical product development? JACC Basic Transl Sci. 2021;6:384–387. doi: 10.1016/j.jacbts.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kadakia KT, Asaad M, Adlakha E, et al. Virtual clinical trials in oncology-overview, challenges, policy considerations, and future directions. JCO Clin Cancer Inform. 2021;5:421–425. doi: 10.1200/CCI.20.00169. [DOI] [PubMed] [Google Scholar]
- 35. Momino K, Akechi T, Yamashita T, et al. Psychometric properties of the Japanese version of the Concerns About Recurrence Scale (CARS-J) Jpn J Clin Oncol. 2014;44:456–462. doi: 10.1093/jjco/hyu032. [DOI] [PubMed] [Google Scholar]
- 36. Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: A systematic review. Psychooncology. 2013;22:978–986. doi: 10.1002/pon.3114. [DOI] [PubMed] [Google Scholar]
- 37.Mynors-Wallis L. Problem-solving Treatment for Anxiety and Depression: A Practical Guide. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 38. Watanabe N, Horikoshi M, Yamada M, et al. Adding smartphone-based cognitive-behavior therapy to pharmacotherapy for major depression (FLATT project): Study protocol for a randomized controlled trial. Trials. 2015;16:293. doi: 10.1186/s13063-015-0805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SMILE Project. https://smile-project.org/ [Google Scholar]
- 40. Vickberg SM. The Concerns About Recurrence Scale (CARS): A systematic measure of women's fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25:16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- 41. Akechi T, Momino K, Iwata H. Brief screening of patients with distressing fear of recurrence in breast cancer survivors. Breast Cancer Res Treat. 2015;153:475–476. doi: 10.1007/s10549-015-3537-6. [DOI] [PubMed] [Google Scholar]
- 42. Kugaya A, Akechi T, Okuyama T, et al. Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol. 1998;28:333–338. doi: 10.1093/jjco/28.5.333. [DOI] [PubMed] [Google Scholar]
- 43. Okuyama T, Akechi T, Yamashita H, et al. Reliability and validity of the Japanese version of the Short-Form Supportive Care Needs Survey Questionnaire (SCNS-SF34-J) Psychooncology. 2009;18:1003–1010. doi: 10.1002/pon.1482. [DOI] [PubMed] [Google Scholar]
- 44. Taku K, Calhoun LG, Tedeschi RG, et al. Examining posttraumatic growth among Japanese university students. Anxiety Stress Coping. 2007;20:353–367. doi: 10.1080/10615800701295007. [DOI] [PubMed] [Google Scholar]
- 45. Wagner LI, Tooze JA, Hall DL, et al. Targeted eHealth intervention to reduce breast cancer survivors' fear of recurrence: Results from the FoRtitude randomized trial. J Natl Cancer Inst. 2021;113:1495–1505. doi: 10.1093/jnci/djab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Furukawa TA, Noma H, Caldwell DM, et al. Waiting list may be a nocebo condition in psychotherapy trials: A contribution from network meta-analysis. Acta Psychiatr Scand. 2014;130:181–192. doi: 10.1111/acps.12275. [DOI] [PubMed] [Google Scholar]
- 47. Michopoulos I, Furukawa TA, Noma H, et al. Different control conditions can produce different effect estimates in psychotherapy trials for depression. J Clin Epidemiol. 2021;132:59–70. doi: 10.1016/j.jclinepi.2020.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.22.00699.