PURPOSE
We sought to evaluate the independent and interactive associations of planned treatment duration, celecoxib use, physical activity, body mass index (BMI), diabetes mellitus, and vitamin B6 with oxaliplatin-induced peripheral neuropathy (OIPN) among patients with stage III colon cancer enrolled in a clinical trial.
METHODS
We conducted a prospective, observational study of 2,450 patients with stage III colon cancer enrolled in the CALGB/SWOG 80702 trial, randomly assigned to 6 versus 12 cycles of adjuvant fluorouracil, leucovorin, and oxaliplatin chemotherapy with or without 3 years of celecoxib. OIPN was reported using the Common Terminology Criteria for Adverse Events (CTCAE) during and following completion of chemotherapy and the FACT/GOG-NTX-13 15-17 months after random assignment. Multivariate analyses were adjusted for baseline sociodemographic and clinical factors.
RESULTS
Patients assigned to 12 treatment cycles, relative to 6, were significantly more likely to experience higher-grade CTCAE- and FACT/GOG-NTX-13-reported neuropathy and longer times to resolution, while neither celecoxib nor vitamin B6 intake attenuated OIPN. Exercising ≥ 9 MET-hours per week after treatment relative to < 9 was associated with improvements in FACT/GOG-NTX-13-reported OIPN (adjusted difference in means, 1.47; 95% CI, 0.49 to 2.45; P = .003). Compared with patients with baseline BMIs < 25, those with BMIs ≥ 25 were at significantly greater risk of developing higher-grade CTCAE-reported OIPN during (adjusted odds ratio, 1.18; 95% CI, 1.00 to 1.40; P = .05) and following completion (adjusted odds ratio, 1.23; 95% CI, 1.01 to 1.50; P = .04) of oxaliplatin treatment. Patients with diabetes were significantly more likely to experience worse FACT/GOG-NTX-13-reported neuropathy relative to those without (adjusted difference in means, –2.0; 95% CI, –3.3 to –0.73; P = .002). There were no significant interactions between oxaliplatin treatment duration and any of these potentially modifiable exposures.
CONCLUSION
Lower physical activity, higher BMI, diabetes, and longer planned treatment duration, but not celecoxib use or vitamin B6 intake, may be associated with significantly increased OIPN severity.
INTRODUCTION
Conflicting findings have been reported regarding the clinical and sociodemographic factors predisposing patients with colorectal cancer (CRC) to oxaliplatin-induced peripheral neuropathy (OIPN).1,2 Only receipt of a higher cumulative dose has consistently been associated with greater neurotoxicity,2-5 whereas discordant findings have been reported regarding the contributions of body mass index (BMI)6-10 and diabetes mellitus.10-14 Nonsteroidal anti-inflammatory drug (NSAID) use,15 physical activity,16 and vitamin B617 have additionally been posited to influence OIPN in CRC, but little evidence currently exists to substantiate these hypotheses.
CONTEXT
Key Objective
What are the associations of planned duration of oxaliplatin-based treatment, celecoxib use, physical activity, body mass index, diabetes mellitus, and vitamin B6 intake with oxaliplatin-induced peripheral neuropathy (OIPN)?
Knowledge Generated
Among patients with stage III colon cancer enrolled in a clinical trial, lower physical activity, higher body mass index, comorbid diabetes, and longer planned treatment duration correlated with significantly increased OIPN severity. No significant associations were observed with celecoxib use or vitamin B6 intake.
Relevance (A.H. Ko)
-
These findings can help guide discussions with patients about modifiable lifestyle factors to help reduce the risk of OIPN, as well as identify individuals at higher risk of OIPN in whom closer monitoring and more aggressive mitigation strategies (including possibly a lower threshold for oxaliplatin dose reduction) may be warranted.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD.
We therefore sought to evaluate the independent and interactive associations of planned treatment duration, celecoxib use, physical activity, BMI, diabetes, and vitamin B6 intake with self-reported OIPN during and following treatment in a prospective cohort of patients with stage III resected colon cancer enrolled in a randomized clinical trial (RCT) of adjuvant fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy.
METHODS
Study Population
Patients in this prospective cohort study were recruited from the United States and Canada as participants in the National Cancer Institute (NCI)–sponsored Cancer and Leukemia Group B (CALGB; now part of Alliance for Clinical Trials in Oncology)/Southwest Oncology Group (SWOG) 80702 adjuvant therapy trial for stage III colon cancer (ClinicalTrials.gov identifier: NCT01150045). The trial used a 2 × 2 factorial design to test the primary hypothesis of the superiority of celecoxib compared with placebo and the secondary hypothesis of the noninferiority of 3 months (six treatments) compared with 6 months (12 treatments) of chemotherapy as part of an international pooling project.18 The results of the primary and secondary survival hypotheses have been reported.18-20 Participants separately consented to a self-administered questionnaire collecting dietary and lifestyle behaviors twice: once within 6 weeks after random assignment during receipt of adjuvant therapy (questionnaire 1 [Q1]), and again 14-16 months after random assignment during the recurrence surveillance period (questionnaire 2 [Q2]).
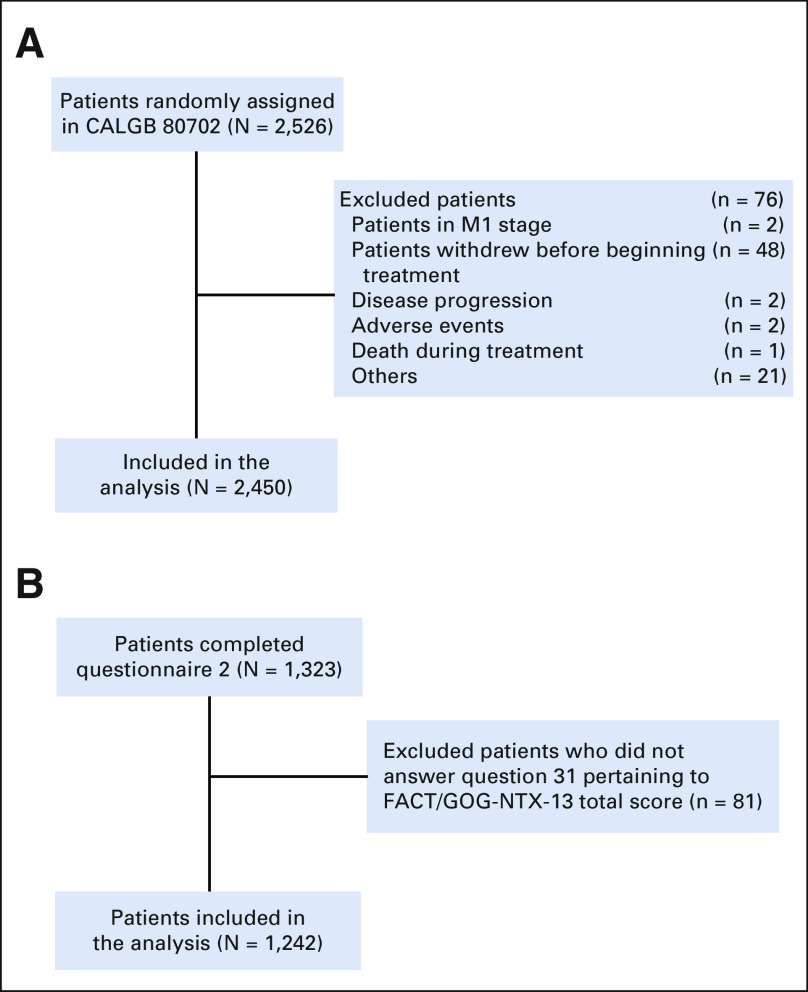
Eligibility required patients to have had a histologically documented, margin-negative resected colon adenocarcinoma, with tumors having had either at least one pathologically confirmed positive lymph node or an N1C designation, as defined in the seventh edition of the American Joint Committee on Cancer staging manual.21 Participants had normal hepatic, renal, and hematologic laboratory values; an Eastern Cooperative Oncology Group performance status of 0-2; and no evidence of metastatic disease. Figure 1 describes the derivation of the final sample sizes of 2,450 and 1,242 patients included in this study for self-reported neuropathy analyses using two independent instruments, respectively.
FIG 1.
Derivation of the study cohort for (A) CTCAE-reported neuropathy and (B) FACT/GOG-NTX-13–reported neuropathy. CTCAE, Common Terminology Criteria for Adverse Events.
Evaluation of Physical Activity, BMI, Diabetes, and Vitamin B6 Intake
Physical activity, BMI, diabetes, and vitamin B6 intake were assessed during both questionnaires 1 and 2, with BMI additionally reported by treatment sites at study entry (baseline). Survey questions regarding physical activity and food intake have previously been described and validated.22,23 Vitamin B6 intake was calculated by multiplying the vitamin B6 content of the specified portion of each food item, including supplements, in the questionnaires by consumption frequency and then summing across all items. Patients self-reported diabetes diagnoses and use of medications for diabetes management through the questionnaires. Cumulative averages for physical activity, BMI, and vitamin B6 intake were calculated using the following equation, consistent with prior analyses24-26:
Neuropathy Assessment
Neuropathy was evaluated through two patient-reported instruments. One tool was the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 criteria, which uses a five-point grading scale as follows: 0 = normal; 1 = asymptomatic, loss of deep tendon reflexes, or paresthesia; 2 = moderate symptoms, limiting instrumental activities of daily living; 3 = severe symptoms, limiting self-care activities of daily living; and 4 = life-threatening consequences, urgent intervention indicated. Assessment occurred every 3 months for 3 years following chemotherapy commencement, and subsequently every 6 months for 6 years after random assignment or until disease recurrence, whichever came first. The rating from each reporting period reflects the worst grade experienced during that term. We report maximum grades of CTCAE-reported neuropathy (1) during and (2) after completion of oxaliplatin treatment.
In Q2—completed a median of 12 months (interquartile range [IQR], 10-13 months) from the last dose of oxaliplatin—neuropathy was additionally evaluated through the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity-13 (FACT/GOG-NTX-13) subscale,27 which evaluates the degree and impact of sensory and motor neurotoxicity symptoms in the preceding 7 days. Items are assessed on a five-point scale—ranging from 0 (not at all) to 4 (very much)—and inverse-summed such that a higher total score correlates with a lower neurotoxicity burden.
Statistical Analysis
The findings from the CALGB 80702 trial regarding the primary end point of disease-free survival have previously been reported,18 and treatment groups were well balanced for baseline characteristics.
Associations of planned treatment duration, celecoxib use, diabetes, physical activity, BMI, and vitamin B6 intake with FACT/GOG-NTX-13-reported neuropathy were estimated using linear regression. For CTCAE-reported neuropathy, proportional odds logistic regression was used to evaluate these associations with the maximum-grade neuropathy experienced during and after completion of oxaliplatin treatment. BMI reported at baseline and physical activity and vitamin B6 intake reported in Q1 were used for during oxaliplatin treatment analyses; cumulative averaging of measurements for BMI, physical activity, and vitamin B6 intake were used for post-treatment completion analyses. BMI was dichotomized to categories of ≥ 25 kg/m2 (ie, overweight/obese) or < 25 kg/m2. Physical activity was dichotomized to categories of ≥ 9 or < 9 metabolic equivalent of task hours per week (MET‐h/wk) on the basis of prior data22 suggesting inflection of a protective hazard ratio at this level. Interaction tests were performed using the Wald test on the cross-product of the variable of interest and CTCAE- or FACT/GOG-NTX-13–reported neuropathy. Models were adjusted for age, sex, tumor-stage, node-stage, performance status, planned treatment duration (3 v 6 months of adjuvant FOLFOX), and celecoxib addition. Missing covariates were imputed by recoding into the majority category (tumor-stage) as the missing proportion was < 5%. All statistical tests were two-sided, and P values ≤ 0.05 were considered statistically significant. All analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC).
Patient registration and clinical data collection were managed and their analyses performed by the Alliance Statistics and Data Management Center. Statistical analyses were based on the study database frozen on August 10, 2020. Data quality was ensured through data review by the Alliance Statistics and Data Management Center and the study chairs (J.A.M. and A.F.S.) following Alliance policies.
All patients signed study-specific informed consent, which was approved by the NCI Cancer Treatment Evaluation Program and each participating site's institutional review board.
RESULTS
Baseline Characteristics According to Planned Treatment Duration
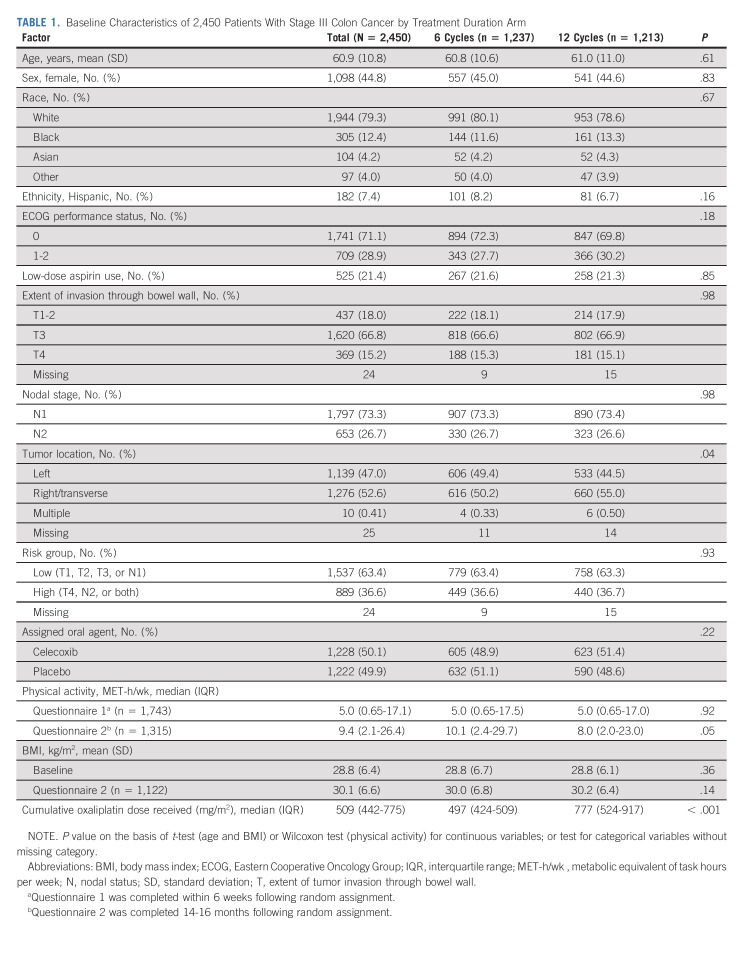
Table 1 summarizes baseline characteristics of the study cohort by planned treatment duration. Relative to patients randomly assigned to six treatments, those randomly assigned to 12 treatments were more likely to have a more proximal tumor, report engaging in less physical activity in Q2, and receive a significantly higher cumulative oxaliplatin dose.
TABLE 1.
Baseline Characteristics of 2,450 Patients With Stage III Colon Cancer by Treatment Duration Arm
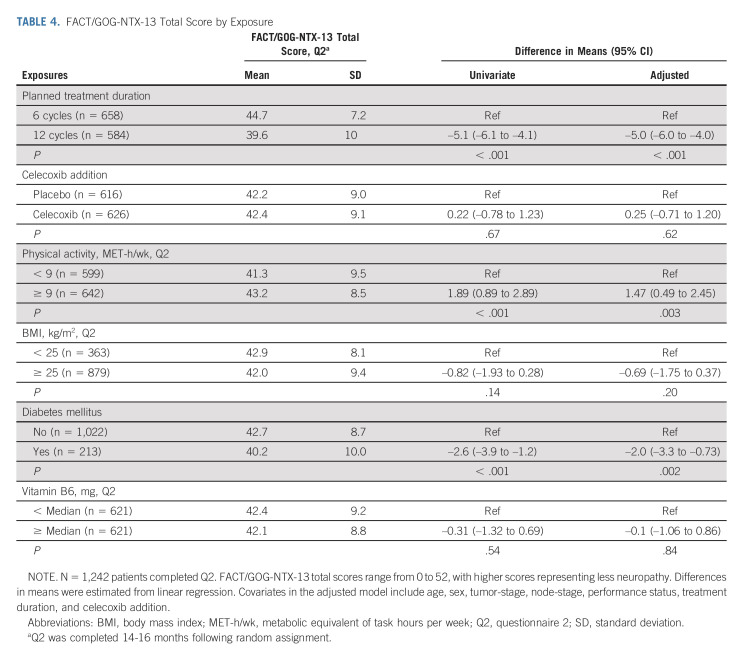
Within our cohort of 2,450 patients for CTCAE-reported neuropathy, 99.8% had at least one neuropathy measurement during chemotherapy treatment and 96.3% had at least one follow-up neuropathy measurement after completion of oxaliplatin-based therapy. Median post-treatment follow-up was 58 months (IQR, 40-68 months). One thousand two hundred forty-two patients completed the FACT/GOG-NTX-13 neurotoxicity measure. Median time from end of oxaliplatin treatment to Q2 completion was 12 months (IQR, 10-13 months). Figure 2 shows the distribution of FACT/GOG-NTX-13 scores by planned treatment duration (Fig 2A), celecoxib addition (Fig 2B), physical activity (Fig 2C), BMI (Fig 2D), diabetes (Fig 2E), and vitamin B6 intake (Fig 2F).
FIG 2.
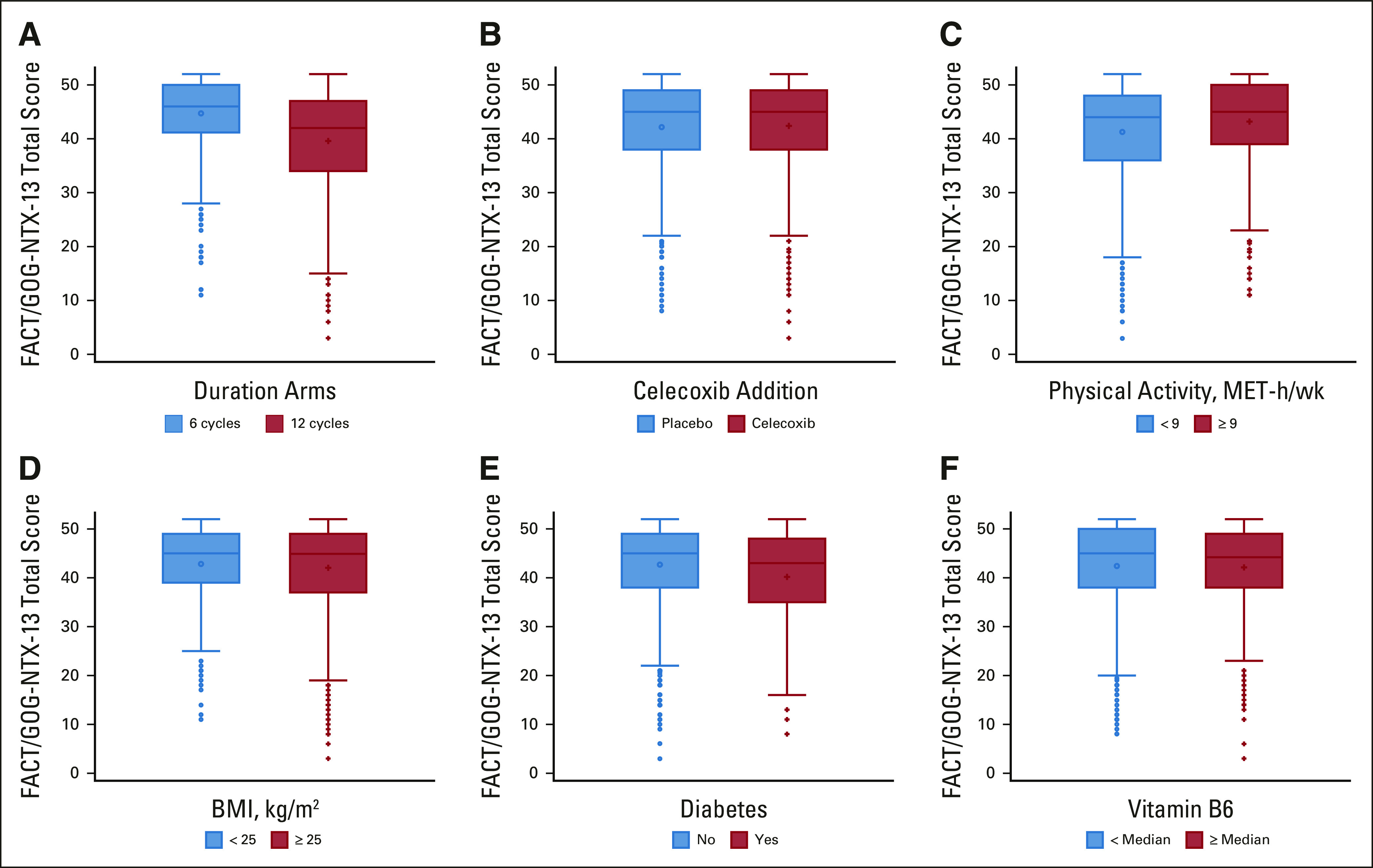
FACT/GOG-NTX-13 total score at median 12 months (IQR, 10-13 months) from last oxaliplatin dose by (A) planned treatment duration, (B) celecoxib use, (C) physical activity, (D) BMI, (E) comorbid diabetes, and (F) vitamin B6 intake. The bottom and top edges of the box indicate the IQR, the marker inside the box indicates the mean value, and the line inside the box indicates the median value. The upper and lower fences are maximum and minimum values, respectively, and points beyond the upper and lower fences are outliers. BMI, body mass index; IQR, interquartile range.
Planned Treatment Duration and OIPN
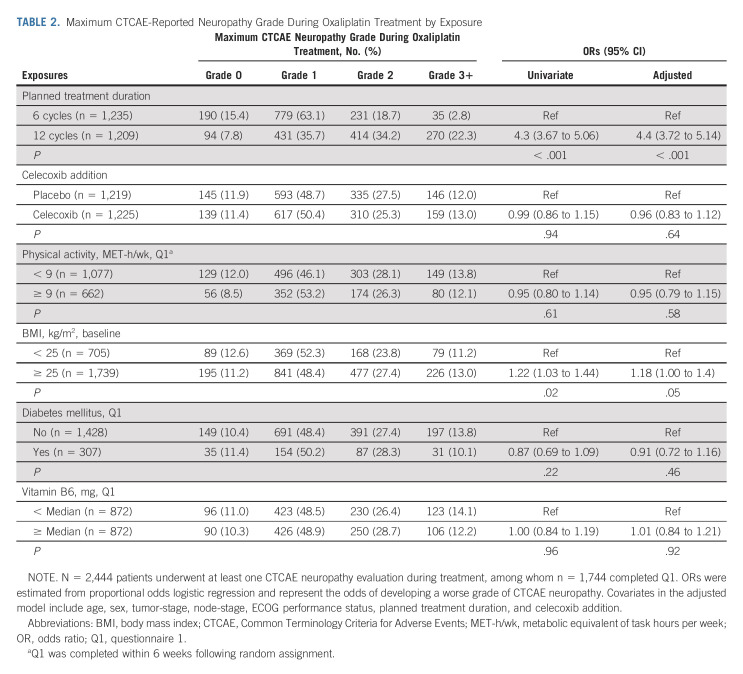
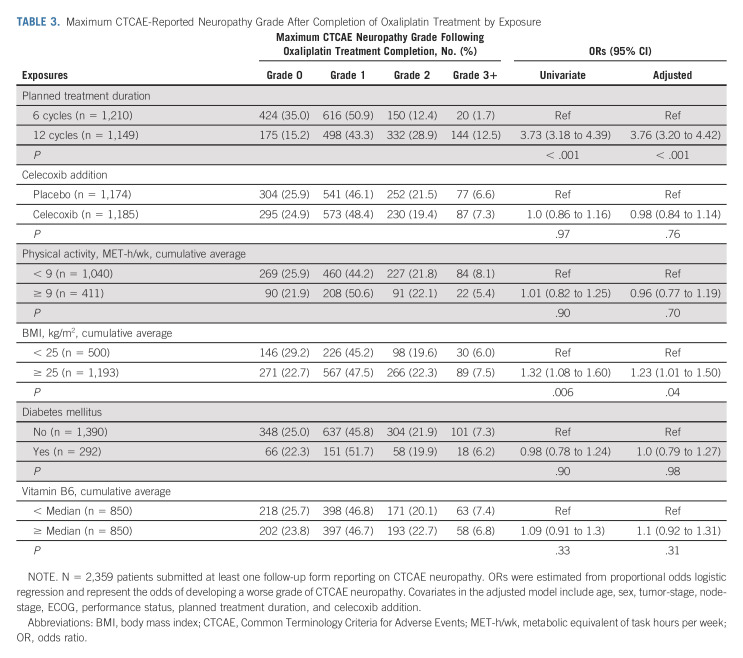
Figure 3A presents the proportion of evaluated patients with grades 1, 2, or 3+ CTCAE-reported neuropathy at each follow-up time point by planned treatment duration. Relative to patients randomly assigned to six treatments, those randomly assigned to 12 treatments were approximately four times as likely to experience a higher grade of CTCAE-reported neuropathy during (adjusted odds ratio [OR], 4.4; 95% CI, 3.72 to 5.14; P < .001; Table 2) and after completion (adjusted OR, 3.76; 95% CI, 3.20 to 4.42; P < .001) of oxaliplatin treatment (Table 3); they were also more likely to develop worse FACT/GOG-NTX-13-reported neuropathy severity (adjusted difference in means, –5.0; 95% CI, –6.0 to –4.0; P < .001; Table 4).
FIG 3.
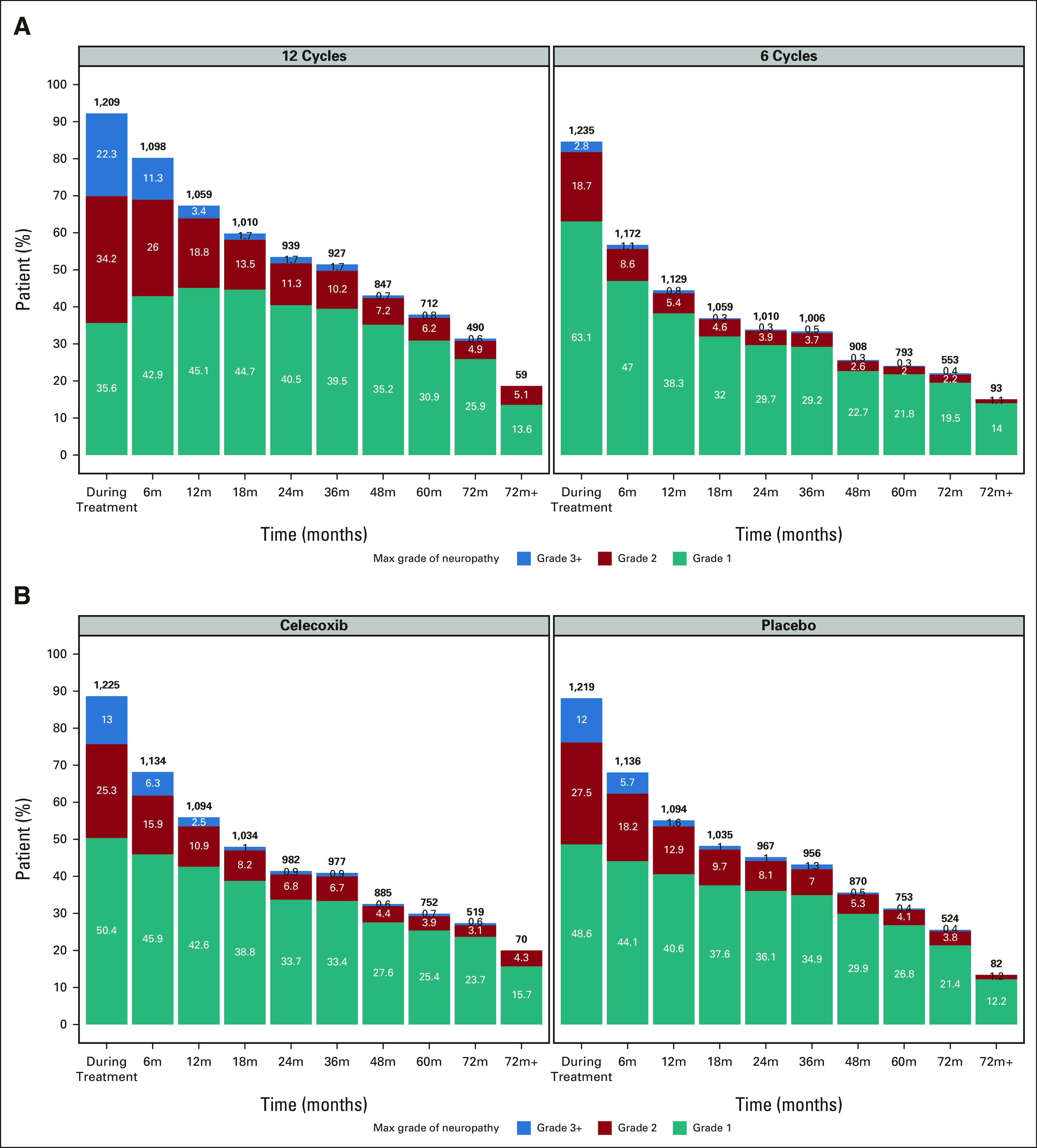
CTCAE-reported neuropathy by (A) planned treatment duration and (B) celecoxib use. The maximum grade of neuropathy during the follow-up period is presented. x-axis represents follow-up time points (eg, 6m represents the period from end of chemotherapy to 6 months after chemotherapy), and y-axis represents the proportion of evaluated patients. Numbers within each bar are the percentage of patients in each category; numbers on top of each bar are the total number of patients. Normal (grade 0) patients are not shown. CTCAE, Common Terminology Criteria for Adverse Events.
TABLE 2.
Maximum CTCAE-Reported Neuropathy Grade During Oxaliplatin Treatment by Exposure
TABLE 3.
Maximum CTCAE-Reported Neuropathy Grade After Completion of Oxaliplatin Treatment by Exposure
TABLE 4.
FACT/GOG-NTX-13 Total Score by Exposure
Patients randomly assigned to 12 treatments who had developed any degree of CTCAE-reported neuropathy during treatment additionally experienced significantly longer times to resolution (ie, to grade 0) of neuropathic symptoms (median, 14 months; IQR, 8-27 months) than patients randomly assigned to six treatments (median, 9 months; IQR, 3-17.5 months; median difference, 5 months; 95% CI, 4 to 6; P < .001). There were no significant interactions by planned duration of FOLFOX therapy between CTCAE- or FACT/GOG-NTX-13-measured neuropathy severity and celecoxib addition (Pinteraction = .61 and .06, respectively), physical activity (Pinteraction = .18 and .46, respectively), BMI (Pinteraction = .82 and .23, respectively), diabetes (Pinteraction = .67 and .20, respectively), or vitamin B6 intake (Pinteraction = .47 and .20, respectively).
Celecoxib and OIPN
Patients randomly assigned to receive celecoxib experienced similar rates of CTCAE-reported neuropathy during and after completion of oxaliplatin treatment (Tables 2 and 3) and of FACT/GOG-NTX-13-reported neuropathy (Table 4). There were no significant differences over time by celecoxib randomization interaction analyses (Fig 3B).
Other Potential Mediators of OIPN
As shown in Table 4, patients who exercised ≥ 9 MET-h/wk were statistically significantly more likely to experience lower FACT/GOG-NTX-13–reported neuropathy severity (mean score, 43.2; standard deviation [SD], 8.5) than those exercising < 9 MET-h/wk (mean score, 41.3; SD, 9.5; difference in means, 1.47; 95% CI, 0.49 to 2.45; P = .003). However, the association between physical activity and CTCAE-reported neuropathy grade was nonsignificant during both treatment and follow-up (Tables 2 and 3).
Compared with patients with a BMI < 25, those with a BMI ≥ 25 were more likely to experience higher grade CTCAE-reported neuropathy during (adjusted OR, 1.18; 95% CI, 1.00 to 1.40; P = .05) and after completion (adjusted OR, 1.23; 95% CI, 1.01 to 1.50; P = .04) of oxaliplatin treatment (Tables 2 and 3). By contrast, we observed no significant differences in FACT/GOG-NTX-13–reported neuropathy severity by BMI (Table 4).
As shown in Table 4, patients with diabetes were significantly more likely to experience worse FACT/GOG-NTX-13–reported neuropathy severity (mean, 40.2; SD, 10) relative to those without diabetes (mean, 42.7; SD, 8.7; difference in means, –2.00; 95% CI, –3.30 to –0.73; P = .002). However, we observed no differences in CTCAE-reported neuropathy grade during or after completion of oxaliplatin treatment by presence or absence of diabetes (Tables 2 and 3).
Compared with patients consuming less than the cohort median of vitamin B6 intake, those consuming an amount equal to or greater than it experienced similar grades of CTCAE-reported neuropathy during and after completion of oxaliplatin treatment as well as similar FACT/GOG-NTX-13-measured neuropathy severity (Tables 2–4).
DISCUSSION
In this prospective cohort of patients with stage III resected colon cancer enrolled in a postoperative adjuvant FOLFOX chemotherapy clinical trial, we found that longer planned treatment duration and less physical activity were associated with both worse CTCAE- and FACT/GOG-NTX-13-measured OIPN severity, while higher BMI correlated only with the former and comorbid diabetes mellitus the latter. Celecoxib addition and vitamin B6 intake were not associated with differential OIPN outcomes. To our knowledge, this is the first investigation into the associations of physical activity and vitamin B6 intake with OIPN in CRC and the largest prospective study on the correlations of celecoxib use, BMI, and diabetes with OIPN in CRC. Our findings highlight key patient, lifestyle, and treatment risk factors associated with increased OIPN severity.
The literature remains highly conflicted regarding what factors predispose patients to greater OIPN risk and severity. Increasing cumulative dose is the only characteristic that has consistently been correlated with greater chronic neurotoxicity.2-5 We similarly observed that patients randomly assigned to 12 treatments, relative to 6 treatments, were more likely to experience higher-grade neuropathy and longer times to resolution.
NSAID coadministration is another iatrogenic factor that may potentially influence the development of peripheral neuropathy via inhibition of cyclooxygenase 2–dependent prostaglandin E2.28,29 Indeed, a retrospective analysis of 52 patients with CRC treated with oxaliplatin had found NSAID use to be associated with improved sensory OIPN.15 However, in our prospective analysis of significantly greater statistical power, we found no association between random assignment to receive celecoxib and OIPN severity.
Little is known about the role of physical activity in modulating the risk of chemotherapy-induced peripheral neuropathy (CIPN) specifically in the context of CRC; however, prior studies generally underscore that physical activity may improve CIPN.30-34 Although similar findings have been reported in a cohort comprised in part by a small number of patients with CRC who had received oxaliplatin,16 no study to date has to our knowledge specifically evaluated the association between physical activity and OIPN in CRC. To our knowledge, this study is the first to report that increased physical activity is associated with decreased rates and severity of OIPN in CRC, as assessed through two independent patient-reported instruments. Exercise has been shown to alleviate chronic inflammation35 and may therefore ameliorate the neuroinflammation necessary for CIPN pathogenesis.36,37 Physical activity can also induce functional and morphologic changes in the brain such that processing of peripheral sensation is altered,38,39 potentially opposing central sensitization of peripheral neuropathy40 and thereby resulting in decreased CIPN independent of peripheral neuropathology.41 It should be noted, however, that as patients did not report the reasons for which they engaged in specific levels of physical activity, the directionality of the observed relationship between physical activity and OIPN is unclear.
Although several studies have previously examined the relationship between BMI and OIPN in CRC, they have reported conflicting findings. One retrospective investigation6 observed an inverse relationship between BMI and OIPN; however, four prospective studies7-10 of approximately 100 patients with CRC each reported a positive correlation. In our significantly larger cohort, we similarly demonstrate that a higher BMI is associated with increased severity of CTCAE-reported—although not FACT/GOG-NTX-13-measured—neuropathy. Obesity is not only independently associated with an increased risk for idiopathic neuropathy42; treatment doses are typically on the basis of body surface area—a measure of obesity—and thus the higher doses received by patients with greater BMIs may be contributing to the observed worse OIPN.
The association between diabetes mellitus and OIPN in CRC is also unclear. Although one study reported diabetes to correlate with an increased risk for OIPN,10 others have found no significant associations.11-13 Interestingly, Uwah et al14 observed that patients with diabetes developed OIPN at a lower cumulative oxaliplatin dose, although the severity of neuropathy was similar between those with and without diabetes. The findings from these studies are limited by their retrospective designs and relatively small subgroup sizes of patients with diabetes. In our prospective cohort including a much larger number of patients with diabetes, we found diabetes to be associated with greater FACT/GOG-NTX-13-reported, but not CTCAE-measured, OIPN. Although diabetic neuropathy and CIPN are distinct entities with independent pathogenic mechanisms, existing peripheral nerve injury from chronic hyperglycemia43 may be aggravated by or potentiate the clinical manifestation of oxaliplatin-associated neurotoxicity.
Vitamin B6 is a key factor in neuronal function and survival,44 and has been reported to improve neurotoxicity among patients with ovarian cancer receiving cisplatin.45 Although one prior study17 had examined the impact of a vitamin B complex supplement on CIPN among patients with cancer, only two patients in their cohort had colon cancer and the findings were not delineated to inform on their specific chemotherapy regimens. To our knowledge, our study is the first to report that vitamin B6 intake is not associated with OIPN severity in CRC, as evaluated by two independent patient-reported instruments.
Assessing OIPN in CRC through an RCT confers several strengths. By studying patients enrolled in a clinical trial, we potentially reduced the biases introduced by differences in access to health care resources unavoidable in population-based cancer registries. Moreover, as all patients in this study met the same enrollment criteria and received adjuvant FOLFOX-based chemotherapy, confounding by patient or treatment characteristics was minimized. Finally, all patients had stage III colon cancer, minimizing the effect of disease stage heterogeneity.
Our study is not without limitations. Patients who choose to enroll in clinical trials may differ from the general population: they must meet specific eligibility criteria, be chosen as appropriate candidates, and have the motivation to participate. However, CALGB 80702 enrolled patients from both community and academic centers across North America, thereby lowering the likelihood of biased sampling. There is additionally no current gold standard for OIPN evaluation, and certain facets of peripheral neuropathy—such as motor involvement and subclinical nerve damage—have been demonstrated to be suboptimally assessed via commonly used clinical tools.46,47 CTCAE is a standard tool widely used in the identification of adverse events in cancer clinical trials, and the validity of the FACT/GOG-NTX-13 subscale has been specifically demonstrated in the context of OIPN measurement among patients with CRC.27 The accuracy and reliability of symptomatic adverse events have been shown to be improved when patient-reported, with clinicians potentially under-reporting up to 50% of symptomatic adverse events.48-50 Although reporting neuropathy through the CTCAE was the standard at the time of when CALGB 80702 was being conducted, the NCI PRO-CTCAE is now more commonly incorporated in clinical trials. The presence of other residual confounding cannot be excluded; however, our findings remained consistent even after controlling for both known and suspected patient outcome predictors. We further demonstrate the potential for discordance between different measures of patient self-reported outcomes, and thereby emphasize the importance of accurate and comprehensive measurements, given the associated potential for earlier detection of treatment-related toxicities.51
In conclusion, we observed that several clinical, comorbidity, and lifestyle factors—including longer planned treatment duration with oxaliplatin, lower physical activity, higher BMI, and comorbid diabetes mellitus—are significantly negatively associated with OIPN severity or duration in this cohort of patients with stage III CRC treated within an RCT. Notably, celecoxib addition was not associated with differential OIPN outcomes. More than 90% of patients with CRC treated with oxaliplatin may develop OIPN,52 resulting in impairments in daily functioning, mental health, and quality of life.8,53-55 As patients with CRC comprise one of the largest groups of cancer survivors,56 improving our understanding of preventing and managing chronic OIPN is of especially high importance. Shorter chemotherapy durations in the management of CRC have been shown to be similarly effective as longer treatments,22,23,57 and our findings lend support for potential additional benefits through decreased chemotherapy-related toxicity and improved treatment adherence and quality of life. Our study highlights the need to more effectively identify, monitor, and manage the various patient and treatment risk factors associated with increased OIPN. Indeed, there is an ongoing randomized phase II/III trial evaluating the use of duloxetine to prevent OIPN in patients with CRC (ClinicalTrials.gov identifier: NCT04137107).
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network, Kronos Bio
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Felix Couture
Consulting or Advisory Role: Bristol Myers Squibb, Novartis Canada Pharmaceuticals Inc
Philip Kuebler
Consulting or Advisory Role: Advarra
Smitha Krishnamurthi
Research Funding: Bristol Myers Squibb (Inst), Aravive (Inst), Pfizer (Inst), Natera (Inst)
DeQuincy Lewis
Stock and Other Ownership Interests: Cellular Therapeutics, Verastem, Veru, Pieris Pharmaceuticals, Oncternal Therapeutics, Inc, Spectrum Pharmaceuticals, Curis, Precision Biosciences
Benjamin Tan
Research Funding: Eisai (Inst), Exelixis (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Adaptimmune (Inst), TYME (Inst), Agios (Inst)
Richard M. Goldberg
Stock and Other Ownership Interests: Advanced Chemotherapy Technologies
Consulting or Advisory Role: Taiho Pharmaceutical, AstraZeneca, Bayer, G1 Therapeutics, Compass Therapeutics, UpToDate, Eisai/H3 Biomedicine, Sorrento Therapeutics, Adaptimmune, IQVIA, GlaxoSmithKline, Merck
Expert Testimony: Taiho Pharmaceutical, Genentech/Roche
Alan Venook
Consulting or Advisory Role: Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Exelixis, BridgeBio Pharma, Bayer Health, Gilead Sciences, Exact Sciences, Bristol Myers Squibb Foundation/Janssen
Research Funding: Amgen
Patents, Royalties, Other Intellectual Property: Royalties from Now-UpToDate for authoring and maintaining two chapters
Eileen M. O'Reilly
Consulting or Advisory Role: Adicet Bio, AstraZeneca, Alnylam, Autem Medical, BeiGene, Berry Genomics, CytomX Therapeutics, Eisai, Exelixis, Genentech/Roche, Genoscience Pharma, Helio Health, Incyte, Ipsen, Legend Biotech, Merck, Nerviano Medical Sciences, QED Therapeutics, RedHill Biopharma, Yiviva, Novartis, Rafael Pharmaceuticals, Seattle Genetics, Boehringer Ingelheim, IDEAYA Biosciences, Noxxon Pharma, Thetis Pharma, BioSapien, Cend Therapeutics, Flatiron Health
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Silenseed (Inst), Arcus Ventures (Inst), BioNTech (Inst), Elicio Therapeutics (Inst), Parker Institute for Cancer Immunotherapy (Inst)
Uncompensated Relationships: Thetis Pharma
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences, Cogent Biosciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst), Gritstone Bio (Inst), SQZ Biotechnology (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Merck
Research Funding: Boston Biomedical (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The National Cancer Institute was involved in the design of the study and review of the manuscript. Pfizer participated in initial protocol development and review and approval of the final manuscript. Pfizer provided celecoxib and placebo tablets. Pfizer was not involved in the collection, management, analysis, or interpretation of the data. Neither Pfizer nor the National Cancer Institute had the right to veto publication or control the decision to which journal the article was submitted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology; https://acknowledgments.alliancefound.org); UG1CA189858, UG1CA233180, UG1CA233290, UG1CA233320, UG1CA233337, UG1CA233339, UG1CA189954, and U10CA180863 to the Canadian Cancer Trials Group; UG1CA233234 to the ECOG–ACRIN Cancer Research Group; U10CA180868 to NRG Oncology; and U10CA180888 and UG1CA233163 to the SWOG Cancer Research Network from the National Cancer Institute of the National Institutes of Health. Dr Meyerhardt is supported by the Douglas Gray Woodruff Chair fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, and the George Stone Family Foundation.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data are from the Alliance for Clinical Trials in Oncology. Investigators may request access to these data per Alliance protocol as outlined below and as detailed at https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=%2FPublic%2FDatasharing. Per NCI National Clinical Trials Network (NCTN) guidelines, any investigator may submit a request for data from published Alliance or legacy ACOSOG, CALGB, or NCCTG trials. To submit a data request, the investigator should complete an Alliance Data Sharing Request Form and send it by e-mail to gro. NTCNecnailla@stpecnoc. Once received, the request will be forwarded to the Alliance Statistics and Data Center (SDC). The SDC will confirm the availability of the data. Once the SDC confirms availability, the investigator will be asked to provide documentation of institutional review board (IRB) approval or exemption from their institution, as well as to submit an Alliance data release agreement. Once the IRB documentation and the data release agreement are received from the requesting investigator, the SDC will be notified that the requested data may be released. Questions about the process may be directed to gro. NTCNecnailla@stpecnoc.
AUTHOR CONTRIBUTIONS
Conception and design: Seohyuk Lee, Chao Ma, Qian Shi, Philip Kuebler, Richard M. Goldberg, Anthony F. Shields, Jeffrey A. Meyerhardt
Financial support: Jeffrey A. Meyerhardt
Administrative support: Qian Shi, Richard M. Goldberg, Jeffrey A. Meyerhardt
Provision of study materials or patients: Felix Couture, Philip Kuebler, Smitha Krishnamurthi, Benjamin Tan, Richard M. Goldberg, Alan Venook, Charles Blanke, Jeffrey A. Meyerhardt
Collection and assembly of data: Seohyuk Lee, Chao Ma, Felix Couture, Philip Kuebler, Smitha Krishnamurthi, DeQuincy Lewis, Benjamin Tan, Richard M. Goldberg, Alan Venook, Anthony F. Shields, Jeffrey A. Meyerhardt
Data analysis and interpretation: Seohyuk Lee, Chao Ma, Qian Shi, Pankaj Kumar, Smitha Krishnamurthi, Benjamin Tan, Richard M. Goldberg, Alan Venook, Charles Blanke, Eileen M. O'Reilly, Anthony F. Shields, Jeffrey A. Meyerhardt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Potential Mediators of Oxaliplatin-Induced Peripheral Neuropathy from Adjuvant Therapy in Stage III Colon Cancer: Findings from CALGB (Alliance)/SWOG 80702
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network, Kronos Bio
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Felix Couture
Consulting or Advisory Role: Bristol Myers Squibb, Novartis Canada Pharmaceuticals Inc
Philip Kuebler
Consulting or Advisory Role: Advarra
Smitha Krishnamurthi
Research Funding: Bristol Myers Squibb (Inst), Aravive (Inst), Pfizer (Inst), Natera (Inst)
DeQuincy Lewis
Stock and Other Ownership Interests: Cellular Therapeutics, Verastem, Veru, Pieris Pharmaceuticals, Oncternal Therapeutics, Inc, Spectrum Pharmaceuticals, Curis, Precision Biosciences
Benjamin Tan
Research Funding: Eisai (Inst), Exelixis (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Adaptimmune (Inst), TYME (Inst), Agios (Inst)
Richard M. Goldberg
Stock and Other Ownership Interests: Advanced Chemotherapy Technologies
Consulting or Advisory Role: Taiho Pharmaceutical, AstraZeneca, Bayer, G1 Therapeutics, Compass Therapeutics, UpToDate, Eisai/H3 Biomedicine, Sorrento Therapeutics, Adaptimmune, IQVIA, GlaxoSmithKline, Merck
Expert Testimony: Taiho Pharmaceutical, Genentech/Roche
Alan Venook
Consulting or Advisory Role: Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Exelixis, BridgeBio Pharma, Bayer Health, Gilead Sciences, Exact Sciences, Bristol Myers Squibb Foundation/Janssen
Research Funding: Amgen
Patents, Royalties, Other Intellectual Property: Royalties from Now-UpToDate for authoring and maintaining two chapters
Eileen M. O'Reilly
Consulting or Advisory Role: Adicet Bio, AstraZeneca, Alnylam, Autem Medical, BeiGene, Berry Genomics, CytomX Therapeutics, Eisai, Exelixis, Genentech/Roche, Genoscience Pharma, Helio Health, Incyte, Ipsen, Legend Biotech, Merck, Nerviano Medical Sciences, QED Therapeutics, RedHill Biopharma, Yiviva, Novartis, Rafael Pharmaceuticals, Seattle Genetics, Boehringer Ingelheim, IDEAYA Biosciences, Noxxon Pharma, Thetis Pharma, BioSapien, Cend Therapeutics, Flatiron Health
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Silenseed (Inst), Arcus Ventures (Inst), BioNTech (Inst), Elicio Therapeutics (Inst), Parker Institute for Cancer Immunotherapy (Inst)
Uncompensated Relationships: Thetis Pharma
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences, Cogent Biosciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst), Gritstone Bio (Inst), SQZ Biotechnology (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Merck
Research Funding: Boston Biomedical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Staff NP, Cavaletti G, Islam B, et al. Platinum-induced peripheral neurotoxicity: From pathogenesis to treatment. J Peripher nervous Syst. 2019;24(suppl 2):S26–S39. doi: 10.1111/jns.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pulvers JN, Marx G. Factors associated with the development and severity of oxaliplatin-induced peripheral neuropathy: A systematic review. Asia Pac J Clin Oncol. 2017;13:345–355. doi: 10.1111/ajco.12694. [DOI] [PubMed] [Google Scholar]
- 3. Besora S, Santos C, Izquierdo C, et al. Rechallenge with oxaliplatin and peripheral neuropathy in colorectal cancer patients. J Cancer Res Clin Oncol. 2018;144:1793–1801. doi: 10.1007/s00432-018-2691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsu SY, Huang WS, Lee SH, et al. Incidence, severity, longitudinal trends and predictors of acute and chronic oxaliplatin-induced peripheral neuropathy in Taiwanese patients with colorectal cancer. Eur J Cancer Care (Engl) 2019;28:e12976. doi: 10.1111/ecc.12976. [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi K, Kusaba H, Makiyama A, et al. The risk factors for oxaliplatin-induced peripheral sensory neuropathy and thrombocytopenia in advanced gastric cancer. Cancer Chemother Pharmacol. 2018;82:625–633. doi: 10.1007/s00280-018-3652-2. [DOI] [PubMed] [Google Scholar]
- 6. Alejandro LM, Behrendt CE, Chen K, et al. Predicting acute and persistent neuropathy associated with oxaliplatin. Am J Clin Oncol. 2013;36:331–337. doi: 10.1097/COC.0b013e318246b50d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahriari-Ahmadi A, Fahimi A, Payandeh M, et al. Prevalence of oxaliplatin-induced chronic neuropathy and influencing factors in patients with colorectal cancer in Iran. Asian Pac J Cancer Prev. 2015;16:7603–7606. doi: 10.7314/apjcp.2015.16.17.7603. [DOI] [PubMed] [Google Scholar]
- 8. Kerckhove N, Selvy M, Lambert C, et al. Colorectal cancer survivors suffering from sensory chemotherapy-induced peripheral neuropathy are not a homogenous group: Secondary analysis of patients' profiles with oxaliplatin-induced peripheral neuropathy. Front Pharmacol. 2021;12:744085. doi: 10.3389/fphar.2021.744085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ben Mahmoud IT, Ben Said A, Berguiga S, et al. Incidence and risk factors associated with development of oxalipatin-induced acute peripheral neuropathy in colorectal cancer patients. J Oncol Pharm Pract. doi: 10.1177/10781552211068138. epub ahead of print on December 17, 2021. [DOI] [PubMed] [Google Scholar]
- 10. Ottaiano A, Nappi A, Tafuto S, et al. Diabetes and body mass index are associated with neuropathy and prognosis in colon cancer patients treated with capecitabine and oxaliplatin adjuvant chemotherapy. Oncology. 2016;90:36–42. doi: 10.1159/000442527. [DOI] [PubMed] [Google Scholar]
- 11. Vincenzi B, Frezza AM, Schiavon G, et al. Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer. 2013;21:1313–1319. doi: 10.1007/s00520-012-1667-5. [DOI] [PubMed] [Google Scholar]
- 12. Ramanathan RK, Rothenberg ML, de Gramont A, et al. Incidence and evolution of oxaliplatin-induced peripheral sensory neuropathy in diabetic patients with colorectal cancer: A pooled analysis of three phase III studies. Ann Oncol. 2010;21:754–758. doi: 10.1093/annonc/mdp509. [DOI] [PubMed] [Google Scholar]
- 13. Soveri LM, Lamminmäki A, Hänninen UA, et al. Long-term neuropathy and quality of life in colorectal cancer patients treated with oxaliplatin containing adjuvant chemotherapy. Acta Oncol. 2019;58:398–406. doi: 10.1080/0284186X.2018.1556804. [DOI] [PubMed] [Google Scholar]
- 14. Uwah AN, Ackler J, Leighton JC, Jr, et al. The effect of diabetes on oxaliplatin-induced peripheral neuropathy. Clin Colorectal Cancer. 2012;11:275–279. doi: 10.1016/j.clcc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 15. Kanbayashi Y, Hosokawa T, Okamoto K, et al. Statistical identification of predictors for peripheral neuropathy associated with administration of bortezomib, taxanes, oxaliplatin or vincristine using ordered logistic regression analysis. Anticancer Drugs. 2010;21:877–881. doi: 10.1097/CAD.0b013e32833db89d. [DOI] [PubMed] [Google Scholar]
- 16. Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support Care Cancer. 2018;26:615–624. doi: 10.1007/s00520-017-3875-5. [DOI] [PubMed] [Google Scholar]
- 17. Schloss JM, Colosimo M, Airey C, et al. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN) Support Care Cancer. 2017;25:195–204. doi: 10.1007/s00520-016-3404-y. [DOI] [PubMed] [Google Scholar]
- 18. Meyerhardt JA, Shi Q, Fuchs CS, et al. Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: The CALGB/SWOG 80702 (Alliance) randomized clinical trial. JAMA. 2021;325:1277–1286. doi: 10.1001/jama.2021.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177–1188. doi: 10.1056/NEJMoa1713709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. André T, Meyerhardt J, Iveson T, et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): Final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020;21:1620–1629. doi: 10.1016/S1470-2045(20)30527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 22. Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 23. Meyerhardt JA, Heseltine D, Campos H, et al. Assessment of a dietary questionnaire in cancer patients receiving cytotoxic chemotherapy. J Clin Oncol. 2005;23:8453–8460. doi: 10.1200/JCO.2005.02.5460. [DOI] [PubMed] [Google Scholar]
- 24. Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 25. Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 26. Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: Findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaiser K, Lyleroehr M, Shaunfield S, et al. Neuropathy experienced by colorectal cancer patients receiving oxaliplatin: A qualitative study to validate the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity scale. World J Gastrointest Oncol. 2020;12:205–218. doi: 10.4251/wjgo.v12.i2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma W, Quirion R. Does COX2-dependent PGE2 play a role in neuropathic pain? Neurosci Lett. 2008;437:165–169. doi: 10.1016/j.neulet.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 29. Vo T, Rice ASC, Dworkin RH. Non-steroidal anti-inflammatory drugs for neuropathic pain: How do we explain continued widespread use? Pain. 2009;143:169–171. doi: 10.1016/j.pain.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 30. Lin W-L, Wang R-H, Chou F-H, et al. The effects of exercise on chemotherapy-induced peripheral neuropathy symptoms in cancer patients: A systematic review and meta-analysis. Support Care Cancer. 2021;29:5303–5311. doi: 10.1007/s00520-021-06082-3. [DOI] [PubMed] [Google Scholar]
- 31. Dhawan S, Andrews R, Kumar L, et al. A randomized controlled trial to assess the effectiveness of muscle strengthening and balancing exercises on chemotherapy-induced peripheral neuropathic pain and quality of life among cancer patients. Cancer Nurs. 2020;43:269–280. doi: 10.1097/NCC.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 32. Schwenk M, Grewal GS, Holloway D, et al. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: A randomized controlled trial. Gerontology. 2016;62:553–563. doi: 10.1159/000442253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: A multicenter, randomized controlled trial. Support Care Cancer. 2018;26:1019–1028. doi: 10.1007/s00520-017-4013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mols F, Beijers AJM, Vreugdenhil G, et al. Chemotherapy-induced peripheral neuropathy, physical activity and health-related quality of life among colorectal cancer survivors from the PROFILES registry. J Cancer Survivorship. 2015;9:512–522. doi: 10.1007/s11764-015-0427-1. [DOI] [PubMed] [Google Scholar]
- 35. Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 36. Lees JG, Makker PG, Tonkin RS, et al. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer. 2017;73:22–29. doi: 10.1016/j.ejca.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 37. Wang XM, Lehky TJ, Brell JM, et al. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59:3–9. doi: 10.1016/j.cyto.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holschneider DP, Yang J, Guo Y, et al. Reorganization of functional brain maps after exercise training: Importance of cerebellar-thalamic-cortical pathway. Brain Res. 2007;1184:96–107. doi: 10.1016/j.brainres.2007.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kleckner IR, Zhang J, Touroutoglou A, et al. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav. 2017;1:0069. doi: 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cathcart-Rake EJ, Hilliker DR, Loprinzi CL. Chemotherapy-induced neuropathy: Central resolution of a peripherally perceived problem? Cancer. 2017;123:1898–1900. doi: 10.1002/cncr.30650. [DOI] [PubMed] [Google Scholar]
- 42. Callaghan BC, Reynolds E, Banerjee M, et al. Central obesity is associated with neuropathy in the severely obese. Mayo Clin Proc. 2020;95:1342–1353. doi: 10.1016/j.mayocp.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: Where are we now and where to go? J Diabetes Investig. 2011;2:18–32. doi: 10.1111/j.2040-1124.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baltrusch S. The role of neurotropic B vitamins in nerve regeneration. Biomed Res Int. 2021;2021:9968228. doi: 10.1155/2021/9968228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiernik PH, Yeap B, Vogl SE, et al. Hexamethylmelamine and low or moderate dose cisplatin with or without pyridoxine for treatment of advanced ovarian carcinoma: A study of the Eastern Cooperative Oncology Group. Cancer Invest. 1992;10:1–9. doi: 10.3109/07357909209032783. [DOI] [PubMed] [Google Scholar]
- 46. Molassiotis A, Cheng HL, Lopez V, et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019;19:132. doi: 10.1186/s12885-019-5302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burgess J, Ferdousi M, Gosal D, et al. Chemotherapy-induced peripheral neuropathy: Epidemiology, pathomechanisms and treatment. Oncol Ther. 2021;9:385–450. doi: 10.1007/s40487-021-00168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Atkinson TM, Li Y, Coffey CW, et al. Reliability of adverse symptom event reporting by clinicians. Qual Life Res. 2012;21:1159–1164. doi: 10.1007/s11136-011-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33:910–915. doi: 10.1200/JCO.2014.57.9334. [DOI] [PubMed] [Google Scholar]
- 50. Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 51. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. PAIN. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 53. Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 54. Tofthagen C, Donovan KA, Morgan MA, et al. Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21:3307–3313. doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bennett BK, Park SB, Lin CS, et al. Impact of oxaliplatin-induced neuropathy: A patient perspective. Support Care Cancer. 2012;20:2959–2967. doi: 10.1007/s00520-012-1428-5. [DOI] [PubMed] [Google Scholar]
- 56. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 20192019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 57. Iveson TJ, Sobrero AF, Yoshino T, et al. Duration of adjuvant doublet chemotherapy (3 or 6 months) in patients with high-risk stage II colorectal cancer. J Clin Oncol. 2021;39:631–641. doi: 10.1200/JCO.20.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are from the Alliance for Clinical Trials in Oncology. Investigators may request access to these data per Alliance protocol as outlined below and as detailed at https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=%2FPublic%2FDatasharing. Per NCI National Clinical Trials Network (NCTN) guidelines, any investigator may submit a request for data from published Alliance or legacy ACOSOG, CALGB, or NCCTG trials. To submit a data request, the investigator should complete an Alliance Data Sharing Request Form and send it by e-mail to gro. NTCNecnailla@stpecnoc. Once received, the request will be forwarded to the Alliance Statistics and Data Center (SDC). The SDC will confirm the availability of the data. Once the SDC confirms availability, the investigator will be asked to provide documentation of institutional review board (IRB) approval or exemption from their institution, as well as to submit an Alliance data release agreement. Once the IRB documentation and the data release agreement are received from the requesting investigator, the SDC will be notified that the requested data may be released. Questions about the process may be directed to gro. NTCNecnailla@stpecnoc.