Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
The open-label phase Ib/II Study 111/KEYNOTE-146 of daily lenvatinib 20 mg plus pembrolizumab 200 mg once every 3 weeks showed promising efficacy and tolerable safety in patients with previously treated advanced endometrial carcinoma (EC; primary data cutoff date: January 10, 2019). This updated analysis reports long-term follow-up efficacy and safety data from 108 patients with previously treated EC included in the primary analysis. End points included objective response rate, duration of response, progression-free survival, overall survival, and safety. Investigators performed tumor assessments per immune-related RECIST. At the updated data cutoff date (August 18, 2020), the median study follow-up duration was 34.7 months (95% CI, 30.9 to 41.2), the objective response rate was 39.8% (95% CI, 30.5 to 49.7), and the median duration of response was 22.9 months (95% CI, 10.2 to not estimable). The median progression-free survival and overall survival were 7.4 months (95% CI, 5.2 to 8.7) and 17.7 months (95% CI, 15.5 to 25.8), respectively. Treatment-related treatment-emergent adverse events of any grade occurred in 104 (96.3%) patients. The most common grade ≥ 3 treatment-related treatment-emergent adverse events were hypertension (33.3%), elevated lipase (9.3%), fatigue (8.3%), and diarrhea (7.4%). The results demonstrate extended efficacy and tolerability of lenvatinib plus pembrolizumab in this cohort of patients with previously treated advanced EC.
INTRODUCTION
Endometrial carcinoma (EC), the most common gynecologic cancer in the United States,1 has a 5-year relative survival rate of 20% in patients with distant metastases.2 Lenvatinib (an oral multikinase inhibitor of vascular endothelial growth factor receptors 1-3, fibroblast growth factor receptors 1-4, platelet-derived growth factor receptor α, RET, and KIT3-6) is approved in the United States and Canada in combination with pembrolizumab (an anti–programmed death receptor-1 monoclonal antibody7) for the treatment of patients with advanced EC that is not microsatellite instability-high (MSI-H) or mismatch-repair deficient (dMMR) who have disease progression after prior systemic (the United States) or platinum-based systemic (Canada) therapy and are not candidates for curative surgery or radiation.8-11 The combination is also approved in Europe for treating patients with advanced EC who have disease progression after platinum-containing therapy and are not candidates for curative surgery or radiation.12,13
The phase III Study 309/KEYNOTE-775 demonstrated significantly longer progression-free survival (PFS) and overall survival (OS) with lenvatinib plus pembrolizumab versus the physician's choice of chemotherapy in patients (intention-to-treat population and patients with mismatch-repair proficient [pMMR] tumors) with previously treated advanced endometrial cancer.14 The earlier open-label, single-arm, phase Ib/II Study 111/KEYNOTE-146 (ClinicalTrials.gov identifier: NCT02501096), which evaluated lenvatinib plus pembrolizumab in patients with previously treated advanced EC (primary analysis data cutoff date: January 10, 2019) showed promising efficacy and a tolerable safety profile, irrespective of mismatch-repair (MMR) status.15 The objective response rate (ORR) by investigators per immune-related RECIST (irRECIST)16 was 38.9% (95% CI, 29.7 to 48.7); the median duration of response (DOR) was 21.2 months (95% CI, 7.6 to not estimable [NE]). PFS by investigator assessment per irRECIST and OS were 7.4 months (95% CI, 5.3 to 8.7) and 16.7 months (95% CI, 15.0 to NE), respectively. Grade 3-4 treatment-related treatment-emergent adverse events (TEAEs) occurred in 69.4% of patients. To our knowledge, in this first extended follow-up analysis of lenvatinib plus pembrolizumab in patients with previously treated advanced EC, we report long-term efficacy and safety results from Study 111/KEYNOTE-146.
METHODS
Study 111/KEYNOTE-146 procedures have been published.15,17 Eligible patients were age 18 years and older with histologically confirmed advanced EC, an Eastern Cooperative Oncology Group performance status ≤ 1, and life expectancy ≥ 12 weeks. Patients received ≤ 2 prior systemic therapies (unless discussed with the sponsor). Patients received lenvatinib 20 mg orally once daily and pembrolizumab 200 mg intravenously once every 3 weeks in 3-week cycles. End points for the primary analysis included ORR at week 24 (primary end point) and ORR, DOR, PFS, OS, disease control rate, and clinical benefit rate (secondary end points). End points for this follow-up analysis included ORR, DOR, PFS, OS, and safety. Tumors were evaluated at baseline, every 6 weeks for the first 24 weeks, and every 9 weeks thereafter; in this analysis, all assessments were by investigators per irRECIST. All previously treated patients included in the efficacy analysis and analyzed at the primary data cutoff were included in this updated analysis (data cutoff date: August 18, 2020). Efficacy end points (assessed in all patients who entered the study treatment period) were reported for all patients, patients who were non–MSI‐H/pMMR, and patients who were MSI-H/dMMR. Treatment-related TEAEs (assessed in patients who received any amount of study drug) were graded using the Common Terminology Criteria for Adverse Events version 4.03. This study was approved by each research site's institutional review board or ethics committee.
RESULTS
Patients
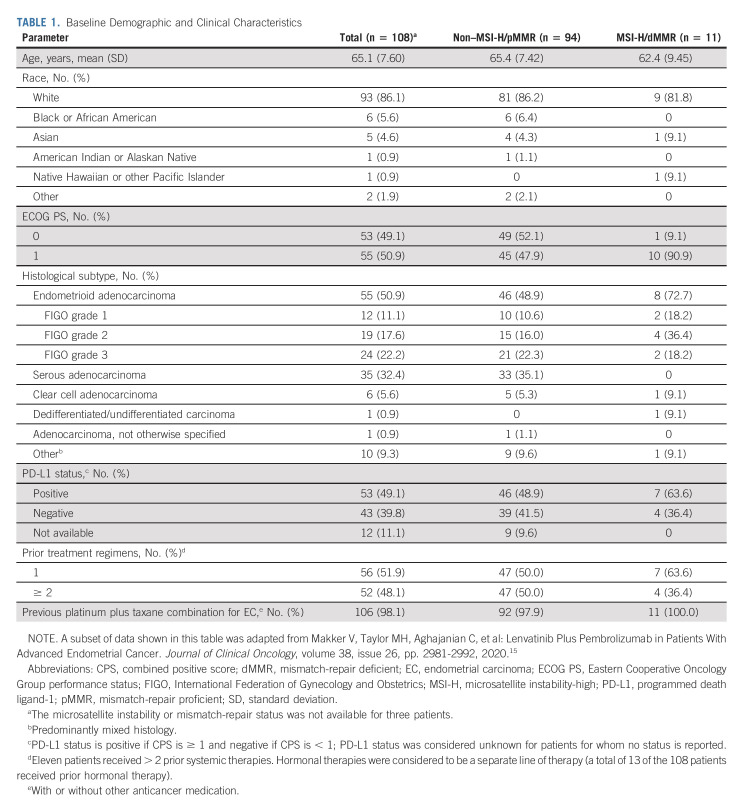
Baseline characteristics are summarized in Table 1. At the updated data cutoff date, 32 (29.6%) patients were on study treatment or in survival follow-up; the remaining 76 (70.4%) patients discontinued the study because of death (n = 71), consent withdrawal (n = 4), or other reason (n = 1). Six (5.6%) patients were on study treatment (either on both drugs or lenvatinib only). The median follow-up was 34.7 months (95% CI, 30.9 to 41.2).
TABLE 1.
Baseline Demographic and Clinical Characteristics
Efficacy
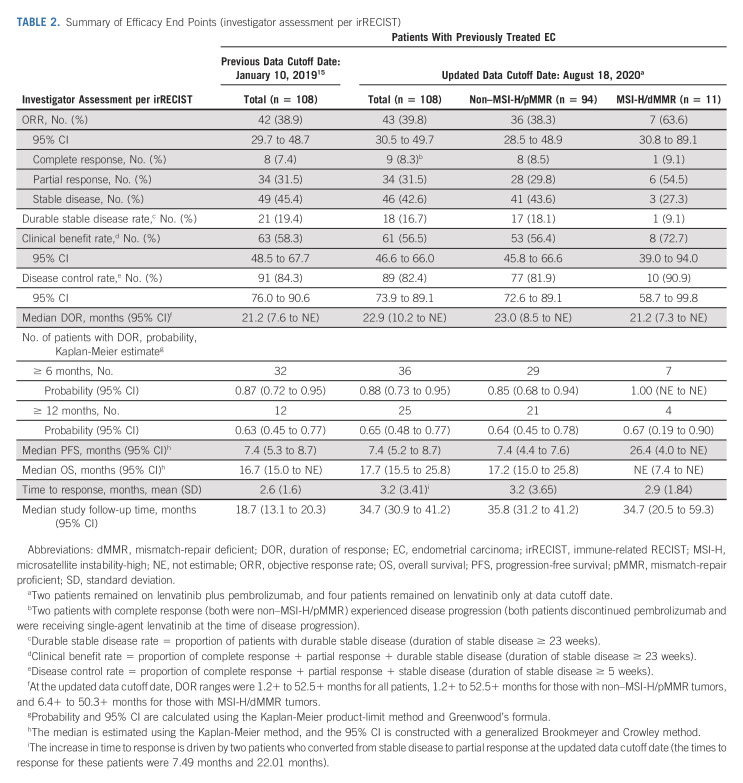
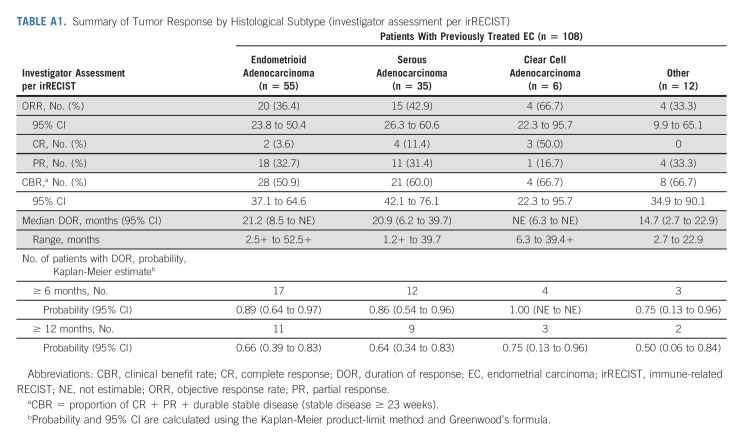
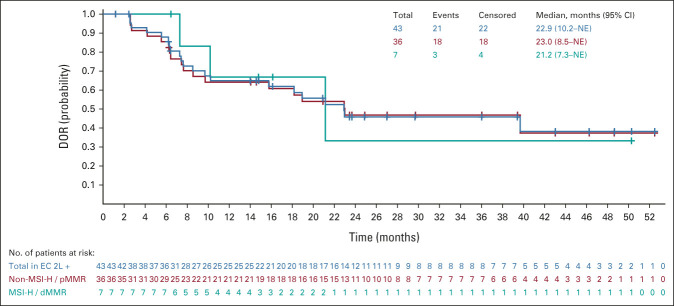
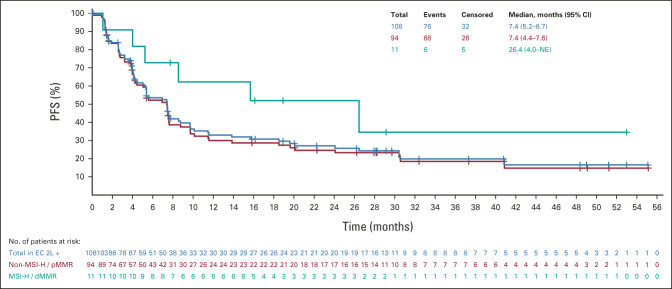
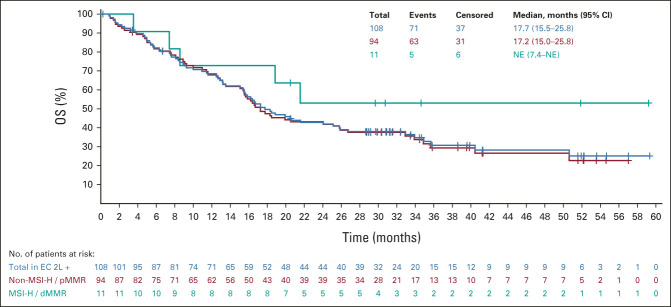
All analyses were updated using the new cutoff date (August 18, 2020). An efficacy summary is presented in Table 2. The ORR was 39.8% (95% CI, 30.5 to 49.7), with nine complete responses and 34 partial responses. The median DOR was 22.9 months (95% CI, 10.2 to NE), with the upper range of DOR ongoing at 4 years (Table 2, Appendix Fig A1, online only). Tumor responses were observed regardless of histological subtype (Appendix Table A1, online only). Overall, median PFS and OS were 7.4 months (95% CI, 5.2 to 8.7) and 17.7 months (95% CI, 15.5 to 25.8), respectively (Appendix Figs A2 and A3, online only).
TABLE 2.
Summary of Efficacy End Points (investigator assessment per irRECIST)
In patients with non–MSI-H/pMMR and MSI-H/dMMR tumors, the ORR was 38.3% and 63.6%, respectively (Table 2); the median DOR was 23.0 months (95% CI, 8.5 to NE) and 21.2 months (95% CI, 7.3 to NE), respectively. Upper ranges of DOR were ongoing at 4 years for both subgroups (Table 2, Appendix Fig A1). The median PFS in patients with non–MSI-H/pMMR and MSI-H/dMMR tumors was 7.4 months (95% CI, 4.4 to 7.6) and 26.4 months (95% CI, 4.0 to NE; Appendix Fig A2), respectively; the median OS was 17.2 months (95% CI, 15.0 to 25.8), and NE (95% CI, 7.4 to NE) in patients with non–MSI-H/pMMR and MSI-H/dMMR tumors, respectively (Appendix Fig A3).
Safety
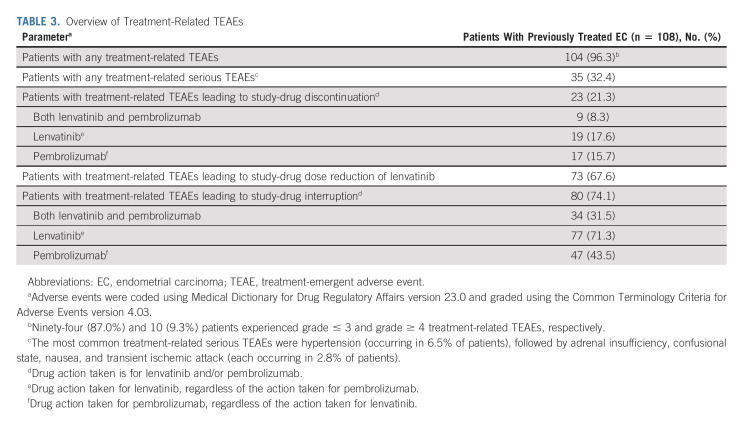
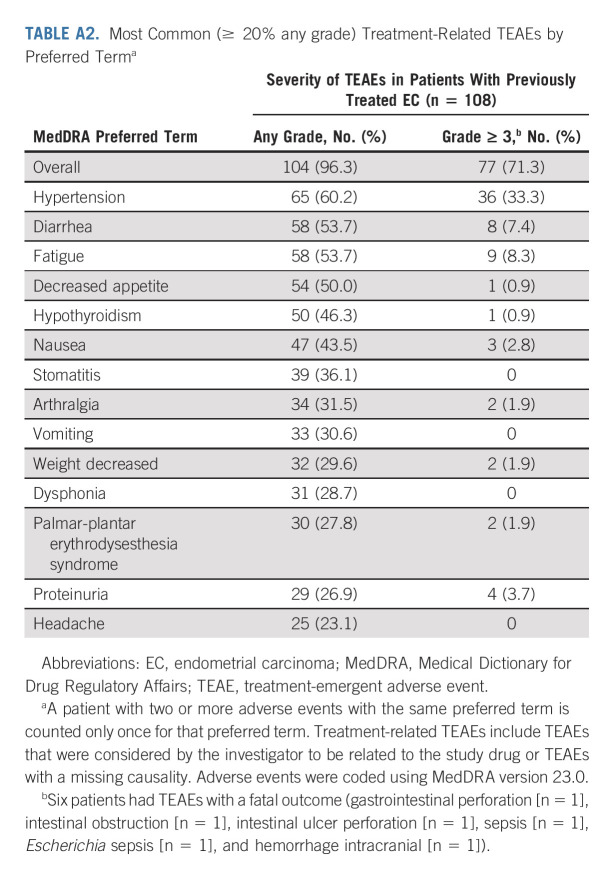
In the overall population, the median dose intensity of lenvatinib was 13.84 mg once per day; the median number of pembrolizumab treatment cycles was 10. An overview of treatment-related TEAEs is presented in Table 3. Any-grade treatment-related TEAEs occurred in 104 (96.3%) patients. Grade ≤ 3 and grade ≥ 4 treatment-related TEAEs occurred in 94 (87.0%) and 10 (9.3%) patients. Serious treatment-related TEAEs occurred in 35 (32.4%) patients (hypertension was the most common serious treatment-related TEAE [6.5% of patients]). Treatment-related TEAEs led to discontinuation of at least one study drug in 23 (21.3%) patients, discontinuation of both study drugs in nine (8.3%) patients, lenvatinib dose reductions in 73 (67.6%) patients, and interruption of one study drug or both study drugs in 80 (74.1%) patients. Any grade treatment-related TEAEs occurring in ≥ 20% of patients are shown in Appendix Table A2 (online only). The most common grade ≥ 3 treatment-related TEAEs were hypertension (33.3%), elevated lipase (9.3%), fatigue (8.3%), and diarrhea (7.4%). Aside from two treatment-related deaths reported in the primary analysis,15 no additional treatment-related deaths occurred. Treatment-related serious TEAEs are presented in Appendix Table A3 (online only).
TABLE 3.
Overview of Treatment-Related TEAEs
DISCUSSION
This follow-up analysis showed deep and durable tumor responses (with one additional complete response compared with the primary analysis15) in patients with previously treated advanced EC who received lenvatinib plus pembrolizumab. Tumor responses were observed regardless of histological subtype or MMR status. The combination continued to show compelling PFS and OS benefits in comparison with what would be expected on the basis of historical data for this treatment setting.18,19 Lenvatinib plus pembrolizumab had a manageable safety profile that was generally comparable with established profiles of the individual monotherapies.8,9,20-23
Recently, a confirmatory phase III trial demonstrated significantly longer PFS (median 7.2 v 3.8 months; hazard ratio, 0.56; 95% CI, 0.47 to 0.66; P < .001) and OS (median 18.3 v 11.4 months; hazard ratio, 0.62; 95% CI, 0.51 to 0.75; P < .001) with lenvatinib plus pembrolizumab versus the physician's choice of chemotherapy in the intention-to-treat all-comer population of patients with previously treated advanced endometrial cancer (Study 309/KEYNOTE-775).14 Treatment benefits were observed irrespective of MMR status. The extended follow-up of the phase II part of Study 111/KEYNOTE-146 provides data on the long-term efficacy and safety of this regimen. Although Study 111/KEYNOTE-146 was limited in that it was a single-arm study with a relatively small number of patients and enrollment sites, the results were confirmed in the randomized phase III global Study 309/KEYNOTE-775. The median PFS (7.4 months), median OS (17.7 months), and ORR (39.8%) in the overall population of Study 111/KEYNOTE-146 at extended follow-up were comparable with the efficacy findings of Study 309/KEYNOTE-775.14 Similar to efficacy, safety results were also consistent with those of the primary analysis15 and Study 309/KEYNOTE-775.14 These results confirm the benefit of the combination in patients with previously treated advanced EC when lenvatinib is initiated at the recommended starting dose of 20 mg orally once daily (in combination with pembrolizumab 200 mg intravenously once every 3 weeks) and the individualized patient approach of dose interruption/modification or discontinuation is implemented.
APPENDIX 1.
Definitions of Efficacy End Points
Objective response rate is defined as the proportion of patients who had a best overall response of confirmed complete response or confirmed partial response at the time of data cutoff.
Duration of response (DOR) is defined as the time from the date of the first documentation of confirmed complete response or confirmed partial response (whichever occurred first) to the date of disease progression or death (whichever occurred first). Patients who had no record of disease progression or did not die before the data cutoff date were censored at the last available tumor assessment.
Clinical benefit rate is defined as the proportion of patients who had a best overall response of confirmed complete response, confirmed partial response, or durable confirmed stable disease (duration of confirmed stable disease ≥ 23 weeks).
Disease control rate is defined as the proportion of patients who had a best overall response of confirmed complete response, confirmed partial response, or confirmed stable disease (duration of confirmed stable disease ≥ 5 weeks).
Durable stable disease rate is defined as the proportion of patients whose best overall response was confirmed stable disease and the duration of confirmed stable disease was ≥ 23 weeks.
Progression-free survival is defined as the time from the first study dose date to the date of first documentation of confirmed disease progression or death (whichever occurred first). Patients who did not experience disease progression or death were censored at the date of the last available tumor assessment.
Overall survival is measured from the start date of the treatment period until date of death from any cause. Patients who were lost to follow-up and the patients who were alive at the date of data cutoff were censored at the date the patient was last known to be alive.
Statistical Methods
DOR: DOR among responders is defined as the time from the date that a confirmed response was first documented as the evidence of complete response or partial response until the date of the first documentation of disease progression or date of death from any cause, whichever occurs first. The median was estimated using the Kaplan-Meier method, and the 95% CI was constructed using a generalized Brookmeyer and Crowley method. All tumor assessments were considered, provided the patients did not start a new anticancer therapy.
Progression-free survival: The median was estimated using the Kaplan-Meier method, and the 95% CI was constructed using a generalized Brookmeyer and Crowley method. All tumor assessments were considered, provided the patients did not start a new anticancer therapy.
Overall survival: The median was estimated using the Kaplan-Meier method, and the 95% CI was constructed using a generalized Brookmeyer and Crowley method.
TABLE A1.
Summary of Tumor Response by Histological Subtype (investigator assessment per irRECIST)
TABLE A2.
Most Common (≥ 20% any grade) Treatment-Related TEAEs by Preferred Terma
TABLE A3.
Treatment-Related Treatment-Emergent Serious Adverse Events (> 1% in all patients with EC)a
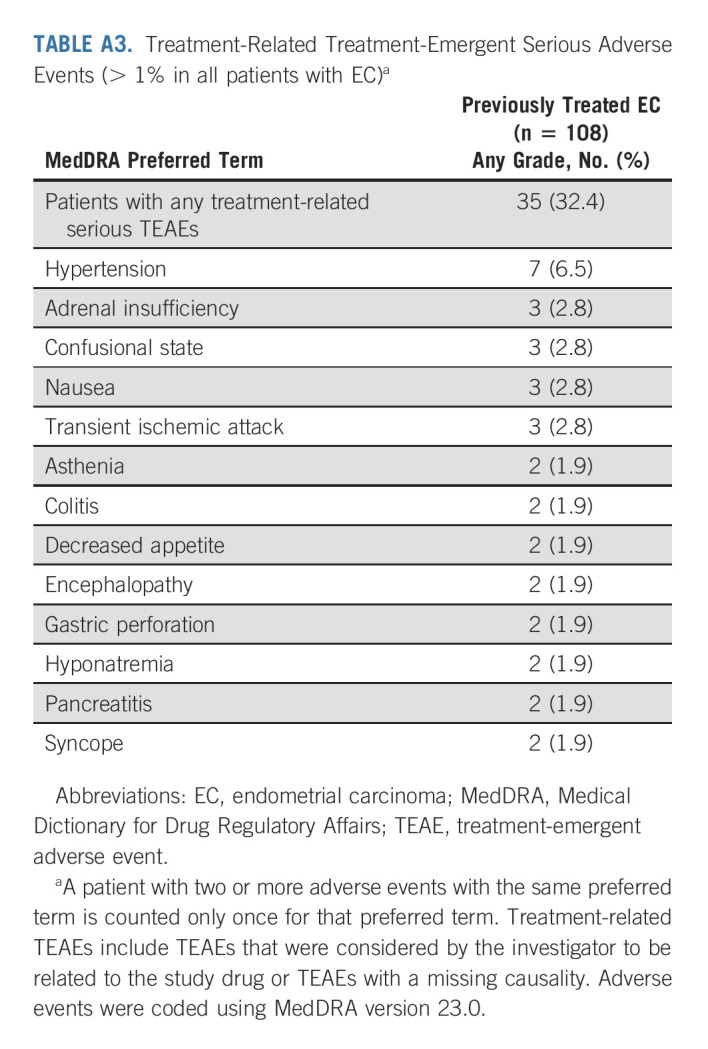
FIG A1.
Kaplan-Meier plot of DOR (DOR among responders is defined as the time from the date that a confirmed response was first documented as the evidence of complete response or partial response until the date of the first documentation of disease progression or date of death from any cause, whichever occurs first; investigator assessment per irRECIST). The median was estimated using the Kaplan-Meier method, and the 95% CI was constructed using a generalized Brookmeyer and Crowley method. All tumor assessments were considered, provided the patients did not start a new anticancer therapy. Of the 22 patients in the overall population who were censored, 16 had no progression and no death at the time of data cutoff, three had death or progression after more than one missing assessment, and three had started a new anticancer treatment before radiological progression per irRECIST. 2L, second-line; dMMR, mismatch-repair deficient; DOR, duration of response; EC, endometrial carcinoma; irRECIST, immune-related RECIST; MSI-H, microsatellite instability-high; NE, not estimable; pMMR, mismatch-repair proficient.
FIG A2.
Kaplan-Meier plot of PFS (the median was estimated using the Kaplan-Meier method, and the 95% CI was constructed using a generalized Brookmeyer and Crowley method; investigator assessment per immune-related RECIST). All tumor assessments were considered, provided the patients did not start a new anticancer therapy. 2L, second-line; dMMR, mismatch-repair deficient; EC, endometrial carcinoma; MSI-H, microsatellite instability-high; NE, not estimable; PFS, progression-free survival; pMMR, mismatch-repair proficient.
FIG A3.
Kaplan-Meier plot of OS (the median was estimated using the Kaplan-Meier method, and the 95% CI was constructed using a generalized Brookmeyer and Crowley method). 2L, second-line; dMMR, mismatch-repair deficient; EC, endometrial carcinoma; MSI-H, microsatellite instability-high; NE, not estimable; OS, overall survival; pMMR, mismatch-repair proficient.
Vicky Makker
Consulting or Advisory Role: Eisai, Merck, Karyopharm Therapeutics, Takeda, ArQule, IBM, GlaxoSmithKline, Clovis Oncology, Faeth Therapeutics, Novartis, Duality, ITeos Therapeutics, Kartos Therapeutics, Lilly
Research Funding: Lilly (Inst), AstraZeneca (Inst), Eisai (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Karyopharm Therapeutics (Inst), Takeda (Inst), Clovis Oncology (Inst), Bayer (Inst), Zymeworks (Inst), Duality (Inst), Faeth Therapeutics (Inst)
Travel, Accommodations, Expenses: Eisai, Merck, Karyopharm Therapeutics
Other Relationship: IBM
Carol Aghajanian
Consulting or Advisory Role: Eisai, AstraZeneca/Merck, Repare Therapeutics
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
Allen L. Cohn
Honoraria: Amgen
Expert Testimony: Department of Justice
Margarita Romeo
Consulting or Advisory Role: Tesaro/GSK, AstraZeneca, Roche
Research Funding: Eisai (Inst), Aprea AB (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer, MSD
Marcia S. Brose
Honoraria: Bayer, Eisai, Lilly
Consulting or Advisory Role: Bayer, Eisai, Blueprint Medicines, Loxo, Exelixis, Lilly, AADi
Research Funding: Bayer (Inst), Eisai (Inst), Exelixis (Inst), Blueprint Medicines (Inst), Loxo (Inst), Lilly (Inst)
Lea Dutta
Employment: Eisai
Corina E. Dutcus
Employment: Eisai
Jie Huang
Employment: Eisai
Stock and Other Ownership Interests: Eisai
Emmett V. Schmidt
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Robert Orlowski
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme, OncoSec, Nektar, Bluebird Bio, 2seventy bio, Bristol Myers Squibb
Research Funding: Merck Sharp & Dohme
Matthew H. Taylor
Honoraria: Bristol Myers Squibb Foundation, Eisai, Bayer, Merck, Pfizer, Regeneron, Roche, Blueprint Medicines
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Loxo, Bayer, Blueprint Medicines, Novartis, Sanofi, Cascade Prodrug, Merck, Pfizer, Exelixis, Immune-Onc Therapeutics, Regeneron
Speakers' Bureau: Bristol Myers Squibb, Eisai, Merck, Blueprint Medicines
Research Funding: Bristol Myers Squibb (Inst), Eisai (Inst), Pfizer (Inst), Merck (Inst), Moderna Therapeutics (Inst), Loxo/Bayer (Inst), Blueprint Medicines (Inst), Seattle Genetics (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at The Society for Immunotherapy of Cancer annual meeting 2021, Washington, DC and virtual, November 10-14, 2021.
SUPPORT
Supported by Eisai Inc, Nutley, NJ, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ. Medical writing support was provided by Irene Minkina, PhD, of Oxford PharmaGenesis Inc, Newtown, PA, with funding by Eisai Inc, Nutley, NJ, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The data will not be available for sharing at this time because the data are commercially confidential. However, Eisai will consider written requests to share the data on a case-by-case basis.
AUTHOR CONTRIBUTIONS
Conception and design: Vicky Makker, Carol Aghajanian, Corina E. Dutcus, Emmett V. Schmidt
Provision of study materials or patients: Carol Aghajanian, Allen L. Cohn, Margarita Romeo, Mark Messing
Collection and assembly of data: Vicky Makker, Allen L. Cohn, Margarita Romeo, Raquel Bratos, Marcia S. Brose, Mark Messing, Lea Dutta, Corina E. Dutcus, Matthew H. Taylor
Data analysis and interpretation: Vicky Makker, Carol Aghajanian, Allen L. Cohn, Marcia S. Brose, Mark Messing, Lea Dutta, Corina E. Dutcus, Jie Huang, Robert Orlowski, Matthew H. Taylor
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
A Phase Ib/II Study of Lenvatinib and Pembrolizumab in Advanced Endometrial Carcinoma (Study 111/KEYNOTE-146): Long-Term Efficacy and Safety Update
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Vicky Makker
Consulting or Advisory Role: Eisai, Merck, Karyopharm Therapeutics, Takeda, ArQule, IBM, GlaxoSmithKline, Clovis Oncology, Faeth Therapeutics, Novartis, Duality, ITeos Therapeutics, Kartos Therapeutics, Lilly
Research Funding: Lilly (Inst), AstraZeneca (Inst), Eisai (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Karyopharm Therapeutics (Inst), Takeda (Inst), Clovis Oncology (Inst), Bayer (Inst), Zymeworks (Inst), Duality (Inst), Faeth Therapeutics (Inst)
Travel, Accommodations, Expenses: Eisai, Merck, Karyopharm Therapeutics
Other Relationship: IBM
Carol Aghajanian
Consulting or Advisory Role: Eisai, AstraZeneca/Merck, Repare Therapeutics
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
Allen L. Cohn
Honoraria: Amgen
Expert Testimony: Department of Justice
Margarita Romeo
Consulting or Advisory Role: Tesaro/GSK, AstraZeneca, Roche
Research Funding: Eisai (Inst), Aprea AB (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer, MSD
Marcia S. Brose
Honoraria: Bayer, Eisai, Lilly
Consulting or Advisory Role: Bayer, Eisai, Blueprint Medicines, Loxo, Exelixis, Lilly, AADi
Research Funding: Bayer (Inst), Eisai (Inst), Exelixis (Inst), Blueprint Medicines (Inst), Loxo (Inst), Lilly (Inst)
Lea Dutta
Employment: Eisai
Corina E. Dutcus
Employment: Eisai
Jie Huang
Employment: Eisai
Stock and Other Ownership Interests: Eisai
Emmett V. Schmidt
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Robert Orlowski
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme, OncoSec, Nektar, Bluebird Bio, 2seventy bio, Bristol Myers Squibb
Research Funding: Merck Sharp & Dohme
Matthew H. Taylor
Honoraria: Bristol Myers Squibb Foundation, Eisai, Bayer, Merck, Pfizer, Regeneron, Roche, Blueprint Medicines
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Loxo, Bayer, Blueprint Medicines, Novartis, Sanofi, Cascade Prodrug, Merck, Pfizer, Exelixis, Immune-Onc Therapeutics, Regeneron
Speakers' Bureau: Bristol Myers Squibb, Eisai, Merck, Blueprint Medicines
Research Funding: Bristol Myers Squibb (Inst), Eisai (Inst), Pfizer (Inst), Merck (Inst), Moderna Therapeutics (Inst), Loxo/Bayer (Inst), Blueprint Medicines (Inst), Seattle Genetics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society : Key Statistics for Endometrial Cancer. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html [Google Scholar]
- 2.American Cancer Society : Survival Rates for Endometrial Cancer. https://www.cancer.org/cancer/endometrial-cancer/detection-diagnosis-staging/survival-rates.html [Google Scholar]
- 3.Okamoto K, Ikemori-Kawada M, Jestel A, et al. : Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett 6:89-94, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stjepanovic N, Capdevila J: Multikinase inhibitors in the treatment of thyroid cancer: Specific role of lenvatinib. Biologics 8:129-139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tohyama O, Matsui J, Kodama K, et al. : Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014:638747, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Matsui J, Matsushima T, et al. : Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 6:18, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwok G, Yau TC, Chiu JW, et al. : Pembrolizumab (Keytruda). Hum Vaccin Immunother 12:2777-2789, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenvima (lenvatinib) [prescribing information]. Nutley, NJ, Eisai Inc, 2021 [Google Scholar]
- 9.Keytruda (pembrolizumab) [prescribing information]. Whitehouse Station, NJ, Merck Sharp & Dohme Corp, 2022 [Google Scholar]
- 10.Lenvima (lenvatinib) [product monograph]. Mississauga, ON, Canada, Eisai Limited, 2021 [Google Scholar]
- 11.Keytruda (pembrolizumab) [product monograph]. Kirkland, QC, Canada, Merck Canada, 2021 [Google Scholar]
- 12.Lenvima (lenvatinib) [summary of product characteristics]. Frankfurt am Main, Germany, Eisai GmbH, 2021 [Google Scholar]
- 13.Keytruda (pembrolizumab) [summary of product characteristics]. Haarlem, the Netherlands, Merck Sharp & Dohme B.V., 2022 [Google Scholar]
- 14.Makker V, Colombo N, Casado Herráez A, et al. : Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386:437-448, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makker V, Taylor MH, Aghajanian C, et al. : Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 38:2981-2992, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seymour L, Bogaerts J, Perrone A, et al. : iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143-e152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makker V, Rasco D, Vogelzang NJ, et al. : Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 20:711-718, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghajanian C, Sill MW, Darcy KM, et al. : Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. J Clin Oncol 29:2259-2265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMeekin S, Dizon D, Barter J, et al. : Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol 138:18-23, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motzer RJ, Hutson TE, Glen H, et al. : Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16:1473-1482, 2015 [DOI] [PubMed] [Google Scholar]
- 22.O'Malley DO, Marabelle A, De Jesus-Acosta A, et al. : Pembrolizumab in patients with MSI-H advanced endometrial cancer from the KEYNOTE-158 study. Ann Oncol 30:v425-v426, 2019. (suppl 5; abstr 1044P) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlumberger M, Tahara M, Wirth LJ, et al. : Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621-630, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be available for sharing at this time because the data are commercially confidential. However, Eisai will consider written requests to share the data on a case-by-case basis.