PURPOSE
Zanubrutinib is a potent, irreversible next-generation Bruton tyrosine kinase (BTK) inhibitor designed to maximize BTK occupancy and minimize off-target kinase inhibition. We hypothesized that complete/sustained BTK occupancy may improve efficacy outcomes and increased BTK specificity may minimize off-target inhibition-related toxicities.
PATIENTS AND METHODS
ALPINE (ClinicalTrials.gov identifier: NCT03734016) is a global, randomized, open-label phase III study of zanubrutinib versus ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia. The primary end point was investigator-assessed overall response rate (ORR). The preplanned interim analysis was scheduled approximately 12 months after the first 415 patients were enrolled.
RESULTS
Between November 1, 2018, and December 14, 2020, 652 patients were enrolled. We present the interim analysis of the first 415 enrolled patients randomly assigned to receive zanubrutinib (n = 207) or ibrutinib (n = 208). At 15 months of median follow-up, ORR (partial or complete response) was significantly higher with zanubrutinib (78.3%; 95% CI, 72.0 to 83.7) versus ibrutinib (62.5%; 95% CI, 55.5 to 69.1; two-sided P < .001). ORR was higher with zanubrutinib versus ibrutinib in subgroups with del(17p)/TP53 mutations (80.5% v 50.0%) and del(11q) (83.6% v 69.1%); 12-month progression-free survival in all patients was higher with zanubrutinib (94.9%) versus ibrutinib (84.0%; hazard ratio, 0.40; 95% CI, 0.23 to 0.69). Atrial fibrillation rate was significantly lower with zanubrutinib versus ibrutinib (2.5% v 10.1%; two-sided P = .001). Rates of cardiac events, major hemorrhages, and adverse events leading to treatment discontinuation/death were lower with zanubrutinib.
CONCLUSION
Zanubrutinib had a significantly higher ORR, lower atrial fibrillation rate, and improved progression-free survival and overall cardiac safety profile versus ibrutinib. These data support improved efficacy/safety outcomes with selective BTK inhibition.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the western world with an incidence of 4.2/100,000 persons per year.1 Chemoimmunotherapy has improved outcomes for patients with CLL,2 but the advent of nonchemotherapy-based treatments, including B-cell receptor inhibitors, has transformed CLL treatment.3
CONTEXT
Key Objective
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib is a standard treatment option for untreated and relapsed/refractory chronic lymphocytic leukemia (CLL) and is associated with adverse events which may be attributed to off-target kinase inhibition. Zanubrutinib, a highly selective next-generation BTK inhibitor, was designed to maximize BTK occupancy and minimize off-target inhibition. To our knowledge, ALPINE (ClinicalTrials.gov identifier: NCT03734016) is the first head-to-head study of zanubrutinib versus ibrutinib in patients with relapsed/refractory CLL. This interim analysis evaluates the efficacy and safety of zanubrutinib versus ibrutinib in patients with relapsed/refractory CLL.
Knowledge Generated
Initial data from the ALPINE study suggest that zanubrutinib may improve efficacy outcomes, and its increased specificity may minimize off-target, inhibition-related toxicities.
Relevance (S. Lentzsch)
-
Owing to the favorable benefit risk profile for zanubrutinib shown in the ALPINE trial, zanubrutinib is preferred over ibrutinib for the treatment of most patients with relapsed/refractory CLL/small lymphocytic lymphoma.*
*Relevance section written by JCO Associate Editor Suzanne Lentzsch, MD, PhD.
A first-generation Bruton tyrosine kinase inhibitor (BTKi), ibrutinib, is a standard treatment option for untreated and relapsed/refractory CLL.3 However, ibrutinib is associated with adverse events (AEs) which may be attributed to off-target kinase inhibition, such as hemorrhage, atrial fibrillation, ventricular arrhythmias, and hypertension.4-6 Approximately 16%-23% of patients discontinue ibrutinib because of treatment-related toxicities.7-10 In RESONATE, a landmark study comparing ibrutinib versus ofatumumab in patients with relapsed/refractory CLL or small lymphocytic lymphoma (SLL), 37% discontinued ibrutinib because of progressive disease (PD) and high-risk patients (del[17p] and/or TP53 mutation) treated with ibrutinib had median progression-free survival (PFS) of 40.7 versus 56.9 months in patients without mutations.9
Zanubrutinib (BGB-3111), a potent, irreversible next-generation BTKi, is more selective for BTK inhibition and exhibits less off-target kinase activity than ibrutinib.11,12 In a phase I/II zanubrutinib study (BGB-3111-AU-003) in patients with CLL/SLL (82%; 101 of 123 relapsed/refractory), zanubrutinib demonstrated complete and sustained BTK occupancy in peripheral blood mononuclear cells and lymph nodes of all patients.12 At a median follow-up of 54.1 months (treatment-naive) and 43.7 months (relapsed/refractory), 21% discontinued zanubrutinib because of PD and 10% because of AEs, and the rate of atrial fibrillation/flutter was 4.9%.13 Estimated 2-year PFS was 90% in treatment-naive and 91% in relapsed/refractory patients, with a median follow-up of 47.2 months.13 For patients with del(17p)/TP53, PFS was 82% at 2 years. In patients with relapsed/refractory CLL, the overall response rate (ORR) was 95%.13
Achieving complete BTK inhibition in blood and lymph nodes is hypothesized to provide meaningful and sustained responses in CLL/SLL.12 It is hypothesized that higher selectivity reduces toxicities and high BTK occupancy maximizes efficacy, potentially increasing the likelihood of achieving deep and sustained remission in CLL.12 We, therefore, initiated a global multicenter, open-label phase III, head-to-head study of zanubrutinib or ibrutinib in patients with relapsed/refractory CLL.
PATIENTS AND METHODS
Study Design
ALPINE (ClinicalTrials.gov identifier: NCT03734016) is a global, randomized, open-label phase III study of zanubrutinib versus ibrutinib in patients with relapsed/refractory CLL. The results of a preplanned interim analysis scheduled approximately 12 months after the enrollment of the first 415 patients are presented. The trial was approved by the institutional review board or independent ethics committee at each study site and conducted in accordance with the principles of the Declaration of Helsinki, International Council on Harmonisation guidelines, the principles of Good Clinical Practice, and all applicable regulatory requirements. All participants gave written informed consent.
Patients
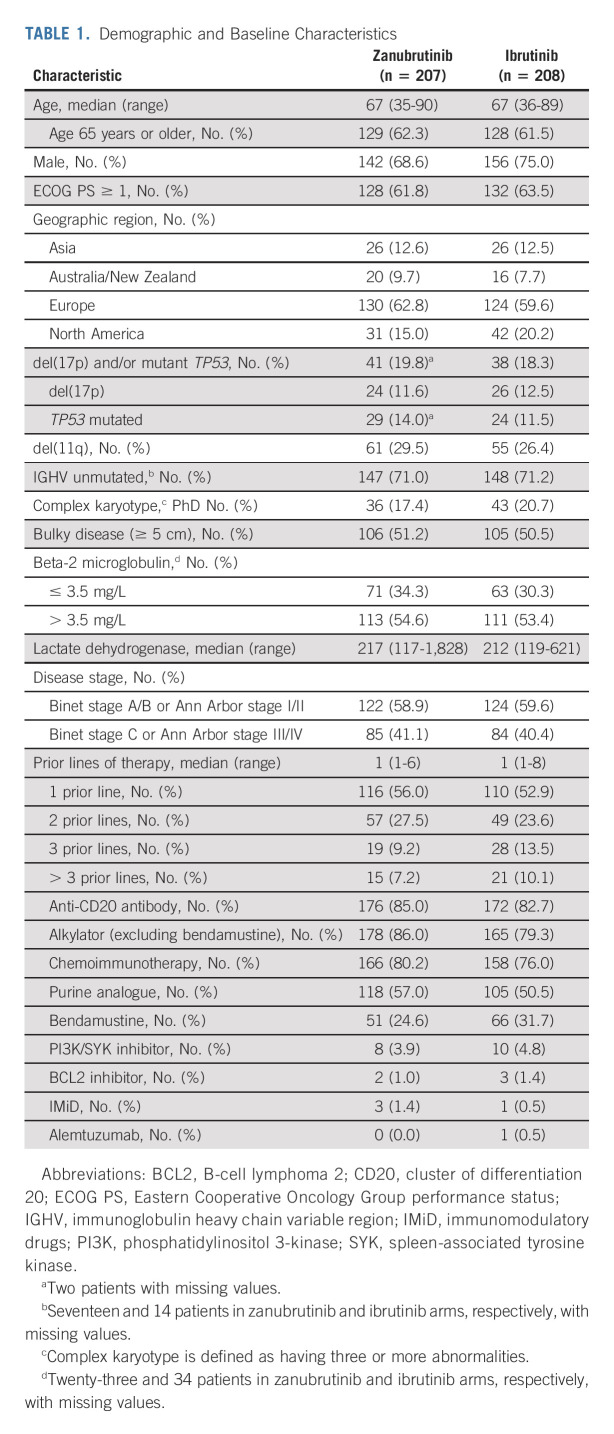
Eligible patients had a confirmed diagnosis of CLL or SLL that met International Workshop on CLL (iwCLL) criteria,14 required treatment, were relapsed after, or refractory to, at least one prior line of therapy, were age 18 years or older, and had measurable disease by imaging. Patients were required to have an Eastern Cooperative Oncology Group performance status score of 0, 1, or 2; adequate bone marrow and organ function; and a life expectancy of ≥ 6 months. Patients with bleeding disorders, active infections, stroke/intracranial hemorrhage, recent prior malignancies, or major surgery were ineligible. Patients with ongoing treatment with warfarin, strong CYP3A inhibitors/inducers, corticosteroids, or previously treated with BTKis were excluded (Data Supplement, online only).
Random Assignment and Treatment
Patients were randomly assigned 1:1 to receive zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily until PD or unacceptable toxicity (Data Supplement). Study drugs were administered open-label because dosing schedules and suggested dose modifications differed. Patients were randomly assigned by an interactive web response system on the basis of a computer-generated random assignment schedule, and patients were stratified by age (younger than 65 v 65 years or older), geographic region (China v non-China), refractory status, and del(17p)/TP53 mutation status (Table 1). Patients were stratified by geographic region to account for potential differences in standards of care and prior treatments.
TABLE 1.
Demographic and Baseline Characteristics
Outcomes
Efficacy end points were analyzed with the intent-to-treat analysis set and only included the first 415 patients randomly assigned in the study. The safety analysis set included all patients who received any dose of the study drug among the first 415 randomly assigned patients. Safety data from all enrolled patients will be available at the final analysis.
The primary efficacy end point was investigator-assessed ORR, defined as complete response (CR) or partial response (PR) per regulatory authority requirement. Disease response was assessed per iwCLL 2008 criteria every 3 months for 2 years then every 6 months,14 with modification for treatment-related lymphocytosis15 for patients with CLL and per Lugano classification16 for patients with SLL (Protocol, online only). To support the primary analysis, ORR per blinded independent central review (ICR) and rate of PR with lymphocytosis (PR-L) or higher were analyzed.
Secondary end points included PFS, atrial fibrillation/flutter rate, duration of response, rate of PR-L or higher, overall survival (OS), time to treatment failure, and patient-reported outcomes. AEs were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.03. Hematologic AEs were graded according to the CLL Working Group grading scheme (Data Supplement).14 Arrhythmia signs/symptoms were reviewed at every visit.
Statistical Analysis
A sample size of 600 was estimated to provide > 90% power to demonstrate noninferiority of zanubrutinib to ibrutinib in ORR, with a noninferiority margin of 0.8558 on the response ratio scale (zanubrutinib rate divided by ibrutinib rate), an assumed response ratio of 1.02 (72%/70%), and a one-sided alpha level of 0.025. The prespecified interim analysis included the first 415 randomly assigned patients. On April 20, 2021, the independent data monitoring committee reviewed the interim analysis and confirmed that the prespecified statistical boundary for noninferiority in ORR had been crossed based on a stratified Wald test of the response ratio. Subsequent formal hypothesis testing was performed, including the superiority of ORR per a prespecified hypothesis testing approach (Protocol).
RESULTS
Patient Population
Between November 1, 2018, and December 14, 2020, 652 patients were enrolled from 113 sites across 15 countries in North America, Europe, and Asia-Pacific. The interim analysis only included the first 415 patients enrolled in the study; the 415th patient was randomly assigned on December 20, 2019. Patients were randomly assigned 1:1 to zanubrutinib (n = 207) or ibrutinib (n = 208; Fig 1). Demographics and disease characteristics were balanced at baseline, except for more female patients on zanubrutinib versus ibrutinib (31% v 25%; Table 1). The median age was 67 years (range, 35-90 years), 83% were White, 12% were Asian, 51% of patients entered the study with bulky disease, 50% with cytopenias, and 19% had del(17p) and/or TP53 mutations. The median prior lines of therapy was one (range, 1-8); 9% of patients received more than three lines of therapy (Table 1). A total of 80% and 76% of patients on zanubrutinib and ibrutinib, respectively, had previously received chemoimmunotherapy. At data cutoff (December 31, 2020), 87.4% and 75.5% of patients in the zanubrutinib and ibrutinib arms, respectively, were still receiving treatment. Three patients in the zanubrutinib arm and one patient in the ibrutinib arm did not receive any drug and were excluded from the safety analyses.
FIG 1.
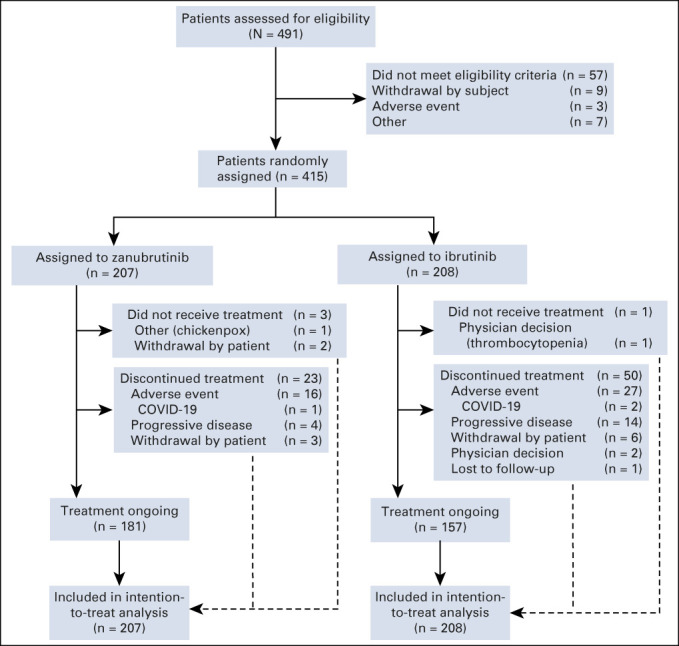
ALPINE study CONSORT diagram. A total of 491 patients had been screened at the time of random assignment of the 415th patient. All 415 randomly assigned patients were included in the efficacy analyses.
Efficacy
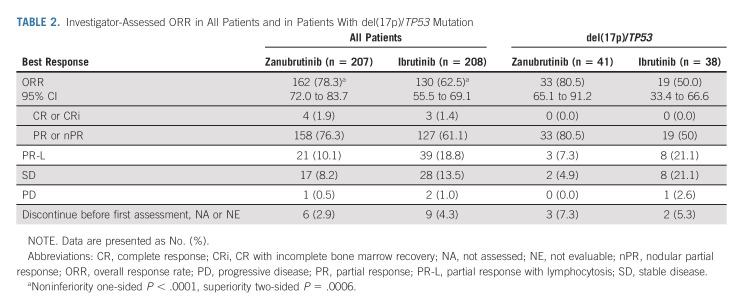
After 15.3 months of median follow-up (range, 0.1-26.0 months), investigator-assessed ORR (CR and PR) was significantly higher with zanubrutinib versus ibrutinib (78.3% v 62.5%; two-sided P < .001; Table 2). Consistent benefit in favor of zanubrutinib was observed in all prespecified patient subgroups (Fig 2). A higher ORR among patients with del(17p)/TP53 mutation was observed with zanubrutinib versus ibrutinib (80.5% v 50.0%; 95% CI rate difference, 10.5 to 50.5; Table 2). Patients with del(11q) achieved an ORR of 83.6% with zanubrutinib and 69.1% with ibrutinib (Fig 2).
TABLE 2.
Investigator-Assessed ORR in All Patients and in Patients With del(17p)/TP53 Mutation
FIG 2.
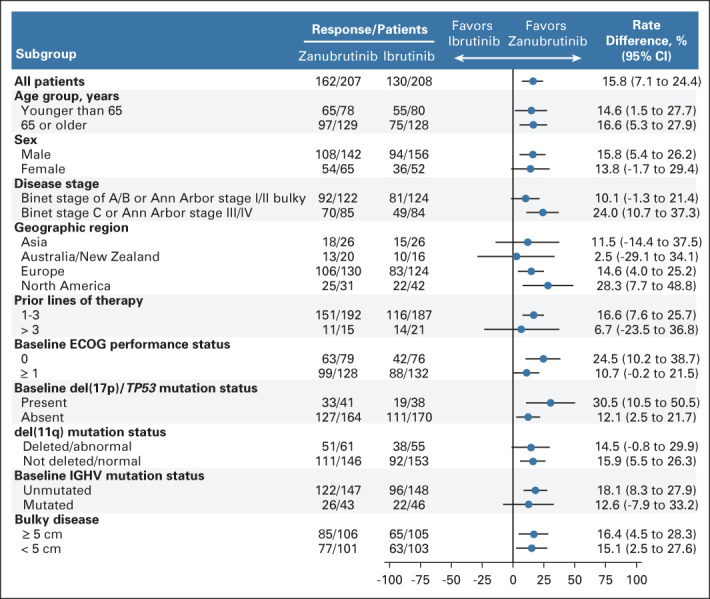
Subgroup analysis for ORR. Forest plot showing ORR in prespecified baseline and disease subgroups. The rate difference (unstratified estimates of the differences in response rates) and 95% CI (normal approximations) are shown as a blue dot and black bars. A rate difference of greater than zero represents response rates favoring zanubrutinib. ECOG PS, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy chain variable region; ORR, overall response rate.
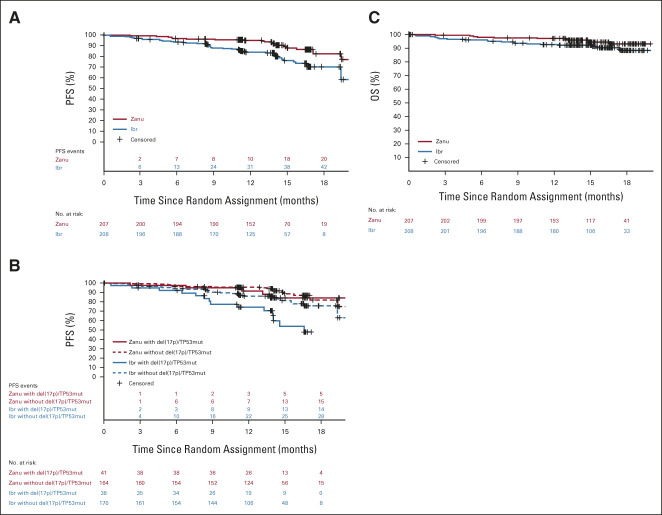
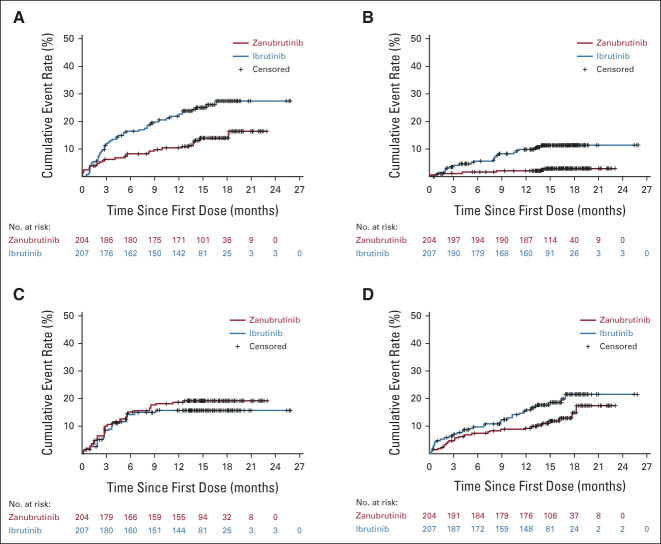
By ICR, ORR met noninferiority (76.3% v 64.4%; P < .001) but not superiority (P = .0121, two-sided significance level 0.0099). In patients with del(17p)/TP53 mutation, ORR by ICR was 80.5% with zanubrutinib versus 55.3% with ibrutinib (Data Supplement). The concordance rate for best overall response of PR or higher between ICR and investigator was 94.2% and 93.3% for the zanubrutinib and ibrutinib arms, respectively; the concordance rate of PR-L or higher was 91.3% and 92.8%, respectively. Patients achieving a best overall response of PR-L or higher were 88.4% with zanubrutinib and 81.3% with ibrutinib by investigator. Transient lymphocytosis was observed with both BTKi; lymphocytosis returned to baseline in approximately 3 months for zanubrutinib and 4 months for ibrutinib (Data Supplement). Investigator-assessed PFS at 12 months was 94.9% (95% CI, 90.7 to 97.2) with zanubrutinib and 84.0% (95% CI, 78.1 to 88.5) with ibrutinib. At data cutoff, 21 and 44 PFS events occurred in the zanubrutinib and ibrutinib arms, respectively (Data Supplement). Hazard ratio for PD or death was 0.40 (95% CI, 0.23 to 0.69; for descriptive purposes only P < .001; Fig 3A). Similar 12-month PFS was seen in ICR-assessed data (92.2% in the zanubrutinib arm, 82.4% in the ibrutinib arm; Data Supplement). Twelve-month PFS was favorable for patients treated with zanubrutinib regardless of del(17p)/TP53 mutation status (91.5% v 74.4%; Fig 3B). A formal PFS final analysis will be conducted once 205 PFS events are observed. Among responders, the estimated proportion of responses with a duration ≥ 12 months was higher for zanubrutinib (89.8%; 95% CI, 78.1 to 95.4) than ibrutinib (77.9%; 95% CI, 64.7 to 86.7). At data cutoff, nine events (five PD and four deaths without PD) among 162 zanubrutinib responders and 16 events (14 PD and two deaths without PD) among 130 ibrutinib responders were observed. A total of 7.2% of patients died before data cutoff. The 12-month OS rate was 97.0% and 92.7% with zanubrutinib and ibrutinib (hazard ratio, 0.54; 95% CI, 0.25 to 1.16), respectively (Fig 3C).
FIG 3.
Investigator-assessed PFS and OS. (A) Kaplan-Meier estimates of PFS for all patients. (B) Kaplan-Meier estimates of PFS for patients with or without del(17p)/TP53 mutations. (C) Kaplan-Meier estimates of OS for all patients. Ibr, ibrutinib; OS, overall survival; PFS, progression-free survival; Zanu, zanubrutinib.
Safety
Median treatment duration with zanubrutinib (15.3 months [range, 0.4-23.0 months]) and ibrutinib (14.6 months [range, 0.1-25.9 months]) was similar. One or more AEs occurred in 95.6% and 99.0% of patients receiving zanubrutinib and ibrutinib, respectively.
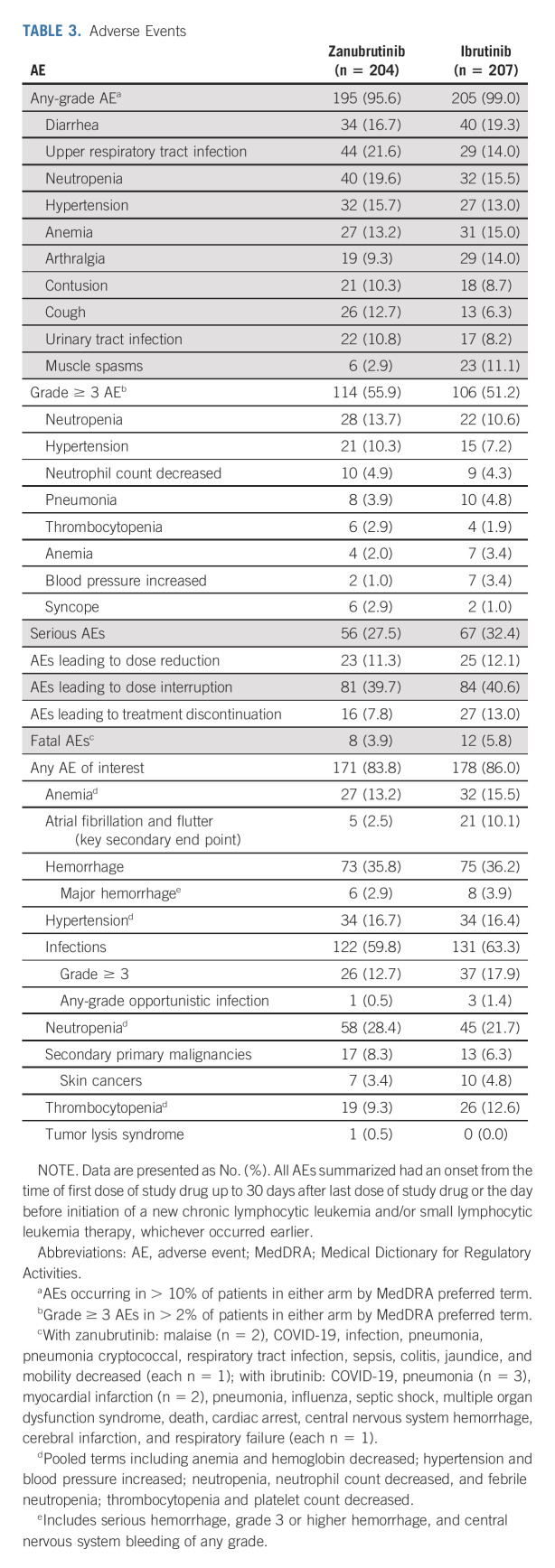
Fatal AEs were reported in 8 and 12 patients receiving zanubrutinib and ibrutinib, respectively (Table 3; Data Supplement). Fewer patients on zanubrutinib experienced AEs leading to treatment discontinuation versus ibrutinib (7.8% v 13.0%; Data Supplement). Infection events were the most common AEs of interest leading to treatment discontinuation (2.9%, each arm). Arthralgia was reported in 9.3% and 14.0% of patients receiving zanubrutinib and ibrutinib, respectively. Diarrhea was reported in 16.7% and 19.3% of patients receiving zanubrutinib and ibrutinib, respectively (Table 3). Overall cardiac disorder AEs occurred at a lower incidence with zanubrutinib (13.7%) versus ibrutinib (25.1%; Fig 4A). Cardiac disorders leading to treatment discontinuation occurred in 3.4% of patients in the ibrutinib arm (Data Supplement). Grade ≥ 3 AEs were experienced in 55.9% and 51.2% of patients receiving zanubrutinib and ibrutinib, respectively. Common grade ≥ 3 AEs are listed in Table 3.
TABLE 3.
Adverse Events
FIG 4.
Time to adverse events of interest and cardiac disorders. Kaplan-Meier plot of time to (A) cardiac disorders, on the basis of System Organ Class in Medical Dictionary for Regulatory Activities (includes atrial fibrillation and atrial flutter); (B) atrial fibrillation/flutter; (C) grade 3 or higher neutropenia; (D) grade 3 or higher infection. For these plots, patients without the events of interest were censored on the last date of safety follow-up (30 days after treatment discontinuation or initiation of a new anticancer therapy or death, whichever is earliest).
Adverse Events of Interest
The rate of any-grade atrial fibrillation/flutter was significantly lower with zanubrutinib versus ibrutinib (2.5% v 10.1%; two-sided P = .001, prespecified alpha of 0.0099; Fig 4B). Grade ≥ 3 atrial fibrillation/flutter occurred in two (1.0%) and four (1.9%) patients, and atrial fibrillation/flutter occurred in four (2.1%) and 18 (9.3%) patients with no history of atrial fibrillation/flutter receiving zanubrutinib versus ibrutinib, respectively. Neutropenia was reported at a higher frequency (28.4% v 21.7%; Table 3) with zanubrutinib versus ibrutinib; 18.6% and 15.0% of patients on zanubrutinib and ibrutinib, respectively, experienced grade ≥ 3 neutropenia (Fig 4C). A total of 15.2% of patients on zanubrutinib and 11.1% of patients on ibrutinib received colony-stimulating growth factor as concomitant treatment. One patient discontinued zanubrutinib because of neutropenia (Data Supplement), and one case of febrile neutropenia occurred in a patient treated with ibrutinib. Grade ≥ 3 infections occurred in 12.7% and 17.9% of patients receiving zanubrutinib and ibrutinib, respectively (Fig 4D). The most common grade ≥ 3 infection (pneumonia) occurred in 3.9% and 4.8% of patients receiving zanubrutinib and ibrutinib, respectively. Eleven patients in each arm experienced COVID-19–related AEs (Data Supplement).
Any-grade hemorrhagic events occurred with similar frequency in the zanubrutinib and ibrutinib arms (35.8% v 36.2%, respectively). Major hemorrhagic events occurred in 2.9% and 3.9% of patients receiving zanubrutinib and ibrutinib, respectively, including one case of CNS hemorrhage in a patient treated with ibrutinib (Table 3). A total of 16.7% and 16.4% of patients in each arm experienced any-grade hypertensive AEs. Seventeen and 13 patients were diagnosed with a second primary malignancy with zanubrutinib and ibrutinib, respectively. The most common second primary malignancy was nonmelanoma skin cancer, observed in 3.4% and 4.8% of patients on zanubrutinib and ibrutinib, respectively. One patient developed grade 3 tumor lysis syndrome at commencement of zanubrutinib treatment.
DISCUSSION
Zanubrutinib is a potent, irreversible next-generation BTKi designed to maximize BTK occupancy and minimize off-target inhibition of tyrosine-protein kinase and epidermal growth factor receptor-family kinases. We hypothesized that complete/sustained BTK occupancy may improve efficacy outcomes, and increased BTK specificity may minimize off-target inhibition-related toxicities.
Zanubrutinib was first compared with ibrutinib in Waldenström macroglobulinemia in the randomized, phase III trial, ASPEN.17 ALPINE is the second randomized, phase III trial comparing zanubrutinib with ibrutinib and compares approximately 600 patients (planned) with relapsed/refractory CLL/SLL. This preplanned interim analysis was conducted approximately 12 months after enrolling the first 415 patients.
Except for sex, demographic and baseline disease characteristics, including del(17p)/TP53 mutations, were balanced between each arm in the intent-to-treat analysis set and in the first 415 randomly assigned patients.
The primary study end point, ORR (CR or PR) as assessed by investigator, was significantly higher with zanubrutinib compared with ibrutinib in all patients, in molecular subgroups, including del(11q), and in the difficult-to-treat del(17p)/TP53. Multiple sensitivity analyses of ORR (Data Supplement), and a high concordance rate of ORR between investigator assessments and ICR, support the favorable results for zanubrutinib.
In the ALPINE study, the rate of PR-L or higher for patients treated with ibrutinib (81% with a median follow-up of 15.4 months) was comparable with the reported cumulative best response at 15 months of 89% in the RESONATE study.18 A key secondary end point, PFS, although currently immature, was notably prolonged with zanubrutinib compared with ibrutinib, and a consistent trend for improvement with zanubrutinib compared with ibrutinib was seen in OS and secondary efficacy end points, including duration of response (Fig 3; Data Supplement) and time to treatment failure (data not shown). The PFS rate seen with ibrutinib (84.0% at 12 months) was comparable with that in the RESONATE study (84% at 12 months).19
Lower rates of AEs leading to discontinuation or death with zanubrutinib versus ibrutinib in this study were consistent with those reported in the ASPEN study, in which AEs leading to discontinuation with zanubrutinib versus ibrutinib were 4% versus 9% and AEs leading to death were 1% versus 2%, respectively.17 The rate of atrial fibrillation, a prespecified key secondary end point, was significantly lower with zanubrutinib compared with ibrutinib (2.5% v 10.1%; two-sided P = .001). This difference is consistent with data reported in the ASPEN study, in which the rate of atrial fibrillation was significantly lower with zanubrutinib versus ibrutinib (2.0% v 15.3%; one-sided P = .0004) and is consistent with multiple clinical settings and study populations.17 No patients in the zanubrutinib arm had cardiac AEs that led to treatment discontinuation, compared with seven patients in the ibrutinib arm (atrial fibrillation [two patients], cardiac arrest, cardiac failure, myocardial infarction, palpitations, and ventricular fibrillation [one patient each]). One hypothesis suggests that ibrutinib therapy can lead to atrial fibrillation and cardiac damage through the off-target inhibition of C-terminal Src kinase.20 Additionally, ibrutinib inhibits ERBB2/HER2, which results in cardiac myocyte dysfunction and reduced heart contractile efficiency.21 Zanubrutinib demonstrates less inhibition of C-terminal Src kinase than does ibrutinib.11,22
Although not significant, diarrhea, a bothersome AE that may contribute to treatment discontinuation, was less common in patients treated with zanubrutinib (16.7%) compared with ibrutinib (19.3%). These results differ from the ASPEN study in which all-grade diarrhea was significantly less common in patients treated with zanubrutinib (21%) than with ibrutinib (32%).17 In the ASPEN study, the rate of hypertension was lower with zanubrutinib (11%) compared with ibrutinib (16%); the incidence of hypertension in patients treated with zanubrutinib leveled off at 18 months of treatment, whereas patients treated with ibrutinib remained at continuous risk for hypertension with a clear separation of the time-to-event curves with longer time on treatment.17 Here, we observed a similar rate of hypertension across both arms with hypertension leading to treatment discontinuation in 0 (0.0%) and 1 (0.5%) patient treated with zanubrutinib and ibrutinib, respectively; a difference in the rate of hypertension between arms may only appear with longer duration of treatment and follow-up.
The study has several limitations. Owing to the preliminary nature of the survival analysis and the short follow-up, PFS and OS data should be interpreted with caution. A formal PFS final analysis will be conducted when 205 PFS events are observed. The choice of ORR, defined as PR or higher, by investigator assessment as a primary end point is consistent with the tumor response criteria in the iwCLL guidelines but can be a limitation because excluding PR-L responses from the ORR excludes patients who may be benefiting clinically from treatment.14 The primary end point was selected on the basis of agreement with regulatory authorities and expert investigators in CLL clinical trials. The open-label design of the study may have introduced bias. To mitigate potential investigator bias, ICR review was blinded, and multiple sensitivity analyses of ORR were conducted.
The ALPINE study showed a superior ORR, improved PFS, and lower rates of atrial fibrillation/flutter in patients with relapsed/refractory CLL/SLL treated with zanubrutinib compared with ibrutinib. Initial data support the hypothesis that complete/sustained BTK occupancy may improve efficacy outcomes and increased specificity may minimize off-target inhibition-related toxicities. The interim analysis demonstrated a favorable benefit-risk profile for zanubrutinib in the treatment of patients with relapsed/refractory CLL/SLL.
ACKNOWLEDGMENT
We thank study patients, their supporters, and investigators and clinical research staff at study centers. Medical writing and editorial assistance were provided, under the direction of the authors, by Laura S. Moye, PhD and Miriam Cohen, PhD, ISMPP CMPP of Bio Connections, LLC, (Chicago, IL), supported by BeiGene.
Kenneth Wu
Employment: BeiGene, Genentech/Roche
Stock and Other Ownership Interests: BeiGene, Genentech/Roche
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, BeiGene, Pharmacyclics, Roche/Genentech, Loxo/Lilly
Consulting or Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag (Inst), AbbVie (Inst), BeiGene (Inst)
Tadeusz Robak
Honoraria: AstraZeneca, BeiGene, Janssen
Consulting or Advisory Role: AstraZeneca, BeiGene, Janssen Oncology
Research Funding: AstraZeecaa, BeiGene, Janssen
Jessica Yecies
Employment: BeiGene, Day One Biopharmaceuticals, Kaiser Permanente, GRAIL
Stock and Other Ownership Interests: BeiGene, GRAIL, Day One Biopharmaceuticals
Mazyar Shadman
Employment: Bristol Myers Squibb
Consulting or Advisory Role: AbbVie, Genentech, AstraZeneca, Sound Biologics, Cellectar, Pharmacyclics, BeiGene, Bristol Myers Squibb/Celgene, MorphoSys, Innate Pharma, Kite, a Gilead Company, Adaptive Biotechnologies, Epizyme, Fate Therapeutics, Lilly, Regeneron, Adaptimmune, MustangBio, TG Therapeutics, MEI Pharma
Research Funding: Pharmacyclics (Inst), Acerta Pharma (Inst), Merck (Inst), TG Therapeutics (Inst), BeiGene (Inst), Celgene (Inst), Genentech (Inst), MustangBio (Inst), AbbVie (Inst), Sunesis Pharmaceuticals (Inst), Bristol Myers Squibb/Celgene, Genmab (Inst), Vincerx Pharma (Inst)
Adam Idoine
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Susan M. O'Brien
Employment: University of California, Irvine
Honoraria: Celgene, Janssen, Pharmacyclics, Gilead Sciences, Pfizer, Amgen, Astellas Pharma, GlaxoSmithKline, Aptose Biosciences, Vaniam Group, AbbVie, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Eisai, TG Therapeutics, NOVA Research Company
Consulting or Advisory Role: Amgen, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, AbbVie/Genentech, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Astellas Pharma, Gilead Sciences, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis Pharmaceuticals
Research Funding: Acerta Pharma (Inst), Regeneron (Inst), Gilead Sciences (Inst), Pfizer (Inst), TG Therapeutics (Inst), Pharmacyclics (Inst), Kite, a Gilead Company (Inst), Sunesis Pharmaceuticals (Inst), Lilly (Inst), Caribou Biosciences (Inst)
Travel, Accommodations, Expenses: Celgene, Janssen, Gilead Sciences, Regeneron, Janssen Oncology
Jiří Mayer
Research Funding: BeiGene
Barbara Eichhorst
Honoraria: Roche, AbbVie, Janssen-Cilag, AstraZeneca, Miltenyi Biotec, BeiGene, MSD
Consulting or Advisory Role: Janssen-Cilag, AbbVie, AstraZeneca, Lilly
Research Funding: AbbVie, Janssen, BGI, Gilead Sciences, AstraZeneca
Travel, Accommodations, Expenses: Roche, AbbVie, Gilead Sciences, Janssen
Habte A. Yimer
Employment: Texas Oncology/US Oncology
Stock and Other Ownership Interests: Karyopharm Therapeutics
Speakers' Bureau: Janssen, AstraZeneca, BeiGene, GlaxoSmithKline, Karyopharm Therapeutics, Amgen, Pharmacyclics
Nicole Lamanna
Consulting or Advisory Role: Celgene, Genentech, AbbVie, ProNAi, Pharmacyclics, Juno Therapeutics, Roche, Janssen, AstraZeneca, Gilead Sciences, BeiGene, Loxo/Lilly
Research Funding: Genentech/AbbVie (Inst), Infinity Pharmaceuticals (Inst), Gilead Sciences (Inst), ProNAi (Inst), BeiGene (Inst), Verastem (Inst), Juno Therapeutics (Inst), TG Therapeutics (Inst), Acerta Pharma/AstraZeneca (Inst), Loxo (Inst), Oncternal Therapeutics, Inc (Inst), MingSight (Inst), Octapharm (Inst)
Meng Ji
Employment: BeiGene Beijing, Takeda
Stock and Other Ownership Interests: BeiGene
Wojciech Jurczak
Consulting or Advisory Role: Roche, AstraZeneca, BeiGene
Research Funding: Acerta Pharma, TG Therapeutics, Sandoz-Novartis, Roche, Takeda, Epizyme, Janssen-Cilag, BeiGene, MorphoSys, MEI Pharma
Peter Hillmen
Employment: Apellis Pharmaeuticals
Honoraria: Janssen, AbbVie, Roche, AstraZeneca, BeiGene, Swedish Orphan Biovitrum, Pharmacyclics
Research Funding: Janssen (Inst), Pharmacyclics (Inst), Roche (Inst), Gilead Sciences (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Janssen, AbbVie
Alessandra Ferrajoli
Consulting or Advisory Role: Giants of Cancer Care, AstraZeneca, Janssen Oncology
Patents, Royalties, Other Intellectual Property: Celgene, Acerta Pharma, BeiGene
Jane Huang
Employment: BeiGene, Prelude Therapeutics
Leadership: 858 Therapeutics, Protara Therapeutics, Prelude Therapeutics, BeiGene
Stock and Other Ownership Interests: Protara Therapeutics, BeiGene, 858 Therapeutics, Prelude Therapeutics
Consulting or Advisory Role: BeiGene
Jennifer R. Brown
Consulting or Advisory Role: AbbVie, Acerta/Astra-Zeneca, BeiGene, Bristol Myers Squibb/Celgene/Juno, Catapult Therapeutics, Lilly, Genentech/Roche, HUTCHMED, iOnctura, Janssen, MEI Pharma, MorphoSys, Novartis, Pharmacyclics
Research Funding: BeiGene (Inst), Gilead Sciences (Inst), Loxo/Lilly (Inst), MEI Pharma (Inst), Secura Bio (Inst), Sun Pharma (Inst), TG Therapeutics (Inst)
Martin Šimkovič
Honoraria: Janssen-Cilag, AbbVie/Genentech, AstraZeneca
Consulting or Advisory Role: AbbVie
Speakers' Bureau: Janssen-Cilag, AbbVie, AstraZeneca
Travel, Accommodations, Expenses: Gilead Sciences, AbbVie, Janssen-Cilag, AstraZeneca
Robert Weinkove
Stock and Other Ownership Interests: Fisher & Paykel Healthcare
Consulting or Advisory Role: Janssen, AbbVie
Research Funding: Janssen (Inst)
Travel, Accommodations, Expenses: Janssen
Uncompensated Relationships: Wellington Zhaotai Therapies Limited
Tommi Salmi
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Hematology Association virtual meeting, June 9-17, 2021, and the International Workshop on Chronic Lymphocytic Leukemia virtual meeting, September 17-20, 2021.
SUPPORT
Supported by BeiGene. Investigators and BeiGene collaborated on protocol development, data collection, and interpretation. BeiGene performed statistical analyses.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The study protocol and statistical analysis plan are provided in the Data Supplement. All authors had access to the original data for the analyses described here. On request and subject to certain criteria, conditions, and exceptions, BeiGene will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. Data requests may be submitted to DataDisclosure@beigene.com.
AUTHOR CONTRIBUTIONS
Conception and design: Barbara Eichhorst, Jennifer R. Brown, Nicole Lamanna, Susan M. O'Brien, Constantine Tam, Lugui Qiu, Tommi Salmi, Meng Ji, Kenneth Wu, Jane Huang
Administrative support: Adam Idoine
Provision of study materials or patients: Peter Hillmen, Barbara Eichhorst, Jennifer R. Brown, Maciej Kazmierczak, Keshu Zhou, Martin Šimkovič, Jiří Mayer, Amanda Gillespie-Twardy, Mazyar Shadman, Alessandra Ferrajoli, Peter S. Ganly, Robert Weinkove, Sebastian Grosicki, Andrzej Mital, Tadeusz Robak, Habte A. Yimer, Wojciech Jurczak
Collection and assembly of data: Peter Hillmen, Barbara Eichhorst, Jennifer R. Brown, Nicole Lamanna, Susan M. O'Brien, Constantine S. Tam, Lugui Qiu, Maciej Kazmierczak, Keshu Zhou, Martin Šimkovič, Jiří Mayer, Amanda Gillespie-Twardy, Mazyar Shadman, Alessandra Ferrajoli, Peter S. Ganly, Robert Weinkove, Sebastian Grosicki, Andrzej Mital, Tadeusz Robak, Anders Österborg, Habte A. Yimer, Tommi Salmi, Meng Ji, Jessica Yecies, Adam Idoine, Jane Huang, Wojciech Jurczak
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: Interim Analysis of a Randomized Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kenneth Wu
Employment: BeiGene, Genentech/Roche
Stock and Other Ownership Interests: BeiGene, Genentech/Roche
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, BeiGene, Pharmacyclics, Roche/Genentech, Loxo/Lilly
Consulting or Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag (Inst), AbbVie (Inst), BeiGene (Inst)
Tadeusz Robak
Honoraria: AstraZeneca, BeiGene, Janssen
Consulting or Advisory Role: AstraZeneca, BeiGene, Janssen Oncology
Research Funding: AstraZeecaa, BeiGene, Janssen
Jessica Yecies
Employment: BeiGene, Day One Biopharmaceuticals, Kaiser Permanente, GRAIL
Stock and Other Ownership Interests: BeiGene, GRAIL, Day One Biopharmaceuticals
Mazyar Shadman
Employment: Bristol Myers Squibb
Consulting or Advisory Role: AbbVie, Genentech, AstraZeneca, Sound Biologics, Cellectar, Pharmacyclics, BeiGene, Bristol Myers Squibb/Celgene, MorphoSys, Innate Pharma, Kite, a Gilead Company, Adaptive Biotechnologies, Epizyme, Fate Therapeutics, Lilly, Regeneron, Adaptimmune, MustangBio, TG Therapeutics, MEI Pharma
Research Funding: Pharmacyclics (Inst), Acerta Pharma (Inst), Merck (Inst), TG Therapeutics (Inst), BeiGene (Inst), Celgene (Inst), Genentech (Inst), MustangBio (Inst), AbbVie (Inst), Sunesis Pharmaceuticals (Inst), Bristol Myers Squibb/Celgene, Genmab (Inst), Vincerx Pharma (Inst)
Adam Idoine
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Susan M. O'Brien
Employment: University of California, Irvine
Honoraria: Celgene, Janssen, Pharmacyclics, Gilead Sciences, Pfizer, Amgen, Astellas Pharma, GlaxoSmithKline, Aptose Biosciences, Vaniam Group, AbbVie, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Eisai, TG Therapeutics, NOVA Research Company
Consulting or Advisory Role: Amgen, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, AbbVie/Genentech, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Astellas Pharma, Gilead Sciences, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis Pharmaceuticals
Research Funding: Acerta Pharma (Inst), Regeneron (Inst), Gilead Sciences (Inst), Pfizer (Inst), TG Therapeutics (Inst), Pharmacyclics (Inst), Kite, a Gilead Company (Inst), Sunesis Pharmaceuticals (Inst), Lilly (Inst), Caribou Biosciences (Inst)
Travel, Accommodations, Expenses: Celgene, Janssen, Gilead Sciences, Regeneron, Janssen Oncology
Jiří Mayer
Research Funding: BeiGene
Barbara Eichhorst
Honoraria: Roche, AbbVie, Janssen-Cilag, AstraZeneca, Miltenyi Biotec, BeiGene, MSD
Consulting or Advisory Role: Janssen-Cilag, AbbVie, AstraZeneca, Lilly
Research Funding: AbbVie, Janssen, BGI, Gilead Sciences, AstraZeneca
Travel, Accommodations, Expenses: Roche, AbbVie, Gilead Sciences, Janssen
Habte A. Yimer
Employment: Texas Oncology/US Oncology
Stock and Other Ownership Interests: Karyopharm Therapeutics
Speakers' Bureau: Janssen, AstraZeneca, BeiGene, GlaxoSmithKline, Karyopharm Therapeutics, Amgen, Pharmacyclics
Nicole Lamanna
Consulting or Advisory Role: Celgene, Genentech, AbbVie, ProNAi, Pharmacyclics, Juno Therapeutics, Roche, Janssen, AstraZeneca, Gilead Sciences, BeiGene, Loxo/Lilly
Research Funding: Genentech/AbbVie (Inst), Infinity Pharmaceuticals (Inst), Gilead Sciences (Inst), ProNAi (Inst), BeiGene (Inst), Verastem (Inst), Juno Therapeutics (Inst), TG Therapeutics (Inst), Acerta Pharma/AstraZeneca (Inst), Loxo (Inst), Oncternal Therapeutics, Inc (Inst), MingSight (Inst), Octapharm (Inst)
Meng Ji
Employment: BeiGene Beijing, Takeda
Stock and Other Ownership Interests: BeiGene
Wojciech Jurczak
Consulting or Advisory Role: Roche, AstraZeneca, BeiGene
Research Funding: Acerta Pharma, TG Therapeutics, Sandoz-Novartis, Roche, Takeda, Epizyme, Janssen-Cilag, BeiGene, MorphoSys, MEI Pharma
Peter Hillmen
Employment: Apellis Pharmaeuticals
Honoraria: Janssen, AbbVie, Roche, AstraZeneca, BeiGene, Swedish Orphan Biovitrum, Pharmacyclics
Research Funding: Janssen (Inst), Pharmacyclics (Inst), Roche (Inst), Gilead Sciences (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Janssen, AbbVie
Alessandra Ferrajoli
Consulting or Advisory Role: Giants of Cancer Care, AstraZeneca, Janssen Oncology
Patents, Royalties, Other Intellectual Property: Celgene, Acerta Pharma, BeiGene
Jane Huang
Employment: BeiGene, Prelude Therapeutics
Leadership: 858 Therapeutics, Protara Therapeutics, Prelude Therapeutics, BeiGene
Stock and Other Ownership Interests: Protara Therapeutics, BeiGene, 858 Therapeutics, Prelude Therapeutics
Consulting or Advisory Role: BeiGene
Jennifer R. Brown
Consulting or Advisory Role: AbbVie, Acerta/Astra-Zeneca, BeiGene, Bristol Myers Squibb/Celgene/Juno, Catapult Therapeutics, Lilly, Genentech/Roche, HUTCHMED, iOnctura, Janssen, MEI Pharma, MorphoSys, Novartis, Pharmacyclics
Research Funding: BeiGene (Inst), Gilead Sciences (Inst), Loxo/Lilly (Inst), MEI Pharma (Inst), Secura Bio (Inst), Sun Pharma (Inst), TG Therapeutics (Inst)
Martin Šimkovič
Honoraria: Janssen-Cilag, AbbVie/Genentech, AstraZeneca
Consulting or Advisory Role: AbbVie
Speakers' Bureau: Janssen-Cilag, AbbVie, AstraZeneca
Travel, Accommodations, Expenses: Gilead Sciences, AbbVie, Janssen-Cilag, AstraZeneca
Robert Weinkove
Stock and Other Ownership Interests: Fisher & Paykel Healthcare
Consulting or Advisory Role: Janssen, AbbVie
Research Funding: Janssen (Inst)
Travel, Accommodations, Expenses: Janssen
Uncompensated Relationships: Wellington Zhaotai Therapies Limited
Tommi Salmi
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Eichhorst B, Robak T, Montserrat E, et al. : Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v78-v84, 2015. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, et al. : Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 376:1164-1174, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Kipps TJ, Stevenson FK, Wu CJ, et al. : Chronic lymphocytic leukaemia. Nat Rev Dis Primers 3:16096, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger JA, Tedeschi A, Barr PM, et al. : Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373:2425-2437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Brown JR, O'Brien S, et al. : Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 371:213-223, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barf T, Covey T, Izumi R, et al. : Acalabrutinib (ACP-196): A covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther 363:240-252, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Sharman JP, Black-Shinn JL, Clark J, et al. : Understanding ibrutinib treatment discontinuation patterns for chronic lymphocytic leukemia. Blood 130:4060, 2017 [Google Scholar]
- 8.Mato AR, Nabhan C, Thompson MC, et al. : Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica 103:874-879, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munir T, Brown JR, O'Brien S, et al. : Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol 94:1353-1363, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghia P, Owen C, Robak T, et al. : Ibrutinib treatment in the first-line setting for patients with chronic lymphocytic leukemia: Up to 7 years of follow-up in the resonate-2 study. EHA EP636, 2021 (abstr). https://library.ehaweb.org/eha/2021/eha2021-virtual-congress/325396/paolo.ghia.ibrutinib.treatment.in.the.first-line.setting.for.patients.with.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1
- 11.Guo Y, Liu Y, Hu N, et al. : Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton's tyrosine kinase. J Med Chem 62:7923-7940, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Tam CS, Trotman J, Opat S, et al. : Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 134:851-859, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cull G, Burger JA, Opat S, et al. : Zanubrutinib for treatment-naive and relapsed/refractory chronic lymphocytic leukaemia: Long-term follow-up of the phase I/II AU-003 study. Br J Haematol 196:1209-1218, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, et al. : Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111:5446-5456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Byrd JC, Rai KR, et al. : Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol 30:2820-2822, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam CS, Opat S, D'Sa S, et al. : A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: The ASPEN study. Blood 136:2038-2050, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrd JC, Hillmen P, O'Brien S, et al. : Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood 133:2031-2042, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JR, Hillmen P, O'Brien S, et al. : Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia 32:83-91, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao L, Salem JE, Clauss S, et al. : Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation 142:2443-2455, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berglof A, Hamasy A, Meinke S, et al. : Targets for ibrutinib beyond B cell malignancies. Scand J Immunol 82:208-217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honigberg LA, Smith AM, Sirisawad M, et al. : The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 107:13075-13080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol and statistical analysis plan are provided in the Data Supplement. All authors had access to the original data for the analyses described here. On request and subject to certain criteria, conditions, and exceptions, BeiGene will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. Data requests may be submitted to DataDisclosure@beigene.com.