Abstract
Background
Patients with multiple sclerosis (MS) are commonly treated with anti-CD20 therapies. Reduced seroconversion following COVID-19 vaccination in patients receiving certain anti-CD20 therapies has been reported; however, the immune response following natural infection is poorly characterised. This study aimed to retrospectively evaluate COVID-19 antibody responses after vaccination and natural infection in patients treated with anti-CD20 therapies.
Methods
We performed a retrospective review evaluating COVID-19 seroconversion and anti-spike glycoprotein antibody titres in double-vaccinated patients with MS, or related neuroinflammatory conditions, treated with anti-CD20 therapies (N = 30) with a confirmed history of natural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (n = 14) or without infection (control; n = 16). This single-centre study was performed at the Yale Multiple Sclerosis Center, where patients treated with anti-CD20 therapies (ocrelizumab, n = 21; rituximab, n = 5; ofatumumab, n = 4) were systematically checked for SARS-CoV-2 anti-spike antibody levels throughout the pandemic. Data were collected from March 2020 to March 2022. All patients had received at least two doses of a Food and Drug Administration (FDA)-approved COVID-19 vaccine. Qualitative anti-spike antibody seropositivity was determined based on test-specific laboratory reference ranges. For a subset of patients (n = 18), quantitative anti-spike antibody levels were assessed via DiaSorin LIAISON® chemiluminescence immunoassay (positive titre was defined as ≥ 13). Vaccination and infection dates were also recorded, and patients were monitored for adverse COVID-19-related health effects.
Results
Overall, 15/30 (50.0%) patients seroconverted following double vaccination. After infection, 13/14 (92.9%) seroconverted, while 6/16 (37.5%) uninfected patients seroconverted after vaccination. For the 18 patients with quantitative anti-spike antibody titres, mean titre post-vaccination was 37.4. Mean antibody titres were significantly higher after infection: 540.3 versus 20.1 in the control group (p < 0.05). Of the 14 infected patients, 13 had mild COVID-19 symptoms and one was asymptomatic. No hospitalisations or deaths were reported.
Conclusions
This study reports that SARS-CoV-2 anti-spike antibody titres in double-vaccinated MS patients treated with anti-CD20 therapies were significantly increased post-infection compared with the control group. Patients treated with anti-CD20 therapy who had confirmed infections displayed mild or asymptomatic infection. These results provide reassurance that anti-CD20 therapies in double-vaccinated patients do not preclude an appropriate SARS-CoV-2 antibody response post-infection.
Keywords: Multiple sclerosis, COVID-19, Seroconversion, Anti-CD20 therapy, Disease-modifying therapy, Vaccination
1. Introduction
The COVID-19 (coronavirus disease 2019) vaccination programme has been a critical tool for countering the pandemic, both at an individual and at a public health level. An effective immunological vaccine strategy relies on activating both humoral and cellular responses to reduce transmission and disease severity (Sadarangani et al., 2021). In multiple sclerosis (MS) and related neuroinflammatory conditions, disease-modifying therapies (DMTs) – in particular, ocrelizumab, ofatumumab and rituximab, which are anti-CD20 therapies (also known as B-cell depletion therapies [BCDTs]) – are frequently used in disease management (Brancati et al., 2021; Roach and Cross, 2020). The immunomodulatory effects of DMTs result in a substantially compromised immune system; the elimination of B-cell populations by anti-CD20 therapies specifically impairs the humoral response to vaccination (Apostolidis et al., 2021; Roach and Cross, 2020). Patients with MS and related neuroinflammatory conditions receiving anti-CD20 therapies are at an increased risk of infections and impaired responses to vaccinations (Bar-Or et al., 2020; Krajnc et al., 2022). Accordingly, concerns about the implications of limited seroconversion in patients treated with anti-CD20 therapies following COVID-19 vaccination have been reported (Bar-Or et al., 2020; Cross et al., 2022; Georgieva et al., 2022; Iannetta et al., 2021; Levit et al., 2022; Smets et al., 2021; Tallantyre et al., 2022). However, there is currently less information available regarding seroconversion in patients treated with anti-CD20 therapies following natural infection, largely owing to the inability to distinguish severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein seropositivity due to vaccination from natural infection. More information on the implications of treatment with anti-CD20 therapy on vaccination responses and outcomes is needed to inform and develop effective management strategies for patients with MS and related neuroinflammatory conditions, and to help to address heightened anxieties regarding possible attenuated vaccine responses (Baker et al., 2020; Levit et al., 2022). This retrospective analysis evaluated qualitative and quantitative seroconversion rates in patients treated with anti-CD20 therapies, comparing responses following vaccination with responses following natural infection.
2. Materials and methods
2.1. Patients
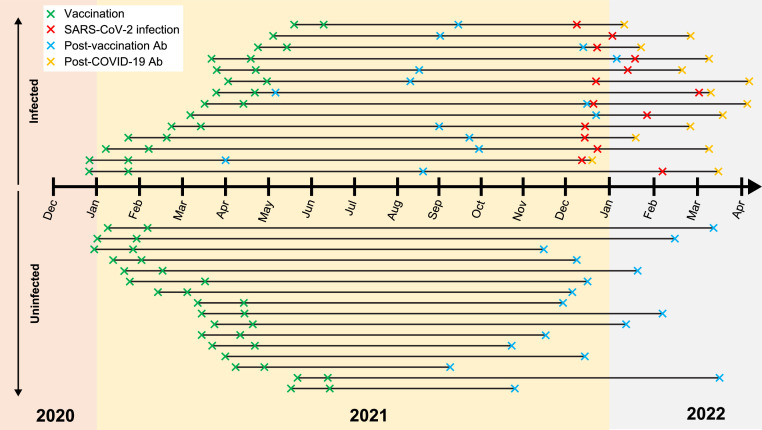
We performed a retrospective, single-centre review study at the Yale Multiple Sclerosis Center, where patients treated with anti-CD20 therapies were systematically and routinely checked for SARS-CoV-2 anti-spike glycoprotein antibody titres throughout the pandemic. Data were collected from March 2020 to March 2022 on patients treated with anti-CD20 therapies (ocrelizumab, n = 21; rituximab, n = 5; ofatumumab, n = 4) with a diagnosis of MS or related neuroinflammatory conditions (including autoimmune encephalitis) who were double-vaccinated (and considered to be fully vaccinated at the time of data collection) with a US Food and Drug Administration (FDA)-approved COVID-19 vaccine (either with two doses of an mRNA vaccine [Pfizer-BioNTech BNT172b2 or Moderna mRNA-1273] or with a single dose of Johnson & Johnson JNJ-78436735 and an mRNA booster vaccine) and had sufficient seroconversion data available for analysis. The full analysis comprised 30 patients; 16 (53.3%) of these patients had no history of natural SARS-CoV-2 infection (control cohort) and 14 (46.7%) had a confirmed history of natural SARS-CoV-2 infection (Table 1 , Fig. 1 , Supplemental Table 1). All patients with a confirmed history of infection had been infected from December 2021 onwards, during the Omicron BA.1 variant wave (Fig. 1, Supplemental Table 1). This work was carried out in accordance with the Declaration of Helsinki (World Medical Association, 2018) and exemption from ethics approval given the nature of the study was provided by the Yale University Institutional Review Board.
Table 1.
Patient demographics and infection and treatment history.
| Double-vaccinated patients without natural infection | Double-vaccinated patients with confirmed history of natural infection | |
|---|---|---|
| N | 16 | 14 |
| Female, n (%) | 10 (62.5) | 7 (50.0) |
| Race, n (%) | ||
| White/Caucasian | 14 (87.5) | 12 (85.7) |
| Black/African American | 2 (12.5) | 1 (7.1) |
| South Asian | 0 (0.0) | 1 (7.1) |
| Age, years, mean (SD) | 51.6 (11.5) | 47.6 (16.1) |
| Seroconverted after double vaccination, n (%) | 6 (37.5) | 9 (64.3) |
| Seroconverted after SARS-CoV-2 infection, n (%) | N/A | 13 (92.9) |
| Anti-CD20 therapy, n (%) | ||
| Ocrelizumab | 13 (81.3) | 8 (57.1) |
| Rituximab | 3 (18.8) | 2 (14.3) |
| Ofatumumab | 0 (0.0) | 4 (28.6) |
| Vaccine brand, n (%) | ||
| Moderna | 10 (62.5) | 8 (57.1) |
| Pfizer | 5 (31.3) | 4 (28.6) |
| Johnson & Johnson | 1 (6.3) | 2 (14.3) |
N/A, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Fig. 1.
Timeline of vaccination, infection and antibody draw dates. Where only one vaccination date is provided, patients received a single dose of Johnson & Johnson JNJ-78436735 vaccine. Dates are provided in Supplemental Table 1. Ab, antibody draw; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
2.2. Analyses
All patients were evaluated for qualitative anti-spike antibody seropositivity through chemiluminescence immunoassays. Seropositivity was based on test-specific laboratory reference ranges, which were dependent on which chemiluminescence immunoassay was used. Immunoassays included SARS-CoV-2 Total Antibody, Spike, Semi-quantitative (Quest Diagnostics, Greenwich, CT, USA) and LIAISON® (DiaSorin, Saluggia, Italy).
For patients with confirmed COVID-19, the final antibody measurement before infection was used in the post-vaccination analyses. For these patients, this antibody measurement occurred anywhere between 2 weeks to nearly 10 months after the second vaccination (Fig. 1, Supplemental Table 1). For patients without infection, the final antibody measurement before the data cut-off point of March 2022 was used. For these patients, this measurement occurred anywhere between 19 weeks to just over 13 months after the second vaccination (Fig. 1, Supplemental Table 1). Patients with confirmed COVID-19 also had a measurement of their SARS-CoV-2 spike antibodies performed anywhere between 1 and 16 weeks after infection, at their first follow-up visit after infection (Fig. 1, Supplemental Table 1).
All dates of vaccination, infection, and infusion or injection with anti-CD20 therapy were recorded via retrospective chart review at the Yale Multiple Sclerosis Center, and patients were monitored for adverse COVID-19-related health effects at each follow-up visit. Follow-up visits were every 6 months indefinitely. SARS-CoV-2 infection was confirmed either through polymerase chain reaction (PCR) or self-reported rapid antigen testing. Patients with self-reported confirmation informed us via telephone or at the next follow-up visit of their infection, any COVID-19-related symptoms and the date of confirmed infection. A systematic notes review of all patient charts was performed in March 2022.
A subset of 18 patients (10 with a confirmed history of symptomatic infection by PCR or rapid antigen test; eight without a history of infection) were quantitatively assessed for anti-spike antibody titres using the LIAISON chemiluminescence immunoassay. This subset of patients was available for quantitative assessment because their data were obtained from a single, fully quantitative chemiluminescence LIAISON immunoassay, thereby reducing inter-assay variability and ambiguity. A positive titre was defined as ≥ 13. Statistical analysis was performed in RStudio (RStudio, Boston, MA, USA) using the R tool developed by the R Core Team (The R Foundation, Vienna, Austria). Student's t-test was used to determine significant differences in antibody titres for patients with and without infection, and Fisher's exact test was used to evaluate the frequency of seropositivity among all patients.
3. Results
3.1. Qualitative seropositivity
Overall, 15 (50.0%) of the 30 patients had seroconverted post-vaccination. Of the 16 patients with no history of natural infection, six (37.5%) had seroconverted post-vaccination. Of the 14 patients with a confirmed history of infection, 13 (92.9%) had seroconverted post-infection; this represents a significantly higher proportion compared with those with no history of infection who had seroconverted post-vaccination (6/16 [37.5%]; p = 0.002).
3.2. Quantitative titres
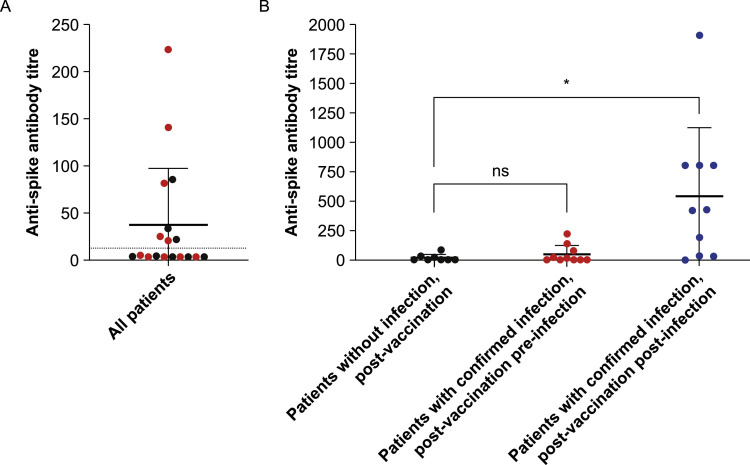
Overall, eight (44.4%) of the 18 patients in the quantitative analysis subset had positive anti-spike antibody titres post-vaccination but pre-infection (seropositivity threshold was defined as ≥ 13). However, all of these positive titres were considered to be relatively low (Fig. 2 A). The mean post-vaccination titre for all 18 patients in the subset was 37.4, and there was no significant difference in titre between the patients with a confirmed history of infection (51.2) and those without a history of infection (20.1) (p = 0.25) (Fig. 2 B). For the 10 patients in the subset with a confirmed history of infection (9/10 of whom seroconverted post-infection), the mean post-infection titre was 540.3. This was significantly higher than that for the eight patients in the subset without a history of infection, whose mean post-vaccination titre was 20.1 (p < 0.05) (Fig. 2 B).
Fig. 2.
Anti-spike antibody titres in quantitative analysis subset. (A) Post-vaccination titres for all patients in the quantitative analysis subset (n = 18), including patients with no confirmed history of natural infection (n = 8; black) and patients with confirmed history of natural infection (n = 10; red). The dotted line indicates the seropositivity threshold (anti-spike antibody titre ≥ 13). (B) Post-vaccination titres for patients with no confirmed history of natural infection (n = 8; black), post-vaccination pre-infection titres for patients with confirmed history of natural infection (n = 10; red) and post-vaccination post-infection titres for patients with confirmed history of natural infection (n = 10; blue). * p < 0.05. ns, not significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Patient health
Among the 14 patients with a confirmed history of infection, 13 patients had mild symptoms and one patient was asymptomatic. There were no hospitalisations or deaths recorded during the analysis period.
4. Discussion
These results demonstrate that a significantly greater proportion of the patients who experienced increased immune stimulation via a confirmed history of natural SARS-CoV-2 infection in addition to double vaccination exhibited qualitative seropositivity compared with the patients without a history of SARS-CoV-2 infection. The patients with a confirmed history of infection also exhibited a significant increase in anti-spike antibody titres compared with the patients without a history of infection. All patients with a confirmed history of infection experienced mild symptoms or were asymptomatic and none required hospitalisation. Further, there were no notable differences in COVID-19 symptoms between patients who had seroconverted post-vaccination and those who had not. These results provide reassurance to clinicians and patients that humoral immune responses to COVID-19 vaccination and natural infection in patients treated with anti-CD20 therapies may not be as poor as previously thought (Bar-Or et al., 2020; Cross et al., 2022; Faissner et al., 2022; Georgieva et al., 2022; Hughes et al., 2021; Iannetta et al., 2021; Milo et al., 2022; Smets et al., 2021; Tallantyre et al., 2022), and add to the growing body of evidence supporting the continued use of COVID-19 vaccination in pandemic and infection management strategies in patients treated with anti-CD20 therapies (National Multiple Sclerosis Society, 2022).
This growing body of evidence provides further encouragement and indicates that cellular immune responses – in addition to humoral immune responses – to COVID-19 vaccinations may be sufficient in the majority of DMT-treated patients with MS. However, these responses are reduced in comparison with untreated, healthy controls, in line with what is expected for immunosuppressive therapies. A large, multicentre study in Israel (Milo et al., 2022) assessed both humoral and cellular immune responses in DMT-treated patients with MS following three doses of the Pfizer mRNA vaccine, compared with untreated healthy vaccinated controls. Overall, cellular and humoral responses in the DMT-treated patients with MS were encouraging and not significantly worse than in the control cohort. Among the patients treated with anti-CD20 therapies, there was a reduction in humoral seropositivity seen in those treated with ocrelizumab compared with the control cohort. However, the investigators also reported a correlation between improved seroconversion and the timing of vaccination around the 6-monthly infusion schedule, indicating that vaccination more than 5 months post-infusion is optimal (Milo et al., 2022). Reassuring cellular responses after vaccination were also observed in a small study in Germany focusing on immune responses to mRNA vaccination in ofatumumab-treated patients with MS (Faissner et al., 2022). Although humoral responses were found to be impaired in ofatumumab-treated patients compared with healthy controls, the cellular immune response remained robust and notably included a strong type 1 T-helper cell presence (Faissner et al., 2022). Similar results were observed in a large, prospective, multicentre study in Italy (Tortorella et al., 2022) that compared humoral- and T-cell specific responses in DMT-treated patients with MS following two doses of an mRNA vaccine with those of untreated healthy vaccinated controls. Overall, cellular and humoral responses in the DMT-treated patients with MS were reassuring, although the ocrelizumab-treated patients did exhibit significantly reduced antibody response rates and titres compared with the control cohort (Tortorella et al., 2022). However, it should be noted that the attenuated vaccine response in ocrelizumab-treated patients is an expected outcome and is also seen in response to common vaccines, such as those for tetanus, pneumococcus and influenza (Bar-Or et al., 2020). Furthermore, there is a potential confounding effect of the MS disease state itself contributing to impaired immune responses, in addition to immunosuppression due to anti-CD20 therapies or other DMTs (Bar-Or et al., 2020; Graves and Killestein, 2022).
Our work adds to the growing body of evidence indicating that patients with MS receiving immunosuppressive anti-CD20 therapies are not at greater risk of serious COVID-19 when compared with the general risk in the wider public, both before and after vaccination (Cross et al., 2022; Hughes et al., 2021). In a large (n = 1703) multicentre sub-study of the ALITHIOS trial, most COVID-19 cases in ofatumumab-treated patients with MS were mild or moderate (222 [90.6%] of 245 cases) (Cross et al., 2022). Additionally, 98.4% (241) of all patients with COVID-19 had recovered by the point of study cut-off, with none of these patients permanently discontinuing ofatumumab treatment. Furthermore, of the 476 ofatumumab-treated patients in the ALITHIOS trial who were considered fully vaccinated at the time of the study, only seven (1.5%) had breakthrough COVID-19. Of these seven patients, five had mild or moderate symptoms, one had severe disease and one had life-threatening disease, with all seven patients recovering from the disease (Cross et al., 2022). Similar reassuring findings relating to COVID-19 disease severity were reported in a large analysis of ocrelizumab-treated patients with MS (Hughes et al., 2021). Despite not assessing for COVID-19 severity after vaccination, this study found that of the 358 cases of COVID-19 across the analysed clinical trial and post-marketing cohorts of ocrelizumab-treated MS patients, 49.7% (178) were asymptomatic, mild or moderate, and 70.9% (254) of patients had recovered, or were recovering, at the time of the analysis (Hughes et al., 2021).
Limitations of the current study include the small cohort size and short follow-up period. The lack of availability of antibody titres in patients without a history of infection who received a subsequent third dose of the COVID-19 booster vaccine also limits the study; this would have provided a more equivalent number of antigen exposures to the double-vaccinated patients with a confirmed history of natural SARS-CoV-2 infection, and could have therefore served as a more accurate comparison. Future work should therefore focus on comparing antibody titres after triple vaccination with titres after double vaccination plus natural infection. Additionally, immune responses after COVID-19 vaccination and natural infection are well known to wane over time (Feikin et al., 2022; Ferdinands et al., 2022; Goldberg et al., 2022; Pérez-Alós et al., 2022). This is an important consideration for the results of our study as post-vaccination antibody titres were measured over a broad range of different times for each participant following double vaccination. These post-vaccination measurements were also generally measured after a longer time period post-vaccination when compared with the time period between natural infection and the post-infection antibody measurements. Therefore, to factor in waning immunity, future work should analyse post-vaccination and post-infection antibody titres in MS patients treated with anti-CD20 therapies at similar time points after vaccination and infection, respectively. Finally, the accepted definition of ‘fully vaccinated’ at the time of data collection was two doses of an mRNA vaccine (Pfizer or Moderna) or a single Johnson & Johnson vaccine with an mRNA booster vaccine (Centers for Disease Control and Prevention, 2022). However, this definition has evolved over time, and guidance now indicates additional booster vaccines depending on medical history.
5. Conclusions
Ultimately, this work provides reassurance that anti-CD20 therapy in patients with MS and related neuroinflammatory conditions does not preclude an appropriate humoral response to COVID-19 vaccination and natural infection, contributing to the growing body of evidence informing MS and neuroinflammatory disease management and care during the COVID-19 pandemic. Additionally, this work suggests that increased frequency of immune stimulation plays an important role in developing a robust humoral response. Future work should be directed at investigating the implications of reinfection, especially given the poor immune-boosting effects of the Omicron variant (Reynolds et al., 2022). It should also compare the immunostimulatory effects of triple vaccination relative to those of double vaccination plus natural infection, as well as examine the maintenance and longevity of antibody titres in this patient population in order to better define the duration of the humoral response to COVID-19 vaccination and infection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CRediT authorship contribution statement
Conceptualisation: SS. Data curation: SD, EL. Formal analysis: EL. Funding acquisition: SS. Investigation: SD, EL. Methodology: SS, EL. Project administration: SS. Resources: SS. Software: EL. Supervision: SS. Validation: SD, EL. Visualisation: SS, SD, EL, Oxford PharmaGenesis. Writing – original draft: Oxford PharmaGenesis. Writing – review and editing: SS, SD, EL, Oxford PharmaGenesis.
Data statement
Data may be made available upon reasonable request to the authors.
Declaration of Competing Interest
EL and SD have no declarations of interest to report. SS has served on scientific advisory boards for Bristol Myers Squibb, F. Hoffmann-La Roche Ltd., Forepont Capital Partners, Genentech, Inc., Horizon Therapeutics plc and TG Therapeutics, Inc., and received research support from BeCare MS Link and MedDay Pharmaceuticals SA. She has also received compensation for consulting services from, served on scientific advisory boards for and received speaker honorarium from Alexion Pharmaceuticals, Inc., Biogen, Inc., Bristol Myers Squibb, EMD Serono, Inc., Horizon Therapeutics, Inc., Novartis AG, Genentech, Inc. and Sanofi Genzyme. She is also CEO of Global Consultant MD. She also serves on the steering committee of Horizon Therapeutics, Inc. and Genentech, Inc.
Acknowledgements
Medical writing support was provided by Adeline Rosenberg, MSc, of Oxford PharmaGenesis, Oxford, UK, and Mark Elms, PhD, of PharmaGenesis London, London, UK, and was funded by Novartis AG.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2023.104574.
Appendix. Supplementary materials
References
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., Markowitz C.E., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Roberts C.A.K., Pryce G., Kang A.S., Marta M., Reyes S., Schmierer K., Giovannoni G., Amor S. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020;202:149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., Evershed J., Fox E.J., Herman A., Manfrini M., McNamara J., Robertson D.S., Stokmaier D., Wendt J.K., Winthrop K.L., Traboulsee A. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancati S., Gozzo L., Longo L., Vitale D.C., Drago F. Rituximab in multiple sclerosis: are we ready for regulatory approval? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.661882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2022. Stay up to date with your COVID-19 vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html#recommendations [Accessed 8 July 2022].
- Cross A.H., Delgado S., Habek M., Davydovskaya M., Ward B.J., Cree B.A.C., Totolyan N., Pingili R., Mancione L., Hu X., Sullivan R., Su W., Zielman R., Gupta A.D., Montalban X., Winthrop K. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol. Ther. 2022;11:741–758. doi: 10.1007/s40120-022-00341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner S., Heitmann N., Plaza-Sirvent C., Trendelenburg P., Ceylan U., Motte J., Bessen C., Urlaub D., Watzl C., Overheu O., Reinacher-Schick A., Hellwig K., Pfaender S., Schmitz I., Gold R. Immune response in ofatumumab treated multiple sclerosis patients after SARS-CoV-2 vaccination. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.980526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., Groome M.J., Huppert A., O'Brien K.L., Smith P.G., Wilder-Smith A., Zeger S., Knoll M.D., Patel M.K. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinands J.M., Rao S., Dixon B.E., Mitchell P.K., DeSilva M.B., Irving S.A., Lewis N., Natarajan K., Stenehjem E., Grannis S.J., Han J., McEvoy C., Ong T.C., Naleway A.L., Reese S.E., Embi P.J., Dascomb K., Klein N.P., Griggs E.P., Liao I.C., Yang D.H., Fadel W.F., Grisel N., Goddard K., Patel P., Murthy K., Birch R., Valvi N.R., Arndorfer J., Zerbo O., Dickerson M., Raiyani C., Williams J., Bozio C.H., Blanton L., Link-Gelles R., Barron M.A., Gaglani M., Thompson M.G., Fireman B. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022;379 doi: 10.1136/bmj-2022-072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva Z.G., Dffinger R., Kumararatne D., Coles A.J., McCarthy C. Diminished seroconversion following a single SARS-COV-2 vaccine in ocrelizumab-treated relapsing-remitting multiple sclerosis patients. Mult. Scler. 2022;28:1126–1130. doi: 10.1177/13524585211046786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L.S., Ash N., Alroy-Preis S., Huppert A., Milo R. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N. Engl. J. Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J.S., Killestein J. Reading the "T" leaves of COVID-19 vaccine responses in multiple sclerosis. Neurology. 2022;98:177–178. doi: 10.1212/WNL.0000000000013166. [DOI] [PubMed] [Google Scholar]
- Hughes R., Whitley L., Fitovski K., Schneble H.M., Muros E., Sauter A., Craveiro L., Dillon P., Bonati U., Jessop N., Pedotti R., Koendgen H. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta M., Landi D., Cola G., Malagnino V., Teti E., Fraboni D., Buccisano F., Grelli S., Coppola L., Campogiani L., Andreoni M., Marfia G.A., Sarmati L. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnc N., Bsteh G., Berger T., Mares J., Hartung H.P. Monoclonal antibodies in the treatment of relapsing multiple sclerosis: an overview with emphasis on pregnancy, vaccination, and risk management. Neurotherapeutics. 2022;19:753–773. doi: 10.1007/s13311-022-01224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levit E., Longbrake E.E., Stoll S.S. Seroconversion after COVID-19 vaccination for multiple sclerosis patients on high efficacy disease modifying medications. Mult. Scler. Relat. Disord. 2022;60 doi: 10.1016/j.msard.2022.103719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo R., Staun-Ram E., Karussis D., Karni A., Hellmann M.A., Bar-Haim E., Miller A., Israeli Neuroimmunology Study Group on Covid-19 Vaccination in Multiple Sclerosis Humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis: an Israeli multi-center experience following 3 vaccine doses. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.868915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Multiple Sclerosis Society, 2022. COVID-19 vaccine guidance for people living with MS. https://www.nationalmssociety.org/coronavirus-covid-19-information/covid-19-vaccine-guidance [Accessed 16 June 2022].
- Pérez-Alós L., Armenteros J.J.A., Madsen J.R., Hansen C.B., Jarlhelt I., Hamm S.R., Heftdal L.D., Pries-Heje M.M., Møller D.L., Fogh K., Hasselbalch R.B., Rosbjerg A., Brunak S., Sørensen E., Larsen M.A.H., Ostrowski S.R., Frikke-Schmidt R., Bayarri-Olmos R., Hilsted L.M., Iversen K.K., Bundgaard H., Nielsen S.D., Garred P. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022;13:1614. doi: 10.1038/s41467-022-29225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C.J., Pade C., Gibbons J.M., Otter A.D., Lin K.M., Munoz Sandoval D., Pieper F.P., Butler D.K., Liu S., Joy G., Forooghi N., Treibel T.A., Manisty C., Moon J.C., Semper A., Brooks T., McKnight A., Altmann D.M., Boyton R.J., COVIDsortium Investigators, COVIDsortium Immune Correlates Network Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377:eabq1841. doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach C.A., Cross A.H. Anti-CD20 B cell treatment for relapsing multiple sclerosis. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.595547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets I., Reyes S., Baker D., Giovannoni G. Blunted vaccines responses after ocrelizumab highlight need for immunizations prior to treatment. Mult. Scler. Relat. Disord. 2021;50 doi: 10.1016/j.msard.2021.102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Vickaryous N., Anderson V., Asardag A.N., Baker D., Bestwick J., Bramhall K., Chance R., Evangelou N., George K., Giovannoni G., Godkin A., Grant L., Harding K.E., Hibbert A., Ingram G., Jones M., Kang A.S., Loveless S., Moat S.J., Robertson N.P., Schmierer K., Scurr M.J., Shah S.N., Simmons J., Upcott M., Willis M., Jolles S., Dobson R. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2022;91:89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella C., Aiello A., Gasperini C., Agrati C., Castilletti C., Ruggieri S., Meschi S., Matusali G., Colavita F., Farroni C., Cuzzi G., Cimini E., Tartaglia E., Vanini V., Prosperini L., Haggiag S., Galgani S., Quartuccio M.E., Salmi A., Repele F., Altera A.M.G., Cristofanelli F., D’Abramo A., Bevilacqua N., Corpolongo A., Puro V., Vaia F., Capobianchi M.R., Ippolito G., Nicastri E., Goletti D., INMI COVID-19 Vaccine Study Group Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98:e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association, 2018. WMA Declaration of Helsinki – Ethical principles for medical research involving human subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/[Accessed 16 June 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.