Summary Abstract
Background:
Accumulating evidence from clinical trials suggests that a lower (restrictive) hemoglobin threshold (<8% g/dL) for red blood cell (RBC) transfusion, compared with a higher (liberal) threshold (≥10 g/dL) is safe. However, in anemic patients with acute myocardial infarction (MI), maintaining a higher hemoglobin level may increase oxygen delivery to vulnerable myocardium resulting in improved clinical outcomes. Conversely, RBC transfusion may result in increased blood viscosity, vascular inflammation, and reduction in available nitric oxide resulting in worse clinical outcomes. We hypothesize that a liberal transfusion strategy would improve clinical outcomes as compared to a more restrictive strategy.
Methods:
We will enroll 3500 patients with acute MI (type 1, 2, 4b or 4c) as defined by the Third Universal Definition of MI and a hemoglobin <10 g/dL at 144 centers in the United States, Canada, France, Brazil, New Zealand, and Australia. We randomly assign trial participants to a liberal or restrictive transfusion strategy. Participants assigned to the liberal strategy receive transfusion of RBCs sufficient to raise their hemoglobin to at least 10 g/dL. Participants assigned to the restrictive strategy are permitted to receive transfusion of RBCs if the hemoglobin falls below 8 g/dL or for persistent angina despite medical therapy. We will contact each participant at 30 days to assess clinical outcomes and at 180 days to ascertain vital status. The primary endpoint is a composite of all-cause death or recurrent MI through 30 days following randomization. Secondary endpoints include all-cause mortality at 30 days, recurrent adjudicated MI, and the composite outcome of all-cause mortality, nonfatal recurrent MI, ischemia driven unscheduled coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), or readmission to the hospital for ischemic cardiac diagnosis within 30 days. The trial will assess multiple tertiary endpoints.
Conclusions:
The MINT trial will inform RBC transfusion practice in patients with acute MI.
Keywords: red blood cell transfusion, myocardial infarction, hemoglobin, anemia
Background
Blood transfusion is a common medical intervention, with 118.5 million units of blood collected worldwide each year.1 Physicians often transfuse patients based on a specific hemoglobin threshold, although it is unclear at what threshold the benefits of increasing hemoglobin outweighs the risks of transfusion.
In most clinical settings, trials have demonstrated that a restrictive transfusion strategy using a hemoglobin threshold of 7–8 g/dL is as effective as a liberal transfusion strategy using a hemoglobin threshold of 9–10 g/dL.2 A recent systematic review published in the Cochrane database identified 48 trials involving 21,433 participants that evaluated transfusion triggers in a variety of populations.2 This meta-analysis documented that a restrictive strategy is associated with 41% fewer transfusions (risk ratio 0.59, 95% confidence interval 0.53 to 0.66) and no difference in 30-day mortality (risk ratio 0.99, 95% confidence interval 0.86 to 1.15) as compared with a liberal transfusion strategy. Prior to initiating MINT, only two small randomized controlled trials comparing liberal and restrictive transfusion strategies in the setting of acute coronary events had been published. The first was a pilot trial with 45 patients3, and the second was the MINT pilot with 110 patients.4 Both small studies noted a trend toward increased mortality in the restrictive group; however, the number of patients and events were inadequate to guide transfusion practice in the population of patients with acute MI. Indeed, all transfusion practice guidelines clearly stated that there were too few patients with MI enrolled in clinical trials to provide clinical guidance.5, 6 Anemia in this setting is not only common but has been shown to be associated with adverse outcomes such as mortality rates7, 8, It is also biologically plausible that patients with acute coronary events may require greater blood supply to the myocardium. With such justification and limited high quality studies, we designed and undertook the MINT trial to fill existing knowledge gaps.9, 10
Methods
Trial Aims and Objectives
The MINT trial was designed to determine whether a liberal transfusion strategy with a threshold of 10 g/dL reduces the composite outcome of all-cause mortality or nonfatal recurrent MI through 30 days, compared with a restrictive transfusion strategy with a threshold of 7 to 8 g/dL among patients with an acute MI and a hemoglobin concentration less than 10 g/dL.
Study Design
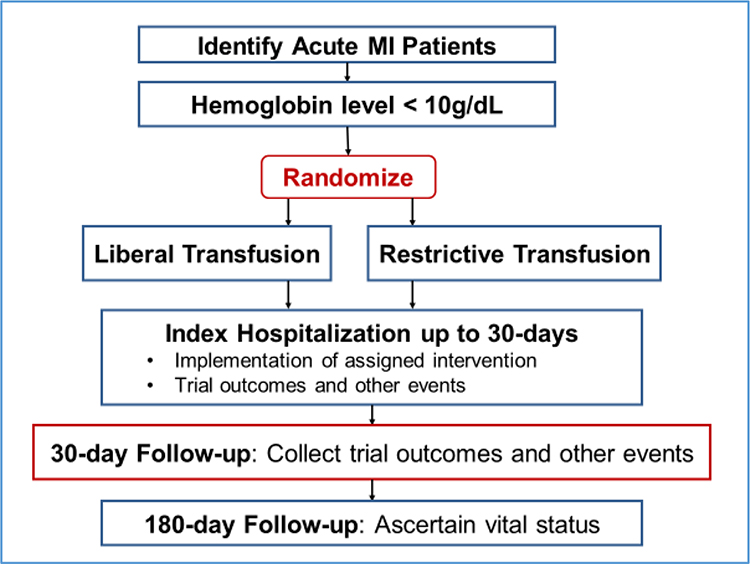
The MINT trial is a randomized, open-label, two group multicenter clinical trial. The trial is being conducted in approximately 144 clinical sites in the United States, Canada, France, Brazil, New Zealand, and Australia (see Supplementary Appendix 5 for list of trial sites). Eligible consenting patients who have had an acute MI and have a hemoglobin < 10 g/dL are randomized to receive either the liberal or the restrictive transfusion strategy using a centralized web-based system. Treatment is allocated with a 1:1 ratio using a permuted block design, with random variable block sizes, stratified by clinical site. The trial intervention is delivered during the index hospitalization, and primary and secondary outcomes are collected through 30-days post-randomization. Follow-up continues for 6 months. An overview of the trial enrollment, treatment and follow-up processes is shown in Figure 1.
Figure 1. Patient Flow for the MINT Trial.
MINT was designed as a pragmatic trial.11 The trial uses broad eligibility criteria based on standard clinical criteria for diagnosing MI and anemia, tests two transfusion strategies that are routinely used in current medical practice, and assesses trial outcomes that are clinically meaningful and objectively measured. Further, assessment of adherence to the transfusion protocol is based on routinely drawn hemoglobin measures, an abbreviated follow-up schedule and streamlined data collection, and the analysis strategy includes all available outcome data and utilizes the intention to treat principle. A description of the MINT design using the domains from the PRECIS-2 wheel12 is shown in Supplementary Appendix 1.
Population
The eligible study population includes adult patients with ST-segment elevation MI or non ST-segment elevation MI consistent with the Third Universal Definition of Myocardial Infarction criteria13 that occurs on admission or during the index hospitalization, and anemia defined as a hemoglobin concentration less than 10 g/dL at the time of randomization. Patients with Type 1 (spontaneous), Type 2 (oxygen supply/demand mismatch), Type 4b (stent thrombosis at angiography), and Type 4c (in-stent restenosis without evidence of thrombus) are eligible for the trial, and MI type is determined by the enrollment site team (Table 1). Patients scheduled for cardiac surgery during the current admission are excluded.
Table 1.
MINT Eligibility Criteria
| Inclusion Criteria: | |
|
| |
| 1) | 18 years of age or older; |
| 2) | with either ST-segment elevation myocardial infarction or non-ST-segment elevation myocardial infarction consistent with the Third Universal Definition of Myocardial Infarction and Type 1 (spontaneous), Type 2 (oxygen supply/demand mismatch), Type 4b (stent thrombosis at angiography), or Type 4c (in-stent restenosis)13 that occurs on admission or during the index hospitalization, |
| 3) | with a hemoglobin concentration less than 10 g/dL at the time of random allocation, and |
| 4) | 4) the patient’s attending physician, with expertise in cardiovascular care, believes that both of the transfusion strategies are consistent with good medical care for the patient as determined by his/her clinical judgement. |
|
| |
| Exclusion Criteria: | |
|
| |
| 1) | uncontrolled acute bleeding at the time of randomization defined as the need for uncrossed or non-type specific blood; |
| 2) | decline blood transfusion; |
| 3) | scheduled for cardiac surgery during the current admission; |
| 4) | are receiving only palliative treatment; |
| 5) | if known that follow-up will not be possible at 30 days; |
| 6) | if previously participated in MINT |
| 7) | if currently enrolled in a competing study that interferes with the intervention or follow-up of MINT or enrolled in a competing study that has not been approved by the local IRB; or |
| 8) | if the attending physician does not believe the patient is an appropriate candidate for the trial for any reason. |
Given the pragmatic nature of MINT, several approaches to screening study patients are used. Study staff are encouraged to review results of troponin tests performed in hospitalized patients daily. Medical records for patients with values meeting study enrollment criteria are reviewed and assessed for eligibility. Staff may also screen admissions to the cardiac intensive care unit, cardiac service, and patients scheduled for cardiac catheterization. Study staff contact the physician of each eligible patient to confirm clinical equipoise between transfusion strategies and to obtain permission to approach the patient about trial enrollment. Once physician approval has been obtained, the patient is approached to seek informed consent to participate in the trial. Surrogate consent, in accordance with local ethics rules, can be sought for each patient who is not able to grant consent. Enrollment of patients with COVID-19 infection is not encouraged but is permitted at the discretion of the individual study sites.
Transfusion Strategies
The trial compares two common approaches to transfusion therapy: a restrictive and a liberal approach.
Restrictive Transfusion Strategy:
Patients randomized to the restrictive transfusion strategy are to receive a transfusion if the hemoglobin concentration falls below 8 g/dL and are strongly recommended to receive transfusion if the hemoglobin concentration is below 7 g/dL. Transfusion is also allowed when anginal symptoms (i.e., retrosternal chest discomfort, chest discomfort described as pressure or heaviness) are determined by the patient’s treating physician to be related to anemia and are not controlled with anti-anginal medications. Enough blood is given to increase the hemoglobin concentration to above 7 to 8 g/dL or to relieve anginal symptoms.
Liberal Transfusion Strategy:
Patients randomly allocated to the liberal transfusion strategy receive one unit of packed RBCs following randomization and receive enough blood to raise the hemoglobin concentration to 10 g/dL or above any time during the index hospitalization that the hemoglobin concentration is detected to be below 10 g/dL. A post transfusion hemoglobin measurement showing a hemoglobin level of at least 10 g/dL must be obtained.
For both strategies, blood is administered one unit at a time followed by a hemoglobin measurement. The transfusion strategy is followed throughout the index hospitalization up to 30 days, discharge, or death. A patient in either group may be transfused at any time without a measured hemoglobin level if the patient is actively bleeding (e.g., brisk gastrointestinal bleeding) and the treating physician believes an emergency transfusion is needed. A patient in either group with a history of congestive heart failure or low ejection fraction may receive diuretics prior to or after transfusion, and transfusion may be delayed until the patient can safely tolerate the additional volume. Patients with end stage renal disease may receive transfusion during dialysis if requested by treating physician. The transfusion protocol may be suspended for 24 hours if the patient undergoes a surgical procedure. The trial centrally monitors transfusions and hemoglobin levels on an ongoing basis.
Assessments of Laboratory Markers
Information collected as part of the trial includes hemoglobin and troponin levels and electrocardiogram (ECG) readings at specified time points while the patient is in the hospital (Table 2). The blood samples are normally drawn with daily morning phlebotomy. The required collection time points for hemoglobin levels and ECGs are within 24 hours prior to randomization (eligibility hemoglobin level), and on days 1, 2 and 3 following randomization. The required time points for the troponin levels are within 24 hours prior to randomization and at 12 hours, and on days 1, 2 and 3 following randomization. All hemoglobin and troponin levels performed for clinical purposes during the index hospitalization are collected by the trial.
Table 2.
Information collected at various time points
| Screening | Baseline | Randomization | 12 hours Post Randomization | Day 1 | Day 2 | Day 3 | Daily Upto 30 Days | Hospital Discharge/30 Days | 30 Day Follow-up | 6 Month Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Confirm Eligibility | X | ||||||||||
| Informed Consent | X | ||||||||||
| Hemoglobin Levela | Xb,c | Xb | Xb | Xb | X | ||||||
| Troponin Levela | Xb,c | Xb | Xb | Xb | Xb | X | |||||
| ECGa | Xb,c | Xb | Xb | Xb | X | ||||||
| Demographics | X | ||||||||||
| Medical History | X | ||||||||||
| Medications | X | ||||||||||
| Implement Transfusion Strategy | XX | ||||||||||
| RBC Transfusionsd | XX | ||||||||||
| Study Outcomes | X | X | Xe | ||||||||
| Quality of Life (EQ-5D) | X | ||||||||||
| Vital Status | X | X | X | ||||||||
| Assessment of AEs and Ups Safety Monitoring | XX | ||||||||||
in addition to required time points, all performed for clinical reasons are collected
required, if still in hospital
within the 24 hours prior to randomization
including unit information (e.g, leukoreduction)
mortality outcome only
Participant Follow-up
Study staff review medical records and follow participants during the index hospitalization for up to 30 days following randomization. At hospital discharge or 30 days following randomization, whichever comes first, the site submits data related to the hospitalization including health status, laboratory results, electrocardiograms performed, and post-randomization clinical events.
Study staff at the clinical sites contact the patient at 30 days to ascertain vital status, administer the quality-of-life questionnaire, and determine if there has been a subsequent hospital admission or emergency room visit (Table 2). Staff obtain and review the medical records for each readmission and record all relevant data and identify the study outcomes. If there is a suspected acute coronary syndrome event, the site submits de-identified copies of the documentation from the index admission or the readmission for review. Study staff perform a final follow-up contact at 6 months following randomization to ascertain the patient’s vital status.
Outcome Definitions
The prespecified trial endpoints are presented in Table 3. The primary endpoint is the composite outcome of all-cause mortality and recurrent MI at 30 days following randomization. Myocardial recurrent MI in the 30-day window is classified by the Clinical Events Classification committee (CEC) using the Third Universal Definition of Myocardial Infarction.11 The diagnosis of recurrent MI requires a rise in the troponin value of at least 20% from the trough with additional evidence of myocardial ischemia (new ECG changes, imaging evidence, clinical history). (See Supplementary Appendix 2). The definitions of secondary and tertiary outcomes are included in Supplementary Appendix 3. Clinical outcomes other than MI are not centrally adjudicated.
Table 3.
MINT Trial Endpoints
| Primary Endpoint: Composite of all-cause mortality and recurrent MI through 30 days. | |
|
| |
| Secondary Endpoints: | |
|
| |
| 1) | all-cause mortality through 30 days, |
| 2) | recurrent MI through 30 days, |
| 3) | the composite outcome of all-cause mortality, nonfatal recurrent MI, ischemia driven unscheduled coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), or readmission to the hospital for ischemic cardiac diagnosis within 30 days. |
|
| |
| Tertiary Endpoints: | |
|
| |
| 1) | all-cause mortality, nonfatal recurrent MI, or unstable angina (i.e. acute coronary syndrome) within 30 days; |
| 2) | ischemia driven unscheduled coronary revascularization within 30 days; |
| 3) | unscheduled readmission to hospital for ischemic cardiac diagnosis within 30 days; |
| 4) | congestive heart failure within 30 days; |
| 5) | unscheduled readmission to hospital for any reason within 30 days; |
| 6) | individual thrombotic/hemorrhagic outcomes of stroke, pulmonary embolism or deep venous thrombosis, and bleeding within 30 days; |
| 7) | individual infectious outcomes of pneumonia, and blood stream infection within 30 days; |
| 8) | individual in-hospital outcomes of length of hospital stay following randomization and number of days in intensive care unit; |
| 9) | patient reported quality of life using the EuroQol questionnaire (EQ-5D) at 30 days; |
| 10) | all-cause mortality at 6-months following randomization. |
Recurrent MI is diagnosed when a suspected myocardial ischemic event is reported by the investigators at the clinical sites and confirmed by the CEC masked to assignment. In addition, all cardiac troponin values from all randomized participants are reviewed by the CEC to detect abnormal biomarker patterns that meet the protocol biomarker definition of a 20% rise from trough values or an increase above the 99th percentile upper reference limit for patients that have an initial normal cardiac troponin value. When surveillance criteria for a suspected MI are met, the CEC requests the medical records and ECGs that are temporally related to the abnormal values. The CEC adjudicates occurrences of MI masked to assignment. Recurrent MI is diagnosed when biomarker and clinical or ECG criteria of myocardial ischemia meet the 3rd Universal Definition of MI definition. MI is classified according to MI type as specified in the Third Universal Definition of Myocardial Infarction and whether MI type was ST segment elevation MI (STEMI) non-ST segment elevation MI (NSTEMI), or cannot be determined. The definition of recurrent MI will include all MI types. A sensitivity analysis is planned that will exclude procedural MIs (types 4a and 5). The cause of death is classified by the site personnel into one of three categories: cardiovascular death (e.g., congestive heart failure, arrhythmia), non-cardiovascular death (e.g., infection, cancer), or undetermined cause of death. Information about the specific cause of death will also be collected and compared by treatment groups.
We are collecting other events that are not designated primary, secondary, or tertiary outcomes including transfusion reactions (transfusion related acute lung injury, acute hemolytic transfusion reaction, transfusion associated sepsis, anaphylactic transfusion reaction, urticarial transfusion reaction, febrile non-hemolytic reaction) cardiac arrhythmias (i.e., atrial fibrillation/flutter, ventricular tachycardia, ventricular fibrillation and others), thrombotic ischemic events complications of MI (chest pain or stable angina, mechanical complications of MI, pericarditis, pericardial effusion/tamponade, syncope, cardiomyopathy), other (acute respiratory failure, acute renal failure, urinary tract infection).
Safety and Compliance Monitoring
The trial centrally monitors key aspects of protocol compliance including enrollment, retention, adherence to assigned intervention, deviations from the protocol, missing data, and adherence to adverse event reporting requirements and event adjudication procedures. Sites submit a serious adverse event form for unexpected serious adverse events occurring within 30 days of randomization and for unexpected deaths occurring within 6 months of randomization. A designated Medical Safety Officer determines the severity, expectedness, and relatedness of the event to the study protocol masked to the transfusion strategy. An external Data and Safety Monitoring Board (DSMB) consisting of members appointed by the National Heart, Lung, and Blood Institute (NHLBI) monitors the trial. On a semi-annual basis, the DSMB reviews trial conduct, study endpoints, and adverse events. The DSMB annually reviews interim analyses of the primary endpoint by assigned treatment group annually using the Lan-DeMets approach and O’Brien Fleming monitoring boundaries. An interim analysis to assess sample size assumptions was conducted in January 2020 when 1028 participants were enrolled, and the DSMB recommended that the trial should continue with the planned sample size of 3500 patients.
Since MINT compares two established transfusion strategies with different resource and cost implications, a null result from a well-powered trial would be important for establishing treatment guidelines and policy. As a result, the original Data Safety Monitoring Plan stated that no interim futility analyses would be conducted. Given the delay in enrollment due to COVID pandemic, a futility analysis was requested by NHLBI in February 2022. A plan to present the conditional power for detecting superiority of either transfusion strategy and the conditional power for detecting non-inferiority of the restrictive strategy was submitted; conditional power is based on the observed trial data collected thus far, hypothesized treatment effect sizes, and the proposed trial sample size. The submitted futility analysis plan was approved by a blinded statistician at NHLBI in May 2022. The DSMB reviewed the futility analysis at July and August 2022 meetings and recommended that the trial complete enrollment to the target sample size of 3500 patients. The NHLBI accepted the DSMB’s recommendation to complete enrollment.
Organization and Participating Centers
The Rutgers Robert Wood Johnson School of Medicine, Division of General Internal Medicine is responsible for overall clinical coordination of the trial (CCC). Each country has subsidiary coordinating center. The Canadian Coordinating Center is at the Centre de Recherche du Centre Hospitalier de l’Université de Montréal, Montréal Québec. The Brazilian Coordinating Center is at the Brazilian Clinical Research Institute (BCRI), Sao Paulo. The European Coordinating Center is at FACT (French Alliance for Cardiovascular clinical Trials), Paris. The New Zealand Coordinating Center is at Green Lane Coordinating Centre Ltd, Auckland and the Australian Coordinating Center is at Flinders University in Adelaide.
The Epidemiology Data Center at the University of Pittsburgh is responsible for the data coordination of the trial (DCC). FACT collects primary data for participants enrolled in Europe and transmits the data to the University of Pittsburgh.
The primary decision-making body of the MINT trial is the Executive Committee that includes the PIs of the CCC and DCC and the lead clinical investigators from the United States, Canada, Europe, Brazil, New Zealand and Australia. The Executive Committee identifies high-level study design and implementation issues and will serve as the Publications Committee. The Steering Committee is composed of a larger group of investigators and staff representing the sites, CCC, and DCC; they meet monthly to provide essential input on operational and scientific issues. A central and independent Clinical Events Classification committee is responsible for adjudication of recurrent MIs.
MINT initially included clinical sites in the U.S. and Canada. A small randomized study conducted in Montreal, Canada in 2016 (unpublished data) served as the “vanguard phase” of the trial, and this vanguard study contributed a total of 45 patients to the MINT trial. In 2020, the trial was expanded to include sites in Brazil, France, New Zealand, Australia, and Spain.
The MINT trial is funded by the National Heart Lung and Blood Institute (U01 HL133817, U01HL132853). The trial protocol is IRB approved through the CCC, and each clinical site has local IRB approval.
Statistical Methods
Sample Size and Power
The composite 30-day rate of death and MI for the combined liberal and restrictive transfusion strategies was 16.4% in the MINT pilot trial.4 Assuming a 16.4% event rate and that <5% of patients will have missing 30-day outcome data, a sample size of N=3500 provides the MINT trial 80% power to detect a 20% relative risk reduction and 90% power to detect a 25% relative reduction for the trial primary endpoint with alpha=0.05. Initial power calculations were performed using a two-sided chi-square test, and we conducted simulations to confirm that a log binomial model with a random intercept for site had comparable power. The target relative risk reduction of 20% corresponds to an absolute risk reduction of 3.6% (i.e., 18.2% versus 14.6%). The risk difference of 3.6% is small enough, such that, if a liberal transfusion threshold were not shown to be superior to a restrictive transfusion threshold, we would recommend that transfusion not be given.
Primary Endpoint Analysis
The intention-to-treat principle will be used for all analyses. The primary endpoint, 30-day death/MI, will be analyzed using a log-binomial regression model with a fixed effect variable for assigned treatment strategy and a random effect for clinical site. A two-sided test with alpha=0.05 will be used for the assigned treatment variable. To address the primary aim, we will report the estimated relative risk and significance level accounting for interim monitoring and imputing missing primary outcome data. Markov Chain Monte Carlo (MCMC) multiple imputation methods will be used to impute the missing values for the primary endpoint. 14 A second log-binomial model with random effects for site will be created from patients with non-missing 30-day primary endpoint data (i.e. no imputation). The proportion of events in each arm, the relative risk and absolute difference will be presented, and 95% confidence intervals will be calculated from the observed data. If superiority of one of the two transfusion strategies is not demonstrated through the primary hypothesis test, the non-inferiority of the restrictive strategy compared to the liberal strategy will be evaluated setting the non-inferiority margin to a 15% relative increase; that is, the restrictive arm will be considered non-inferior to the liberal arm if the 95% confidence interval for the estimated relative risk is < 1.15. This non-inferiority margin is consistent with other major cardiology trials.9
A per protocol analysis, including only patients who undergo transfusion according to their assignment and adjusting for baseline factors that are associated with adherence to the treatment protocol, will be conducted as a sensitivity analysis.
Analysis of Secondary and Tertiary Endpoints
For dichotomous secondary and tertiary endpoints, we will present the relative and absolute risk differences with 95% confidence intervals. Length of hospital stay will be analyzed using Wilcoxon rank sum tests, and six-month mortality will be analyzed with the log rank statistic. The median difference will be presented for length of stay and Kaplan-Meier methods will be used to estimate cumulative mortality at 180 days. We will use t-tests or the Wilcoxon rank sum tests to compare the EQ-5D utility index and health today by assigned treatment. The alpha level will not be adjusted for the multiple secondary and tertiary analyses.
Subgroup Analyses
Subgroup analyses will be performed based on select baseline factors: ST segment elevation MI and non ST segment elevation MI, MI types 1 or 2, baseline hemoglobin level (<8, 8–8.9, ≥9 g/dL), revascularization for treatment of index MI prior to randomization (yes/no), acute anemia (normal hemoglobin at admission, low hemoglobin at admission and no history of chronic anemia, and low hemoglobin at admission and history of chronic anemia), sex (male/female), age (<60, 60–69, 70–79, ≥80 years), heart failure (yes/no), renal function (renal dialysis, no dialysis and eGFR <30, 30–59, ≥60 mL/mi/1.73m2), and diabetes status (yes/no). Among participants from sites in the U.S., Canada, New Zealand and Australia we will also examine subgroups defined by race (White, Black, Non-White and Non-Black Race) and Hispanic ethnicity (yes/no). The 30-day event rates by assigned transfusion strategy will be compared within each pre-defined subgroup. In addition, a log-binomial regression model including treatment assignment, subgroup variable, and the interaction between the subgroup variable and treatment assignment will be created, and the significance of the interaction will be presented. Cox proportional hazards regression models will be created with similar covariates to test whether pre-specified subgroup variables modify the treatment effect on 6-month all-cause mortality.
MINT Site Survey
We created and administered a survey of MINT site investigators to document practice variation of transfusion practices and uncertainty about best transfusion strategies in patients with ischemic heart disease. Among the 81 physicians at 73 hospitals in U.S. and Canada, the response rate was 90.1%. We documented substantial heterogeneity in the preferred hemoglobin transfusion threshold among bedside clinicians for each of several specified clinical scenarios (Supplementary Appendix 4). Investigators most frequently reported using transfusion thresholds of 7 to 8 g/dL in sample cases, but the threshold was somewhat higher in the case with active symptoms. For many of the investigators (>65%), the evidence to support the choice of a transfusion threshold was judged as uncertain or very uncertain. Another 20–25% of the investigators indicated that they were neutral about the strength of the evidence to transfuse at a particular threshold (neither certain nor uncertain). Only 4.1–8.5% of the investigators were certain or very certain about the evidence to support their decision to transfuse in any of the sample cases. When individual physicians were certain about a transfusion threshold for an acute MI case, the chosen thresholds varied among the site physicians and most often clustered close to 8 g/dL.
Discussion
The risks and benefits of a liberal versus a restrictive transfusion strategy remain unresolved in anemic patients presenting with acute MI. Observational data suggests an association between a liberal transfusion strategy and increased mortality.15 Prior to the initiation of the MINT trial, two small trials comparing liberal to restrictive transfusion in patients with acute MI suggested that mortality may be higher in patients treated with restrictive transfusion.3, 4 The REALITY trial was completed after the MINT trial was initiated.9, 10 In 668 patients with acute MI and hemoglobin concentration less than 10 g/dL, the composite outcome of all cause 30-day mortality, recurrent myocardial infarction, stroke, or emergency revascularization for ischemia (or MACE) occurred in 36 (11.0%) in the restrictive transfusion strategy arm and 45 (14.0%) in the liberal transfusion strategy arm (relative risk=0.79, one sided upper 95% confidence limit=1.18, meeting the prespecified criteria for non-inferiority). At 1 year, however, the restrictive strategy did not meet criteria for non-inferiority.10 When these three randomized trials were combined in meta-analysis, the 30-day mortality risk was not significantly different between treatment strategies owing to very wide confidence intervals given the limited sample size (relative risk 1.61, 95% CI,0.38 to 6.88).2
At the start of MINT, we conducted an operational survey in 73 hospitals in U.S. and Canada to evaluate transfusion practice in patients with acute MI. The results from this survey documented variation in transfusion practice based on a high level of uncertainty regarding the best threshold for transfusion in patients with acute MI. Despite the recent publication of REALITY,9 there remains a paucity of evidence helping clinicians decide what degree of anemia should trigger a transfusion in patients with acute MI.
The MINT trial outcomes were selected to assess the clinically important benefits and harms of transfusion and anemia in vulnerable individuals with compromised myocardium. The primary outcome in the MINT trial is the composite of all-cause mortality or recurrent MI within 30 days of randomization. Higher hemoglobin concentrations might decrease ischemic injury and improve myocardial performance by improving oxygen delivery to the myocardium in the acute MI patients, and additionally reduce other sequelae of decreased oxygen delivery to the myocardium: unscheduled coronary revascularization, hospital readmission for ischemic symptoms, unstable angina, arrhythmias, cardiogenic shock, and cardiovascular mortality. Higher hemoglobin levels may also be associated with a greater sense of well-being and a better perceived quality of life. On the other hand, if transfusion results in clinically important fluid overload, immuesnosuppression, increased viscosity, inflammation, microvascular vasoconstriction, and platelet activation, there may be an increase in congestive heart failure, infection, bleeding, stroke, venous thromboembolism, recurrent MI, or cardiovascular mortality, or a detrimental effect on the quality of life. Blood transfusion will likely have its maximum effect within a 30-day time period, but the MINT trial will also assess 6-month mortality to determine if early effects of blood transfusion persist over a longer period.
The trial compares two transfusion strategies routinely used in current medical practice. In the restrictive strategy, blood transfusion is not permitted unless the hemoglobin concentration is less than 8 g/dL (or there is clinical justification such as active bleeding/uncontrolled angina). A blood transfusion is not required if the hemoglobin concentration is less than 8 g/dL but is encouraged if the hemoglobin concentration is less than 7 g/dL. Prior trials performed in critical care patients established the safety of 7 g/dL threshold.16, 17 Discussions with many cardiologists suggested that many clinicians are not comfortable with a threshold as low as 7 g/dL (as used in other settings such as ICU patients); however, individual clinicians may choose to use a threshold of less than 7 g/dL to trigger transfusions. Patients may also be transfused for signs or symptoms when the clinician believes it is necessary, although this occurred infrequently in prior trials that incorporated symptoms into the restrictive transfusion protocol.4, 18
In the liberal transfusion group, a threshold of less than 10 g/dL was chosen because oxygen delivery to the myocardium is flow dependent, and myocardial ischemia may be precipitated or worsened by low hemoglobin concentrations in patients with Type 1 MIs and plaque erosion or rupture as well as in type 2 MIs with oxygen supply/demand mismatch. Studies performed in canines and in patients who decline blood transfusion for religious reasons found that the odds of death rose as the hemoglobin fell below 10 g/dL.19–22 The pragmatic transfusion protocol encourages administering transfusions more slowly or delaying transfusion in patients with congestive heart failure until fluid overload is adequately treated and administering transfusions on the days of dialysis in patients with end stage renal disease. If a patient is hemorrhaging, the transfusion protocol is paused (allowing emergency transfusion) until bleeding is controlled.
Recurrent MI is a component of the 30-day composite primary outcome along with all-cause mortality. Recurrent MI is detected by site study team evaluation of troponin levels and ECGs performed for three days following randomization and by central surveillance of troponin levels. Abnormal post randomization troponin profiles following the index MI event e.g., a new rise in troponin greater than 20% or greater than the upper reference limit if initial values have returned to baseline triggers review of medical records for clinical signs, symptoms, and tests. These findings are used by clinical events committee blinded to transfusion group to adjudicate the diagnose recurrent MI.
The interventions in the MINT trial are not masked due to the logistical complexities for blinding a transfusion intervention. As a result, it is critical that the trial maintains good adherence to the assigned treatment protocol, the endpoints are objective, and the trial endpoint ascertainment is nearly complete. Recurrent MI is adjudicated blinded to assignment although other secondary outcomes are recorded by sites unblinded. Most outcomes are based on objective test results such as radiographic images.
The trial encountered enrollment challenges with the COVID-19 pandemic. To sustain recruitment, the trial was expanded from the U.S. and Canada to include sites in Brazil, France, New Zealand, Australia, and Spain. These additional sites have increased the recruitment capacity and generalizability of the trial as well as the complexity of conducting a trial with different healthcare systems, languages, and time zones.
Summary
In patients with acute MI, the risks and benefits of RBC transfusion may be different than in other patient populations. Anemia, if untreated, may result in increased risks of further myocardial ischemia, injury, or infarction. Transfusions, on the other hand, may result in increased risks of volume overload and other adverse effects. Thus, it is unclear whether the benefits of immediate correction of anemia with transfusion are outweighed by the potential side effects of transfusion. Regardless of whether liberal transfusion strategy is proven to be superior or not, the MINT trial will inform clinical practice and RBC transfusion guidelines in patients with acute MI.
Supplementary Material
Funding
This trial is supported by the National Heart Lung and Blood Institute U01 HL 133817-Clinical Coordinating Center, U01HL132853 - Data Coordinating Center. The vanguard phase was supported by a peer-reviewed grant 342193 from the Canadian Blood Services and the Canadian Institutes of Health Research (CIHR) Institute of Circulatory and Respiratory Health ClinicalTrials.gov Identifier: NCT02981407
Footnotes
Disclosures
JLC: DSMB member for Cerus Corporation project
MMB: DSMB member for Cerus Corporation project
JDA: Research funding MicroPort, Boston Scientific. Advisory Boards Philips, Medtronic. Consulting Abbott, Shockwave, Penumbra, Recor.
SGG: Research grant support (e.g., steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from: Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Merck, Novartis, Novo Nordisk A/C, Pendopharm/Pharmascience, Pfizer, Regeneron, Sanofi, Servier, Tolmar Pharmaceuticals, Valeo Pharma; and salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, PERFUSE Research Institute, TIMI Study Group (Brigham Health)
AMG: None.
MHB: None
Philippe Gabriel Steg: has received research grants from Bayer, Merck, Sanofi, Servier; has been a speaker or consultant for Amarin, Amgen, AstraZeneca, Bayer, Bristol-Myers-Squibb, Janssen, Lexicon, Merck, Novartis, Novo-Nordisk, PhaseBio, Pfizer, Regeneron, Sanofi, Servier. He is a Senior Associate Editor for Circulation
APD: Consultant Velakor Biotherapeutics Inc.
RDL: Grants from Bristol-Myers Squibb, GlaxoSmithKline plc., Medtronic, Pfizer, Bayer, Sanofi; consulting fees from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Novo Nordisk, GlaxoSmithKline plc., Medtronic, Merck, Pfizer, Portola, Sanofi; and honoraria for lectures from Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Novo Nordisk, and Bayer.
PCH: DSMB member of Cerus Corporation project
“The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Blood Transfusion. In; 2021.
- 2.Carson JLS SJ; Roubinian N; Fergussion DA; Triulizi DJ; Doree C; Hebert PH; Trivella M Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Sys Rev 2021;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108(8):1108–11. [DOI] [PubMed] [Google Scholar]
- 4.Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013;165(6):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016;316(19):2025–2035. [DOI] [PubMed] [Google Scholar]
- 6.Mueller MM, Van Remoortel H, Meybohm P, Aranko K, Aubron C, Burger R, et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019;321(10):983–997. [DOI] [PubMed] [Google Scholar]
- 7.Alexander KP, Chen AY, Wang TY, Rao SV, Newby LK, LaPointe NM, et al. Transfusion practice and outcomes in non-ST-segment elevation acute coronary syndromes. Am Heart J 2008;155(6):1047–53. [DOI] [PubMed] [Google Scholar]
- 8.Salisbury AC, Amin AP, Reid KJ, Wang TY, Masoudi FA, Chan PS, et al. Hospital-acquired anemia and in-hospital mortality in patients with acute myocardial infarction. American heart journal 2011;162(2):300–309 e3. [DOI] [PubMed] [Google Scholar]
- 9.Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand-Zaleski I, et al. Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial. JAMA 2021;325(6):552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Juanatey JR, Lemesle G, Puymirat E, Ducrocq G, Cachanado M, Arnaiz JA, et al. One-Year Major Cardiovascular Events After Restrictive Versus Liberal Blood Transfusion Strategy in Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Trial. Circulation 2022;145(6):486–488. [DOI] [PubMed] [Google Scholar]
- 11.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62(5):464–75. [DOI] [PubMed] [Google Scholar]
- 12.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60(16):1581–98. [DOI] [PubMed] [Google Scholar]
- 14.van Buuren SG-O K mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 2011;45(3):1–67. [Google Scholar]
- 15.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 2008;36(9):2667–74. [DOI] [PubMed] [Google Scholar]
- 16.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group [see comments]. New England Journal of Medicine 1999;340(6):409–417. [DOI] [PubMed] [Google Scholar]
- 17.Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014;371(15):1381–91. [DOI] [PubMed] [Google Scholar]
- 18.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365(26):2453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson HT, Kessinger JM, McFarland WJ, Jr., Laks H, Geha AS. Response of the hypertrophied heart to acute anemia and coronary stenosis. Surgery 1978;84(1):8–15. [PubMed] [Google Scholar]
- 20.Hagl S, Heimisch W, Meisner H, Erben R, Baum M, Mendler N. The effect of hemodilution on regional myocardial function in the presence of coronary stenosis. Basic Research in Cardiology 1977;72(4):344–364. [DOI] [PubMed] [Google Scholar]
- 21.Wilkerson DK, Rosen AL, Sehgal LR, Gould SA, Sehgal HL, Moss GS. Limits of cardiac compensation in anemic baboons. Surgery 1988;103(6):665–670. [PubMed] [Google Scholar]
- 22.Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet 1996;348(9034):1055–60. [DOI] [PubMed] [Google Scholar]
- 23.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66(4):403–69. [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3(4):692–4. [DOI] [PubMed] [Google Scholar]
- 25.CDC/NHSN surveillance definitions for specific types of infections. In: CDC; 2016. [Google Scholar]
- 26.van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15(5):708–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.