Abstract
Vaccination is useful for the prevention of COVID-19 and is recommended for everyone, especially people with a weakened immune system. However, various neurological complications have been reported following vaccination. Here, we report the case of a 25 years-old, Japanese man, who presented with acute encephalopathy two days after the second dose of the COVID-19 vaccine. He had been treated with nivolumab, a medication used to treat cancer. He had a high fever and was confused upon admission, and the antibody test was positive for anti-myelin oligodendrocyte glycoproteins. Abnormal signal intensity at the splenium corporis callosi was observed on diffusion-weighted imaging of the brain. We diagnosed him with autoimmune encephalitis and initiated intravenous methylprednisolone, after which, the patient’s symptoms rapidly subsided.
Keywords: COVID-19 vaccination, Encephalopathy, Immune checkpoint inhibitor, Anti-myelin oligodendrocyte glycoproteins antibody, Methylprednisolone
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported to the World Health Organization in December 2019, and the disease was named Coronavirus Disease (COVID-19). The pandemic has affected many people and has changed the world. Treatments including antiviral (remdesivir, favipiravir, etc.) and anti-inflammatory drugs (dexamethasone, etc.), and different types of vaccines, like mRNA, viral vector, and recombinant protein vaccines, for the prevention of COVID-19, have been rapidly developed. Vaccination is recommended for everyone, especially people with a weakened immune system such as the elderly and the immunocompromised. These individuals are at a higher risk of severe symptoms if exposed to COVID-19.
However, various neurological complications such as Guillain-Barré syndrome, encephalitis, and seizures have been reported after vaccination, though a causal relationship is yet to be established [1]. Here, we report a case of acute encephalopathy following the COVID-19 vaccine. The patient had also received treatment with nivolumab for cancer.
2. Case report
Informed consent was obtained from the patient for this journal article. A 25-year-old Japanese man received the second dose of COVID-19 vaccination (Pfizer Inc. NY). Two days later, he was brought to the emergency department because of a high fever and altered mental status. The patient had a history of left parotid cancer and underwent tumor resection and postoperative radiation therapy, three years ago. Multiple lung metastases were also observed and chemotherapy was initiated. The first line of treatment —cisplatin and fluorouracil— caused a side effect of hearing impairment; thus, it was replaced with a second line of treatment —nivolumab. The last dose was administered three weeks before his presentation at the emergency department. The cancer was in remission.
At the emergency department, he had a high fever (40.4 ℃) and was confused and agitated. He opened his eyes but could not communicate (Glasgow Coma Scale score of E4V2M5). A general physical examination was unremarkable. Paralysis, ataxia, or involuntary movements were not detected. The neck was supple. The patient tested negative for the SARS-CoV-2 antigen. Blood tests showed a normal number of leukocytes, increased C-reactive protein (9.04 mg/dl), mildly elevated serum levels of hepatic enzymes, and abnormalities of the fibrinolytic system (d-dimer 4.5 μg/ml).
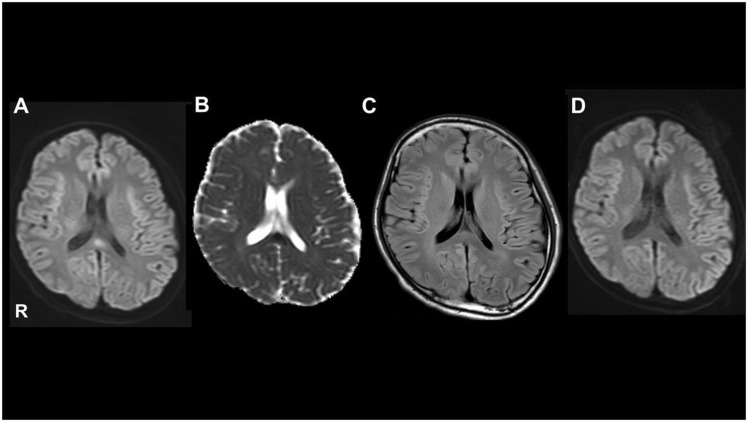
Microbiological studies for herpes simplex virus, hepatitis B virus, hepatitis C virus, human T-cell leukemia virus type 1, influenza, syphilis, and fungi, yielded a negative result. Tests for autoantibodies (SS-DNA, DS-DNA, SS-A, SS-B, PR3-ANCA, MPO-ANCA, ANA, anti-thyroid peroxidase, anti-thyroglobulin, anti-aqua-porin-4, and anti-glutamic acid decarboxylase) were also negative. However, a cell based assay revealed that the test for anti-myelin oligodendrocyte glycoprotein (MOG) was positive. The cerebrospinal fluid showed a protein level of 60 mg/dl and a cell number of 4/μl. Oligoclonal bands were positive. We detected high intensity at the splenium corporis callosi using diffusion weighted imaging and low intensity on apparent diffusion coefficient on an initial brain Magnetic Resonance Imaging (MRI) examination ( Fig. 1). Electroencephalogram showed that the dominant rhythm was 8 Hz and there was intermittent semirhythmic theta wave in the bifrontal-parietal regions, but no paroxysmal activity; hence we ruled out status epilepticus.
Fig. 1.
Brain magnetic resonance imaging findings. Diffusion-weighted imaging (DWI) showed hyperintensity in the splenium corporis callosi (A), and lower intensity in the apparent diffusion coefficient (B). Fluid-attenuated inversion recovery showed no abnormalities (C). DWI showed resolution of the abnormal signals three weeks later (D).
We diagnosed the patient with autoimmune encephalitis based on these results and initiated intravenous methylprednisolone (1 g/day for three days) on the day of admission. This therapy led to immediate clinical improvement, and his cognitive status improved remarkably. He became alert and could communicate well. He was discharged 11 days after admission, with no subsequent complications. A follow-up brain MRI performed 3 weeks later showed resolution of the abnormal signals previously observed.
3. Discussion
We report the case of an anti-MOG antibody-positive encephalopathy patient who had been treated with nivolumab and developed encephalopathy following a second dose of COVID-19 vaccine. We considered that the vaccination could have stimulated autoreactive T-cell clones and that nivolumab, an immune checkpoint inhibitor, promoted humoral immunity by acting on B cells. This would lead to the production of anti-MOG antibody and result in encephalopathy. In addition, steroid therapy was remarkably effective.
Since the introduction of the COVID-19 vaccine, neurological complications have been reported, such as Guillain- Barré syndrome, encephalitis, and seizure [1]. The relationship between neuroinflammation and the COVID-19 vaccine is not adequately clear. However, similar conformational structures between the vaccination and self-antigen could stimulate autoreactive T-cell clones, which would ultimately cause neuroinflammation. In this case, steroid treatment was effective, suggesting that the pathogenesis of neurological complications was mainly of immunological origins.
In this case, the patient tested positive for the anti-MOG antibody. Previous studies have reported of patients who developed anti-MOG antibody-related diseases after vaccination, and it was suggested that vaccination may have activated a predisposition for the disease [2]. Although the mechanism has not been clarified, it has been suggested that cross-reactivity between vaccine antigens and myelin proteins causes autoimmune demyelination, and specific mechanisms involved in the pathogenesis include expansion and stimulation of auto-reactive T cell clones and enhanced antigen presentation [3].
Nivolumab is an immune checkpoint inhibitor (ICI) that upregulates the costimulatory T-cell activation pathway. One of the most frequent side effects of nivolumab, when compared to other ICIs, was encephalitis with various symptoms and imaging findings. In these cases, too, steroid treatment was highly effective, but the median time between the first treatment and onset of symptoms was 65 (4−630) days, which was atypical in our case (1430 days).
Moreover, demyelination can be induced through the modification of immune checkpoint pathways in animal models [4]. Nivolumab, a kind of ICIs (anti-PD-1 antibody), stimulates follicular T helper cells (Tfh) through connections to PD-1, expressed on Tfh. Tfh promotes humoral immunity by acting on B cells [5] and can contribute to autoantibody production, which could cause encephalopathy. In this case, a combination of these three mechanisms, namely COVID-19 vaccination, use of nivolumab, and the presence of anti-MOG antibodies, might have caused encephalitis. Moreover, the vaccination in this case was the mRNA type, which is the first of its kind in the world. Since the mechanism for adverse events of this type of vaccination have not yet been characterized, such cases should be accumulated and analyzed.
4. Conclusion
COVID-19 vaccination is recommended; however, vaccination may induce an autoimmune neurological disorder. Moreover, it is likely to enhance immunological mechanisms for patients with ICIs, such as nivolumab. Further analysis of cases is essential for establishing suitable management strategies for the COVID-19 pandemic.
CRediT authorship contribution statement
MT examined, evaluated the patient. MT and MN designed the study and wrote the draft. MT and RI contributed to the acquisition and analysis of the clinical data and the interpretation of the clinical data. MN, SA, and HM participated in the design of the case-report and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of interest and sources of funding
No funding was received for this study. The authors declare no potential conflicts of interest.
References
- 1.Lu L., Xiong W., Mu J., Zhang Q., Zhang H., Zou L., Li W., He L., Sander J.W., Zhou D. The potential neurological effect of the COVID-19 vaccines: a review. Acta Neurol. Scand. 2021;144:3–12. doi: 10.1111/ane.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.S. Jarius, K. Ruprecht, I. Kleiter, N. Borisow, N. Asgari, K. Pitarokoili, F. Pache, O. Stich, L.A. Beume, M.W. Hümmert, M. Ringelstein, C. Trebst, A. Winkelmann, A. Schwarz, M. Buttmann, H. Zimmermann, J. Kuchling, D. Franciotta, M. Capobianco, E. Siebert, C. Lukas, M. Korporal-Kuhnke, J. Haas, K. Fechner, A.U. Brandt, K. Schanda, O. Aktas, F. Paul, M. Reindl, B. Wildemann, 2016. in cooperation with the Neuromyelitis Optica Study Group. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome, in: J. Neuroinflammation. 13 (2016) 280. https://doi.org/10.1186/s12974–016-0718–0. [DOI] [PMC free article] [PubMed]
- 3.Kumar N., Graven K., Joseph N.I., Johnson J., Fulton S., Hostoffer R., Abboud H. Case report: postvaccination anti-myelin oligodendrocyte glycoprotein neuromyelitis optica spectrum disorder: a case report and literature review of postvaccination demyelination. Int. J. MS Care. 2020;22:85–90. doi: 10.7224/1537-2073.2018-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira M.C.B., de Brito M.H., Simabukuro M.M. Central nervous system demyelination associated with immune checkpoint inhibitors: review of the literature. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.538695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J., Hou S., Fang Q., Liu X., Liu X., Qi H. PD-1 controls follicular T helper cell positioning and function. Immunity. 2018;49(2):264. doi: 10.1016/j.immuni.2018.06.012. 274 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]