PURPOSE
Patients with bulky stage I/II classic Hodgkin lymphoma (cHL) are typically treated with chemotherapy followed by radiation. Late effects associated with radiotherapy include increased risk of second cancer and cardiovascular disease. We tested a positron emission tomography (PET)–adapted approach in patients with bulky, early-stage cHL, omitting radiotherapy in patients with interim PET-negative (PET−) disease and intensifying treatment in patients with PET-positive (PET+) disease.
METHODS
Eligible patients with bulky disease (mass > 10 cm or 1/3 the maximum intrathoracic diameter on chest x-ray) received two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by interim fluorodeoxyglucose PET (PET2). Patients with PET2–, defined as 1-3 on the 5-point scale, received four additional cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine. Patients with PET2+ received four cycles of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone followed by 30.6 Gy involved-field radiation.
RESULTS
Of 94 evaluable patients, 53% were female with median age 30 years (range, 18-58 years). Eight-five (90%) had stage II disease, including 48 (51%) with stage IIB/IIBE. Seventy-eight (78%) were PET2– and 21 (22%) were PET2+. The predominant toxicity was neutropenia, with 9% of patients developing febrile neutropenia and one developing sepsis. The primary end point of 3-year progression-free survival (PFS) was 93.1% in PET2– and 89.7% in PET2+ patients. Three-year overall survival was 98.6% and 94.4%, respectively. The estimated hazard ratio comparing PFS of patients with PET2+ and patients with PET2− was 1.03 (85% upper bound 2.38) and was significantly less than the null hypothesis of 4.1 (one-sided P = .04).
CONCLUSION
Our study of PET-adapted therapy in bulky stage I/II cHL met its primary goal and was associated with an excellent 3-year PFS rate of 92.3% in all patients, with the majority being spared radiotherapy and exposure to intensified chemotherapy.
INTRODUCTION
Bulky mediastinal adenopathy is a well-established risk factor in patients with early-stage classic Hodgkin lymphoma (cHL).1,2 Historically, such patients have been treated with chemotherapy followed by radiation. The US Eastern Cooperative Oncology Group ECOG 2496 study randomly assigned 264 patients with bulky mediastinal disease to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) versus Stanford V followed by consolidation with 36 Gy of involved-field radiation.3 The 5-year failure-free survival rate was 86% versus 79% (P = .22), respectively.
CONTEXT
Key Objective
Our study was, to our knowledge, the first trial focused specifically on patients with bulky stage I/II classic Hodgkin lymphoma testing a positron emission tomography (PET)–adapted approach with omission of radiotherapy in early PET-negative patients and intensification of therapy in PET-positive patients.
Knowledge Generated
The majority of patients were interim PET-negative and achieved excellent progression-free survival with doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy without radiation. For the fewer than 25% of patients who were interim PET-positive, outcomes were similar with intensified chemotherapy followed by radiation.
Relevance (J.W. Friedberg)
-
This National Clinical Trials Network prospective study extends a growing experience eliminating radiation therapy for most patients with early-stage Hodgkin lymphoma to the setting of bulky disease presentations. Prolonged follow-up of these studies, along with registry cohorts, will enable risk/benefit discussions with patients and an individualized approach to therapy.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Late effects of radiotherapy to the chest are well documented and include risk of secondary malignancies, particularly breast cancer in women treated under the age of 30 years.4,5 Cardiovascular complications include accelerated coronary artery disease, as well as arrhythmias, valvular disease, and pericardial disease. Radiation-associated pneumonitis and fibrosis may also occur in these patients. Modern radiotherapy techniques, in combination with the use of lower doses and smaller fields, will likely result in lower rates of late toxicity, but the impact of these advances will take years to fully evaluate and no technique will completely avoid radiation (RT) to the breast and heart in patients with extensive mediastinal disease.
Given the prognostic impact of interim positron emission tomography (PET) scans after two cycles of chemotherapy (PET2) on progression-free survival, a number of trials have evaluated risk-adapted therapy with de-escalation of treatment in patients with PET2-negative (PET2−) and intensified approaches in patients with PET2-positive (PET2+).6-10 Cancer and Leukemia Group B (CALBG) 50604 tested this strategy in 149 patients with nonbulky stage I and II disease.11 The 3-year progression-free survival (PFS) rate in the 135 (91%) patients with PET2− treated with four cycles of ABVD without radiotherapy was 91%. The 14 (9%) patients with PET2+ received two cycles of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (escBEACOPP) followed by involved-field radiation therapy (IFRT) and experienced a 3-year PFS rate of 66%.
In CALGB 50801, the first study to our knowledge focusing exclusively on patients with bulky stage I and II disease, we hypothesized that a PET-adapted strategy would be effective and limit the use of mediastinal radiotherapy in this important and particularly high-risk group of patients in terms of late effects. After two cycles of ABVD, patients with negative PET scans would achieve acceptable PFS with chemotherapy alone, and patients with positive PET scans would receive intensified chemotherapy followed by radiotherapy and achieve PFS closer to that of patients with PET− than what had previously been observed with chemotherapy alone. CALGB is now part of the Alliance for Clinical Trials in Oncology.
METHODS
Eligibility
Previously untreated patients with stage I and II cHL with tumor bulk, defined as a mass of > 10 cm in largest diameter or a ratio of > 0.33 of the maximum intrathoracic diameter on standing posteroanterior chest x-ray, with normal organ function including normal ejection fraction and diffusion capacity of lung for carbon monoxide (DLCO) ≥ 60% were eligible. Patients who had received up to one prior cycle of ABVD could enroll, as long as all baseline eligibility criteria were met. All patients signed an institutional board–reviewed protocol-specific informed consent before enrollment.
Study Design
The study was a single-arm, phase II trial with response-adapted therapy on the basis of interim PET. Patients were treated with two cycles of full-dose ABVD without delay regardless of neutrophil count. Patients underwent centrally reviewed PET days 23-25 following cycle 2 day 1. Patients with a negative PET, defined as 1-3 on the Deauville 5 point scale (PS), received four additional cycles of ABVD. Patients with a positive interim PET (4 and 5) were treated with escalated BEACOPP for four cycles followed by 30 Gy involved-field radiotherapy, as described in the Protocol (online only).
Patients underwent PET at the end of therapy (3-8 weeks in patients with PET2− and 12-16 weeks after radiotherapy in patients with PET2+) and were followed thereafter with computed tomography (CT) scans every 3 months for 1 year, every 6 months for years 2 and 3, and then annually for a maximum of 5 years. PET scans were reviewed centrally by a member of an expert team of PET-CT readers with an adjudicator from the same pool in the case of disagreement with the local physician.
Statistical Analysis
The primary end point was progression-free survival (PFS), defined as the time from study entry to the first evidence of disease progression or death from any cause, censoring patients alive without disease progression at the last disease evaluation. Secondary end points included complete remission rate (CR) and adverse events. Response was measured using the Cheson criteria.12 OS was defined as the time from study entry until death from any cause, censoring patients alive at date of last contact. Adverse events were graded according to the CTCAE v4.0 criteria.
The study was designed with two objectives: to maintain PFS in patients with PET2− without the use of RT and to improve PFS for patients with PET2+ by intensifying chemotherapy with consolidative RT. The statistical design tested that PFS for patients with PET2+ treated with subsequent escBEACOPP followed by RT was not grossly inferior to the PFS of patients with PET2− treated with an additional four cycles of ABVD. The original study design specifications are presented in the Protocol Document. Because of a lower annual accrual of 14 patients, the sample size was decreased to 93 eligible patients, in which 42 events were required under the same PFS assumptions with 80% power. Finally, with few events but mature follow-up, the protocol was amended to allow for the primary end point analysis after patients had been followed for 3 years.
For the primary end point analysis, the 85% upper confidence bound on the HR comparing PFS of patients with PET2+ and patients with PET2− was obtained from a univariable Cox proportional hazards model, and if that upper bound was <4.1, then escBEACOPP followed by radiation was deemed a promising treatment strategy for patients with PET2+ disease after two cycles of ABVD. Likewise, if the 85% lower confidence bound on the PFS estimate at 3 years for patients with PET2+ excluded 40%, then that would support the use of this treatment strategy in patients with PET2+. For each group, PFS and OS distributions were estimated using the Kaplan-Meier method, and 3-year estimates were also provided with two-sided 95% CIs. CR rates were estimated with exact 95% CIs. Frequency tables of worst-grade adverse events summarized safety data. Patient demographics were summarized and compared between the patients with PET2+ and patients with PET2− using the Wilcoxon rank sum test for continuous demographic factors and Fisher's exact test for categorical demographic factors.
All patients with confirmed eligibility and PET status determined after two cycles of ABVD are included in analyses. Data collection was performed by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies. All analyses were performed by the Alliance Statistics and Data Management Center using SAS version 9.4 (Cary, NC). Data were locked for this analysis as of April 16, 2021.
RESULTS
Enrollment and Patient Characteristics
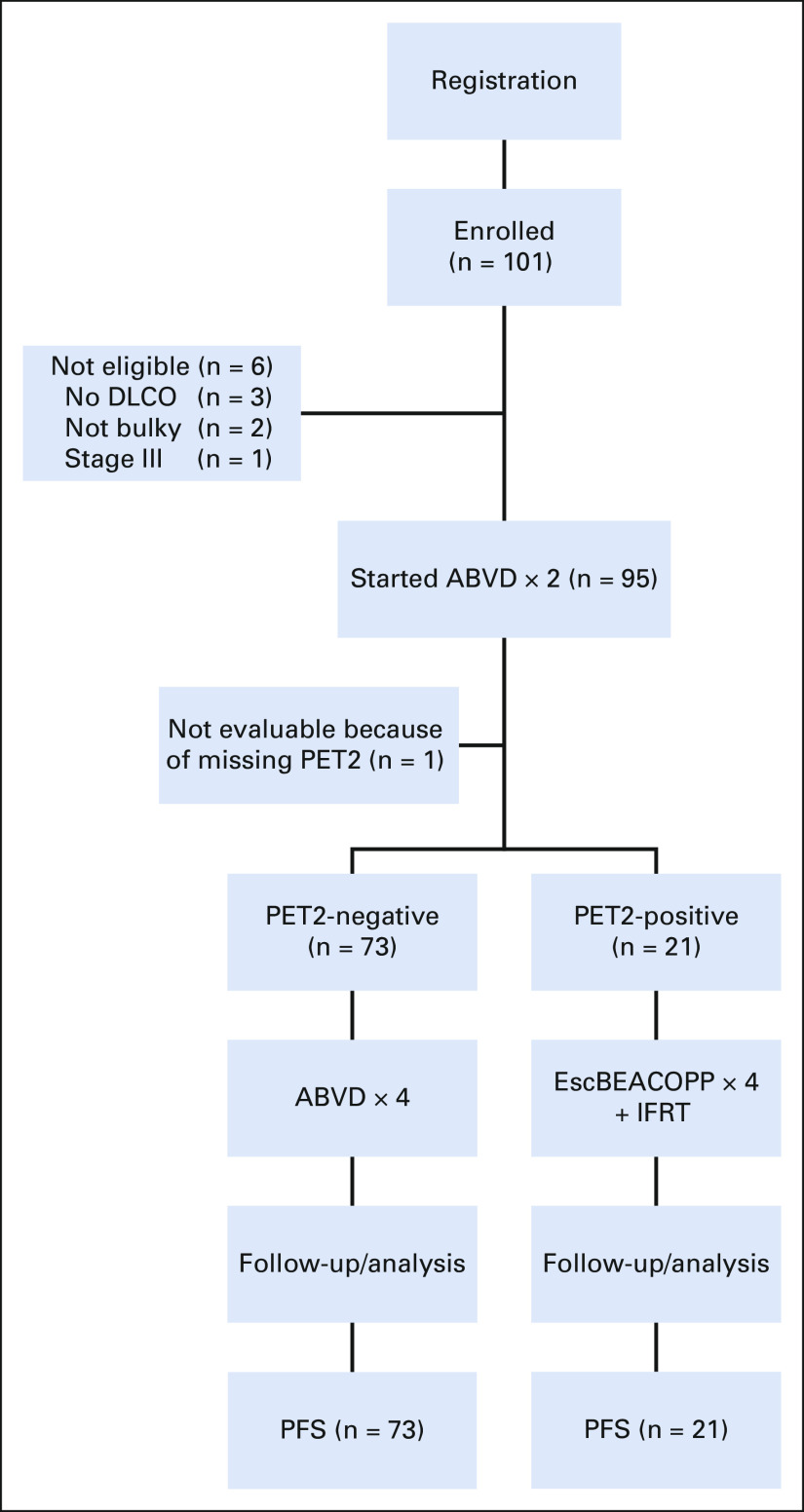
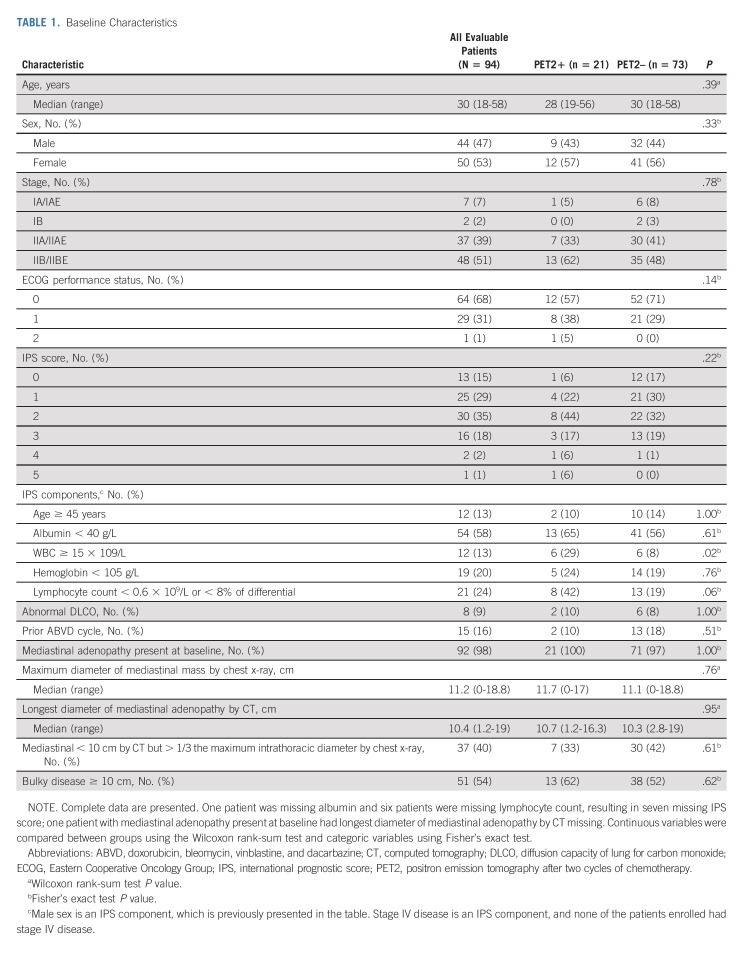
Between May 2010 and October 2017, 101 patients were enrolled to this study (Fig 1). Six patients did not have eligibility confirmed, including three without baseline DLCO, two who did not meet the definition of bulky disease, and one who had stage IIIB disease. In addition, one patient did not undergo PET scanning following cycle 2 of ABVD, resulting in 94 eligible and evaluable patients for the safety and efficacy analyses. Baseline patient characteristics for all 94 patients and by PET status following cycle 2 are shown in Table 1. Median age was 30 years (range, 18-58 years). Of 94 patients, 21 (22%) were PET2+ and 73 (78%) PET2– after two cycles of ABVD. WBC count of at least 15 × 109/L at baseline was significantly associated with PET2+ status (P = .02), and lymphopenia at baseline was moderately associated with PET2+ status (P = .06).
FIG 1.
Flow diagram. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; DLCO, diffusion capacity of lung for carbon monoxide; escBEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; IFRT, involved-field radiation therapy; PET2, positron emission tomography after two cycles of chemotherapy; PFS, progression-free survival.
TABLE 1.
Baseline Characteristics
Tolerability and Adverse Events
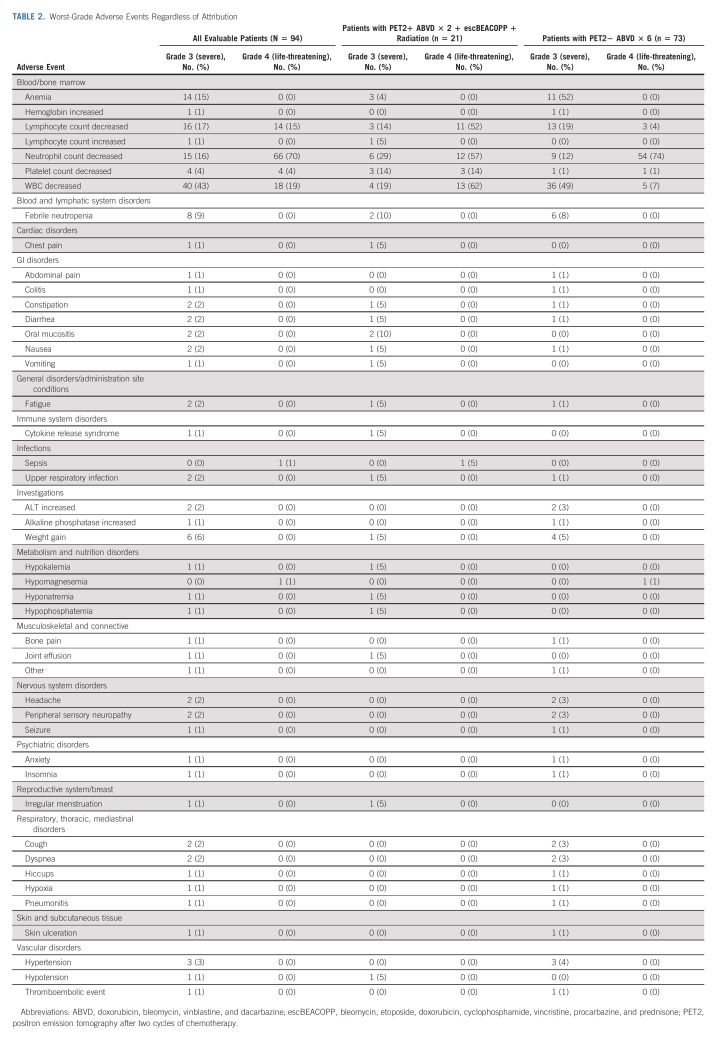
Most patients of the 94 patients who were evaluable for safety analyses completed therapy per protocol (93%): 96% for PET2– and 81% for PET2+. Three patients who were PET2– discontinued therapy early; the reason for discontinuation was due to adverse events in one patient, refusal of further treatment in one patient, and not returning to the site for one patient. Of the four patients who were PET2+, two discontinued because of progressive disease, one for adverse events, and one patient declined further therapy after cycle 5. 70% of PET2– patients and 76% of PET2+ patients (P = .79) received all six cycles of bleomycin without dose modifications. There was one case each of grade 3 pneumonitis and hypoxia and two cases of grade 3 dyspnea without any grade 4 pulmonary toxicity.
No unexpected adverse events were identified for patients treated with ABVD or escBEACOPP, and no grade 5 adverse events were reported. Grade 3 and 4 adverse events, irrespective of attribution, are shown in Table 2.
TABLE 2.
Worst-Grade Adverse Events Regardless of Attribution
Outcome of Therapy
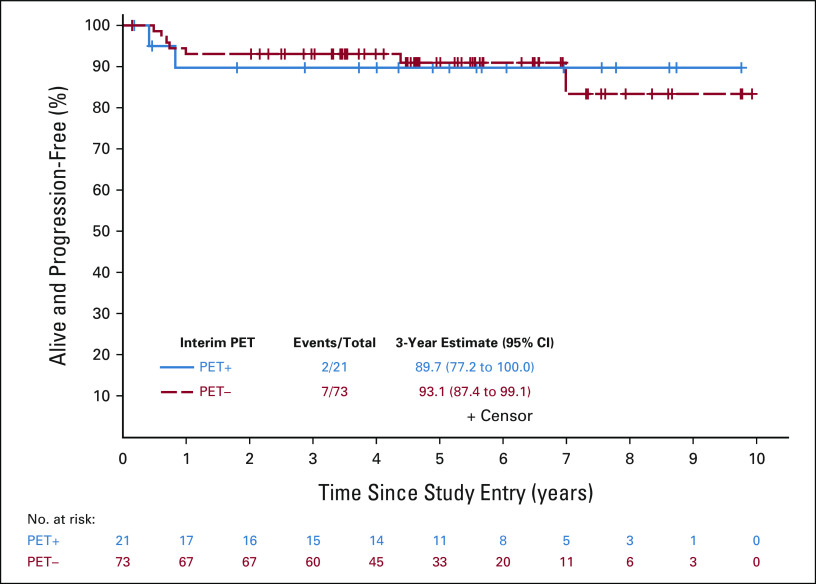
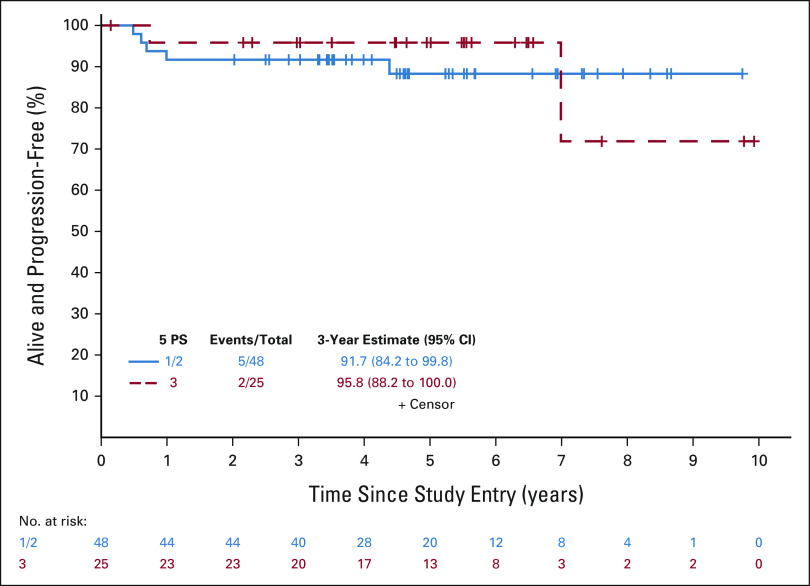
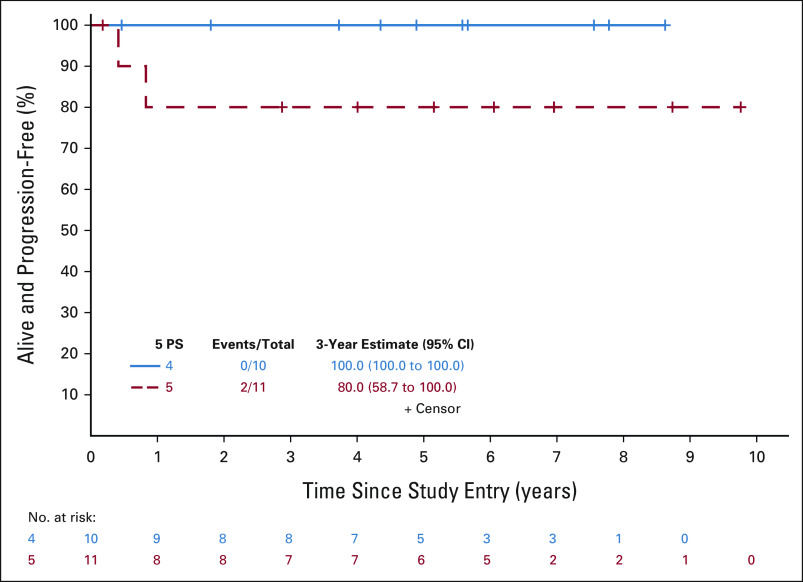
Primary objectives of this study were to use a PET-adapted approach to maintain PFS in patients with PET2− without the use of RT and to improve outcomes in PET2+ by intensifying therapy. We assessed whether PFS for patients with PET2+ receiving escBEACOPP was not inferior to that of patients with PET2− receiving ABVD, compared with historical differences in PFS between patients with PET2+ and patients with PET2− receiving ABVD. There were 94 patients (73 PET2– and 21 PET2+) who were eligible and evaluable for the efficacy analyses. With a median follow-up of 60 months, there were seven PFS events (five progressions and two deaths) in 73 patients with PET2− and two PFS events (both progressions) in 21 patients with PET2+ (Fig 2). In the PET2− patients, three recurred in the bulky site only and two recurred in nonbulky sites. The estimated hazard ratio comparing PFS of patients with PET2+ and patients with PET2− was 1.03 (85% upper bound 2.38), and was significantly less than the null hypothesis of 4.1 (one-sided P = .04). PFS estimates at 3 years were 93.1% (95% CI, 87.4 to 99.1; 85% lower bound: 90%) and 89.7% (95% CI, 77.2 to 100; 85% lower bound: 82.9%) in patients with PET2− and patients with PET2+, respectively. For patients who were PET2–, the 3-year PFS estimates were 92% (95% CI, 84 to 100) and 96% (95% CI, 88 to 100) for patients with 1/2 versus 3 on the 5 PS, respectively (Fig 3). The 3-year estimate was 100% (95% CI, 100 to 100) for those with 4 on the 5 PS and 80% (95% CI, 59 to 100) for those with 5 (Fig 4). Specific to patients with PET2+, the 85% lower bound on the PFS estimate was 82.9%, and was significantly higher than the null hypothesis of 40% (one-sided P < .0001). The study therefore met its primary goal. Among all patients, the 3-year PFS estimate was 92.3% (95% CI, 87.0 to 98.0).
FIG 2.
PFS for interim PET– and PET+ patients. The PFS event summary (number of PFS events/total number of patients) as well as the 3-year PFS estimate and 95% CI are presented for the PET– (dashed red line) and PET+ (solid blue line) groups. PET, positron emission tomography; PFS, progression-free survival.
FIG 3.
PFS for interim Deauville 5 PS 1/2 or 3 patients. The PFS event summary (number of PFS events/total number of patients) as well as the 3-year PFS estimate and 95% CI are presented for the 5 PS = 1/2 (solid blue line) and 3 (dashed red line) groups. PFS, progression-free survival; PS, point scale.
FIG 4.
PFS for interim Deauville 5 PS 4 or 5 patients. The PFS event summary (number of PFS events/total number of patients) as well as the 3-year PFS estimate and 95% CI are presented for the 5 PS = 4 (solid blue line) and 5 (dashed red line) groups. PFS, progression-free survival; PS, point scale.
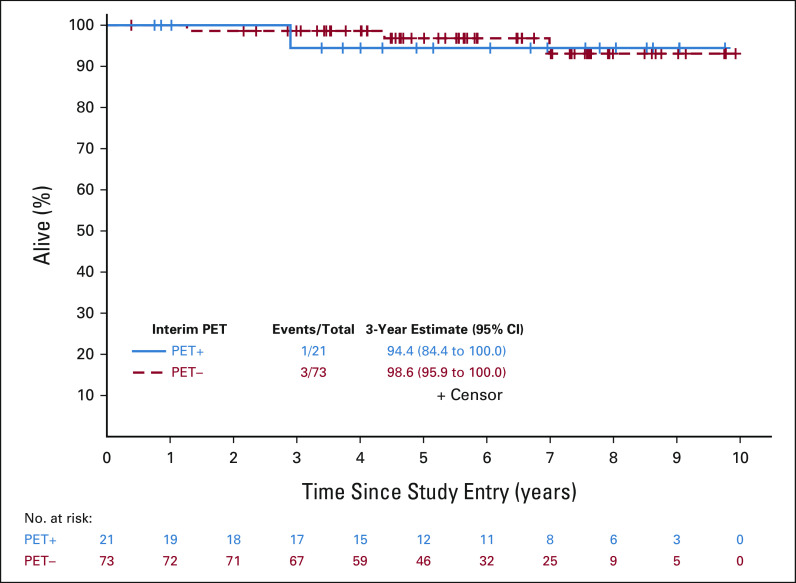
Secondary end points of the study included CR (at any point after enrollment) and OS. Of patients with PET2−, 100% (95% CI, 95 to 100) had a best response of CR, whereas 76% (95% CI, 53 to 92) of patients with PET2+ had a best response of CR, for an overall CR rate of 95% (95% CI, 88 to 98). With a median follow-up of 69 months for survival, there have been four deaths. The one death occurring in a patient with PET2+ was due to progressive disease, complicated by pneumonia, nearly 3 years after enrollment. The three deaths occurring in patients with PET2− were due to recurrence 1.3 years after enrollment, unrelated anaplastic astrocytoma occurring 4.4 years after enrollment, and unrelated chronic obstructive pulmonary disease occurring 7.0 years after enrollment. The 3-year OS estimates for patients with PET2− and PET2+ were 98.6% (95% CI, 95.9 to 100) and 94.4% (95% CI, 84.4 to 100), respectively (Fig 5). Overall, the 3-year overall survival estimate was 97.7% (95% CI, 94.7 to 100).
FIG 5.
OS for interim PET+ and PET– patients. The OS event summary (number of OS events/total number of patients) as well as the 3-year OS estimate and 95% CI are presented for the PET+ (solid blue line) and PET– (dashed red line) groups. OS, overall survival; PET, positron emission tomography.
DISCUSSION
In this PET-adapted study of patients with bulky stage I and II cHL, outcomes were excellent with a 3-year PFS rate of 93.1% in PET2– patients treated with six cycles of ABVD without RT and 89.7% in patients with PET2+ treated with two cycles of ABVD and four cycles of escBEACOPP followed by RT. Our results in patients with PET2− were similar to the results reported in the RATHL study, which included patients with stage II disease with bulk and/or B symptoms, as these patients are treated as advanced stage with chemotherapy alone in some centers. Interim PET– was also defined by scores of 1-3 on the 5 PS.10 The 3-year PFS rate in 119 patients with stage II bulky disease who received six cycles of ABVD or two cycles of ABVD and four cycles of doxorubicin, vinblastine, and dacarbazine (AVD) was 91.5%. The outcome of patients who were PET2+ with early-stage bulky disease was not reported. In addition, in the unfavorable arm of the EORTC H10 study, 515 of 1,196 patients had bulky disease.9 The 5-year PFS rate of patients with a negative PET2 (defined as 1-2 on the 5 PS) who received six cycles of ABVD was 90% compared with 92% in those receiving ABVD for four cycles followed by IFRT. For the PET2+ group (3-5 on the 5 PS) who received two cycles of ABVD, two cycles of escBEACOPP, and IFRT, the 5-year PFS rate was 91%. Outcomes in the subgroup of patients with bulky disease were not reported separately.
Historically, the German Hodgkin Study Group uses escBEACOPP × two followed by ABVD × two followed by IFRT as its standard approach in patients with early-stage unfavorable Hodgkin lymphoma.13 In the recently reported HD17 study, patients were randomly assigned to standard therapy versus a PET-adapted approach.14 Patients who achieved a negative PET scan after chemotherapy, defined as 1-2 on the 5 PS, were randomly assigned to receive radiotherapy versus observation. The 5-year PFS rate in the standard versus the experimental arms was 97% versus 96% in the PET-adapted arm. Approximately 33% of patients were PET+ (3-5) after completing all chemotherapy with 94% of patients progression-free. In a post hoc analysis of patients with 4 or 5 on the 5 PS, the 5-year PFS rate was 82%.
Interestingly, the outcome of patients with PET2+ in the current study appeared better than that reported in CALGB 50604, which also included patients with nonbulky stage I/II disease.11 Only 14 of 149 evaluable patients in CALGB 50604 were PET2+ (4-5) after two cycles of ABVD and received two cycles of escBEACOPP followed by involved-field radiotherapy. The 3-year PFS rate in this group was 67%. PET2– patients treated with a total of four cycles of ABVD experienced a similar 3-year PFS rate of 91% compared with 93% in our study. In CALGB 50604, patients with a score of 3 on PET scan (n = 22) had inferior PFS compared with the 113 patients with score 1-2, with a 3-year PFS rate of 77% versus 94%, respectively. In our study, patients with a 5 PS score of 3 did not have an inferior PFS compared with those with a score of 1-2. Possible explanations for the difference seen for patients with PET2+ in these two studies include small sample size or possibly the use of four versus two cycles of escalated BEACOPP in the current study.
Our study has several limitations. The trial was a moderately sized phase II study, and patients were not randomly assigned to standard therapy compared with a PET-adapted approach. In addition, the number of patients with PET2+ after two cycles of ABVD (n = 21) was small. Allowing patients who initiated up to one full cycle of ABVD before enrolling, however, may have reduced selection bias and allowed for patients with symptomatic disease needing urgent therapy to participate in the study.
Given that more than half of the patients with bulky mediastinal disease treated in the trial were women and the median age was 30 years, a PET-adapted approach with omission of radiotherapy in interim negative patients is appealing. More than 70% of evaluable patients (69 patients with PET2− who did not relapse) were not exposed to radiotherapy, escBEACOPP, or high-dose chemotherapy. Modern radiotherapy techniques with lower doses, smaller fields, and higher precision in targeting disease and avoiding surrounding tissue are likely to reduce late morbidity and mortality. The extent of reduction in toxicity, however, will take many years to fully assess. In an analysis of more than 3,900 patients with cHL followed for a median of 19.1 years, the cumulative incidence of a second cancer at 40 years was 48.5%.15 In addition, the risk of second solid cancers did not decrease when comparing patients treated between 1965 and 1988 compared with patients treated between 1989 and 2000, despite significant decreases in both dose and extent of radiotherapy in the later era. With regard to cardiovascular risk, the cumulative risk of cardiovascular disease was 50% at 40 years in a cohort of 2,524 cHL survivors.16 For patients treated with mediastinal radiotherapy, the risk was 2.7-fold for coronary disease, 6.6-fold for valvular disease, and 2.7-fold for heart failure. Proton therapy may mitigate risk, although further study is necessary.17
Varying approaches to balancing the risks and benefits of therapy have been used with consideration of the number of cycles and intensity of chemotherapy, the use of radiotherapy, and the risk of recurrence with need for stem-cell transplantation. In our study and the unfavorable arm of H10, using interim PET-adapted therapy, 22% and 25% of patients, respectively, required escalation to BEACOPP (four cycles 50801 and two cycles H10) and involved-field radiotherapy, with similar PFS rates of approximately 90% at 3 and 5 years. In HD17, using positive end-of-treatment PET threshold after two cycles of escBEACOPP and two cycles of ABVD of ≥ 3 (32% of patients), the 5-year PFS rate was 96.5% without radiotherapy. The outcome of patients with bulky disease was not specifically reported in H10 or HD17.
With the approval of the highly active agents brentuximab vedotin and programed cell death protein 1 inhibitors (pembrolizumab and nivolumab), recent and ongoing clinical trials have incorporated these agents in the upfront setting in both advanced-stage and limited-stage cHL. ECHELON-1 (brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine [BV-AVD] compared with ABVD) and the ongoing North American Intergroup study comparing BV-AVD to nivolumab plus AVD in patients with stage III/IV disease are not PET-adapted.18 Kumar et al19 recently published their results in early-stage unfavorable patients, including those with bulky disease. One hundred seventeen patients who were PET-negative after four cycles of BV-AVD were treated in sequential cohorts with 30 Gy involved-site radiotherapy, 20 Gy involved-site radiotherapy, 30 Gy consolidation volume radiotherapy, and no radiotherapy. Overall, the 2-year PFS rate was 94% without significant difference among the four cohorts. Bulky disease in this study was defined as > 7 cm, as opposed to 10 cm or >1/3 the maximal intrathoracic diameter in our study, thus affecting comparison of the trials.
In this study, the first to our knowledge focused exclusively on patients with early-stage bulky cHL who are particularly vulnerable to the late effects of radiotherapy, the outcome of both patients with PET2− and patients with PET2+ was excellent. The study met its primary goal and the data have important implications for clinical practice. The majority of patients received ABVD only, an inexpensive and well-tolerated regimen with very low risk of late effects. Our study was initiated before publication of the RATHL study, but elimination of bleomycin in patients with bulky early-stage cHL with PET2– disease is appropriate. The majority of patients with PET2+ remained disease-free without the need for high-dose chemotherapy with autologous stem-cell transplant. Future studies should focus on incorporating novel agents in early-stage patients who are unlikely to achieve durable remissions with chemotherapy alone and reduce exposure to regimens such as escBEACOPP, which may have negative impact on fertility and bone marrow stem cells. Moving beyond using the 5-point scale to assess response to minimize the risk of false-positive PET, particularly in patients with bulky disease, by incorporating novel imaging strategies, such as changes in total metabolic tumor volume, and using cell-free DNA, may allow for better risk-adapted approaches to maximize cure.
Ann S. LaCasce
Consulting or Advisory Role: Seagan
Speakers' Bureau: Research to Practice
Research Funding: Sanofi (Inst), Forty Seven (Inst), Celgene (Inst), Seattle Genetics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate royalties
Amy S. Ruppert
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Consulting or Advisory Role: Telios
Lale Kostakoglu
Honoraria: Lantheus
Consulting or Advisory Role: Roche/Genentech, ImaginAb, Point BioPharma
Speakers' Bureau: Lantheus Medical Imaging
Travel, Accommodations, Expenses: Roche
Eric Hsi
Consulting or Advisory Role: Astellas Pharma, CytomX Therapeutics, Novartis
Research Funding: Lilly (Inst), AbbVie (Inst), Virtuoso Therapeutics (Inst)
Jeffrey Bogart
Stock and Other Ownership Interests: Cardan Robotics, Verve Medical
Bruce Cheson
Leadership: SymBio Pharmaceuticals
Consulting or Advisory Role: TG Therapeutics, AbbVie, Pharmacyclics/Janssen, Morphosys, Celgene, Karyopharm Therapeutics, Epizyme, Gilead Sciences, SymBio Pharmaceuticals, Parexel, Lilly, Imaging Endpoints, Tessa Therapeutics, MEI Pharma
Speakers' Bureau: MorphoSys/Incyte, BeiGene, Lilly, TG Therapeutics
Research Funding: TG Therapeutics (Inst), Seattle Genetics (Inst), Bristol Myers Squibb (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: SymBio Pharmaceuticals
Nina Wagner-Johnston
Consulting or Advisory Role: Epizyme, Seattle Genetics, Karyopharm Therapeutics, Grunenthal
Research Funding: Merck (Inst), Novartis/Pfizer (Inst), Genentech (Inst), Astex Pharmaceuticals (Inst), Juno Therapeutics (Inst), Regeneron (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst)
Jeremy Abramson
Honoraria: Regeneron, AstraZeneca, Janssen
Consulting or Advisory Role: Celgene, Novartis, AbbVie, Kite, a Gilead company, Genentech, EMD Serono, MorphoSys, Alimera Sciences, Karyopharm Therapeutics, Bristol Myers Squibb, C4 Therapeutics, BeiGene, AstraZeneca, Incyte, Bluebird Bio, Kymera, Epizyme, Genmab, MustangBio, Ono Pharmaceutical, Century Therapeutics, Lilly, Caribou Biosciences, Janssen, Takeda
Research Funding: Seattle Genetics (Inst), AI Therapeutics (Inst), Bristol Myers Squibb/Celgene (Inst)
Kristie Blum
Honoraria: American Society of Hemotology, Leidos Biomedical Research/NCI
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), BMSi (Inst)
Travel, Accommodations, Expenses: American Society of Hemotology
John P. Leonard
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Gilead Sciences, Epizyme, Bayer, Genentech/Roche, MEI Pharma, AstraZeneca, Merck, Morphosys, Karyopharm Therapeutics, Sutro Biopharma, Genmab, AbbVie, Incyte, Janssen Oncology, Eisai, MustangBio, Second Genome, Astellas Pharma, BeiGene, Calithera Biosciences, Constellation Pharmaceuticals, Lilly, Grail, Pfizer, Novartis, Seattle Genetics
Research Funding: Celgene (Inst), Alliance for Clinical Trials in Oncology (Inst), Takeda (Inst), Pfizer (Inst), National Cancer Institute (Inst), Janssen Oncology (Inst), Epizyme (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: BeiGene
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics, Roche/Genentech, ADC Therapeutics, BTG, Acerta Pharma
Research Funding: Seattle Genetics (Inst), Kite, a Gilead company (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Immune Design (Inst), Forty Seven (Inst), Janssen (Inst), Pharmacyclics (Inst), Millennium (Inst), ADC Therapeutics (Inst), Autolus (Inst), Roche/Genentech (Inst), Pfizer (Inst), Affimed Therapeutics (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgements.alliancefound.org.
PRIOR PRESENTATION
Presented at ASCO, virtual, June 4-8, 2021; and the International Conference on Malignant Lymphoma, virtual, June 18-22, 2021.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882 (to the Alliance for Clinical Trials in Oncology), and U24 CA196171.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Ann S. LaCasce, Travis Dockter, Lale Kostakoglu, Bruce Cheson, Nina Wagner-Johnston, John P. Leonard, Nancy L. Bartlett
Provision of study materials or patients: Lale Kostakoglu, Bruce Cheson, Nina Wagner-Johnston, Jeremy Abramson, Kristie Blum
Collection and assembly of data: Ann S. LaCasce, Travis Dockter, Lale Kostakoglu, Eric Hsi, Jeremy Abramson, Kristie Blum, Nancy L. Bartlett
Data analysis and interpretation: Ann S. LaCasce, Travis Dockter, Amy S. Ruppert, Lale Kostakoglu, Heiko Schöder, Jeffrey Bogart, Bruce Cheson, Jeremy Abramson, Kristie Blum, John P. Leonard, Nancy L. Bartlett
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Positron Emission Tomography–Adapted Therapy in Bulky Stage I/II Classic Hodgkin Lymphoma: CALGB 50801 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ann S. LaCasce
Consulting or Advisory Role: Seagan
Speakers' Bureau: Research to Practice
Research Funding: Sanofi (Inst), Forty Seven (Inst), Celgene (Inst), Seattle Genetics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate royalties
Amy S. Ruppert
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Consulting or Advisory Role: Telios
Lale Kostakoglu
Honoraria: Lantheus
Consulting or Advisory Role: Roche/Genentech, ImaginAb, Point BioPharma
Speakers' Bureau: Lantheus Medical Imaging
Travel, Accommodations, Expenses: Roche
Eric Hsi
Consulting or Advisory Role: Astellas Pharma, CytomX Therapeutics, Novartis
Research Funding: Lilly (Inst), AbbVie (Inst), Virtuoso Therapeutics (Inst)
Jeffrey Bogart
Stock and Other Ownership Interests: Cardan Robotics, Verve Medical
Bruce Cheson
Leadership: SymBio Pharmaceuticals
Consulting or Advisory Role: TG Therapeutics, AbbVie, Pharmacyclics/Janssen, Morphosys, Celgene, Karyopharm Therapeutics, Epizyme, Gilead Sciences, SymBio Pharmaceuticals, Parexel, Lilly, Imaging Endpoints, Tessa Therapeutics, MEI Pharma
Speakers' Bureau: MorphoSys/Incyte, BeiGene, Lilly, TG Therapeutics
Research Funding: TG Therapeutics (Inst), Seattle Genetics (Inst), Bristol Myers Squibb (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: SymBio Pharmaceuticals
Nina Wagner-Johnston
Consulting or Advisory Role: Epizyme, Seattle Genetics, Karyopharm Therapeutics, Grunenthal
Research Funding: Merck (Inst), Novartis/Pfizer (Inst), Genentech (Inst), Astex Pharmaceuticals (Inst), Juno Therapeutics (Inst), Regeneron (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst)
Jeremy Abramson
Honoraria: Regeneron, AstraZeneca, Janssen
Consulting or Advisory Role: Celgene, Novartis, AbbVie, Kite, a Gilead company, Genentech, EMD Serono, MorphoSys, Alimera Sciences, Karyopharm Therapeutics, Bristol Myers Squibb, C4 Therapeutics, BeiGene, AstraZeneca, Incyte, Bluebird Bio, Kymera, Epizyme, Genmab, MustangBio, Ono Pharmaceutical, Century Therapeutics, Lilly, Caribou Biosciences, Janssen, Takeda
Research Funding: Seattle Genetics (Inst), AI Therapeutics (Inst), Bristol Myers Squibb/Celgene (Inst)
Kristie Blum
Honoraria: American Society of Hemotology, Leidos Biomedical Research/NCI
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), BMSi (Inst)
Travel, Accommodations, Expenses: American Society of Hemotology
John P. Leonard
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Gilead Sciences, Epizyme, Bayer, Genentech/Roche, MEI Pharma, AstraZeneca, Merck, Morphosys, Karyopharm Therapeutics, Sutro Biopharma, Genmab, AbbVie, Incyte, Janssen Oncology, Eisai, MustangBio, Second Genome, Astellas Pharma, BeiGene, Calithera Biosciences, Constellation Pharmaceuticals, Lilly, Grail, Pfizer, Novartis, Seattle Genetics
Research Funding: Celgene (Inst), Alliance for Clinical Trials in Oncology (Inst), Takeda (Inst), Pfizer (Inst), National Cancer Institute (Inst), Janssen Oncology (Inst), Epizyme (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: BeiGene
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics, Roche/Genentech, ADC Therapeutics, BTG, Acerta Pharma
Research Funding: Seattle Genetics (Inst), Kite, a Gilead company (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Immune Design (Inst), Forty Seven (Inst), Janssen (Inst), Pharmacyclics (Inst), Millennium (Inst), ADC Therapeutics (Inst), Autolus (Inst), Roche/Genentech (Inst), Pfizer (Inst), Affimed Therapeutics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colonna P, Jais JP, Desablens B, et al. Mediastinal tumor size and response to chemotherapy are the only prognostic factors in supradiaphragmatic Hodgkin's disease treated by ABVD plus radiotherapy: Ten-year results of the Paris-Ouest-France 81/12 trial, including 262 patients. J Clin Oncol. 1996;14:1928–1935. doi: 10.1200/JCO.1996.14.6.1928. [DOI] [PubMed] [Google Scholar]
- 3. Advani RH, Hong F, Fisher RI, et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: A subset analysis of the North American Intergroup E2496 trial. J Clin Oncol. 2015;33:1936–1942. doi: 10.1200/JCO.2014.57.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst. 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 5. Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood. 2002;100:1989–1996. doi: 10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 6. Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 7. Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 8. Rigacci L, Puccini B, Zinzani PL, et al. The prognostic value of positron emission tomography performed after two courses (INTERIM-PET) of standard therapy on treatment outcome in early stage Hodgkin lymphoma: A multicentric study by the fondazione italiana linfomi (FIL) Am J Hematol. 2015;90:499–503. doi: 10.1002/ajh.23994. [DOI] [PubMed] [Google Scholar]
- 9. Andre MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35:1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 10. Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Straus DJ, Jung SH, Pitcher B, et al. CALGB 50604: Risk-adapted treatment of nonbulky early-stage Hodgkin lymphoma based on interim PET. Blood. 2018;132:1013–1021. doi: 10.1182/blood-2018-01-827246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 13. von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin's lymphoma: Final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol. 2012;30:907–913. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 14. Borchmann P, Plutschow A, Kobe C, et al. PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:223–234. doi: 10.1016/S1470-2045(20)30601-X. [DOI] [PubMed] [Google Scholar]
- 15. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med. 2015;373:2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 16. van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 17. Dabaja BS, Hoppe BS, Plastaras JP, et al. Proton therapy for adults with mediastinal lymphomas: The International Lymphoma Radiation Oncology Group guidelines. Blood. 2018;132:1635–1646. doi: 10.1182/blood-2018-03-837633. Erratum: Blood 133:1384-1385, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar A, Casulo C, Advani RH, et al. Brentuximab vedotin combined with chemotherapy in patients with newly diagnosed early-stage, unfavorable-risk Hodgkin lymphoma. J Clin Oncol. 2021;39:2257–2265. doi: 10.1200/JCO.21.00108. [DOI] [PubMed] [Google Scholar]