PURPOSE
Larotrectinib, a highly specific tropomyosin receptor kinase (TRK) inhibitor, previously demonstrated high response rates in single-arm trials of patients with TRK fusion-positive cancer, but there are limited data on comparative effectiveness against standard-of-care (SoC) regimens used in routine health care practice, before widespread adoption of TRK inhibitors as SoC for TRK fusion-positive cancers. Matching-adjusted indirect comparison, a validated methodology that balances population characteristics to facilitate cross-trial comparisons, was used to compare the overall survival (OS) of larotrectinib versus non–TRK-inhibitor SoC.
MATERIALS AND METHODS
Individual patient data from three larotrectinib trials (ClinicalTrials.gov identifiers: NCT02122913, NCT02637687, and NCT02576431) were compared with published aggregate real-world data from patients with locally advanced/metastatic TRK fusion-positive cancer identified in the Flatiron Health/Foundation Medicine database. OS was defined as the time from advanced/metastatic disease diagnosis to death. After matching population characteristics, the analyses included (1) a log-rank test of equality to test whether the two groups were similar before larotrectinib initiation; and (2) estimation of treatment effect of larotrectinib versus non–TRK-inhibitor SoC. These analyses are limited to prognostic variables available in real-world data.
RESULTS
Eighty-five larotrectinib patients and 28 non-TRK-inhibitor SoC patients were included in the analyses. After matching, log-rank testing showed no difference in baseline characteristics between the two groups (P = .31). After matching, larotrectinib was associated with a 78% lower risk of death, compared with non–TRK-inhibitor SoC (adjusted hazard ratio, 0.22 [95% CI, 0.09 to 0.52]; P = .001); median OS was 39.7 months (95% CI: 16.4, NE [not estimable]) for larotrectinib and 10.2 months (95% CI: 7.2, 14.1) for SoC.
CONCLUSION
Matching-adjusted indirect comparison analyses suggest longer OS with larotrectinib, compared with non–TRK-inhibitor SoC, in adult patients with TRK fusion-positive cancer.
INTRODUCTION
Neurotrophic receptor tyrosine kinase genes (NTRK1,2,3) encode tropomyosin receptor kinases (TRK) that regulate development of neuronal function. Chromosomal rearrangements can result in somatic NTRK gene fusions whose encoded TRK fusion proteins are constitutively active/overexpressed and drive oncogenesis.1 Such fusions occur at low frequency in pediatric and adult cancers of the lung, breast, and GI, and in melanomas and sarcomas, and are enriched in infantile fibrosarcoma, secretory carcinoma of the salivary gland, secretory breast carcinoma, and cellular congenital mesoblastic nephroma.2-8
CONTEXT
Key Objective
Larotrectinib is approved for treatment of adult and pediatric patients with tropomyosin receptor kinase (TRK) fusion-positive cancers, on the basis of durable antitumor activity and favorable safety in three single-arm trials. The rarity of TRK fusions is prohibitively too small to conduct head-to-head trials, therefore introducing challenges for decision makers tasked with selecting best available treatments for patients.
Knowledge Generated
Matching-adjusted indirect comparison was used to match population characteristics from patients with advanced/metastatic TRK fusion-positive cancer from three larotrectinib trials to aggregate data from patients in the Flatiron Health/Foundation Medicine database who received standard of care. After matching, no difference was seen in baseline characteristics (P = .31 by log-rank test). Larotrectinib was associated with a 78% lower risk of death and longer median overall survival than standard of care (39.7 v 10.2 months, respectively).
Relevance
We contribute evidence in the absence of a randomized trial, to support clinical decision making and guideline generation within patients with TRK fusion-positive cancers.
Larotrectinib is a highly selective, potent, CNS-active, orally administered, small-molecule, TRK inhibitor, approved in the United States (2018) and European Union (2019), on the basis of the findings from three multicenter, single-arm, clinical trials of patients with locally advanced or metastatic TRK fusion-positive cancer.9,10 Pooled data from those studies (data cutoff: July 2020) showed an investigator-assessed objective response rate of 79%, median duration of response of 35.2 months, median progression-free survival (PFS) of 28.3 months, and median overall survival (OS) of 44.4 months.11 In an expanded analysis of additional patients with longer follow-up (data cutoff: July 2021), independent review committee–assessed objective response rate was 69%, median duration of response was 32.9 months, median PFS was 29.4 months, and median OS was not reached.12 These findings have been practice-changing, exemplified by the development of international consensus guidelines that promote diagnostic approaches and patient selection criteria to identify those expected to benefit most from TRK inhibition.13
Cancers with rare oncogenic drivers often preclude the conduct of randomized, controlled trials (RCTs) because of the small population of patients available for recruitment. Consequently, designing and executing RCTs are challenging, particularly for tumor-agnostic therapies which target a molecular or genomic signature spanning multiple cancer types. In those cases, comparative evidence may be generated using population-adjusted methods, such as matching-adjusted indirect comparison (MAIC).
Several examples are available of unanchored MAIC for comparing outcomes of interventions in trials with no active comparator arm.14-18 MAIC accounts for differences in baseline characteristics between single-arm studies when common comparators are absent, ensuring that comparisons are made within a balanced patient population. By using individual patient data from one interventional trial to match baseline summary statistics from a comparator, MAIC balances the heterogenity in baseline characteristics between populations, thus largely eliminating confounding factors caused by divergent population characteristics.14,19 MAIC is accepted a valid methodology for cross-trial comparisons by England's National Institute for Health and Care Excellence and the European Medicines Agency Committee for Orphan Medicinal Products,20 as well as across many health technology assessment bodies.21,22
Prior to the approval and widespread adoption of TRK inhibitors for treatment of TRK fusion-positive cancers, guidelines-recommended standard of care (SoC) for advanced/metastatic solid tumors varied on the basis of tumor type, consisting of chemotherapy, radiotherapy, surgery, targeted therapies, and/or immuno-oncology agents. We report a MAIC analysis, comparing OS from patients treated with larotrectinib versus patients receiving SoC regimens used in routine health care practice, before widespread acceptance of TRK inhibitors, on the basis of real-world data collected from the Flatiron Health/Foundation Medicine Inc (FMI) clinicogenomic database.
MATERIALS AND METHODS
Selection of SoC Comparator Study
We selected the following published sources for comparative effectiveness studies: Voyager 1/Bazhenova et al,23 Voyager 2/Bridgewater et al,24 Santi et al,25 Zhu et al,26 and Hibar et al27 (previously presented by Demetri et al28). Studies that did not provide sufficient patient data were omitted, and the study by Hibar et al/Demetri et al was selected as the non–TRK-inhibitor SoC comparator.
Data Sources
Patient-level data were collected from an integrated patient population harboring TRK fusion-positive tumors across three clinical trials: a phase I larotrectinib trial (20288/LOXO-TRK-1400), SCOUT, and NAVIGATE. The phase I trial was a dose-escalation, safety and pharmacokinetics study of larotrectinib (50 mg once daily to 200 mg twice daily) in adult patients with advanced solid tumors (including both TRK fusion-positive and fusion-negative). SCOUT is a phase I/II study of larotrectinib (9.6-100 mg/m2 twice a day [maximum of 100 mg]) in pediatric patients with advanced solid or primary CNS tumors. NAVIGATE is a phase II pediatric and adult basket study of larotrectinib (100 mg twice a day), investigating its efficacy in patients with advanced solid tumors harboring a fusion of NTRK1-3. Eligibility criteria for each trial were as previously reported.29,30
For the non–TRK-inhibitor SoC comparator, Hibar et al/Demetri et al used the Flatiron/FMI database to identify NTRK fusion-positive patients who did not receive TRK inhibitors.27,28 Briefly, adult patients were selected using deidentified demographic and clinical data who had ≥ 1 test by next-generation sequencing on tumor tissue and ≥ 1 NTRK fusion-positive test result (defined by presence of a fusion or rearrangement involving NTRK1, NTRK2, or NTRK3, with predicted known or likely functional status; fusion cells were excluded if they had nonprotein coding gene partners or intragenic fusions), locally advanced or metastatic diagnosis between January 2011 and December 2019, no prior treatment with a TRK inhibitor, no visit gap of > 90 days after diagnosis, and no prior unlabeled study drug as part of a clinical trial. This cohort of patients is defined as SoC for this comparison.
Sample Selection
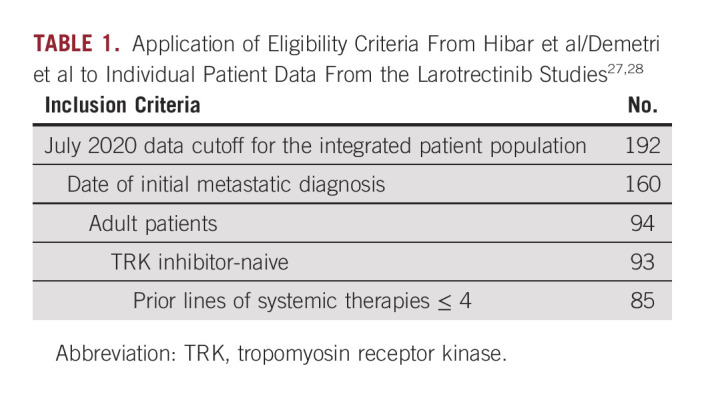
To increase population overlap between the two populations before matching on the selected baseline characteristics, the following inclusion criteria were applied (Table 1).
July 2020 data cutoff for the integrated patient population
Known dates of initial metastatic diagnosis
Age ≥ 18 years
TRK inhibitor-naive
Prior lines of systemic therapies ≤ 4
TABLE 1.
Application of Eligibility Criteria From Hibar et al/Demetri et al to Individual Patient Data From the Larotrectinib Studies27,28
Ethics
Larotrectinib studies were conducted according to ethics principles of the Declaration of Helsinki, good clinical practice, and all applicable country and local regulations. Study protocols were approved by institutional review board or ethics committee of each participating site. For the SoC arm, patients were not directly enrolled; deidentified patient data were collected from the Flatiron/FMI database.
Statistical Methods
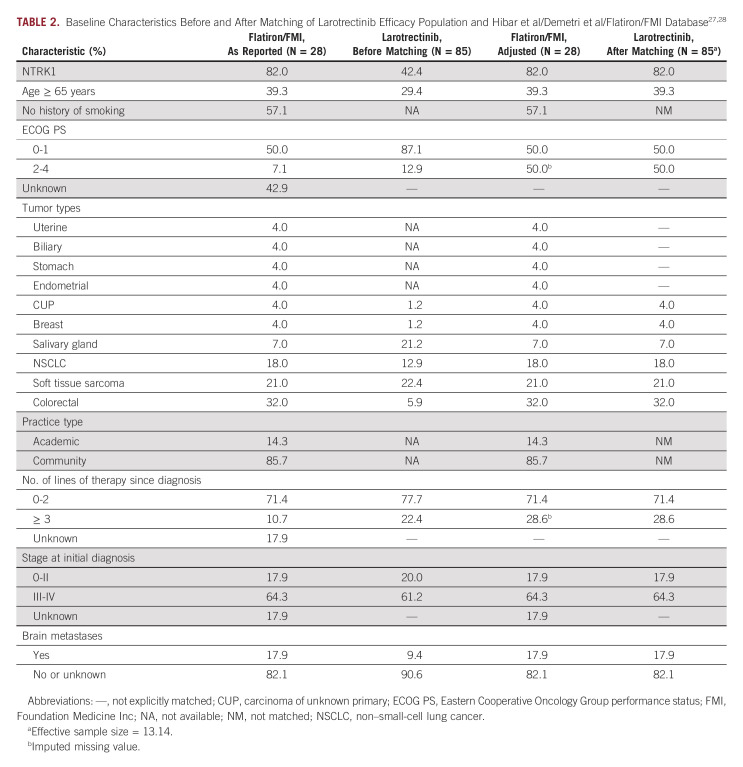
MAIC methodology was used to compare larotrectinib versus non–TRK-inhibitor SoC.21,22 Individual patient data from the larotrectinib trials were matched to the average baseline characteristics from Hibar et al/Demetri et al (Table 2).27,28 Baseline characteristics selected were based on data availability. Hazard ratio (HR) from Cox model was used to compare the two groups.
TABLE 2.
For SoC patients with missing data in the number of lines of therapy since diagnosis (17.9%) and Eastern Cooperative Oncology Group performance status (ECOG PS; 42.9%),27,28 variables were imputed such that patients with missing values were assumed to be in the more severe categories (ie, ≥ 3 lines of therapy; ECOG PS 2-4). These assumptions were made to estimate a conservative HR, which were subsequently tested in a sensitivity analysis that used less conservative assumptions.
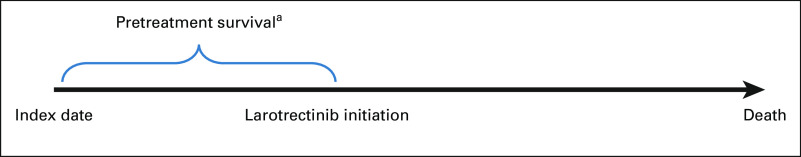
Two analyses were conducted after matching. The first was conducted to validate the performance of matching, that is, if matching were performed adequately, then the two groups will be similar in the pretreatment survival period, defined as the time from locally advanced/metastatic disease diagnosis (index date) to larotrectinib initiation (Fig 1). The second was to estimate the treatment effect of larotrectinib versus SoC on OS (defined as the time from index date to death).
FIG 1.
Pretreatment survival for the log-rank test. aTime from index date to initiation of larotrectinib.
Statistical analyses were conducted in Stata 15, and statistical significance was set a priori at P < .05.
RESULTS
Selection of Comparator Study
We identified five studies that enrolled TRK fusion-positive cancer patients who received non–TRK-inhibitor SoC regimens: Voyager 1/Bazhenova et al,23 Voyager 2/Bridgewater et al,24 Santi et al,25 Zhu et al,26 and Hibar et al/Demetri et al.27,28 Because of limitations of statistical methods used, it was important that the studies identified report Kaplan-Meier (KM) estimates of OS and comprehensive baseline characteristics. Three studies were omitted for further analysis because of the following limitations: Voyager 1/Bazhenova et al23 was missing data on ECOG PS, CNS metastasis, and line of therapy, and had differences/misalignment in index date. Voyager 2/Bridgewater et al24 only reported baseline characteristics for the full cohort, and not for the matched cohort used in the analysis. Santi et al25 lacked sufficient baseline characteristics, and the index date definition could not be aligned with that of larotrectinib. Zhu et al26 did not include OS analysis. The Hibar et al/Demetri et al study provided sufficient data both on baseline characteristics and index date that aligned with the larotrectinib studies, and was selected for further analysis.27,28
Patient Characteristics
A total of 192 patients from the larotrectinib trials were assessed for inclusion in the MAIC. After applying the sample selection criteria (Table 1), 160 patients had complete information on date of initial metastatic diagnosis: 94 were adult patients, 93 were TRK inhibitor-naive, and 85 had ≤ 4 prior lines of systemic therapy. The 85 patients who met all criteria were included in the larotrectinib population. Median time from NTRK testing to larotrectinib initiation was 85 days.
Hibar et al/Demetri et al reported baseline characteristics for 28 patients with TRK fusion-positive tumors.27,28
Baseline characteristics before and after matching in the primary analysis are summarized in Table 2. The weight distribution can be found in Appendix Figure A1 (online only).
Before matching, baseline characteristics were similar in number of lines of prior therapy 0-2 (71.4% v 77.7%) and stage 0-II disease at diagnosis (17.9% v 20.0%), but differed in proportion of patients with NTRKI fusions (82.0% v 42.4%), age ≥ 65 years (39.3% v 29.4%), ECOG PS of 0-1 (50.0% v 87.1%), and brain metastases (17.9% v 9.4%; Table 2). Categorization by tumor type showed more patients in Flatiron/FMI had breast cancer (4.0% v 1.2%) or non–small-cell lung cancer (NSCLC; 18.0% v 12.9%), and fewer patients had salivary gland tumors (7.0% v 21.2%) or soft tissue sarcoma (21.0% v 22.4%).27,28
For the analysis, matching of patient characteristics was conducted to simulate a scenario where there are no differences between the Flatiron/FMI cohort and patients receiving larotrectinib. To accommodate the two unmatched cohorts, matching was performed on the following variables: NTRK1, age, ECOG PS, prior lines of systemic therapy, disease stage, brain metastases, and tumor type (uterine, biliary, stomach, endometrial, cancer of unknown primary, breast, salivary gland, NSCLC, soft tissue sarcoma, and colorectal; Table 2). To accommodate the difference in TRK inhibitor use between cohorts (ie, SoC excluding TRK inhibitors in Flatiron/FMI v larotrectinib studies), the effect of larotrectinib was nullified by readjusting the survival time such that it would reflect the time period between the index date and the date of larotrectinib initiation. Using a log-rank test to validate the performance of matching this pretreatment survival suggested no difference between the two groups (P = .31). The associated KM plot can be found in Appendix Figure A2 (online only). Median pretreatment survival was 10.2 months in the Flatiron/FMI cohort, and 8 months for larotrectinib.
Overall Survival
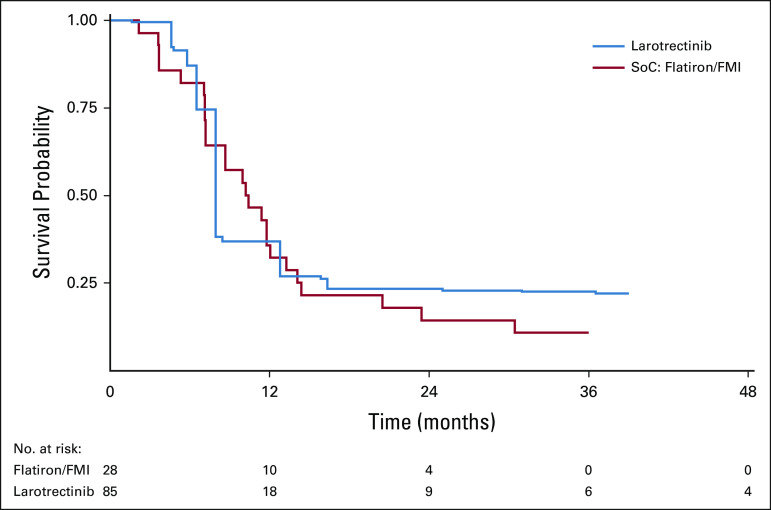
HRs with corresponding 95% CIs were used to assess OS between larotrectinib and non–TRK-inhibitor SoC before and after matching (Table 3). The median OS for TRK fusion-positive patients in the Flatiron/FMI database was 10.2 months (95% CI, 7.2 to 14.1).27,28 Before matching, the median OS for larotrectinib was not reached31; after matching, OS was 39.7 months (95% CI, 16.4 to not estimable [NE]). Larotrectinib was associated with a 78% lower risk of death (HR, 0.22; 95% CI, 0.09 to 0.52; P = .001), which corresponds to a 29.5-month median survival advantage, compared with non–TRK-inhibitor SoC (Table 3; Fig 2). In the afterweighting KM plot (Fig 2), the dip in larotrectinib survival at 16 months is explained by a single patient whose severe disease profile (≥ 3 lines of prior therapy, stage 3/4 disease, ECOG PS 2/4, and comorbid lung cancer with CNS metastasis) resulted in a higher assigned weight, thus amplifying the death event at 16 months.
TABLE 3.
Larotrectinib Versus SoC: Overall Survival From Time of Metastatic/Locally Advanced Diagnosis Index Date
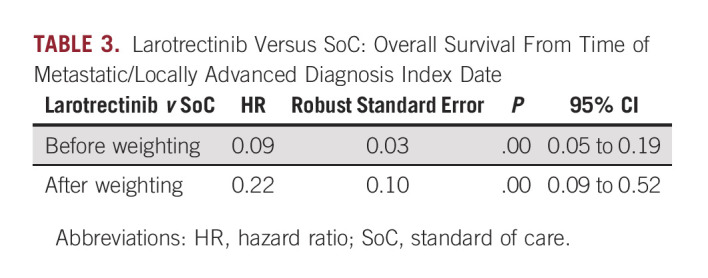
FIG 2.
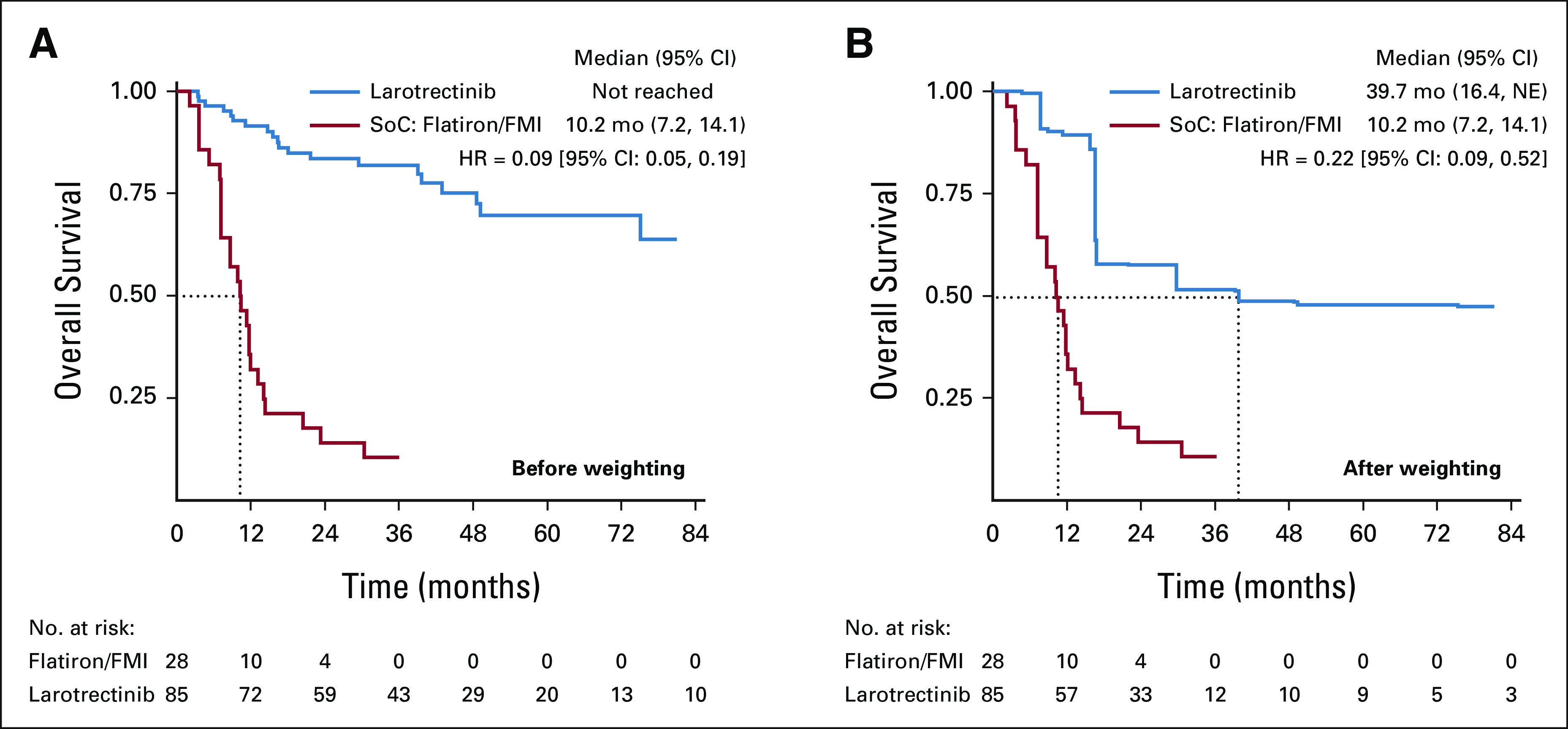
Kaplan-Meier curve of overall survival from time of metastatic/locally advanced diagnosis (A) before and (B) after weighting. FMI, Foundation Medicine Inc; NE, not estimable; SoC, standard of care.
Sensitivity Analysis 1: Use of Alternative Variables for Matching
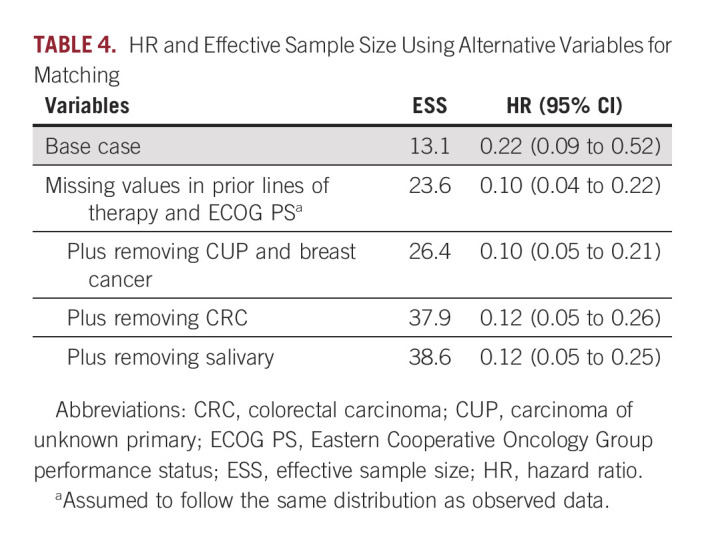
In MAIC, excess matching of variables can reduce the effective sample size (ESS), which can increase uncertainty in the outcome of interest. To test the robustness of the results, the first sensitivity analysis accounted for excessive matching of variables. To address the potential for high uncertainty in the base case analysis, the missing data assumption was relaxed by assuming that the missing values in prior lines of therapy and ECOG PS follow the same distribution as the observed data (Table 4). ESS increased from 13.1 to 23.6; corresponding HR was 0.10 (95% CI, 0.04 to 0.22). Additional removal of carcinoma of unknown primary and breast cancer, colorectal carcinoma, or salivary cancer led to ESS of 26.4, 37.9, and 38.6, respectively; corresponding HRs were 0.10 (95% CI, 0.05 to 0.21), 0.12 (0.05 to 0.26), and 0.12 (0.05 to 0.25). Removal of these variables increased ESS and decreased HR, thus validating the primary analysis wherein ECOG PS and lines of therapy were imputed to more severe disease in larotrectinib patients.
TABLE 4.
HR and Effective Sample Size Using Alternative Variables for Matching
Sensitivity Analysis 2: Restricted Mean Survival Time Analysis
The second sensitivity analysis accounted for the longer follow-up period for larotrectinib than non–TRK-inhibitor SoC. A restricted mean survival time analysis was conducted up to 30.5 months, representing the minimum of the largest observed event time within the SoC arm. A 10.8-month mean survival advantage (95% CI, 7.0 to 14.6; P < .01) was estimated with larotrectinib, compared with SoC (data not shown), suggesting that, on average, a patient treated with larotrectinib who has 30.5 months of follow-up would be predicted to survive approximately 10.8 months longer than with non–TRK-inhibitor SoC. Importantly, this analysis is conservative, and the mean survival benefit is expected to continue to increase with longer follow-up.
DISCUSSION
In the absence of a direct head-to-head comparison between larotrectinib and non–TRK-inhibitor SoC in patients with locally advanced/metastatic TRK fusion-positive cancer, MAIC provides an insightful comparative analysis that can help inform decision making. By using MAIC to balance population characteristics between pooled larotrectinib data and real-world data collected from the Flatiron/FMI database, our analysis suggests that (1) the matching methodology was robust (ie, as demonstrated by log-rank testing that validated the performance of matching pretreatment survival); and (2) larotrectinib is associated with a 78% lower risk of death and 29.5-month survival advantage, compared with non–TRK-inhibitor SoC. These findings were further confirmed using two sensitivity analyses—one that used alternative variables for matching, and the other that restricts the mean survival time.
In selecting a comparator to represent the SoC population, the Flatiron/FMI analysis by Hibar et al/Demetri et al was chosen because, as a US-wide longitudinal database of health care practice data, it provided a vast amount of information on baseline characteristics, as well as aligned on the index date with that used in the larotrectinib studies.27,28 Three of four SoC studies enrolling TRK fusion-positive patients estimated median OS of 10.1-12.7 months, showing consistency across studies and validating the selection of Hibar et al/Demetri et al as the comparator.23,25,27,28 Although the Flatiron/FMI database does not represent a traditional clinical trial comparator, it has previously demonstrated concordance with clinical trial data and has been used as the data source for oncology research across different therapies and indications, including atezolizumab for NSCLC, trastuzumab and lapatinib for human epidermal growth factor receptor 2–positive metastatic breast cancer, and durvalumab and chemotherapy for metastatic urothelial carcinoma.32-34 The dates selected for the Flatiron/FMI patient data (January 2011 to December 2019) were after approval of larotrectinib by the United States and the European Union, but before widespread acceptance of larotrectinib as a SoC for TRK fusion-positive cancers. Exclusion of patients previously treated with TRK inhibitors ensured that the MAIC comparison would restrict the comparison to larotrectinib versus non–TRK-inhibitor SoC. In addition, Hibar et al/Demetri et al adjusted for immortal time bias and left truncation (eg, excluding patients if an event or death occurred before next-generation sequencing testing) to avoid biases in relative effect measure.27,28 Such biases were therefore already accounted for in the comparator data set used in our study.
In the survival analysis by Hibar et al/Demetri et al, presence of NTRK fusions in patient tumors was associated with poor OS, but with no significant differences from patients without NTRK fusions (HR, 1.597; 95% CI, 1.019 to 2.504; P = .059), suggesting that the activity of SoC therapies does not differentiate between NTRK fusion-positive and NTRK fusion-negative tumors.27,28 In our study, the survival advantage seen with larotrectinib (HR, 0.22; 95% CI, 0.09 to 0.52) may be attributed to its mechanism of action as a TRK inhibitor that inhibits constitutively activated, oncogenic TRK fusion proteins.
Several limitations should be noted for this study, notably the small sample size and limited details on prior therapies and other baseline characteristics from the SoC data set.27,28 In the absence of random assignment, some observed or unobserved prognostic factors may be missed. Although MAIC assumes that all effect modifiers are taken into account and every effort was made to adjust for all variables, this analysis was limited to the ones reported in Hibar et al/Demetri et al.27,28 MAIC provides estimates that are accurate with minimal bias even with lower degrees of covariate overlap assuming no failures of assumptions. Next, the use of robust standard error (sandwich estimator) variability is underestimated when ESS are small. However, the actual ESS is likely to be larger than what was reported, as the weights are not fixed and known. Third, Hibar et al/Demetri et al did not include the specific therapies used by patients in the Flatiron/FMI database,27,28 so administration of tumor-specific SoC was assumed. Finally, when comparing trial versus real-world data, the potential impact of trial eligibility criteria on outcomes should be considered. Although TRK inhibitors (larotrectinib and entrectinib) are now considered SoC, they remain inaccessible in many countries because of reimbursement restrictions from lack of comparative evidence. Therefore, despite these limitations, the findings reported herein fill an important knowledge gap regarding the impact of treatment with larotrectinib versus SoC.
In conclusion, our findings suggest that larotrectinib had favorable survival compared to non–TRK-inhibitor SoC in patients with NTRK fusion-positive cancers, after adjusting for the heterogeneity in patient characteristics between trial and registry populations. Although our initial work on data from NTRK fusion-positive patients treated with larotrectinib showed significantly prolonged, intraindividual PFS upon larotrectinib initiation, compared with PFS of prior standard therapy, this new analysis provides an interpatient comparison between well-matched individuals with NTRK fusion-positive cancers receiving either larotrectinib or non–TRK-inhibitor SoC therapy. With confirmation of significant OS difference in a real-world comparison, this analysis represents the second solid piece of evidence, supporting the positive impact of TRK-directed treatment in molecularly selected patients, thus contributing evidence, in the absence of an RCT, to support clinical decision making and guideline generation within these patients.35 Further studies to confirm these findings are warranted.
ACKNOWLEDGMENT
Editorial assistance was provided by Ingrid Koo, PhD, of FleishmanHillard.
APPENDIX
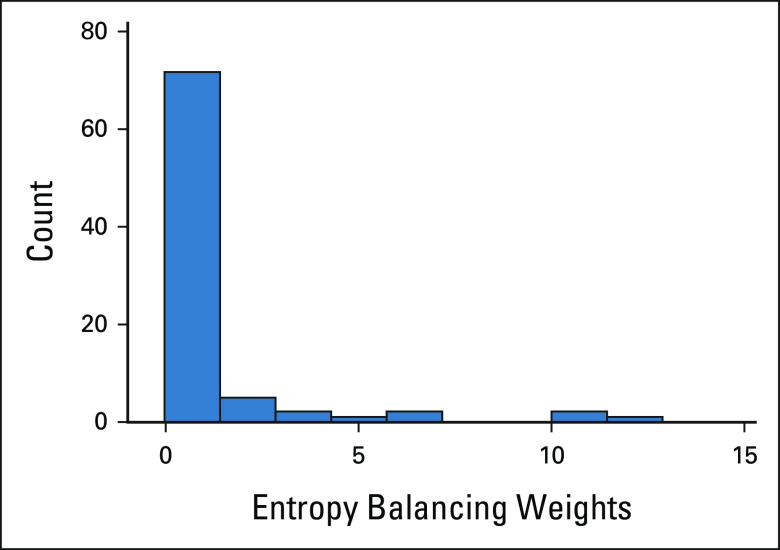
FIG A1.
Histogram of patient weights. Upon diagnosing the weight distribution, most weights were ≤ 1, with some outliers with higher weights. Weight > 1 means that an individual carries more weight in the reweighted population than in the larotrectinib trial population. Weight < 1 means that an individual carries less weight than in the larotrectinib trial population.
FIG A2.
Kaplan-Meier curve of pretreatment survival estimates. FMI, Foundation Medicine Inc; SoC, standard of care.
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp Dohme, Berlin-Chemie, Medac
Consulting or Advisory Role: Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, GSO, AOK Health Insurance, ODC (Oncolgy drug consult), Janssen-Cilag GmbH
Research Funding: AbbVie (Inst), ADC Therapeutics (Inst), Agile Therapeutics (Inst), Alexion Pharmaceuticals (Inst), Amgen (Inst), Apellis Pharmaceuticals (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BerGenBio (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Gilead Sciences (Inst), Glycotope GmbH (Inst), GlaxoSmithKline (Inst), Incyte (Inst), IO Biotech (Inst), Isofol Medical (Inst), Janssen-Cilag (Inst), Karyopharm Therapeutics (Inst), Lilly (Inst), Millennium (Inst), MSD (Inst), Nektar (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Roche (Inst), Springworks Therapeutics (Inst), Taiho Pharmaceutical (Inst), BioNTech (Inst), Ipsen (Inst), Servier/Pfizer (Inst), Immatics (Inst), CPT Cellex Patient Treatment (Inst), Glycostem (Inst)
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Bristol Myers Squibb, Janssen-Cilag
Noman Paracha
Employment: Bayer
Stock and Other Ownership Interests: Bayer
Ulrik Lassen
Honoraria: Bayer, Pfizer, Novartis
Consulting or Advisory Role: Bayer, Pfizer
Research Funding: BMS (Inst), Roche (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Lilly (Inst)
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Sean D. Sullivan
Honoraria: Bayer
Consulting or Advisory Role: Bayer
Research Funding: Bayer (Inst)
Marisca Marian
Employment: Bayer
Nicoletta Brega
Employment: Bayer
Leadership: Bayer
Stock and Other Ownership Interests: Bayer
Travel, Accommodations, Expenses: Bayer
Other Relationship: Bayer
Jesus Garcia-Foncillas
Honoraria: Merck KGaA (Inst), Bayer, Sanofi, Servier (Inst), Novartis
Consulting or Advisory Role: Bayer
Speakers' Bureau: Bayer
Travel, Accommodations, Expenses: Janssen
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at ASCO 2022 annual meeting, Chicago, IL, June 3-7, 2022 (abstr 6579).
SUPPORT
Supported by Bayer.
DATA SHARING STATEMENT
Availability of the data underlying this publication will be based on Bayer's commitment to the EFPIA-PhRMA Principles for responsible clinical trial data sharing. This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing on request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States and European Union as necessary for doing legitimate research. This applies to data on new medicines and indications that have been approved by the regulatory agencies of the European Union and the United States on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to do further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: Noman Paracha, Marisca Marian, Nicoletta Brega
Collection and assembly of data: Noman Paracha, Marisca Marian, Nicoletta Brega
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp Dohme, Berlin-Chemie, Medac
Consulting or Advisory Role: Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, GSO, AOK Health Insurance, ODC (Oncolgy drug consult), Janssen-Cilag GmbH
Research Funding: AbbVie (Inst), ADC Therapeutics (Inst), Agile Therapeutics (Inst), Alexion Pharmaceuticals (Inst), Amgen (Inst), Apellis Pharmaceuticals (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BerGenBio (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Gilead Sciences (Inst), Glycotope GmbH (Inst), GlaxoSmithKline (Inst), Incyte (Inst), IO Biotech (Inst), Isofol Medical (Inst), Janssen-Cilag (Inst), Karyopharm Therapeutics (Inst), Lilly (Inst), Millennium (Inst), MSD (Inst), Nektar (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Roche (Inst), Springworks Therapeutics (Inst), Taiho Pharmaceutical (Inst), BioNTech (Inst), Ipsen (Inst), Servier/Pfizer (Inst), Immatics (Inst), CPT Cellex Patient Treatment (Inst), Glycostem (Inst)
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Bristol Myers Squibb, Janssen-Cilag
Noman Paracha
Employment: Bayer
Stock and Other Ownership Interests: Bayer
Ulrik Lassen
Honoraria: Bayer, Pfizer, Novartis
Consulting or Advisory Role: Bayer, Pfizer
Research Funding: BMS (Inst), Roche (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Lilly (Inst)
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Sean D. Sullivan
Honoraria: Bayer
Consulting or Advisory Role: Bayer
Research Funding: Bayer (Inst)
Marisca Marian
Employment: Bayer
Nicoletta Brega
Employment: Bayer
Leadership: Bayer
Stock and Other Ownership Interests: Bayer
Travel, Accommodations, Expenses: Bayer
Other Relationship: Bayer
Jesus Garcia-Foncillas
Honoraria: Merck KGaA (Inst), Bayer, Sanofi, Servier (Inst), Novartis
Consulting or Advisory Role: Bayer
Speakers' Bureau: Bayer
Travel, Accommodations, Expenses: Janssen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cocco E, Scaltriti M, Drilon A: NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 15:731-747, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stransky N, Cerami E, Schalm S, et al. : The landscape of kinase fusions in cancer. Nat Comm 5:4846, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kheder ES, Hong DS: Emerging targeted therapy for tumors with NTRK fusion proteins. Clin Cancer Res 24:5807-5814, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Skálová A, Vanecek T, Sima R, et al. : Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34:599-608, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Tognon C, Knezevich SR, Huntsman D, et al. : Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2:367-376, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Yang L, Kucherlapati M, et al. : A Pan-cancer compendium of genes deregulated by somatic genomic rearrangement across more than 1,400 cases. Cell Rep 24:515-527, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgeois JM, Knezevich SR, Mathers JA, et al. : Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol 24:937-946, 2000 [DOI] [PubMed] [Google Scholar]
- 8.El Demellawy D, Cundiff CA, Nasr A, et al. : Congenital mesoblastic nephroma: A study of 19 cases using immunohistochemistry and ETV6-NTRK3 fusion gene rearrangement. Pathology 48:47-50, 2016 [DOI] [PubMed] [Google Scholar]
- 9.US FDA Press Release: FDA approves larotrectinib for solid tumors with NTRK gene fusions. 2018. https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions
- 10.Bayer Press Release: Vitrakvi® (larotrectinib) receives first tumor-agnostic approval in EU, 2019. https://www.bayer.com/media/vitrakvir-larotrectinib-receives-first-tumor-agnostic-approval-in-eu/
- 11.Hong DS, DuBois SG, Kummar S, et al. : Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21:531-540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon AE, Hong DS, van Tilburg CM, et al. : Long-term efficacy and safety of larotrectinib in a pooled analysis of patients with tropomyosin receptor kinase (TRK) fusion cancer. J Clin Oncol 40, 2022. (suppl 16; abstr 3100) [Google Scholar]
- 13.Yoshino T, Pentheroudakis G, Mishima S, et al. : JSCO-ESMO-ASCO-JSMO-TOS: International expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol 31:861-872, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Signorovitch JE, Wu EQ, Yu AP, et al. : Comparative effectiveness without head-to-head trials: A method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics 28:935-945, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Di Lorenzo G, Casciano R, Malangone E, et al. : An adjusted indirect comparison of everolimus and sorafenib therapy in sunitinib-refractory metastatic renal cell carcinoma patients using repeated matched samples. Expert Opin Pharmacother 12:1491-1497, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Strand V, Betts KA, Mittal M, et al. : Comparative effectiveness of adalimumab versus secukinumab for the treatment of psoriatic arthritis: A matching-adjusted indirect comparison. Rheumatol Ther 4:349-362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Signorovitch J, Erder MH, Xie J, et al. : Comparative effectiveness research using matching-adjusted indirect comparison: An application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disord. Pharmacoepidemiol Drug Saf 21:130-137, 2012. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 18.Pocoski J, Li N, Ayyagari R, et al. : Matching-adjusted indirect comparisons of efficacy of BAY 81-8973 vs two recombinant factor VIII for the prophylactic treatment of severe hemophilia A. J Blood Med 7:129-137, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signorovitch JE, Sikirica V, Erder MH, et al. : Matching-adjusted indirect comparisons: A new tool for timely comparative effectiveness research. Value Health 15:940-947, 2012 [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency Committee for Orphan Medicinal Products : Polivy (polatuzumab vedotin) Treatment of diffuse large B-cell lymphoma. Orphan Maint Assess Rep, 2020. https://www.ema.europa.eu/en/documents/orphan-maintenance-report/polivy-orphan-maintenance-assessment-report-initial-authorisation_en.pdf [Google Scholar]
- 21.Phillippo DM, Ades AE, Dias S, et al. : Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making 38:200-211, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thom H, Jugl S, Palaka E, et al. : Matching adjusted indirect comparisons to assess comparative effectiveness of therapies: Usage in scientific literature and health technology appraisals. Presented at ISPOR 21st Annual International Meeting, Washington, DC, May 21-25, 2016
- 23.Bazhenova L, Lokker A, Snider J, et al. : TRK fusion cancer: Patient characteristics and survival analysis in the real-world setting. Target Oncol 16:389-399, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridgewater J, Jiao X, Parimi M, et al. : Abstract 394: Prognosis and molecular characteristics of patients with TRK fusion cancer in the 100,000 Genomes Project. Cancer Res 81:394, 2021 [Google Scholar]
- 25.Santi I, Vellekoop H, Huygens S, et al. : 105P—Prognostic value of the NTRK fusion biomarker in the Netherlands. Ann Oncol 32:S401-S402, 2021. (abstr 105P) [Google Scholar]
- 26.Zhu L, Hobbs B, Roszik J, et al. : Investigating the natural history and prognostic nature of NTRK gene fusions in solid tumors. Invest New Drugs 40:157-162, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Hibar DP, Demetri GD, Peters S, et al. : Real-world survival outcomes in patients with locally advanced or metastatic NTRK fusion-positive solid tumors receiving standard-of-care therapies other than targeted TRK inhibitors. PLoS One 17:e0270571, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demetri GD, Peters S, Hibbar DP, et al. : 100P—Characteristics and outcomes of patients (pts) with NTRK fusion-positive (NTRK+) metastatic/locally advanced (LA) solid tumours receiving non-TRK inhibitor (TRKi) standard of care (SoC), and prognostic value of NTRK fusions in clinical practice. Ann Oncol 32:S382-S406, 2021. (abstr 100P) [Google Scholar]
- 29.Drilon A, Laetsch TW, Kummar S, et al. : Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 378:731-739, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong DS, Bauer TM, Lee JJ, et al. : Larotrectinib in adult patients with solid tumours: A multi-centre, open-label, phase I dose-escalation study. Ann Oncol 30:325-331, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott R, van Tilburg CM, Farago AF, et al. : 1955P Survival benefits of larotrectinib in an integrated dataset of patients with TRK fusion cancer. Ann Oncol 31:S1101-S1102, 2020 [Google Scholar]
- 32.Frueh M, Rahma OE, Pachynski RK, et al. : Treatment (tx) characteristics of patients (pts) with locally advanced or metastatic non-small cell lung cancer (NSCLC) receiving atezolizumab (atezo) monotherapy in US clinical practice. Ann Oncol 30:1165, 2019 [Google Scholar]
- 33.Hoyle CK, Klein AB, Patel KA, et al. : Match-adjusted indirect comparison of durvalumab and chemotherapy for locally advanced or metastatic urothelial carcinoma (UC) following failure of platinum-based therapy. Ann Oncol 29:viii323, 2018. (abstr 907P) [Google Scholar]
- 34.Ramagopalan SV, Pisoni R, Zenin A, et al. : Comparative effectiveness of trastuzumab emtansine versus lapatinib plus chemotherapy for HER2+ metastatic breast cancer. J Comp Eff Res 10:595-602, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Bokemeyer C, Vassal G, Italiano A, et al. : Impact of disease evolution on efficacy outcomes from larotrectinib in patients with locally advanced or metastatic tropomyosin receptor kinase fusion-positive solid tumors. JCO Precis Oncol 5:1458-1465, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of the data underlying this publication will be based on Bayer's commitment to the EFPIA-PhRMA Principles for responsible clinical trial data sharing. This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing on request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States and European Union as necessary for doing legitimate research. This applies to data on new medicines and indications that have been approved by the regulatory agencies of the European Union and the United States on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to do further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.