1. INTRODUCTION
Targeted therapies such as ALK inhibitors show dramatic responses in various cancers; however, long‐term administration can be associated with acquired drug resistance. Crizotinib is the first ALK inhibitor reported as salvage therapy for refractory anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma (ALK+ALCL). It has been reported to have high complete response (CR) rates (approximately 80%) for paediatric patients with relapsed/refractory ALK+ALCL[1]. However, crizotinib resistance has been reported as a cause of several acquired ALK mutations, including the L1196M ALK mutation known as the gatekeeper mutation in the ALK kinase domain[2, 3]. To overcome crizotinib resistance, alectinib was developed as a second‐generation ALK inhibitor. It inhibits various crizotinib‐resistant ALK mutations, including L1196M[4]. Alectinib‐resistant ALK mutations have not been reported in patients with ALK+ALCL, and several patients have been reported to have survived more than 1 year with continuous alectinib administration as well‐tolerated maintenance therapy[5, 6]. Here, we report a paediatric patient with relapsed/refractory ALK+ALCL who acquired L1196M ALK mutation and relapsed during alectinib administration.
1.1. Case
A 10‐year‐old boy was admitted to our hospital with a fever and post‐auricular swelling. He was subsequently diagnosed with nucleophosmin (NPM)‐ALK‐rearranged small cell variant of ALK+ALCL and leukemic presentation, whom we have previously reported[7]. We monitored his serum‐soluble interleukin‐2 receptor (sIL‐2R) levels, the number of tumour cells in the blood using flow cytometric analysis (small‐sized tumour cells; CD30‐5‐8+, large‐sized tumour cells; CD30+), and the expression levels of NPM‐ALK mRNA transcripts in the tumour cells by droplet digital polymerase chain reaction (ddPCR) over time as markers of treatment efficacy (Figure S1). Although he showed a refractory treatment course during the ALCL99 chemotherapy protocol, he achieved first CR by alectinib administration and successfully underwent allogeneic bone marrow transplantation (BMT).
Alectinib administration was ceased at the beginning of conditioning therapy in BMT, as previously reported[8]. Engraftment was confirmed 17 days post‐transplantation without NPM‐ALK mRNA transcripts in the peripheral blood; however, he relapsed 40 days post‐transplantation with a small cell variant of ALK+ALCL and leukemic presentation. Re‐administration of alectinib resulted in a second CR. Therefore, he continued alectinib administration to maintain remission. Five months after the first relapse, he complained of a high fever and persistent cough. Computed tomography revealed several pulmonary tumours in his right lobe. Pathological examination revealed that the pulmonary tumours were common type ALK+ALCL that consisted of only large‐sized tumour cells despite alectinib administration. At the second relapse, neither tumour cells nor NPM‐ALK mRNA transcripts were detected in the blood. He underwent vinblastine administration weekly and successfully achieved a third CR.
To elucidate the mechanism for the relapse despite alectinib administration, RNA sequencing analysis was performed for each of the small‐sized tumour cells purified as CD30 negative population at onset, large‐sized tumour cells purified as CD30 positive population at onset, small‐sized tumour cells at the first relapse and large‐sized tumour cells of pulmonary tumours at the second relapse. The cell separation targeting CD30 was performed as previously reported[7]. RNA sequencing analysis revealed that the large‐sized tumour cells of the pulmonary tumour had acquired L1196M mutation in the ALK gene (Table 1). I1461V, K1491R, and D1529E ALK variants, estimated as benign variants in ClinVar, were also detected in all tumour samples from onset to the second relapse. These data indicated that the large‐sized tumour cells that constituted a pulmonary tumour at the second relapse derived from the leukemic lymphoma cells of a small cell variant of ALK+ALCL.
TABLE 1.
SNPs of the ALK gene of each tumour sample by RNA sequence analysis
| Sample | Mutation of ALK gene | ClinVar | Nucleotide change | rs number |
|---|---|---|---|---|
| Small‐sized tumour cells at onset | D1529E | Benign | c.4587C > G | rs1881421 |
| K1491R | Benign | c.4472A > G | rs1881420 | |
| I1461V | Benign | c.4381A > G | rs1670283 | |
| Large‐sized tumour cells at onset | D1529E | Benign | c.4587C > G | rs1881421 |
| K1491R | Benign | c.4472A > G | rs1881420 | |
| I1461V | Benign | c.4381A > G | rs1670283 | |
| Small‐sized tumour cells at the first relapse | D1529E | Benign | c.4587C > G | rs1881421 |
| K1491R | Benign | c.4472A > G | rs1881420 | |
| I1461V | Benign | c.4381A > G | rs1670283 | |
| Large‐sized tumour cells of the pulmonary tumour at the second relapse | L1196M | Likely pathogenic | c.3586C > A | rs1057519784 |
| D1529E | Benign | c.4587C > G | rs1881421 | |
| K1491R | Benign | c.4472A > G | rs1881420 | |
| I1461V | Benign | c.4381A > G | rs1670283 |
Note: Large tumour cells of the pulmonary tumour at the second relapse acquired an L1196M mutation in the ALK gene.
Abbreviation: SNPs, single nucleotide polymorphisms.
Next, to reveal when L1196M ALK mutation emerged in lymphoma cells over time, ddPCR analysis using an L1196M ALK mutation detection probe set (LBx Probe ALK L1196M (A071); Riken Genesis, Tokyo, Japan) was performed for each of the blood cell samples preserved over time and the pulmonary lymphoma sample at the second relapse (Figure 1). As a result, L1196M ALK mutation was detected only in the pulmonary lymphoma sample and not in any of the blood samples, including the sample at the second relapse. Therefore, we concluded that the acquired L1196M ALK mutation induced alectinib resistance.
FIGURE 1.
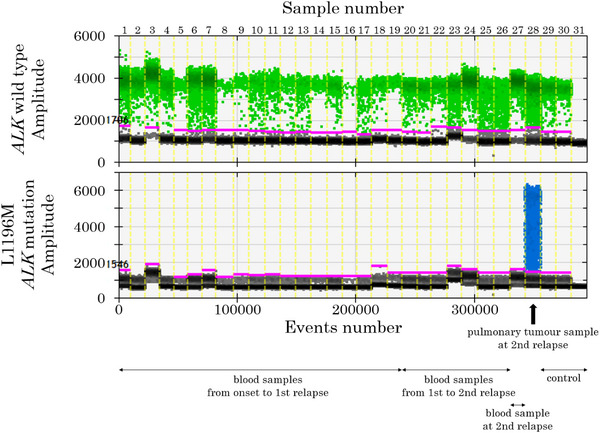
Droplets plot of the digital droplet polymerase chain reaction assay. ALK wild‐type positive and negative droplets are presented as green and grey dots, respectively. L1196M ALK mutation positive and negative droplets are presented as blue and grey dots, respectively. Thirty‐one samples were analysed. Sample number one is the large‐sized tumour cells purified as the CD30 positive population from the blood sample at onset; sample number two is the small‐sized tumour cells purified as the CD30 negative population from the blood cells at onset; sample numbers three to 19 are the blood samples from onset to the first relapse over time, days 1, 7, 13, 21, 28, 33, 41, 52, 73, 79, 91, 96, 112, 118, 126, 142, and 161 from treatment initiation, respectively; sample numbers 20–26 are blood samples from the first to the second relapse over time, days 189, 205, 215, 219, 221, 243, and 272 from treatment initiation, respectively; sample number 27 is the blood sample at the second relapse; sample number 28 is the pulmonary tumour sample; sample number 29 is the lymph node lesion of another patient with common type anaplastic large cell lymphoma; sample number 30 is a blood sample from a healthy donor; sample number 31 is a negative control (distilled water).
2. DISCUSSION
This is the first report of alectinib resistance in a patient with ALK+ALCL caused by ALK mutation. Alectinib has been reported to be effective for crizotinib‐resistant ALK mutation, including L1196M gatekeeper mutation, and shows objective responses in crizotinib‐resistant ALK‐rearranged non‐small cell lung cancers (NSCLCs)[4, 9]. While L1196M ALK mutation was reported to induce alectinib resistance in a patient with ALK‐rearranged NSCLCs[10]. In a previous study that investigated the susceptibility of EML4‐ALK expressing cells with various ALK mutations to each ALK inhibitor, cells with L1196M ALK mutation were found to be more susceptible to alectinib than crizotinib but cells with L1196M ALK mutation were less susceptible to alectinib than cells with wild‐type ALK [10]. Therefore, reduced alectinib susceptibility by acquired L1196M ALK mutation could contribute to a second relapse during alectinib therapy in our patient.
In our patient, the morphology of ALK+ALCL transformed from a small cell variant with leukemic presentation to a common type at the second relapse. Although the mechanisms of morphologic transformation were unclear, morphologic transformation at relapse has been previously reported in a few patients with ALK+ALCL[11, 12]. High ALK amplification is known to decrease susceptibility to ALK inhibitors[9]. Our previous study revealed that large‐sized tumour cells expressed ten‐fold more NPM‐ALK mRNA transcripts than small‐sized tumour cells, indicating that large‐sized tumour cells acquiring L1196M ALK mutation can survive alectinib administration[7].
As continuous alectinib administration may induce ALK gatekeeper mutation, including L1196M, we need to establish an exit strategy for alectinib. Moreover, EML4‐ALK expressing cells with various ALK mutations including L1196M show higher susceptibility to other ALK inhibitors such as ceritinib, brigatinib and lorlatinib, which are off‐label for ALK+ALCL, than alectinib[10]. Therefore, they may become alternative candidates as salvage therapy for refractory ALK+ALCL cases.
3. CONCLUSION
L1196M ALK mutation, a gatekeeper mutation, was acquired during alectinib administration in ALK+ALCL. The establishment of ‘exit strategies’ for ALK inhibitors is warranted to overcome acquired resistant ALK mutation.
AUTHOR CONTRIBUTIONS
K.N. wrote the manuscript. M.T., Y.S., T.F., R.K., T.A. and S.S. provided care to the patient. H.I. provided pathological diagnosis and analysis. Y.I. was the principal investigator and takes primary responsibility for the paper. T.W. is the chief of our department and provided advice on this study.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Supporting Information
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mossé YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol. 2017;35:3215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kay M, Dehghanian F. Exploring the crizotinib resistance mechanism of NSCLC with the L1196M mutation using molecular dynamics simulation. J Mol Model. 2017;23:323. [DOI] [PubMed] [Google Scholar]
- 3. Zdzalik D, Dymek B, Grygielewicz P, Gunerka P, Bujak A, Lamparska‐Przybysz M, et al. Activating mutations in ALK kinase domain confer resistance to structurally unrelated ALK inhibitors in NPM‐ALK‐positive anaplastic large‐cell lymphoma. J Cancer Res Clin Oncol. 2014;140:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–90. [DOI] [PubMed] [Google Scholar]
- 5. Fukano R, Mori T, Sekimizu M, Choi I, Kada A, Saito AM, et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma: an open‐label phase II trial. Cancer Sci. 2020;111:4540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saito S, Tashiro H, Sumiyoshi R, Matsuo T, Yamamoto T, Matsumoto K, et al. Second allogeneic transplantation using umbilical cord blood for a patient with relapsed ALK+ anaplastic large cell lymphoma after allogeneic bone marrow transplantation in the era of ALK inhibitors: a case report. Medicine (Baltimore). 2021;100:e25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noguchi K, Ikawa Y, Takenaka M, Sakai Y, Fujiki T, Kuroda R, et al. Characterisation of two tumour cell populations in the small cell variant of anaplastic lymphoma kinase‐positive anaplastic large cell lymphoma. Br J Haematol. 2022;196:241–3. [DOI] [PubMed] [Google Scholar]
- 8. Nakai R, Fukuhara S, Maeshima AM, Kim SW, Ito Y, Hatta S, et al. Alectinib, an anaplastic lymphoma kinase (ALK) inhibitor, as a bridge to allogeneic stem cell transplantation in a patient with ALK‐positive anaplastic large‐cell lymphoma refractory to chemotherapy and brentuximab vedotin. Clin Case Rep. 2019;7:2500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov. 2016;6:1118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falini B. Anaplastic large cell lymphoma: pathological, molecular and clinical features. Br J Haematol. 2001;114:741–60. [DOI] [PubMed] [Google Scholar]
- 12. Benharroch D, Meguerian‐Bedoyan Z, Lamant L, Amin C, Brugières L, Terrier‐Lacombe MJ, et al. ALK‐positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998;91:2076–84. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


