PURPOSE
Microsatellite instability (MSI) and DNA mismatch repair (MMR) status is an indispensable biomarker in the management of colorectal cancers. We therefore examined the epidemiology of MSI-high/MMR-deficient colorectal cancers in the United States.
METHODS
Adults presenting with colorectal adenocarcinoma in 2018-2019 were identified from the US National Cancer Database. Attributes associated with MSI-high/MMR-deficiency were identified using multivariable logistic regression and reported using average adjusted probabilities (%AAP) and 99.9% CIs. As a secondary aim, the survival associated with MSI/MMR status was assessed.
RESULTS
Among 101,259 colorectal adenocarcinomas in 2018-2019, 82.0% were microsatellite stable/MMR-proficient, 3.8% MSI-low, and 14.2% MSI-high/MMR-deficient—including 16.6%, 19.9%, 12.4%, and 7.3% of stage I, II, III, and IV cancers, respectively. In locoregional cancers, MSI-high/MMR-deficiency was associated with a bimodal age distribution, female sex, right-sided colonic origin, wild-type KRAS, and a prior diagnosis of cancer (all P < .001). By race/ethnicity, colorectal adenocarcinomas were MSI-high/MMR-deficient in 16.9%AAP of non-Hispanic White (99.9% CI, 16.5 to 17.4) patients, compared with 11.3%AAP of non-Hispanic Black (99.9% CI, 10.3 to 12.4), 12.4%AAP of Asian/Pacific Islander (99.9% CI, 10.5 to 14.3), and 15.1%AAP of Hispanic (99.9% CI, 13.4 to 16.7) patients (all P < .001). Histologically, MSI-high/MMR-deficiency was associated with increasing grade, from 11.3%AAP of well-differentiated tumors (99.9% CI, 10.2 to 12.4) to 28.4%AAP of poorly differentiated cases (99.9% CI, 27.1 to 29.8; P < .001). Compared with conventional histology (15.2%AAP, 99.9% CI, 14.8 to 15.6), medullary (41.1%AAP, 99.9% CI, 33.0 to 49.3; P < .001) and mucinous (24.6%AAP, 99.9% CI, 22.8 to 26.3; P < .001) subtypes—but not signet-ring cell histology (15.5%AAP, 99.9% CI, 11.6 to 19.4; P = .79)—were more frequently MSI-high/MMR-deficient when adjusting for clinicopathologic features including grade.
CONCLUSION
Our findings establish the epidemiology, features, and prognostic implications of MSI-high/MMR-deficiency among colorectal adenocarcinoma patients in the United States.
INTRODUCTION
DNA mismatch repair (MMR) deficiency commonly arises because of a loss of MMR protein function, which is caused by germline and/or somatic mutations (including copy-number loss) of MMR genes (namely MLH1, MSH2, MSH6, or PMS2) or somatic hypermethylation of the MLH1 promoter. MMR deficiency leads to an accumulation of unrepaired errors in short repetitive DNA microsatellites (ie, high-level microsatellite instability [MSI]).1 MSI-high/MMR deficiency status has emerged as a potent predictive biomarker for response to programmed cell death-1 (PDCD1; ie, PD-1) checkpoint inhibitors in a range of cancer types.2,3
CONTEXT
Key Objective
Microsatellite instability and DNA mismatch repair (MSI/MMR) status is an important predictive biomarker of response to immune checkpoint inhibitors in patients with colorectal cancer. This study, therefore, examined the molecular pathologic epidemiology and prognostic implications of MSI-high/MMR-deficient colorectal adenocarcinomas across the United States.
Knowledge Generated
In this US national cohort, 16% of newly diagnosed colorectal adenocarcinomas were MSI-high/MMR-deficient, with the highest prevalence among stage I-II tumors. In addition to confirming several well-described associations between colorectal cancer attributes and MSI-high/MMR-deficiency—such as female sex, bimodal age distribution, right-sided colonic origin, poorly differentiated histology, and medullary and mucinous subtypes—MSI/MMR status also varied by patients' race/ethnicity, with MSI-high/MMR deficiency less likely among non-Hispanic Black, Asian/Pacific Islander, and Hispanic patients with colorectal cancer. Well-differentiated and signet-ring cell histologies were not independently associated with MSI-high/MMR deficiency.
Relevance
These findings establish the national-level epidemiology, features, and prognostic implications of MMR-deficiency/MSI-high among patients with colorectal adenocarcinoma in the United States.
The clinical value of MSI/MMR testing is exemplified in colorectal adenocarcinoma, where, in addition to serving as an indispensable predictive biomarker for first-line or second-line PDCD1 (PD-1) checkpoint inhibitors in the metastatic setting, MSI/MMR status also plays a critical role in the initial screening for Lynch syndrome and appears to identify stage II patients who may not benefit from fluorouracil-based adjuvant chemotherapy.4-7 Therefore, we examined the molecular pathologic epidemiology of MSI-high/MMR-deficient colorectal adenocarcinomas across the United States, and, secondarily, the prognostic implications of MSI/MMR status.
METHODS
Patient Population
The data source for this retrospective cohort study was the National Cancer Database (NCDB), a nationwide observational database containing ≥ 71% of newly diagnosed colorectal cancers in the United States.8 Patients who presented with a histologically confirmed invasive colorectal adenocarcinoma in 2018 and 2019 (the latest year of available NCDB data and the first year with data reported for the NCDB's new MSI/MMR data item, and the latest year of available NCDB data, respectively) were identified using International Classification of Diseases-O-3 histologic codes (adenocarcinoma 8140/3, mucinous adenocarcinoma 8480/3, signet-ring cell carcinoma 8490/3, or medullary carcinoma 8510/3) and primary colorectal site codes (C18.0, C18.2-18.9, C19.9, and C20.9). Patients were excluded if they lacked MSI/MMR testing results or lacked complete staging data.
Study Variables
Starting for patients diagnosed in 2018, a new colorectal site–specific data item was implemented by cancer registries for reporting both MSI and MMR status: MSI (NAACCR item no. 3890), which defines microsatellite stable and/or MMR intact (no loss of nuclear expression of MMR proteins) as code 0, MSI-low as code 1, and MSI-high and/or MMR deficiency (loss of nuclear expression of ≥ 1 MMR proteins) as code 2. MSI/MMR not assessed, unknown if assessed, or indeterminate results were all defined as code 9. The NCDB did not report details about the specific MSI/MMR testing modality (eg, immunohistochemistry v polymerase chain reaction–based methods v next-generation sequencing panels; use of mononucleotide markers only v mononucleotide and dinucleotide markers; MLH1 promoter methylation; etc) or strategy (eg, whether immunohistochemistry and/or molecular testing was used), nor were the details reported regarding whether MMR deficiency was sporadic or germline.
Patient and tumor factors of interest were patient age, sex, race/ethnicity, and tumor features including location, sidedness, KRAS mutational status (reported as either wild-type, codon 12/13/61 mutated, codon 146 mutated, other codon mutated, and mutated but codons not specified), pretreatment carcinoembryonic antigen (CEACAM5, ie, CEA) levels, history of prior cancer, and American Joint Committee on Cancer (AJCC) eighth edition pathologic stage group. Right-sided colon was defined as cecum, ascending colon, hepatic flexure, or transverse colon, whereas left-sided was defined as splenic flexure, descending colon, sigmoid, or rectosigmoid junction. Histologic features included subtype (adenocarcinoma, mucinous adenocarcinoma, signet-ring cell carcinoma, or medullary carcinoma), grade, lymphovascular invasion, and perineural invasion. For analyses of stage IV cancers, metastatic site involvement (liver, lung, bone, brain, distant lymph node, and other visceral organs or carcinomatosis) was also included as separate binary variables.
Statistical Analysis
The associations between patient and tumor factors and MSI/MMR status (categorized as either MSI-high/MMR-deficient or microsatellite stable/MSI-low/MMR-proficient) were evaluated separately for patients with locoregional (stage I-III) and metastatic (stage IV) colorectal adenocarcinomas using multivariable logistic regression and reported with corresponding 99.9% CIs. In addition to reporting effect sizes using odds ratios, we also used the multivariable logistic regression models to estimate the average adjusted predicted probability (%AAP; ie, average predicted margin) associated with being MSI-high/MMR-deficient for any given clinicopathologic feature—so as to help with interpretation of the effect sizes associated with each feature.9 To reduce false positives, two-sided P values < .001 were stipulated as statistically significant.
The prognostic roles of MSI-high/MMR-deficient versus MSI-low versus microsatellite stable/MMR-proficient statuses were explored in a secondary analysis, in which overall survival (OS) was estimated via Kaplan-Meier techniques from the date of initial diagnosis to the date of death or last contact, and adjusted for patient, tumor, and first-line treatment characteristics using multivariable Cox proportional hazards regression analysis. A less-conservative two-sided alpha level of .05 was used for the exploratory secondary analysis, with Bonferroni method to correct for multiplicity of testing: the association between MSI/MMR status and OS was tested for each of four stages, corresponding to a significant P value threshold of .006 per test. All analyses were conducted using Stata (v17.1, StataCorp). The NCDB excludes OS data for patients diagnosed in the final year of the released database, so OS data were not yet available for patients diagnosed in 2019. This study was approved by the Mass General Brigham institutional review board (no. 2019P000950) as exempt and performed in accordance with the Declaration of Helsinki. The NCDB Participant User Files contain deidentified national data for which consenting was not applicable.
Ethics Approval and Consent to Participate
This study was not considered human subjects research and was approved by the Mass General Brigham institutional review board (no. 2019P000950) and performed in accordance with the Declaration of Helsinki. The NCDB Participant User Files contain deidentified national data for which consenting was not applicable.
RESULTS
In 2018-2019, 101,259 patients with colorectal adenocarcinoma met study criteria, of whom 82% were reported as microsatellite stable/MMR-proficient (n = 83,051), 3.8% as MSI-low (n = 3,884), and 14.2% as MSI-high/MMR-deficient (n = 14,324). MSI-high/MMR deficiency was detected in 16.6% of stage I, 19.9% of stage II, 12.4% of stage III, and 7.3% of stage IV cancers.
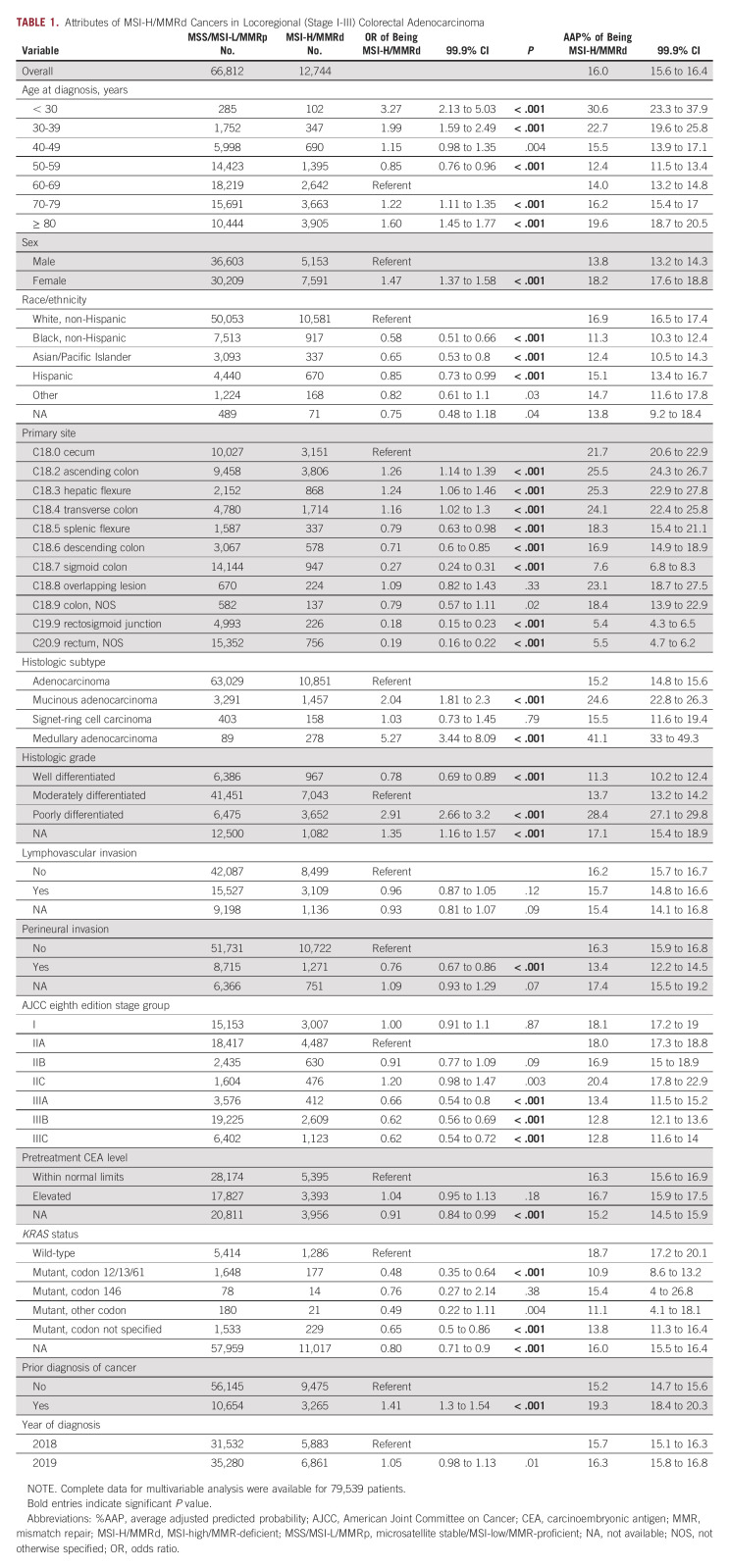
Attributes of MSI-High/MMR Deficiency in Locoregional (stage I-III) Colorectal Adenocarcinomas
Among patients with locoregional cancers (n = 79,556), women were more likely to have MSI-high/MMR-deficient tumors (18.2%AAP, 99.9% CI, 17.6 to 18.8; P < .001) compared with men (13.8%AAP, 99.9% CI, 13.2 to 14.3; Table 1). By age, MSI-high/MMR deficiency demonstrated a bimodal pattern: peaking at 30.6%AAP in < 30-year-olds (99.9% CI, 23.3 to 37.9), falling to 12.4%AAP in 50-59-year-olds (99.9% CI, 11.5 to 13.4), and rising to 19.6%AAP in ≥ 80-year-olds (99.9% CI, 18.7 to 20.5). Additionally, patients who were of non-Hispanic Black (11.3%AAP, 99.9% CI, 10.3 to 12.4; P < .001), Asian/Pacific Islander (12.4%AAP, 99.9% CI, 10.5 to 14.3; P < .001), or Hispanic (15.1%AAP, 99.9% CI, 13.4 to 16.7; P < .001) race/ethnicities less frequently had MSI-high/MMR-deficient cancers than non-Hispanic White patients (16.9%AAP, 99.9% CI, 16.5 to 17.4; referent). A history of a prior cancer diagnosis was also associated with MSI-high/MMR-deficient cancer (19.3%AAP, 99.9% CI, 18.4 to 20.3; v 15.2%AAP without, 99.9% CI, 14.7 to 15.6; P < .001).
TABLE 1.
Attributes of MSI-H/MMRd Cancers in Locoregional (Stage I-III) Colorectal Adenocarcinoma
In terms of histologic features, increasing grade was independently associated with increasing likelihood of MSI-high/MMR deficiency: whereas 11.3%AAP of well-differentiated (99.9% CI, 10.2 to 12.4; P < .001) and 13.7%AAP of moderately differentiated (99.9% CI, 13.2 to 14.2; referent) cancers were MSI-high/MMR-deficient, 28.4%AAP of poorly differentiated cases were (99.9% CI, 27.1 to 29.8; P < .001). This association with increasing grade persisted when only those cases with conventional histology were included in the regression model (data not shown). Additionally, although lymphovascular invasion was not associated with MSI/MMR status, perineural invasion was inversely associated with MSI-high/MMR deficiency (13.4%AAP, 99.9% CI, 12.2 to 14.5; v 16.3%AAP for no perineural invasion, 99.9% CI, 15.9 to 16.8; P < .001). MSI-high/MMR-deficient status was also more commonly associated with the medullary (41.1%AAP, 99.9% CI, 33.0 to 49.3; P < .001) and mucinous (24.6%AAP, 99.9% CI, 22.8 to 26.3; P < .001) histologic subtypes of colorectal adenocarcinoma, compared with 15.2%AAP of conventional histology (99.9% CI, 14.8 to 15.6; referent) and 15.5%AAP of signet-ring cell histology (99.9% CI, 11.6 to 19.4; P = .79). Because signet-ring cell histology overwhelmingly is poorly differentiated, the regression analysis was repeated excluding grade, in which the association between signet-ring cell histology and MSI-high/MMR deficiency strengthened (23.4%AAP, 99.9% CI, 18.2 to 28.5; P < .001 compared with conventional histology).
A higher prevalence of MSI-high/MMR-deficient tumors was observed on the right side of the colon, peaking at 25.5%AAP of ascending colon tumors (99.9% CI, 24.3 to 26.7) and bottoming at 7.6%AAP of sigmoid colon tumors (99.9% CI, 6.8 to 8.3) and 5.4%AAP of rectosigmoid junction tumors (99.9% CI, 4.3 to 6.5). Rectal tumors exhibited the lowest prevalence of MSI-high/MMR deficiency (5.5%AAP, 99.9% CI, 4.7 to 6.2). By AJCC eighth edition stage groups, stage III cancers were less likely to be MSI-high/MMR-deficient (eg, 12.8%AAP of stage IIIC, 99.9% CI, 11.6 to 14.0) than either stage I (18.1%AAP, 99.9% CI, 17.2 to 19.0) or stage II (eg, 20.4%AAP of stage IIC, 99.9% CI, 17.8 to 22.9) cancers. Furthermore, among the 13.3% (n = 10,580) of cases with reported KRAS molecular status, KRAS codon 12/13/61 mutated cancers (10.9%AAP, 99.9% CI, 8.6 to 13.2; P < .001)—but not codon 146 mutated (15.4%AAP, 4.0 to 26.8; P = .38)—were less commonly MSI-high/MMR-deficient compared with KRAS wild-type cancers (18.7%AAP, 99.9% CI, 17.2 to 20.1).
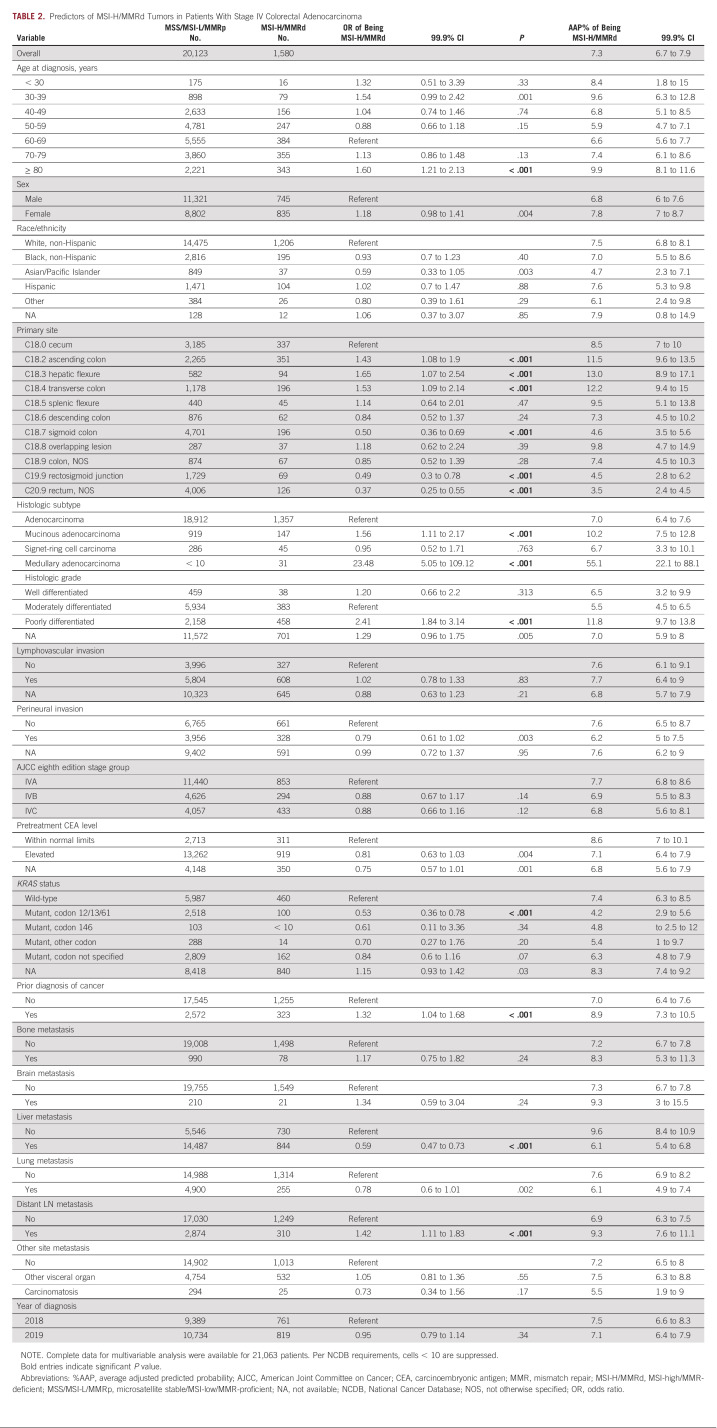
Attributes of MSI-High/MMR Deficiency in Distantly Metastatic (stage IV) Colorectal Adenocarcinomas
Among the n = 21,703 patients who presented with stage IV colorectal adenocarcinoma, 7.3%AAP had MSI-high/MMR-deficient cancers. Unlike locoregional tumors, however, the MSI-high/MMR deficiency levels varied only minimally by patient features (Table 2). The associations that were observed among locoregional tumors between MSI/MMR status and primary tumor site, histologic subtype, KRAS status, and prior diagnosis of cancer were also found in stage IV patients. In terms of metastatic sites, stage IV cases with liver involvement were less likely to be MSI-high/MMR-deficient (6.1%AAP, 99.9% CI, 5.4 to 6.8, v 9.6%AAP without, 99.9% CI, 8.4 to 10.9; P < .001), whereas those with distant lymph node involvement were more likely to be MSI-high/MMR-deficient (9.3%AAP, 99.9% CI, 7.6 to 11.1; v 6.9%AAP without, 99.9% CI, 6.3 to 7.5; P < .001). MSI/MMR status was not otherwise associated with bone or brain metastatic involvement.
TABLE 2.
Predictors of MSI-H/MMRd Tumors in Patients With Stage IV Colorectal Adenocarcinoma
Secondary Analysis of the Prognostic Implications of MSI/MMR Status for Colorectal Adenocarcinoma
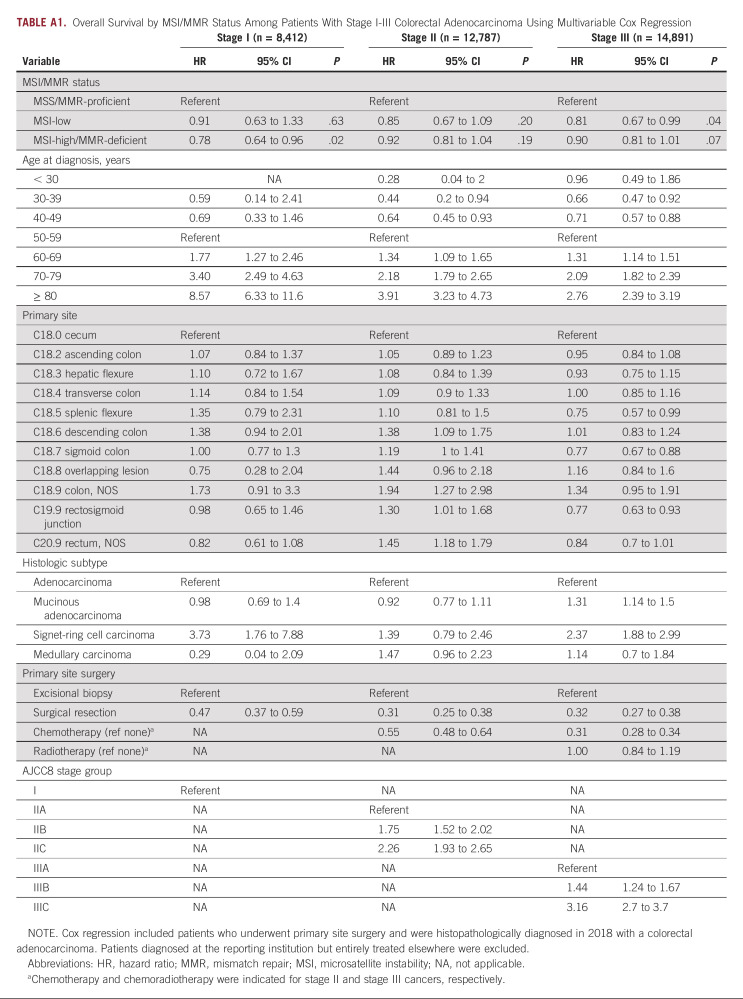
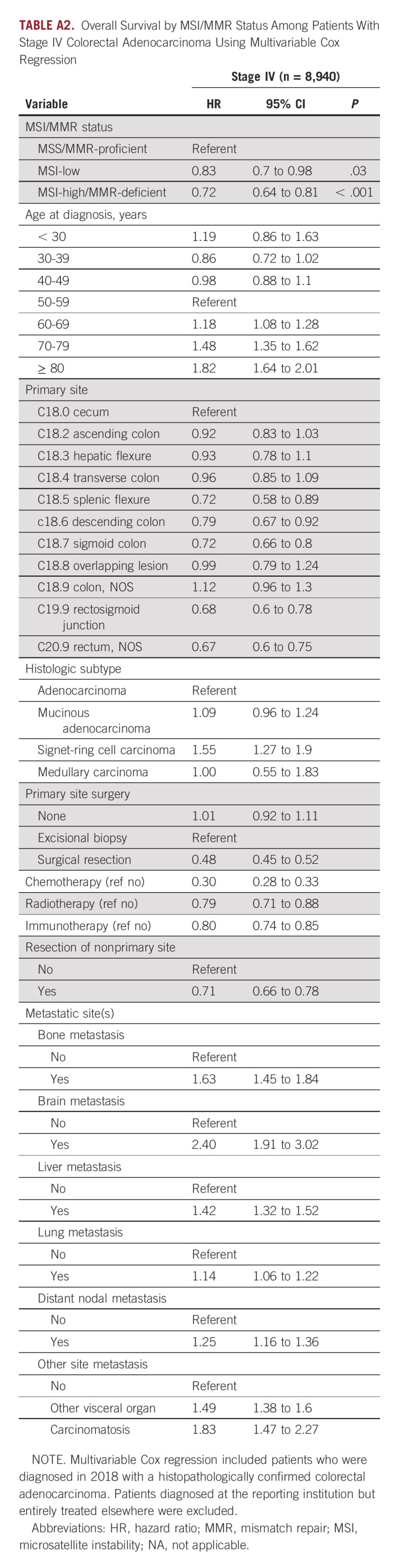
As a secondary analysis, we examined the prognosis associated with MSI/MMR status for patients diagnosed in 2018 (OS data for patients diagnosed in 2019 were not yet reported by the NCDB). We evaluated the OS associated with MSI-high/MMR-deficient cancers, compared with MSI-low and microsatellite stable/MMR-proficient cancers. For locoregional cases, the survival analysis was adjusted for patients' age, primary site, histologic subtype, stage group, and primary therapy (ie, surgery, chemotherapy for stage II-III, and radiotherapy for stage III; n = 36,090; Appendix Table A1) and, for stage IV cases (n = 8,940), further adjusted for metastatic sites, resection of nonprimary sites, and first-line immunotherapy, given that some stage IV patients may have been receiving first-line immune checkpoint inhibitors in the context of clinical trials or in an off-label manner (Appendix Table A2). Of note, the NCDB does not report data for therapy received in the second-line or subsequent-line setting, including immune checkpoint inhibitors. MSI-high/MMR-deficient status was independently associated with improved OS compared with MSS/MMR-proficient cancers in stage IV patients (hazard ratio [HR], 0.72; 95% 99.9% CI, 0.64 to 0.81; P < .001). Among locoregional cancers, the adjusted HR associated with MSI-high/MMR-deficient status was 0.78 (95% 99.9% CI, 0.64 to 0.96; P = .02) for stage I, 0.92 (95% 99.9% CI, 0.81 to 1.04; P = .19) for stage II, and 0.90 (95% 99.9% CI, 0.71 to 1.01; P = .07) for stage III, which did not reach corrected significance for secondary analysis (ie, P < .006). Furthermore, a significant difference in OS between MSI-low and MSS/MMR-proficient cancers was not observed at any stage follow correction for multiple testing, although MSI-low stage IV cancers demonstrated an adjusted HR of 0.83 (95% 99.9% CI, 0.70 to 0.98) compared with MSS/MMR-proficient cancers.
DISCUSSION
Herein, we examined the relationships between colorectal adenocarcinoma patients' clinicopathologic features and their MSI/MMR status across the United States. Overall, 14% of colorectal adenocarcinomas were MSI-high/MMR-deficient and 4% were MSI-low, which is in line with prior estimates from institutional series. Our findings recapitulated many of the well-described features of MSI-high/MMR-deficient tumors, including a predilection for the right side of the colon, higher-grade histology, wild-type KRAS, earlier stages, and female patients.1 Using multivariable logistic regression, we provide estimates for the prevalence of MSI-high/MMR-deficiency associated with each of these attributes. In survival analyses accounting for patients' treatments, MSI-high/MMR-deficient cancers independently displayed a more favorable prognosis compared with MSS/MMR-proficient cancers in stage IV disease—although, owing to the limitations of the NCDB, these survival results were confounded by receipt of immune checkpoint inhibitor therapy in the ≥ second line, which was US Food and Drug Administration–approved in 2017 for patients with MSI-high/MMR-deficient metastatic colorectal cancer who had progressed following chemotherapy. Consequently, it is likely that a majority of MSI-high/MMR-deficient stage IV colorectal cancers in our analysis had received PD-1 inhibitor therapy in the ≥ second line. As additional years of data become available, it will become important to assess how the natural history of MSI-high/MMR-deficient disease changes over the years.
MSI-high/MMR-deficient colorectal cancers displayed a bimodal age distribution, which corresponds with the well-characterized observation that early-onset MSI-high colorectal cancers frequently are due to germline MMR mutations (ie, Lynch syndrome; approximately 30% of cases), whereas late-onset MSI-high cancers are predominantly sporadic (approximately 70% of cases) and associated with CpG island methylator phenotype in older women.1 We have previously shown that older patients with colorectal cancer in the United States were less likely to receive MSI testing and our findings herein underscore the need to consider MSI/MMR testing even among elderly patients, who experience an increased incidence of sporadic MSI-high/MMR-deficient tumors.10
There have been limited molecular epidemiology data with regards to MSI/MMR status by racial/ethnicity status, with some institutional series suggesting a lower incidence of MSI-high/MMR deficiency among colorectal adenocarcinomas in Black non-Hispanic patients and others reporting no differences.11,12 However, in the national data, MSI/MMR status clearly varied independently by race/ethnicity, with colorectal adenocarcinomas in Black non-Hispanic, Asian/Pacific Islander, and Hispanic patients all less likely to be MSI-high/MMR-deficient than in White non-Hispanic patients. Although we previously identified MSI testing disparities by patients' socioeconomic status in the United States, differences specifically because of patients' race/ethnicity were not observed.10 These findings underscore the importance of incorporating patients' race/ethnicity in future research into the genetic mechanisms of MSI-high/MMR deficiency.
In terms of histologic features, poorly differentiated cancers were more than twice as likely to be MSI-high/MMR-deficient as their well-differentiated or moderately differentiated counterparts. It has been suggested that well-differentiated cancers may be more likely to be MSI-high/MMR-deficient than moderately differentiated cancers, but our results portray the opposite—when adjusting for clinicopathologic features, well-differentiated cases were least likely to be MSI-high/MMR-deficient. These findings persisted when evaluating only those cases with conventional histology (data not shown). Furthermore, as previously described,13,14 medullary and mucinous subtypes were more frequently associated with MSI-high/MMR deficiency than conventional histology. Of note, although signet-ring cell histology has been reported in institutional series to be associated with MSI-high/MMR deficiency,15 we did not find that to be the case nationally when considering the patients' other clinicopathologic attributes. Importantly, cases with signet-ring cell histology were predominantly reported as being poorly differentiated. We therefore repeated the multivariable regression model excluding grade and found that the association between signet-ring cell histology and MSI-high/MMR deficiency significantly strengthened. By comparison, medullary and mucinous histologies remained significantly associated with increased MSI-high/MMR-deficiency, irrespective of adjustment for grade.
A distinct characteristic of MSI-high/MMR-deficient colorectal cancer that has been widely observed clinically is its penchant for local growth and invasion—reflected in the national data by a prevalence that peaked in stage I-II cancers (up to 20% of stage IIC cases) and fell to 7% of stage IV cancers.1,14 This finding is likely related to the remarkably elevated neoantigen load of MSI-high/MMR-deficient tumors, which may elicit robust local and systemic antitumoral immune responses that both help eliminate tumor cells that leave the primary and that correlate with a favorable prognosis.16,17 Among stage IV cases, there was a higher MSI-high/MMR-deficiency proportion in our cohort than previously published, potentially reflecting that the NCDB would only capture cases that presented as stage IV disease and not metastatic cases that had previously presented at earlier stages.
Because of the nature of NCDB data, there were several important limitations that constrained our analyses. For example, the NCDB did not permit the analysis of incidence. In addition to a lack of details about MSI/MMR testing methodology, for MSI-high/MMR-deficient cases, there were no further molecular details encoded regarding which MMR gene is mutated, nor about the MLH1 promoter methylation, CIMP, or BRAF mutational statuses of tumors. Data regarding BRAF and NRAS mutational status in colorectal cancers began to be collected by US cancer registries in 2021, but will generally not be publicly available until approximately 3 years following initial collection, nor did the NCDB report data regarding mixed histologic patterns, tumor-infiltrating lymphocytes, serrated precursor lesions, or other histopathologic features in these tumors. Furthermore, the NCDB did not collect data about somatic tumor panels, germline data, or family history of colorectal cancer. The NCDB only reports treatment data from a patient's initial treatment course, and so does not capture immunotherapy (ie, immune checkpoint inhibitors) administered beyond the first-line setting—which is an important confounder for the analysis of MSI/MMR prognosis in stage IV patients. Moreover, the NCDB categorizes all immunotherapeutic agents and monoclonal antibodies (eg, cetuximab and bevacizumab) into a binary immunotherapy variable, without identifying specific agents or doses. For outcomes, only all-cause OS was reported by the NCDB, precluding the evaluation of additional outcomes such as disease-free survival and postrelapse survival for patients with metachronous stage IV disease. Additionally, the number of prognostic variables reported by the NCDB for patients with stage IV disease was limited.
In conclusion, in a US national cohort, 16% of newly diagnosed colorectal adenocarcinomas were MSI-high/MMR-deficient, with the highest prevalence among stage I-II tumors. In addition to confirming several well-described associations between colorectal cancer attributes and MSI-high/MMR-deficiency—such as female sex, bimodal age distribution, right-sided colonic origin, poorly differentiated histology, and medullary and mucinous subtypes—we found that MSI/MMR status also varied by patients' race/ethnicity, with lower MSI-high/MMR deficiency prevalence among non-Hispanic Black, Asian/Pacific Islander, and Hispanic patients. In contrast to some institutional series, well-differentiated and signet-ring cell histologies were not independently associated with MSI-high/MMR-deficiency when adjusting for other clinicopathologic features.
ACKNOWLEDGMENT
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and the hospitals participating in the CoC NCDB are the source of the deidentified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
APPENDIX
TABLE A1.
Overall Survival by MSI/MMR Status Among Patients With Stage I-III Colorectal Adenocarcinoma Using Multivariable Cox Regression
TABLE A2.
Overall Survival by MSI/MMR Status Among Patients With Stage IV Colorectal Adenocarcinoma Using Multivariable Cox Regression
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Merck
Research Funding: Boston Biomedical (Inst)
J. Bryan Iorgulescu
Consulting or Advisory Role: AstraZeneca
No other potential conflicts of interest were reported.
SUPPORT
C.G. is supported by the Dana-Farber Cancer Institute Fellowship, the American Society of Hematology Minority Medical Student Award Program, and the NIH Ruth L. Kirschstein NRSA Individual Predoctoral Fellowship F31 Award (1F31CA239443-01). J.B.I. gratefully acknowledges funding support from the National Cancer Institute (K12CA090354) and the Conquer Cancer Foundation/Sontag Foundation.
DATA SHARING STATEMENT
In accordance with the NCDB data use agreement, data are available by application to the NCDB.
AUTHOR CONTRIBUTIONS
Conception and design: J. Bryan Iorgulescu
Collection and assembly of data: Catherine Gutierrez, J. Bryan Iorgulescu
Data analysis and interpretation: Catherine Gutierrez, Shuji Ogino, J. Bryan Iorgulescu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Merck
Research Funding: Boston Biomedical (Inst)
J. Bryan Iorgulescu
Consulting or Advisory Role: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Vilar E, Gruber SB: Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol 7:153-162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sepulveda AR, Hamilton SR, Allegra CJ, et al. : Molecular biomarkers for the evaluation of colorectal cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol 35:1453-1486, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overman MJ, McDermott R, Leach JL, et al. : Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol 18:1182-1191, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of Pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinicrope FA, Mahoney MR, Smyrk TC, et al. : Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 31:3664-3672, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Colon Cancer (Version 1.2022), https://www.nccn.org/guidelines/category_1
- 8.Mallin K, Browner A, Palis B, et al. : Incident cases captured in the national cancer database compared with those in U.S. population based central cancer registries in 2012–2014. Ann Surg Oncol 26:1604-1612, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Williams R: Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J 12:308-331, 2012 [Google Scholar]
- 10.Papke DJ, Lindeman NI, Schrag D, et al. : Underutilization of guideline-recommended mismatch repair/microsatellite instability biomarker testing in advanced colorectal cancer. Cancer Epidemiol Biomarkers Prev 31:1746-1751, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carethers JM, Murali B, Yang B, et al. : Influence of race on microsatellite instability and CD8+ T cell infiltration in colon cancer. PLoS One 9:e100461, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashktorab H, Ahuja S, Kannan L, et al. : A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget 7:34546-34557, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanza G, Gafà R, Matteuzzi M, et al. : Medullary-type poorly differentiated adenocarcinoma of the large bowel: A distinct clinicopathologic entity characterized by microsatellite instability and improved survival. J Clin Oncol 17:2429-2438, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Wright CL, Stewart ID: Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am J Surg Pathol 27:1393-1406, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kakar S, Smyrk TC: Signet ring cell carcinoma of the colorectum: Correlations between microsatellite instability, clinicopathologic features and survival. Mod Pathol 18:244-249, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Willis JA, Reyes-Uribe L, Chang K, et al. : Immune activation in mismatch repair deficient carcinogenesis: More than just mutational rate. Clin Cancer Res 26:11-17, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballhausen A, Przybilla MJ, Jendrusch M, et al. : The shared frameshift mutation landscape of microsatellite-unstable cancers suggests immunoediting during tumor evolution. Nat Commun 11:4740, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In accordance with the NCDB data use agreement, data are available by application to the NCDB.