Abstract
Several FLT3 inhibitors(i) are available to treat relapsed/refractory (R/R) FLT3‐internal tandem duplicated acute myeloid leukemia (AML). This study analyzes the efficacies of various FLT3i (types 1 and 2) tested in clinical trials in treating R/R AML and high‐risk myelodysplastic syndromes (HR‐MDS). PubMed and EMBASE databases were searched for single/double‐arm phase I/II/III R/R AML or HR‐MDS clinical trials published between 1/1/2000 and 6/1/2021. The outcomes studied were composite response rate (CRc) and overall response rate (ORR). Toxicities were compared based on the organ system. The 28 studies analyzed had 1927 patients. The pooled ORR and (CRc) for all FLT3i were 53% (95% CI, 43%–63%) and 34% (95% CI, 26%–44%). Pooled ORR and CRc were 37% (95% CI, 25%–51%) and 35% (95% CI, 21%–52%) for type 1 and 58% (95% CI, 43%–71%) and 38% (95% CI, 27%–50%) for type 2, respectively. Gastrointestinal (GI) and hematological toxicity occurred in 22% (95% CI, 19%–25.4%) and 74.6% (95% CI, 70%–79%) with type 1 and 13.9% (95% CI, 12%–16%) and 57.7% (95% CI, 54.6%–60.8%) with type 2 FLT3i. QTc prolongation occurred in 2.06% (95% CI, 1.03%–3.65%) with type 1 and 7% (95% CI, 5.3%–9%) with type 2 FLT3i. Type 2 FLT3i had less GI toxicity but more QTc prolongation. Prospective studies are needed to compare the efficacy of type 1 and 2 FLT3i.
Keywords: FLT3 inhibitors, meta‐analysis, relapsed/refractory acute myeloid leukemia, type 1, type 2
1. INTRODUCTION
Next‐generation sequencing has revealed the molecular landscape and complex clonal evolution in acute myeloid leukemia (AML) and high‐risk myelodysplastic syndromes (HR‐MDS) [1]. Defining the molecular background of AML identified recurrent somatic events implicated in leukemogenesis and led to targeted treatment strategies and improved outcomes [2]. Mutations in the FMS‐like tyrosine kinase 3 (FLT3) gene are the most frequent somatic events in newly diagnosed AML patients [3]. FLT3 mutations either occur as the more common (∼30% of patients of de novo AML) internal tandem duplication (ITD), an in‐frame duplication, or the less frequent (∼5%–10% of de novo AML) tyrosine kinase domain (TKD) point mutation [4, 5, 6, 7]. FLT3‐ITD mutations confer adverse prognosis and are considered a driver lesion, especially at a higher allelic ratio [3, 8–10]. Approximately more than half of FLT3‐mutated de novo AML patients tend to harbor the FLT3 mutation at relapse [11]; however, the number at relapse is predicted to be less in the midostaurin era. The substantial presence of FLT3 mutation at relapse suggests that the FLT3‐ITD clone present at the initial diagnosis can undergo clonal expansion resulting in relapsed AML [11]. Considering the prevalence and poor prognosis of FLT3‐ITD mutated AML, targeting the FLT3 signaling pathway became a promising therapeutic approach.
There are two generations of FLT3 inhibitors currently in clinical practice: first‐ (e.g., sorafenib, midostaurin) and second‐generation FLT3 inhibitors (e.g., gilteritinib, quizartinib). First‐generation FLT3 inhibitors are multi‐kinase nonspecific inhibitors, whereas the second generation is more specific and potent FLT3 inhibitors [7]. FLT3 inhibitors are further divided into type 1 (e.g., midostaurin, gilteritinib) versus type 2 (e.g., sorafenib, quizartinib) depending on how they interact with the intracellular kinase domain of the FLT3 receptor (Figure S1). Type 1 binds to the FLT3 receptor in both active and inactive conformation and is active against both FLT3‐ITD and ‐TKD mutations. In comparison, type 2 binds to a hydrophobic region adjacent to the ATP‐binding pocket in the inactive conformation and targets only FLT3‐ITD mutation [7]. Currently, gilteritinib is the only FLT3 inhibitor approved by the Food and Drug Administration (FDA) for treating patients with FLT3‐mutated relapsed/refractory (R/R) AML. Although several FLT3 inhibitors have been studied to treat FLT3‐mutated R/R AML patients [12], there are no direct prospective comparative studies to analyze these different FLT3 inhibitors. Although the extant classification is based on the pharmacological properties and mechanism of action of the drugs, its usefulness in understanding their efficacy and toxicity profile is unclear.
Therefore, we conducted this meta‐analysis wherein we analyzed the efficacy of FLT3 inhibitors as monotherapy in patients with R/R AML and HR‐MDS. We will discuss FLT3 inhibitors individually and elucidate the efficacy of type 1 and type 2 FLT3 inhibitors.
2. METHODS
Standard systematic review methods were used and reported according to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines. The protocol was registered on PROSPERO CRD42021267536. PubMed and EMBASE databases were searched for clinical trials published between 1/1/2000 and 6/1/2021 using keywords and subject headings related to FLT3 inhibitors and AML. Two independent reviewers screened titles/abstracts and full texts, with a third reviewer resolving conflicts. Studies were included if (1) full‐length published journal articles that (2) reported the results of single‐ or double‐arm phase I/II/III clinical trials in patients with R/R AML or HR‐MDS were available. Outcomes of interest were composite response rate (CRc = complete response [CR] + complete response with incomplete count recovery) and overall response rate (ORR).
The heterogeneity test was performed using Cochran's Q test and I 2 values. The presence of heterogeneity was considered if either Cochran's Q test p‐value was <0.10 or I 2 values ≥ 50%. If the heterogeneity tests were significant, a random‐effect model was used. Consequently, a random‐effect model was used to calculate pooled estimates for all outcomes (CRc and ORR). The publication bias was evaluated using a funnel plot and Egger's linear regression test. If significant, the trim‐and‐fill method was used to estimate and adjust for the number and outcomes of missing studies in a meta‐analysis. Egger's linear regression test showed that all outcomes have publication bias, and the trim‐and‐fill method was applied to estimate the adjusted pooled estimates.
3. RESULTS
Database (EMBASE, PubMed) search identified 258 studies for screening. A total of 30 studies were included in the qualitative analysis (Figure 1). Two were excluded for quantitative analysis due to inadequate representation of the population of interest and outcomes assessed for intervention other than FLT3 inhibitors.
FIGURE 1.
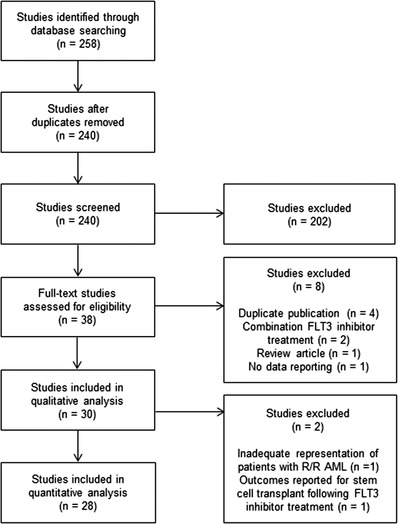
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines diagram. Database (EMBASE, PubMed) search identified 258 studies for screening. A total of 30 studies were included in the qualitative analysis. Two were excluded for quantitative analysis due to inadequate representation of the population of interest and outcomes assessed for intervention other than FLT3 inhibitors.
The study characteristics of all 28 studies involving 1927 patients are summarized in Table 1. The median age of patients included in this meta‐analysis was 62 years (range, 45–71 years). FLT3 inhibitors that were investigated in the included studies were crenolanib (one phase I/II study), EMND‐2076 (one phase I study), FLX925 (one phase I/II study), gilteritinib (one phase I, I/II, and III studies, respectively), lestaurtinib (one phase I/II study), linifanib (one phase I study), midostaurin (one phase I and phase II study, respectively), pacritinib (one phase I study), pexidartinib (one phase I/II study), ponatinib (one phase I study), quizartinib (one phase II study, three phase II studies, and one phase III study), semaxanib (two phase II studies), sorafenib (four phase I studies and one phase II study), sunitinib (one phase I study), and tandutinib (one phase I study).
TABLE 1.
Baseline characteristic of 28 clinical trials included in the meta‐analysis
| Author and year | Identifier | Drug | Study phase/study type | Patients (N)/(MDS) | Age median (range) | Gender (male) |
|---|---|---|---|---|---|---|
| Galanis et al. [13] | NCT01657682 | Crenolanib a | I–II/open label study | 65 | 61 (30–87) | N/A |
| Yee et al. [14] | N/A | EMND‐2076 b | I/open label, randomized | 16 | 69 (43–84) | 20 |
| Daver et al. [15] | NCT02335814 | FLX925 a | I–II/open label study | 51 | 56 (18–71) | 21 |
| Perl et al. [16] | NCT02014558 | Gilteritinib a | I–III/open‐label | 249 | 63 (47–71) | 129 |
| Usuki et al. [17] | NCT02181660 | Gilteritinib a | I/open label, randomized | 19 | 70 (60–81) | 15 |
| Perl et al. [18] | NCT02421939 | Gilteritinib a | III/randomized | 247 | 61.5 (19–85) | 116 |
| Smith et al. [19] | N/A | Lestaurtinib a | I–II/open label single arm | 17 | 56 (18–71) | 9 |
| Wang et al. [20] | N/A | Linifanib b | I/dose escalation | 26 | 54 (24–81) | 16 |
| Stone et al. [21] | N/A | Midostaurin a | I/single arm | 18 (1) | 62 (29–78) | 14 |
| Fischer et al. [22] | NCT00045942 | Midostaurin a | II/open label, randomized | 67 (10) | 62 (18–71) | 49 |
| Jeon et al. [23] | N/A | Pacritinib a | I/pilot | 6 | 56 (33–76) | 4 |
| Smith et al. [24] | NCT01349049 | Pexidartinib a | I–II/single arm open label | 90 | 58 (22–83) | 46 |
| Shah et al. [25] | N/A | Ponatinib b | I/single arm | 12 | 50 (30–72) | 7 |
| Cortes et al. [26] | NCT01565668 | Quizartinib b | II/single‐arm | 333 | 60 (39–73) | 170 |
| Cortes et al. [27] | NCT02039726 | Quizartinib b | III/randomized | 245 | 55 (46–65) | 113 |
| Cortes et al. [28] | NCT00462761 | Quizartinib b | I/randomized | 76 | 60 (23–86) | 46 |
| Cortes et al. [29] | NCT01565668 | Quizartinib b | II/randomized | 76 | 55 (19–77) | 44 |
| Usuki et al. [30] | NCT02675478 | Quizartinib b | I/dose escalation | 16 | 68 (33–91) | 9 |
| Takahash et al. [31] | NCT02984995 | Quizartinib b | II/single‐arm | 27 | 65 (31–81) | 15 |
| Giles et al. [32] | N/A | Semaxinib b | II/single‐arm | 55 (22) | 64 (23–76) | 39 |
| Fiedler et al. [33] | N/A | Semaxinib b | II/single arm | 25 | 65 (27–79) | 26 |
| Man et al. [34] | N/A | Sorafenib b | II/open label single arm | 13 | 45 (13–69) | 2 |
| Borthakur et al. [35] | NCT00217646 | Sorafenib b | I/randomized | 50 (1) | 60 (21–88) | 25 |
| Borthakur et al. [36] | NCT00943943 | Sorafenib b | I/single arm | 28 | 58 (18–85) | 12 |
| Pratz et al. [37] | N/A | Sorafenib b | I/dose escalation | 14 | 63 (37–50) | 8 |
| Crump et al. [38] | N/A | Sorafenib b | I/randomized | 32 (4) | 71 (37–82) | 32 |
| Fiedler et al. [39] | N/A | Sunitinib a | I/single arm | 16 | 64 (55–80) | 6 |
| DeAngelo et al. [40] | MLN518/CT53518 | Tandutinib b | I/single arm | 40 (1) | 70.5 (22–90) | 28 |
Type 1 FLT3 inhibitor.
Type 2 FLT3 inhibitor.
Abbreviations: MDS, myelodysplastic syndromes; N/A, not available.
The efficacy and safety results are described in the following sections.
3.1. Efficacy analysis
3.1.1. All FLT3 inhibitors combined (including type 1 and type 2)
The Cochran's Q test p‐value was less than 0.10 (p < 0.01), and I 2 value was more than 50% (I 2 = 87%), indicating the presence of heterogeneity (Figure 2, top‐left). Thus, random‐effects models were used. Asymmetry test performed using Egger's linear regression test (Figure 2, top‐right) suggested publication bias (p = 0.001; Figure 2, top‐right). The trim‐and‐fill method used to adjust publication bias showed the pooled ORRs to be 53% (95% CI, 43%–63%) (Figure 2, bottom).
FIGURE 2.
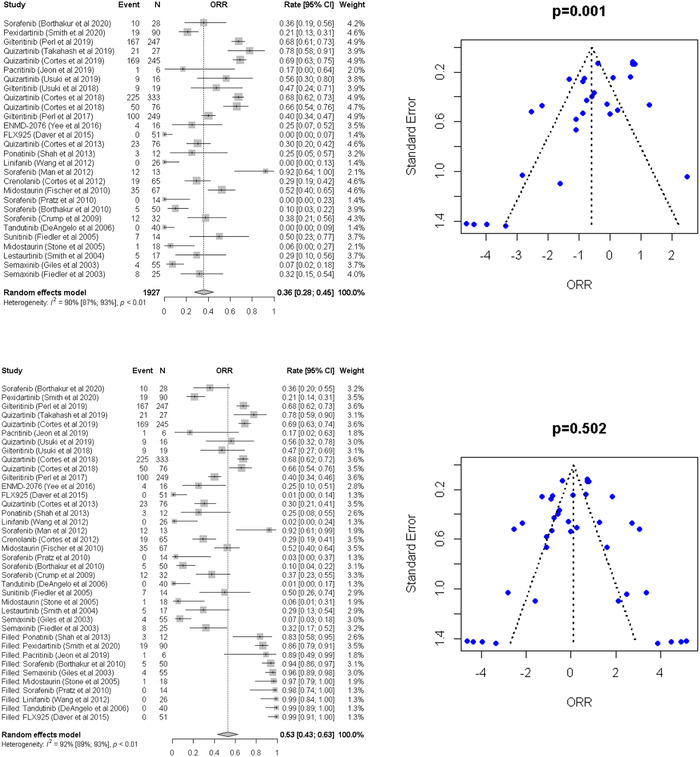
Top: Forest plot of the meta‐analysis and funnel plot for publication bias for overall response rate (ORR). In the funnel plot, the dotted line represents a triangular 95% confidence region and the pooled estimate based on a random‐effect meta‐analysis. Bottom: Forest plot of the meta‐analysis and funnel plot for publication bias for overall response rate (ORR) after adjusting publication bias by the trim‐and‐fill method. In the funnel plot, the dotted line represents a triangular 95% confidence region and the pooled estimate based on a random‐effect meta‐analysis.
The Cochran's Q test p‐value was less than 0.10 (p < 0.01), and I 2 value was more than 50% (I 2 = 87%), indicating the presence of heterogeneity (Figure 3, top‐left). Thus, random‐effects models were used. Asymmetry test performed using Egger's linear regression test (Figure 3, top‐right) suggested publication bias (p < 0.001; Figure 3, top‐right). The trim‐and‐fill method used to adjust publication bias showed the pooled composite response rates (CRc) was 34% (95% CI, 26%–44%) (Figure 3, bottom).
FIGURE 3.
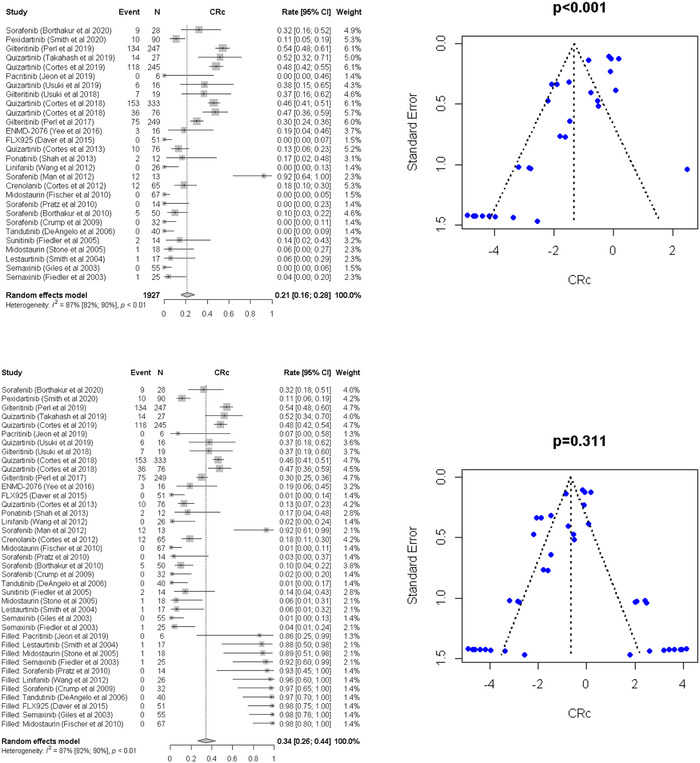
Top: Forest plot of the meta‐analysis and funnel plot for publication bias for overall response rate (ORR) after adjusting publication bias by the trim‐and‐fill method. In the funnel plot, the dotted line represents a triangular 95% confidence region and the pooled estimate based on a random‐effect meta‐analysis. Bottom: Forest plot of the meta‐analysis and funnel plot for publication bias for complete response rate (CRR) after adjusting publication bias by the trim‐and‐fill method. In the funnel plot, the dotted line represents a triangular 95% confidence region and the pooled estimate based on a random‐effect meta‐analysis.
3.1.2. Individual FLT3 inhibitors
The pooled estimates of ORR and CRc of individual FLT3 inhibitors are summarized in Table 2. The pooled response rates were estimated by random effect models when the number of studies was two or more. Quizartinib, sorafenib, and gilteritinib were the most frequently evaluated FLT3 inhibitors, with 6, 5, and 3 studies included in our analysis comprising 773, 137, and 515 patients, respectively.
TABLE 2.
Summary of pooled estimates by type of medication
| Pooled rate (95% CI) a | ||||
|---|---|---|---|---|
| Drug | No. of studies | No. of patients | ORR | CRc |
| Quizartinib | 6 | 773 | 0.61 (0.49,0.72) | 0.40 (0.31,0.51) |
| Gilteritinib | 3 | 515 | 0.52 (0.31,0.73) | 0.40 (0.23,0.60) |
| Sorafenib | 5 | 137 | 0.31 (0.12,0.60) | 0.20 (0.04,0.56) |
| Pexidartinib | 1 | 90 | 0.21 (0.14,0.31) | 0.11 (0.06,0.19) |
| Midostaurin | 2 | 85 | 0.23 (0.02,0.84) | 0.03 (0.00,0.16) |
| Semaxinib | 2 | 80 | 0.16 (0.03,0.53) | 0.02 (0.00,0.11) |
| Crenolanib | 1 | 65 | 0.29 (0.19,0.41) | 0.18 (0.11,0.30) |
| FLX925 | 1 | 51 | 0.01 (0.00,0.14) | 0.01 (0.00,0.14) |
| Tandutinib | 1 | 40 | 0.01 (0.00,0.17) | 0.01 (0.00,0.17) |
| Linifanib | 1 | 26 | 0.02 (0.00,0.24) | 0.02 (0.00,0.24) |
| Lestaurtinib | 1 | 17 | 0.29 (0.13,0.54) | 0.06 (0.01,0.32) |
| ENMD‐2076 | 1 | 16 | 0.25 (0.10,0.51) | 0.19 (0.06,0.45) |
| Sunitinib | 1 | 14 | 0.50 (0.26,0.74) | 0.14 (0.04,0.43) |
| Ponatinib | 1 | 12 | 0.25 (0.08,0.55) | 0.17 (0.04,0.48) |
| Pacritinib | 1 | 6 | 0.17 (0.02,0.63) | 0.07 (0.00,0.58) |
Pooled rates were estimated by random effects models when the number of studies is 2 or more.
Abbreviations: CI, confidence interval; CRc, complete response rate; ORR, overall response rate.
For quizartinib, sorafenib and gilteritinib, the pooled ORR were 61% (95% CI 49%–72%), 31% (95% CI 12%–60%) and 52% (95% CI 31%–73%), whereas the pooled composite response rates (CRc) were 40% (95% CI 31%–51%), 20% (95% CI 4%–56%), and 40% (95% CI 23%–60%), respectively.
3.1.3. Type 1 versus type 2 FLT3 inhibitors
Cochran's Q test p‐values were less than 0.10 (p < 0.01 for both types 1 and 2), and I 2 values were more than 50% (I 2 = 88% for type 1 and 92% for type 2), indicating the presence of heterogeneity. Using Egger's linear regression test, asymmetry tests were performed, suggesting publication bias for type 2 (p = 0.005). After adjusting for publication bias for type 2 using the trim‐and‐fill method, the pooled ORR was calculated to be 37% (95% CI, 25%–51%) for type 1 and 58% (95% CI, 43%–71%) for type 2 (p = 0.258) FLT3 inhibitors. Similarly, pooled CRc was 35% (95% CI, 21%–52%) for type 1 and 38% (95% CI, 27%–50%) for type 2 (p = 0.460) FLT3 inhibitors (Figure S2).
Three prechosen categorical variables (number of prior lines, history of stem cell transplant, and prior use of FLT3 inhibitor) were evaluated for sources of heterogeneity using univariable meta‐regression models before and after correction of publication bias by a trim‐and‐fill method. Before the correction of publication bias, we observed that there were significant associations between the number of prior lines and ORR (p = 0.016), not for the history of stem cell transplant (p = 0.729) and the prior use of FLT3 inhibitor (p = 0.910), and between the history of stem cell transplant and CRc (p = 0.001), not for the number of prior lines (p = 0.374) and the prior use of FLT3 inhibitor (p = 0.327). However, after correction of the publication bias, ORR and CRc were not significantly associated with the number of prior lines, the history of stem cell transplant, and the prior use of FLT3 inhibitor (p = 0.743, 0.447, and 0.776 for ORR; p = 0.362, 0.801, and 0.558 for CRc, respectively).
3.2. Adverse events
Most common adverse events with all FLT3 inhibitors were gastrointestinal (GI) (16.9%; 95% CI, 15.2%–18.6%), hematologic (62.3%; 95% CI, 59.7%–64.9%), and cardiac toxicity, specifically QTc prolongation (4.9%; 95% CI, 3.8%–6.3%) (Table 3). GI toxicity occurred in 22.1% (95% CI, 19%–25.4%) with type 1 and 13.9% (95% CI, 12%–16%) with type 2 inhibitors. Hematologic toxicity was also higher with type 1 than type 2, as it occurred in 74.6% (95% CI, 70%–79%) with type 1 and 57.7% (95% CI, 54.6%–60.8%) with type 2, however; QTc prolongation occurred only in 2% (95% CI, 1%–3.6%) with type 1 and 7% (95% CI, 5.3%–9%) with type 2. The type 2 FLT3 inhibitors that were more associated with prolonged QTc were quizartinib (7.1%; 95% CI, 5.3%–9.2%) and ENMD‐2076 (3.7%; 95% CI, 0.1%–19%). Similarly, the type 1 inhibitors that caused QTc prolongation were gilteritinib (1.9%; 95% CI, 0.9%–3.5%) and pacritinib (7.7%; 95% CI, 0.20%–36%).
TABLE 3.
Major adverse events with FLT3 inhibitors
| Toxicity | All FLT3 inhibitors | Type 1 inhibitors | Type 2 inhibitors |
|---|---|---|---|
| GI toxicity | 16.9% (95% CI, 15.2%–18.6%) | 22.1% (95% CI, 19%–25.4%) | 13.90% (95% CI, 12%–1%) |
| Hematologic toxicity | 62.3% (95% CI, 59.72%–64.94%) | 74.6% (95% CI, 70%–79.%) | 57.7% (95% CI, 54.6%–60.8%) |
| Cardiac toxicity (QTc prolongation) | 4.9% (95% CI, 3.8%–6.3%) | 2% (95% CI, 1%–3.6%) | 7% (95% CI, 5.3%–9%) |
Abbreviation: GI, gastrointestinal.
4. DISCUSSION
FLT3 mutation and preferential targeting in AML have changed the therapeutic armamentarium in the management of AML. Approximately half of the patients have persistence of FLT3 mutated clone at the time of relapse, which makes targeting FLT3 mutation of prime importance in patients with R/R AML. Although gilteritinib (type 1 FLT3 inhibitor) is the only FDA‐approved FLT3 inhibitor to treat patients with FLT3‐mutated R/R AML, a perusal of the literature showed no direct comparisons among FLT3 inhibitors in the treatment of patients with R/R AML. FLT3 inhibitors differ in their mechanism of action depending on their target site on the FLT3 receptor. Whether type 1 FLT3 inhibitors are more effective than type 2 because of relatively broader activity is still yet to be answered. This systematic review and meta‐analyses attempted to understand the differences among different FLT3 inhibitors in general and between type 1 and type 2 FLT3 inhibitors in treating R/R AML and HR‐MDS patients. With the availability of numerous FLT3 inhibitors, what may still be unanswered is the order of sequence in using type 1, and 2 FLT3 inhibitors in de novo and R/R FLT3ITD‐mutated AML.
FLT3 inhibitors were shown to be safe and effective during induction, re‐induction, and post‐allogeneic stem cell transplant in patients with untreated FLT3‐mutated AML in prior systematic reviews and meta‐analyses [41, 42]. However, there are no studies evaluating the safety and efficacy of FLT3 inhibitors as monotherapy in R/R AML. Historically, patients with FLT3‐mutated R/R AML have ORR and CRc rates of 26% and 22% with salvage chemotherapy, respectively [18]. In this study, the pooled ORR and CRc rates in patients treated with FLT3 inhibitors were 53% (95% CI, 43%–63%) and 34% (95% CI, 26%–44%), respectively. Although we acknowledge that most of the studies included in our meta‐analyses were single‐arm/open‐label trials and the limitations of cross‐trial comparisons, the pooled response rates observed in our study suggest that FLT3 inhibitors are quite effective in the treatment of patients with R/R AML who are likely predicted to be chemo‐refractory. Also, it is essential to note that some of the patients included in our meta‐analyses were heavily pre‐treated before their treatment with FLT3 inhibitor.
Type 1 and 2 FLT3 inhibitors differ in their target sites on the FLT3 receptor and mechanisms of resistance. Alotaibi et al. showed that the most common emergent mutations in patients treated with type 1 FLT3 inhibitors were in the RAS/MAPK pathway, whereas FLT3‐D835, IDH1/IDH2, and TP53 were the common emergent escape mutations in patients treated with type 2 FLT3 inhibitors [43]. The latter suggests that type 1 and 2 FLT3 inhibitors have distinct biological implications, which may translate into different efficacies and toxicities. Although the current classification of FLT3 inhibitors based on the pharmacological properties is relevant in understanding their mechanisms of action, toxicity, and the differential emergent patterns of resistance, as well as drug selection based on FLT3‐ITD versus TKD mutation, translating this into clinical efficacy may not be as straightforward. There is significant heterogeneity in properties such as potency, selectivity, and protein binding within the types of FLT3 inhibitors; therefore, a knowledge of the individual drugs is still vital to appropriate drug selection and sequencing them for the patients.
Our analyses showed that the pooled response rates were not significantly different between type 1 and 2 inhibitors. However, there was a trend toward a higher pooled ORR in patients treated with type 2 FLT3 inhibitors (58% vs. 37%). When we look at the individual drugs, appreciating the caveat that cross‐trial comparisons are impractical to derive any definitive conclusions, the suggestion of a numerically higher ORR with type 2 inhibitors becomes less generalizable. Gilteritinib, a type I inhibitor, has a considerable ORR of 52% (95% CI 31%–75%), only behind 61% (95 CI 49%–72%) ORR of quizartinib, which is a type 2 inhibitor. A consistent pattern of efficacy based on the type of inhibitors also fails to emerge when looking at the other drugs, indicating that the numerically higher pooled ORR of type 2 inhibitors is likely driven by quizartinib by virtue of its largest sample size in our study (773 patients from 6 studies). This also underscores that the optimum choice of FLT3 inhibitor in de novo and R/R setting based on emergent resistance mutations and different biological implications can only be answered in a prospective study.
The toxicity profile was variable between type 1 and 2 FLT3 inhibitors. Hematological toxicities were more common in type 1 FLT3 inhibitors (74.6% vs. 57.7%), and QTc were more frequent in type 2 FLT3 inhibitors (7% vs. 2%). This is key information to note as there are ongoing studies evaluating the safety and combination of FLT3 inhibitors with the standard of care regimens such as venetoclax, hypomethylating agent (NCT04140487, NCT04687761), and intensive chemotherapy (NCT04047641, NCT02668653, NCT04027309).
Several limitations in our study should be mentioned. First, we did not include survival estimates in the outcomes assessment as some included studies were early‐phase clinical trials. Also, depending on the time periods of the included studies, the frontline treatment would have been different in our study patients, which would impact the outcomes at the time of relapse. For example, midostaurin was approved by the FDA only in 2017. Some studies included in our systematic review (18/28) were conducted before midostaurin approval. Second, our study did not include FLT3 inhibitors evaluated as combination therapies in patients with R/R AML. We excluded such studies as the current standard of care for patients with FLT3‐mutated R/R AML is gilteritinib monotherapy, and we wanted to deduce the actual efficacy of the FLT3 inhibitor per se. However, it is vital to note that the LACEWING trial, which showed no significant survival benefit of adding azacitidine to gilteritinib, had more than a modicum of benefit with the combination, especially CRc [44]. This highlights the importance of evaluating the efficacy of different FLT3 inhibitors in combination with chemotherapy in treating patients with FLT3‐mutated R/R AML.
In conclusion, our study showed there was a trend to a nonsignificantly higher ORR in patients treated with type 2 FLT3 inhibitors as monotherapy in the treatment of R/R AML, which is broadly an effect of a large sample size rather. However, this will still be an important yet unanswered question about the clinical difference between the two biological classes of FLT3 inhibitors. This underscores the unmet need for prospective studies to compare the efficacies of type 1 and type 2 FLT3 inhibitors in treating patients with FLT3‐mutated AML. Also, with the availability of several FLT3 inhibitors, randomized trials are needed to dissect the choices regarding the class of FLT3 inhibitors to be used in the de novo and R/R AML setting.
AUTHOR CONTRIBUTIONS
Mahesh Swaminathan was involved with study design, curating data, methodology, and writing—original draft. Mai M. Aly involved with study design, curating data, and methodology.
Abdul Moiz Khan was involved in curating data and editing manuscript. Bayan Al Share, Vikram Dhillon, Enxhi lalo and Harry Ramos were involved in curating data, and analysis. Katherine G. Akers involved in study design, extraction of data from scientific databases and creating PRISMA flow diagram. Seongho Kim involved with the study design and statistical analysis. Suresh Balasubramanian involved with study design, methodology, analysis, overall conduct, and manuscript editing.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
FUNDING INFORMATION
The authors received no specific funding for this work.
ETHICS STATEMENT
None.
Supporting information
FiguresS1‐S2ACKNOWLEDGEMENT
ACKNOWLEDGMENTS
BioRender.com was used to create Supplemental Figure 1.
Swaminathan M, Aly MM, Khan AM, Share BA, Dhillon V, Lalo E, et al. Efficacy analysis of different FLT3 inhibitors in patients with relapsed/refractory acute myeloid leukemia and high‐risk myelodysplastic syndrome. eJHaem. 2023;4:165–173. 10.1002/jha2.616
REFERENCES
- 1. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–52. [DOI] [PubMed] [Google Scholar]
- 2. Walter MJ, Payton JE, Ries RE, Shannon WD, Deshmukh H, Zhao Y, et al. Acquired copy number alterations in adult acute myeloid leukemia genomes. Proc Natl Acad Sci USA. 2009;106(31):12950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kottaridis PD, Gale RE, Linch DC. FLT3 mutations and leukaemia. Br J Haematol. 2003;122(4):523–38. [DOI] [PubMed] [Google Scholar]
- 5. Nagel G, Weber D, Fromm E, Erhardt S, Lübbert M, Fiedler W, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population‐based registry study (AMLSG BiO). Ann Hematol. 2017;96(12):1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swaminathan M, Kantarjian HM, Levis M, Guerra V, Borthakur G, Alvarado Y, et al. A phase I/II study of the combination of quizartinib with azacitidine or low‐dose cytarabine for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome. Haematologica. 2021;106(8):2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daver N, Garcia‐Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open‐label, phase II study. Cancer Discov. 2019;9(3):370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hackanson B, Bennett KL, Brena RM, Jiang J, Claus R, Chen S‐S, et al. Epigenetic modification of CCAAT/enhancer binding protein α expression in acute myeloid leukemia. Cancer Res. 2008;68(9):3142–51. [DOI] [PubMed] [Google Scholar]
- 9. Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3‐activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis: presented in part at the 42nd Annual Meeting of the American Society of Hematology, December 1–5, 2000, San Francisco, CA (abstract 2334). Blood: J Am Soc Hematol. 2002;99(12):4326–35. [DOI] [PubMed] [Google Scholar]
- 10. Metzeler KH, Herold T, Rothenberg‐Thurley M, Amler S, Sauerland MC, Görlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood: J Am Soc Hematol. 2016;128(5):686–98. [DOI] [PubMed] [Google Scholar]
- 11. Krönke J, Bullinger L, Teleanu V, Tschürtz F, Gaidzik VI, Kühn MW, et al. Clonal evolution in relapsed NPM1‐mutated acute myeloid leukemia. Blood: J Am Soc Hematol. 2013;122(1):100–8. [DOI] [PubMed] [Google Scholar]
- 12. Wu M, Li C, Zhu X. FLT3 inhibitors in acute myeloid leukemia. J Hematol Oncol. 2018;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galanis A, Ma H, Rajkhowa T, Ramachandran A, Small D, Cortes J, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance‐conferring point mutants. Blood: J Am Soc Hematol. 2014;123(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yee KW, Chen H‐WT, Hedley DW, Chow S, Brandwein J, Schuh AC, et al. A phase I trial of the aurora kinase inhibitor, ENMD‐2076, in patients with relapsed or refractory acute myeloid leukemia or chronic myelomonocytic leukemia. Invest New Drugs. 2016;34(5):614–24. [DOI] [PubMed] [Google Scholar]
- 15. Daver NG, Konopleva M, Kohrt HE, Fridman JS, Johnson D, Jaen JC, et al. First‐in‐human study of FLX925, an orally administered FLT3/CDK4/CDK6 inhibitor, in subjects with relapsed or refractory acute myeloid leukemia (AML). Alexandria, Virginia: American Society of Clinical Oncology; 2015. [Google Scholar]
- 16. Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first‐in‐human, open‐label, phase 1–2 study. Lancet Oncol. 2017;18(8):1061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Usuki K, Sakura T, Kobayashi Y, Miyamoto T, Iida H, Morita S, et al. Clinical profile of gilteritinib in Japanese patients with relapsed/refractory acute myeloid leukemia: an open‐label phase 1 study. Cancer Sci. 2018;109(10):3235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3‐mutated AML. N Engl J Med. 2019;381(18):1728–40. [DOI] [PubMed] [Google Scholar]
- 19. Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, et al. Single‐agent CEP‐701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–76. [DOI] [PubMed] [Google Scholar]
- 20. Wang ES, Yee K, Koh LP, Hogge D, Enschede S, Carlson DM, et al. Phase 1 trial of linifanib (ABT‐869) in patients with refractory or relapsed acute myeloid leukemia. Leuk Lymphoma. 2012;53(8):1543–51. [DOI] [PubMed] [Google Scholar]
- 21. Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small‐molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. [DOI] [PubMed] [Google Scholar]
- 22. Fischer T, Stone RM, DeAngelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS‐like tyrosine kinase 3 receptor (FLT3) and multi‐targeted kinase inhibitor, in patients with acute myeloid leukemia and high‐risk myelodysplastic syndrome with either wild‐type or mutated FLT3. J Clin Oncol. 2010;28(28):4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeon JY, Zhao Q, Buelow DR, Phelps M, Walker AR, Mims AS, et al. Preclinical activity and a pilot phase I study of pacritinib, an oral JAK2/FLT3 inhibitor, and chemotherapy in FLT3‐ITD‐positive AML. Invest New Drugs. 2020;38(2):340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith CC, Levis MJ, Frankfurt O, Pagel JM, Roboz GJ, Stone RM, et al. A phase 1/2 study of the oral FLT3 inhibitor pexidartinib in relapsed/refractory FLT3‐ITD–mutant acute myeloid leukemia. Blood Adv. 2020;4(8):1711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah NP, Talpaz M, Deininger MW, Mauro MJ, Flinn IW, Bixby D, et al. Ponatinib in patients with refractory acute myeloid leukaemia: findings from a phase 1 study. Br J Haematol. 2013;162(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cortes J, Perl AE, Döhner H, Kantarjian H, Martinelli G, Kovacsovics T, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open‐label, multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2018;19(7):889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3‐ITD acute myeloid leukaemia (QuANTUM‐R): a multicentre, randomised, controlled, open‐label, phase 3 trial. Lancet Oncol. 2019;20(7):984–97. [DOI] [PubMed] [Google Scholar]
- 28. Cortes JE, Kantarjian H, Foran JM, Ghirdaladze D, Zodelava M, Borthakur G, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS‐like tyrosine kinase 3–internal tandem duplication status. J Clin Oncol. 2013;31(29):3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cortes JE, Tallman MS, Schiller GJ, Trone D, Gammon G, Goldberg SL, et al. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3‐ITD–mutated, relapsed or refractory AML. Blood Journal Am Soc Hematol. 2018;132(6):598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Usuki K, Handa H, Choi I, Yamauchi T, Iida H, Hata T, et al. Safety and pharmacokinetics of quizartinib in Japanese patients with relapsed or refractory acute myeloid leukemia in a phase 1 study. Int J Hematol. 2019;110(6):654–64. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi T, Usuki K, Matsue K, Ohno H, Sakura T, Imanaka R, et al. Efficacy and safety of quizartinib in Japanese patients with FLT3‐ITD positive relapsed or refractory acute myeloid leukemia in an open‐label, phase 2 study. Int J Hematol. 2019;110(6):665–74. [DOI] [PubMed] [Google Scholar]
- 32. Giles FJ, Stopeck AT, Silverman LR, Lancet JE, Cooper MA, Hannah AL, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood. 2003;102(3):795–801. [DOI] [PubMed] [Google Scholar]
- 33. Fiedler W, Mesters R, Tinnefeld H, Loges S, Staib P, Dührsen U, et al. A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood. 2003;102(8):2763–67. [DOI] [PubMed] [Google Scholar]
- 34. Man CH, Fung TK, Ho C, Han HH, Chow HC, Ma AC, et al. Sorafenib treatment of FLT3‐ITD+ acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood, The Journal of the American Society of Hematology. 2012;119(22):5133–43. [DOI] [PubMed] [Google Scholar]
- 35. Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borthakur G, Zeng Z, Cortes JE, Chen HC, Huang X, Konopleva M, et al. Phase 1 study of combinatorial sorafenib, G‐CSF, and plerixafor treatment in relapsed/refractory, FLT3‐ITD‐mutated acute myelogenous leukemia patients. Am J Hematol. 2020;95(11):1296–303. [DOI] [PubMed] [Google Scholar]
- 37. Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crump M, Hedley D, Kamel‐Reid S, Leber B, Wells R, Brandwein J, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk Lymphoma. 2010;51(2):252–60. [DOI] [PubMed] [Google Scholar]
- 39. Fiedler W, Serve H, Döhner H, Schwittay M, Ottmann OG, O'Farrell A‐M, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–93. [DOI] [PubMed] [Google Scholar]
- 40. DeAngelo DJ, Stone RM, Heaney ML, Nimer SD, Paquette RL, Klisovic RB, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high‐risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108(12):3674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Majothi S, Adams D, Loke J, Stevens S, Wheatley K, Wilson J. FLT3 inhibitors in acute myeloid leukaemia: assessment of clinical effectiveness, adverse events and future research—a systematic review and meta‐analysis. Syst Rev. 2020;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu Q, He S, Yu L. Clinical Benefits and Safety of FMS‐Like Tyrosine Kinase 3 Inhibitors in Various Treatment Stages of Acute Myeloid Leukemia: A Systematic Review, Meta‐Analysis, and Network Meta‐Analysis. Front Oncol. 2021;11:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alotaibi AS, Yilmaz M, Kanagal‐Shamanna R, Loghavi S, Kadia TM, DiNardo CD, et al. Patterns of resistance differ in patients with acute myeloid leukemia treated with type I versus type II FLT3 inhibitors. Blood Cancer Dis. 2021;2(2):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang ES, Montesinos P, Minden MD, Lee J‐H, Heuser M, Naoe T, et al. Phase 3, open‐label, randomized study of gilteritinib and azacitidine vs azacitidine for newly diagnosed FLT3‐mutated acute myeloid leukemia in patients ineligible for intensive induction chemotherapy. Blood. 2021;138:700. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FiguresS1‐S2ACKNOWLEDGEMENT


