Abstract
Objective:
To assess long-term outcomes of patients with advanced-stage ovarian cancer by treatment type.
Methods:
Patients with newly diagnosed stage III-IV ovarian cancer who underwent primary treatment at our tertiary cancer center from 01/01/2015–12/31/2015 were included. We reviewed electronic medical records for clinicopathological, treatment, and survival characteristics.
Results:
Of 153 patients, 88 (58%) had stage III and 65 (42%) stage IV disease. Median follow-up was 65.8 months (range, 3.6–75.3). Eighty-nine patients (58%) underwent primary debulking surgery (PDS), 50 (33%) received neoadjuvant chemotherapy followed by interval debulking surgery (IDS), and 14 (9%) received chemotherapy alone, without surgery (NSx). Median PFS to first recurrence was 26.2 months (range, 20.1–36.2), 13.5 months (range, 12–15.1), and 4.2 months (range, 1.1–5.8) in the PDS, IDS, and NSx groups, respectively (P<.001). At first recurrence/progression, 80 patients (72.7%) were treated with chemotherapy, 28 (25.5%) underwent secondary cytoreductive surgery (CRS) followed by chemotherapy, and 2 (1.8%) received no treatment. Seven patients (4.6%) underwent palliative surgery for malignant bowel obstruction. Overall, 62.7% received 1–3 lines of chemotherapy. The 5-year OS rates were 53.2% (95% CI: 44.7%–61%) for the entire cohort, 71.5% (95% CI: 60.2%–80%) for the PDS group, 35.2% (95% CI: 22.2–48.5%) for the IDS group, and 7.9% (95% CI: 0.5%–29.9%) for the NSx group.
Conclusion:
The longitudinal treatment modalities and outcomes of patients with advanced ovarian cancer described here can be useful for patient counseling, long-term planning, and future comparison studies.
Keywords: ovarian cancer, recurrent ovarian cancer, cytoreductive surgery, treatment lines, survival data
Introduction:
Ovarian cancer is the seventh most common cancer in women worldwide1. At the time of diagnosis, 80% of patients present with advanced-stage disease (stage III-IV)2, with a 5-year survival rate of less than 45%1. Among women 40–79 years of age, ovarian cancer is the fourth leading cause of cancer death3.
Surgery is a mainstay in the comprehensive treatment of advanced ovarian cancer. Survival is greatest with no residual disease4,5. If a complete gross resection (CGR) is not feasible, neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS), with the goal of CGR, may provide the best survival advantage6. In the recurrent setting, surgery is preferred for patients with a long disease-free interval, demonstrated platinum sensitivity, and focal or limited extent of disease. For patients with disease recurrence or progression within 6 months, or who present with extensive disease, chemotherapy is the preferred treatment,7–9 even among patients undergoing tertiary cytoreduction10. Platinum combinations are the most effective treatment option for patients with platinum-sensitive disease.
Ovarian cancer treatment has evolved greatly over the past two decades, and targeted therapies such as vascular endothelial growth factor (VEGF) monoclonal antibodies and poly ADP-ribose polymerase inhibitors (PARPis) are changing the treatment landscape11–14. With a better understanding of the importance of patient selection and timing of surgery, as well as new treatment and maintenance options, survival outcomes have improved significantly. The aim of this study is to provide a detailed analysis of the treatment and long-term follow-up of patients with advanced-stage ovarian cancer who presented to a high-volume cancer center over a 1-year period.
Methods:
We performed a retrospective chart review of patients with advanced-stage ovarian cancer using our institution’s prospectively maintained and curated database, which includes all patients who present to the Gynecologic Oncology Service at Memorial Sloan Kettering Cancer Center (MSK). This study was approved by our Institutional Review Board. All patients with newly diagnosed, previously untreated 2014 International Federation of Gynecology and Obstetrics (FIGO) stage III-IV ovarian, fallopian tube, or primary peritoneal cancer who began initial treatment at MSK between 01/01/2015 and 12/31/2015 were included. Patients who were initially diagnosed at the end of 2014 and started treatment in 2015 were also included. Patients who underwent cytoreductive surgery (CRS) or received chemotherapy prior to presentation to our institution were excluded. Patients with early-stage (stage I-II) ovarian cancer or non-epithelial histology were also excluded.
Data were collected from the electronic medical record. Patient demographics, clinicopathological characteristics, and details of treatment lines including surgery, antineoplastic therapy, and survival data were compiled. Descriptive statistics were provided. Distributions between different treatment groups were tested using the Fisher exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. Progression-free survival (PFS) 1 and 2 were calculated. PFS1 was defined as the time of primary debulking surgery (PDS) or first cycle of chemotherapy to first recurrence, progression of disease (POD), death, or last follow-up, whichever occurred first. PFS2 was defined as the treatment date of either the first cycle of chemotherapy for first recurrence or date of secondary CRS for first recurrence to the second recurrence or POD, death, or last follow-up, whichever occurred first. Overall survival (OS) was defined as the date of PDS or first cycle of chemotherapy to death or last follow-up, whichever occurred first. The Kaplan-Meier method was applied to obtain the median survival time or the survival rate at a specific year. The log-rank test or Wald test based on the Cox proportional hazard model were used to obtain the P value in survival analyses. P values <.05 were considered statistically significant. All tests were 2-sided. All analyses were performed in R 3.6.3 (https://www.R-project.org/).
Results:
A total of 153 patients met the inclusion criteria during the study period. The median follow-up among survivors was 65.8 months (range, 3.6–75.3 months). The median age at diagnosis for the entire cohort was 65 years (range, 30–89 years). The median body mass index (BMI) was 25.3 kg/m2 (range, 16–65.4 kg/m2). The most common histology was high-grade serous, which was found in 126 patients (82.3%). Seventy (55.6%) of the 126 patients with high-grade serous histology had stage III and 56 (44.4%) had stage IV disease at diagnosis. Eighty-eight patients (58%) had stage III disease and 65 patients (42%) had stage IV disease. Twenty-six (21%) of the 122 patients tested had a germline or somatic deleterious BRCA 1/2 alteration. In our entire cohort, 20 patients (13.1%) had a BRCA 1 alteration, 6 (3.9%) had a BRCA2 alteration, 96 (62.7%) had wildtype, and 31 (20.3%) had unknown BRCA status (Table 1).
Table 1.
Clinicopathologic characteristics of the cohort.
| Clinicopathologic characteristic | No. of patients (%) (N=153) |
|---|---|
|
| |
| Age at diagnosis | |
| Median (range), years | 65 (30–89) |
| BMI at diagnosis | |
| Median (range), kg/m2 | 25.3 (16–65.4) |
| FIGO stage at diagnosis | |
| III | 88 (58) |
| IV | 65 (42) |
| BRCA mutation (germline and somatic) | |
| BRCA 1 | 20 (13.1) |
| BRCA 2 | 6 (3.9) |
| Wildtype | 96 (62.7) |
| Unknown | 31 (20.3) |
| Histology | |
| High-grade serous | 126 (82.3) |
| Low-grade serous | 4 (2.6) |
| Clear cell | 5 (3.3) |
| Mucinous | 2 (1.3) |
| Low-grade endometroid | 1 (0.7) |
| Carcinosarcoma | 6 (3.9) |
| Other* | 9 (5.9) |
Mixed histology, treated with cytology only, subtype not classified
BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics
Eighty-nine patients (58%) underwent PDS, 50 (33%) received NACT followed by IDS, and 14 (9%) did not undergo surgery and were treated with chemotherapy alone. NACT followed by IDS was initiated due to extent of disease in 37 patients (74%), pulmonary embolism at diagnosis in 6 patients (12%), poor performance status in 6 patients (12%), and a synchronous diagnosis of ovarian cancer and triple-negative breast cancer in 1 patient (2%) (Table 2). Among the 50 patients who received NACT, 14 (28%) received 3 cycles of NACT, 16 (32%) received 4 cycles, 3 (6%) received 5 cycles, 12 (24%) received 6 cycles, and 5 (10%) received 7 cycles. The median number of NACT cycles was 4 (range, 3–7 cycles). CGR was achieved in 68 (76.4%) of 89 patients who underwent PDS and in 33 (66%) of 50 patients who underwent IDS. In total, 38 patients had RD after PDS and IDS. Gross disease <1 cm was achieved in 18 (20.2%) of 89 patients who underwent PDS and 10 (20%) of 50 patients who underwent IDS. Following PDS and IDS, 3 (3.4%) of 89 patients and 7 (14%) of 50 patients were left with >1 cm RD (ie, suboptimal debulking), respectively (P=.069). The median time from surgery (PDS and IDS) to the start of chemotherapy was 36 days (range, 15–87 days). Five patients did not receive postoperative chemotherapy, and the exact chemotherapy start date was unknown for 14 patients. Postoperative intraperitoneal chemotherapy was administered to 55 patients (36%). No patients received hyperthermic intraperitoneal chemotherapy (HIPEC). In the upfront setting, 5 patients (3.3%) received the anti-angiogenic agent bevacizumab. PARPi/placebo as first-line maintenance therapy was given on a clinical trial to 5 patients (3.3%) (Table 2).
Table 2.
Treatment characteristics of the cohort.
| Treatment characteristic | No. of patients (%) |
|---|---|
|
| |
| Initial treatment (N=153) | |
| PDS | 89 (58) |
| IDS | 50 (33) |
| No surgery, chemotherapy alone | 14 (9) |
| Reason for neoadjuvant chemotherapy (N=50) | |
| Extent of disease | 37 (74) |
| Pulmonary embolism at diagnosis | 6 (12) |
| Poor performance status | 6 (12) |
| Synchronous diagnosis of ovarian and breast cancers | 1 (2) |
| Complete gross resection (CGR) (N=139) | |
| CGR for PDS (n=89) | 68 (76.4) |
| CGR for IDS (n=50) | 33 (66) |
| Residual disease (RD) (N=38) | |
| 1–10 mm RD after PDS | 18 (47.4) |
| 1–10 mm RD after IDS | 10 (26.3) |
| >10 mm RD after PDS | 3 (7.9) |
| >10 mm RD after IDS | 7 (18.4) |
| Time from surgery to chemotherapy | |
| Median time (range), days | 36 (15–87) |
| Intraperitoneal chemotherapy (N=153) | |
| Yes | 55 (36) |
| No | 95 (62.1) |
| Unknown | 3 (1.9) |
| Bevacizumab maintenance, 1st line (N=153) | |
| Yes | 5 (3.3) |
| No | 145 (94.8) |
| Unknown | 3 (1.9) |
| Investigational agent: PARPi/placebo, 1st line (N=153) | |
| Yes | 5 (3.3) |
| No | 145 (94.8) |
| Unknown | 3 (1.9) |
| First recurrence/POD (N=111 * ) | |
| Chemotherapy alone (n=110) | 80 (72.7) |
| No treatment (n=110) | 2 (1.8) |
| Secondary cytoreductive surgery (n=110) | 28 (25.5) |
| CGR (n=28) | 25 (89.3) |
| Any RD (n=28) | 3 (10.7) |
| Bevacizumab maintenance, 2nd line (N= 110) | |
| Yes | 22 (20) |
| No | 86 (78.2) |
| Unknown | 2 (1.8) |
| PARPi maintenance, 2nd line (N=110) | |
| Yes | 23 (20.9) |
| No | 85 (77.3) |
| Unknown | 2 (1.8) |
| Second recurrence/POD (N=84 ^ ) | |
| Chemotherapy alone | 77 (93.9) |
| Declined treatment | 1 (1.2) |
| Tertiary cytoreductive surgery | 4 (4.9) |
| CGR (n=4) | 4 (100) |
| Treatment lines (N=153) | |
| 0 | 1 (0.7) |
| 1 | 42 (27.5) |
| 2 | 26 (17.0) |
| 3 | 28 (18.3) |
| 4 | 15 (9.8) |
| 5 | 10 (6.5) |
| 6 | 14 (9.2) |
| 7 | 9 (5.9) |
| 8 | 3 (2.0) |
| 9 | 1 (0.7) |
| Unknown | 4 (2.6) |
| Palliative surgery for malignant bowel obstruction | 7 (4.6) |
One patient lost to follow-up
Two patients lost to follow-up
PDS, primary debulking surgery; IDS, internal debulking surgery; PARPi, poly ADP-ribose polymerase inhibitor; POD, progression of disease
Among the entire cohort, 35 patients remained disease-free at their last follow-up after upfront treatment alone, and 111 developed a recurrence or POD at the study lock date. Six patients died within 6 months of initial diagnosis. One patient was lost to follow-up. The median PFS1 was 26.2 months (95% CI: 20.1–36.2 months) for patients who underwent PDS, 13.5 months (95% CI: 12–15.1 months) for patients who received NACT followed by IDS, and 4.2 months (95% CI: 1.1–5.8 months) for patients who received chemotherapy only (P<.001) (Figure 1).
Figure 1:
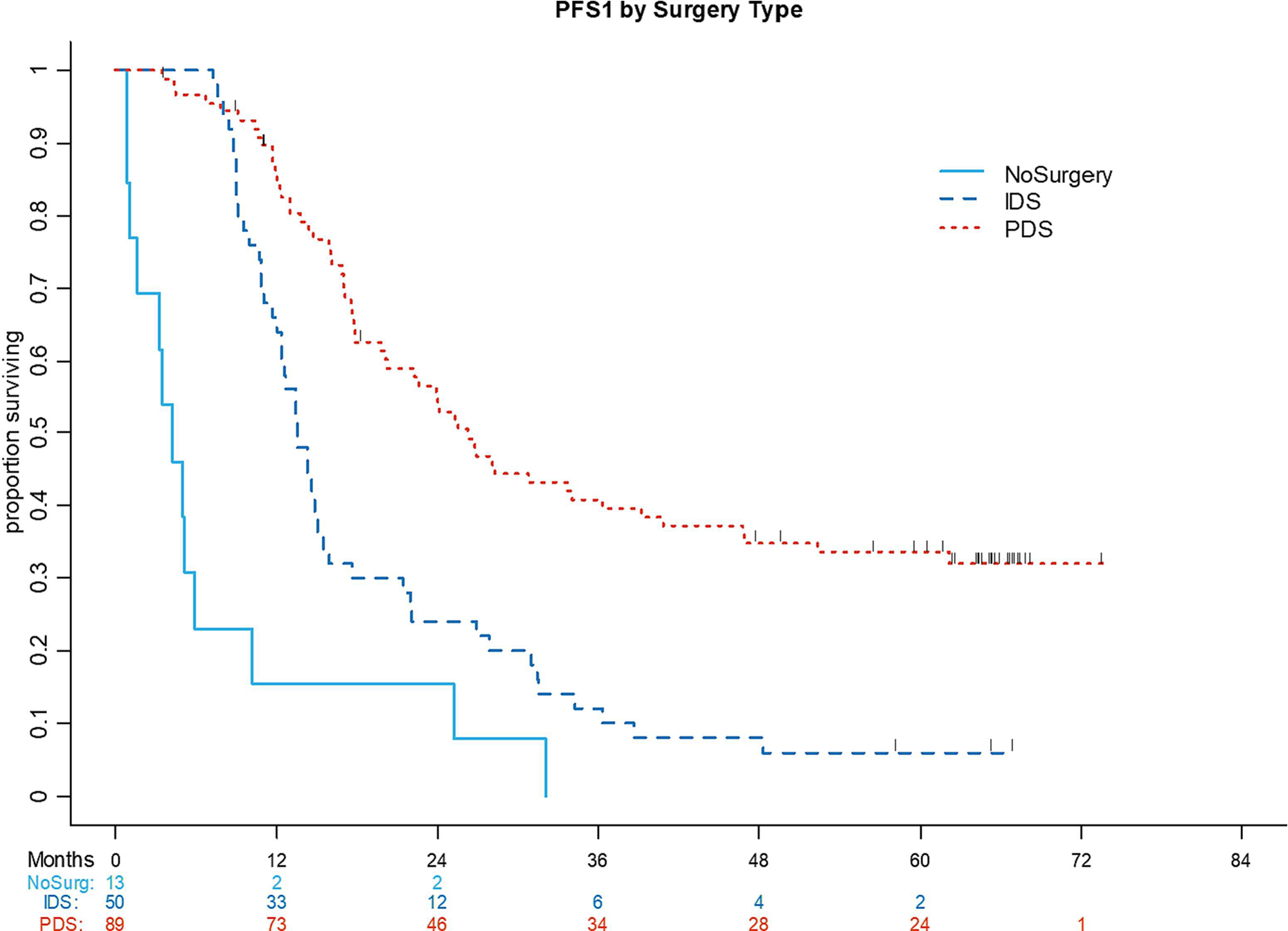
Progression-free survival (PFS) 1 by surgery type (primary debulking surgery, interval debulking surgery, no surgery).
IDS, interval debulking surgery; PDS, primary debulking surgery; NoSurg, no surgery
First recurrence/progression of disease:
Of the 111 patients with first recurrence or POD, 1 was lost to follow-up. At the time of first recurrence or POD, 80 (72.7%) of the 110 patients were treated with chemotherapy alone, 28 (25.5%) underwent secondary CRS followed by platinum-based chemotherapy, and 2 (1.8%) received no further treatment. Among the 28 patients who underwent secondary CRS, CGR was achieved in 25 (89.3%); 1 patient (3.6%) had RD <0.5 mm and 2 (7.1%) had RD >10 mm. As part of an ongoing trial at our institution during the study period, 6 (23.1%) of 26 patients underwent HIPEC at the time of secondary CRS. One patient had an isolated recurrence in the lung and underwent a video-assisted thoracic surgical resection followed by chemotherapy, and 1 patient was diagnosed with an isolated brain metastasis and underwent resection followed by radiotherapy and systemic treatment. The median number of days from secondary CRS to the start of chemotherapy was 32 (range, 16–78 days). All patients received a combination of platinum-based chemotherapy with gemcitabine or pegylated liposomal doxorubicin after secondary CRS; the median number of cycles was 6 (range, 3–8 cycles). One patient (3.6%) was treated with maintenance bevacizumab after secondary CRS and 8 patients (28.6%) received maintenance therapy with a PARPi after undergoing treatment with platinum-based chemotherapy. In total, 22 (20%) of 110 patients were treated with bevacizumab during their second-line treatment and 23 patients (20.9%) received PARPi maintenance therapy (Table 2).
Of the 111 patients with recurrence or POD after second-line treatment, 15 were disease-free at last follow-up and 84 experienced a second recurrence, persistence of disease, or POD and subsequently received third-line treatment. The median PFS2 for the first recurrence or POD cohort was 9.6 months (95% CI: 8.1–12.1 months). The median PFS2 by treatment group was 22.4 months (95% CI: 12.9–26.8 months) for patients who underwent secondary CRS followed by chemotherapy and 7.5 months (95% CI: 5.4–9.4 months) for patients treated with chemotherapy only (P<.001) (Figure 2).
Figure 2:
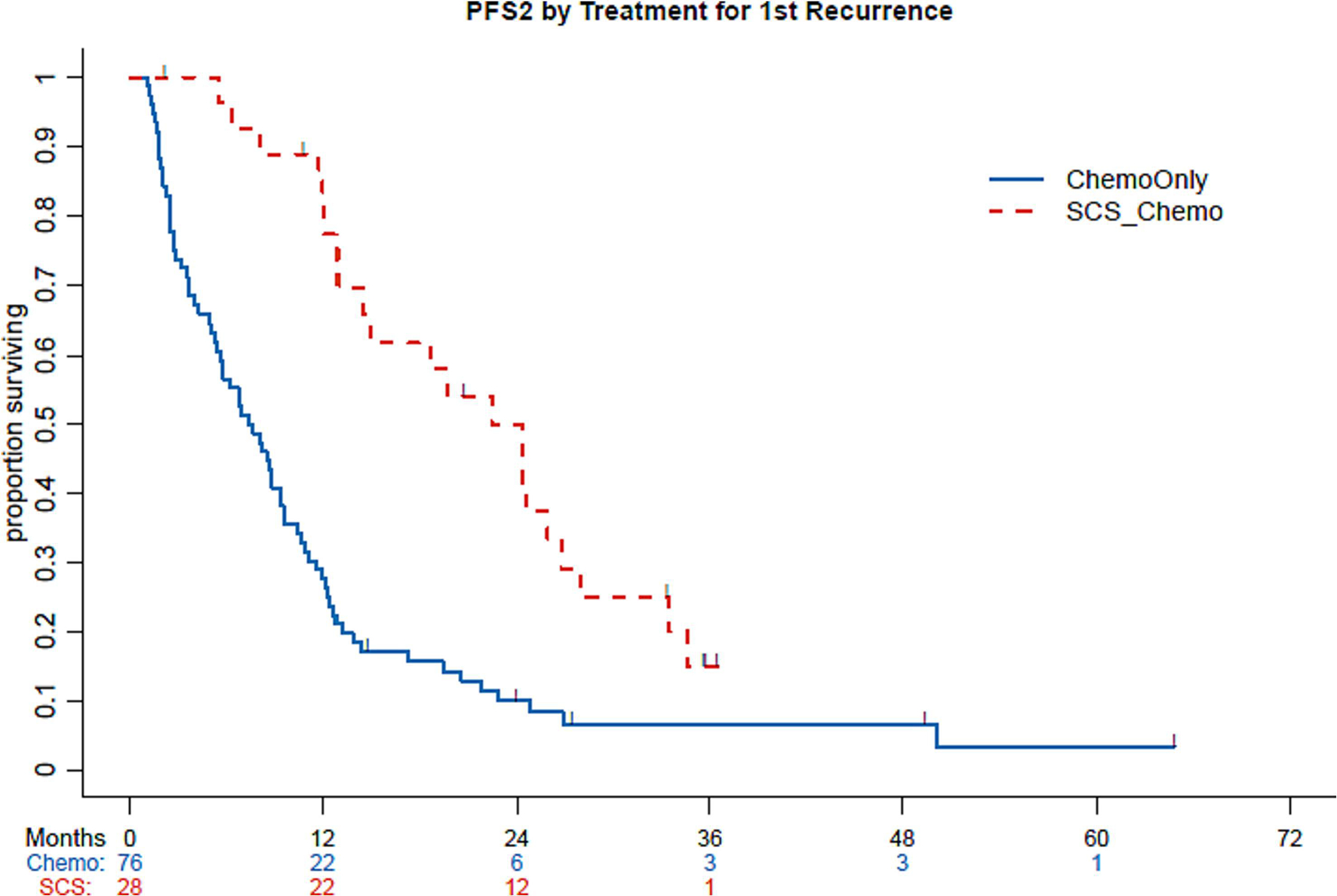
Progression-free survival (PFS) 2 by chemotherapy only and secondary cytoreductive surgery. Chemo, chemotherapy; SCS, secondary cytoreductive surgery
Second recurrence/progression of disease:
Of 84 patients with a second recurrence or POD, 2 were lost to follow-up. Of the 82 remaining patients, 1 (1.2%) declined further treatment, 77 (93.9%) were treated with chemotherapy only, and 4 (4.9%) underwent tertiary CRS. Among the 77 patients treated with chemotherapy alone, 34 (44.2%) received either a platinum-combination regimen or single-agent carboplatin, 34 (44.2%) received bevacizumab maintenance therapy, and 16 (20.8%) received PARPi maintenance therapy; 9 patients (11.6%) were treated on a clinical trial that involved an investigational agent, of whom 2 received immunotherapy.
CGR was achieved in all 4 patients who underwent tertiary CRS. No patients received HIPEC, and the median number of days from tertiary debulking to the start of chemotherapy was 44 (range, 41–46 days). Three of the 4 patients received 6 cycles of platinum-based combination; the fourth patient was treated with liposomal doxorubicin and recurred 5 months after surgery. All 4 patients received maintenance therapy; 2 (50%) received bevacizumab and 2 (50%) received a PARPi.
Third recurrence and beyond/progression of disease:
Fifty-three patients had a third recurrence or POD at the time of analysis. One patient was lost to follow-up. All 52 patients were treated with chemotherapy only and none underwent a quaternary debulking; 14 patients (26.9%) were treated with platinum-based chemotherapy, 21 (40.4%) received additional treatment with bevacizumab, and 3 (5.8%) were treated with a PARPi. Six patients (11.5%) were treated with an investigational agent on a clinical trial, of whom 4 received immunotherapy and 1 received PARPi/placebo.
Thirty-eight patients had a fourth recurrence or POD at the time of analysis. Six patients (15.8%) were treated with platinum-based chemotherapy. Twenty patients (52.6%) received bevacizumab, of whom 17 received non-platinum-based chemotherapy combined with bevacizumab maintenance therapy and 3 received a platinum-based combination with bevacizumab maintenance. One patient (2.6%) was treated on a clinical trial, and another elected for supportive care with home hospice.
Twenty-seven patients had a fifth recurrence or POD. Two patients (7.4%) received platinum-based chemotherapy. Seven patients (25.9%) received bevacizumab maintenance therapy combined with a non-platinum-based regimen. Three patients (11.1%) were treated on a clinical trial. No patients were treated with PARPi.
Thirteen patients had a sixth recurrence or POD, 2 (15.4%) of whom were treated with platinum-based combination chemotherapy. Four patients (30.8%) received bevacizumab maintenance therapy, of whom 2 had a platinum-based chemotherapy combination. No patients were treated with a PARPi.
Four patients had a seventh recurrence or POD, of whom 2 (50%) were treated with single-agent carboplatin. One patient (25%) was treated with post-platinum PARPi maintenance therapy. No patients received treatment with bevacizumab, and 1 (25%) was treated on a clinical trial.
One patient had an eighth recurrence and POD and received irinotecan.
Treatment lines and details:
One patient (0.7%) received no lines of chemotherapy, 42 (27.5%) received 1 line, 26 (17.0%) received 2 lines, 28 (18.3%) received 3 lines, 15 (9.8%) received 4 lines, 10 (6.5%) received 5 lines, 14 (9.2%) received 6 lines, 9 (5.9%) received 7 lines, 3 (2.0%) received 8 lines, 1 (0.7%) received 9 lines, and 4 (2.6%) received an unknown number of lines.
Seven patients (4.6%) underwent palliative surgery for malignant bowel obstruction (MBO). Among the 70 observed deaths in the cohort, 21 patients (30%) had a small bowel obstruction at the end of life. The small bowel obstruction resolved with conservative management in 7 patients (33.3%), and 14 patients (66.7%) required placement of a percutaneous endoscopic gastrostomy (PEG) drainage tube. After PEG placement, the median number of days until death were 32.5 (range, 7–211 days). The median number of lines of chemotherapy among the 70 patients who died was 3 (range, 0–9 lines).
In the entire cohort, 6 patients (3.9%) were diagnosed with brain metastasis, all of whom had high-grade histology, at some point during their disease course. Two (33.3%) of the 6 patients tested positive for a BRC1 mutation and 4 (66.7%) of 6 were BRCA wildtype. One patient (16.7%) underwent a resection of the brain metastasis followed by radiation therapy and systemic treatment, 1 (16.7%) underwent a resection and radiation treatment, 3 (50%) were treated with radiotherapy and chemotherapy, and 1 (16.7%) did not receive any therapy.
Overall survival outcomes:
The median OS was not reached in either the entire cohort or the PDS group. The 5-year OS rate was 53.2% (95% CI: 44.7%–61%) for the entire cohort, 71.5% (95% CI: 60.2%–80%) for the PDS group, 35.2% (95% CI: 22.2–48.5%) for the IDS group, and 7.9% (95% CI: 0.5%–29.9%) for the NSx group. Median OS was 41.7 months (95% CI: 26.7–57.6 months) for the IDS group and 14.6 months (95% CI: 1.1–27.8 months) for patients who had no surgery and chemotherapy only (P<.001) (Figure 3). The 5-year OS rate for patients with stage III disease was 60.1% (95% CI: 48.4%–69.9%) and 44.5% (95% CI: 32%–56.3%) for patients with stage IV disease (P=.081).
Figure 3:
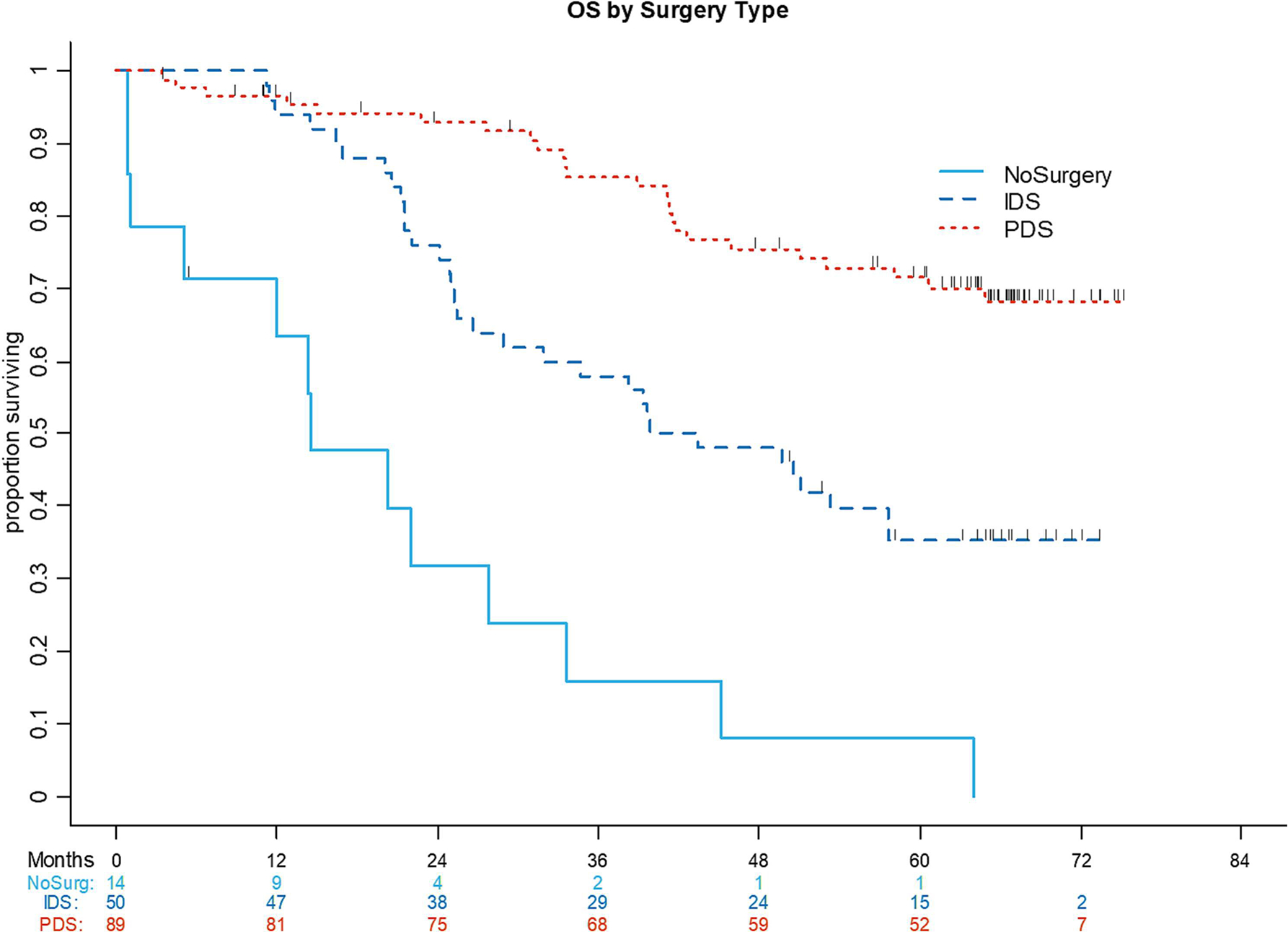
Overall survival (OS) by surgery type (primary debulking surgery, interval debulking surgery, no surgery).
IDS, interval debulking surgery; PDS, primary debulking surgery; NoSurg, no surgery
Discussion:
The disease course for patients diagnosed with ovarian cancer varies and depends on many factors. To our knowledge, this is the first comprehensive, longitudinal, descriptive analysis of treatment lines for patients with newly diagnosed advanced ovarian cancer from initial diagnosis to at least 5 years of follow-up or death.
In the AGO-TR-1 study, the prevalence of a germline deleterious BRCA 1/2 alteration was 20.8%15, which is almost identical to our finding of 21% among patients who underwent genetic testing. The prevalence of deleterious BRCA alterations underlines the importance of genetic testing to enable individualized treatment plans and cascade testing.
Not all patients with newly diagnosed advanced ovarian cancer are suitable candidates for PDS. Some patients with extensive disease require NACT to decrease the disease burden prior to resection, while others are too frail or have experienced a recent event (ie, venous thromboembolism [VTE]) precluding them from undergoing PDS. In our cohort, 12% of patients who received NACT were diagnosed with a pulmonary embolism and/or deep vein thrombosis at presentation, which is comparable to the rate of 13.8% previously reported16. Liu et al reported a median number of 4 cycles of NACT and showed that patients who received 5 or more cycles of NACT had worse OS and PFS than patients who received 3–4 cycles17. The aforementioned study, which was also performed at our institution, includes some patients in our cohort since the study periods overlapped for 6 months. In our study, the median number of NACT cycles before IDS was 4 (range, 3–7 cycles). In our cohort, CGR was achieved in 76.4% and 66% of patients undergoing PDS and IDS, respectively. In the primary setting, CGR is the most important factor for outcome4.
In our cohort, 111 of 153 patients developed recurrence or POD, which is comparable with the literature18. One-quarter of patients with first recurrence underwent secondary CRS in our cohort. Gockley et al described similar findings, with 23% of patients receiving secondary CRS and 77% receiving chemotherapy19. The randomized-controlled DESKTOP III trial reported that the median OS was longer for patients with platinum-sensitive recurrent ovarian cancer undergoing secondary CRS followed by chemotherapy than for patients receiving chemotherapy only (53.7 months versus 46.0 months, respectively). In this trial, patients undergoing secondary CRS resulting in CGR (median OS, 61.9 months) had the most favorable outcome20. In our cohort, the rate of CGR after secondary CRS was 89.3%. At our institution, the “MSKCC Team Ovary” criteria are used to select patients suitable for secondary CRS; these criteria include the disease-free interval (6–12 months, 13–30 months, and >30 months), sites of disease (single or multiple), and the presence or absence of carcinomatosis21. In the DESKTOP III trial, the CGR rate was 75.5%, and the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) score was used for patient selection20. Another randomized control trial (SOC-1) reported a CGR rate of 77%9.
Data on tertiary CRS in patients who recur after secondary CRS is limited. In our cohort, 4.9% of patients with a second recurrence of ovarian cancer underwent tertiary CRS, with a CGR rate of 100%. We previously published oncologic outcomes of patients undergoing tertiary CRS and noted a CGR rate of 89.5%22. Thus, patient selection is of the utmost importance because the oncologic benefit is greatest when CGR is achieved22,23.
Given the high rate of recurrence in ovarian cancer, patients commonly receive multiple lines of chemotherapy. Nearly 63% of patients in our cohort received 1, 2, or 3 lines of systemic chemotherapy at the time of last follow-up, while few received ≥7 lines. Hanker et al found that patients receiving third and fourth lines of chemotherapy have a 3.5-month PFS gain compared to patients not undergoing treatment. The study also reported an impact on OS for third-, fourth-, and fifth-line treatment. They concluded that 3 lines of chemotherapy in the recurrent setting are beneficial, but additional lines may not be helpful24. In the recurrent setting, the treatment drug used depends on the disease-free interval or the time period since last treatment. Platinum-based chemotherapy is favorable to treat patients with ovarian cancer and is used in different combinations until platinum-resistance develops25. In our cohort, platinum-based treatment was administered up to the seventh line of treatment.
The incidence of MBO in patients with ovarian cancer ranges from 20%–51%26. MBO in patients with recurrent ovarian cancer is typically caused by peritoneal carcinomatosis. Patients often present with a MBO at the end-stage of disease; Castro et al showed that the median survival from first episode of MBO to death is 95 days27. In our cohort, 4.6% of patients underwent palliative surgery for MBO. Among patients who died, 30% had a small bowel obstruction at the end of life and 66.7% required placement of a PEG drainage tube.
The incidence of brain metastasis in patients with ovarian cancer is estimated to be 1.01% (range, 0.49%–2.2%)28. In our cohort, 3.9% of patients were diagnosed with brain metastasis. The incidence of brain metastasis in patients with other solid tumor malignancies is much higher29 than in ovarian cancer. We previously reported that approximately 2.5% of patients with ovarian cancer develop brain metastasis, and that patients with a BRCA mutation were more likely to only have isolated brain metastasis30.
The 5-year survival for patients with stage III and IV ovarian cancer reported in the literature is 35%–42% and 20%–26%, respectively31,32. The 5-year OS rate in our cohort was longer at 60.1% for stage III and 44.5% for stage IV disease. Whether or not this difference is due to the demographics of the patients treated at our institution, the location of our institution, the types and lines of surgery and/or chemotherapy, or other factors cannot be determined by this retrospective review.
As mentioned above, not all patients are candidates to be treated with PDS. By comparing the outcome of the PDS and IDS groups, an unavoidable bias between the two groups must be taken under consideration.
Our results emphasize the importance for patients to receive treatment for ovarian cancer at a high-volume center. We recommend that patients be referred to a such center upon diagnosis to achieve the best survival outcome. Treatment and care at a tertiary cancer center assures accessibility to various treatment lines, provides patients the opportunity to participate in clinical trials, and ensures surgical management by highly experienced surgeons.
In conclusion, the 5-year OS for our cohort at an academic, high-volume tertiary care cancer center compares favorably to those described in the literature. Our data provide a comprehensive overview of patients treated at a cancer center with long-term follow-up and can be a useful reference for patient counseling, long-term treatment planning, and future comparison studies, especially as new targeted therapies emerge and become standard in primary therapy.
Highlights.
The 5-year OS rate in our cohort was favorably longer compared to what is described in the literature.
The majority of patients (62.7%) received 1–3 lines of chemotherapy.
Nearly 5% of patients underwent palliative surgery for malignant bowel obstruction.
Longitudinal data on treatment modalities and outcomes are useful for patient counseling, planning, and comparison studies.
Funding:
This research was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Statement
Outside the submitted work, R. O’Cearbhaill reports honoraria from GSK, Bayer, Regeneron, SeaGen, Fresenius Kabi, Immunogen, MJH Life Sciences and Curio; D. Chi reports personal fees from Apyx Medical, Verthermia Inc., Biom ‘Up, and AstraZeneca, as well as recent or current stock/options ownership of Apyx Medical, Verthemia, Intuitive Surgical, Inc., TransEnterix, Inc., Doximity, Moderna, and BioNTech SE; A. Iasonos reports consulting fees from Mylan. The other authors do not have potential conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. May 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 2.Minig L, Zorrero C, Iserte PP, Poveda A. Selecting the best strategy of treatment in newly diagnosed advanced-stage ovarian cancer patients. World J Methodol. Dec 26 2015;5(4):196–202. doi: 10.5662/wjm.v5.i4.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. Jan 2022;72(1):7–33. doi: 10.3322/caac.217085. [DOI] [PubMed] [Google Scholar]
- 4.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. Mar 1 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248 [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. Dec 2006;103(3):1070–6. doi: 10.1016/j.ygyno.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. Sep 2 2010;363(10):943–53. doi: 10.1056/NEJMoa0908806 [DOI] [PubMed] [Google Scholar]
- 7.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. Nov 2006;103(2):559–64. doi: 10.1016/j.ygyno.2006.03.051 [DOI] [PubMed] [Google Scholar]
- 8.Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. Jun 2017;18(6):779–791. doi: 10.1016/S1470-2045(17)30279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. April 2021;22(4):439–449. doi: 10.1016/S1470-2045(21)00006-1 [DOI] [PubMed] [Google Scholar]
- 10.Shih KK, Chi DS. Maximal cytoreductive effort in epithelial ovarian cancer surgery. J Gynecol Oncol. Jun 2010;21(2):75–80. doi: 10.3802/jgo.2010.21.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. Oct 2015;139(1):10–6. doi: 10.1016/j.ygyno.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. May 01 2014;32(13):1302–8. doi: 10.1200/JCO.2013.51.4489 [DOI] [PubMed] [Google Scholar]
- 13.Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. December 19 2019;381(25):2416–2428. doi: 10.1056/NEJMoa1911361 [DOI] [PubMed] [Google Scholar]
- 14.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. December 19 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962 [DOI] [PubMed] [Google Scholar]
- 15.Harter P, Hauke J, Heitz F, et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1). PLoS One. 2017;12(10):e0186043. doi: 10.1371/journal.pone.0186043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basaran D, Boerner T, Suhner J, et al. Risk of venous thromboembolism in ovarian cancer patients receiving neoadjuvant chemotherapy. Gynecol Oncol. Oct 2021;163(1):36–40. doi: 10.1016/j.ygyno.2021.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YL, Zhou QC, Iasonos A, et al. Pre-operative neoadjuvant chemotherapy cycles and survival in newly diagnosed ovarian cancer: what is the optimal number? A Memorial Sloan Kettering Cancer Center Team Ovary study. Int J Gynecol Cancer. Dec 2020;30(12):1915–1921. doi: 10.1136/ijgc-2020-001641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pignata S, S CC, Du Bois A, Harter P, Heitz F. Treatment of recurrent ovarian cancer. Ann Oncol. Nov 1 2017;28(suppl_8):viii51–viii56. doi: 10.1093/annonc/mdx441 [DOI] [PubMed] [Google Scholar]
- 19.Gockley A, Melamed A, Cronin A, et al. Outcomes of secondary cytoreductive surgery for patients with platinum-sensitive recurrent ovarian cancer. Am J Obstet Gynecol. Dec 2019;221(6):625 e1–625 e14. doi: 10.1016/j.ajog.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 20.Harter P, Sehouli J, Vergote I, et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N Engl J Med. Dec 2 2021;385(23):2123–2131. doi: 10.1056/NEJMoa2103294 [DOI] [PubMed] [Google Scholar]
- 21.Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. May 1 2006;106(9):1933–9. doi: 10.1002/cncr.21845 [DOI] [PubMed] [Google Scholar]
- 22.Manning-Geist BL, Chi DS, Long Roche K, et al. Tertiary cytoreduction for recurrent ovarian carcinoma: An updated and expanded analysis. Gynecol Oncol. Aug 2021;162(2):345–352. doi: 10.1016/j.ygyno.2021.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fotopoulou C, Zang R, Gultekin M, et al. Value of tertiary cytoreductive surgery in epithelial ovarian cancer: an international multicenter evaluation. Ann Surg Oncol. Apr 2013;20(4):1348–54. doi: 10.1245/s10434-012-2673-z [DOI] [PubMed] [Google Scholar]
- 24.Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. Oct 2012;23(10):2605–2612. doi: 10.1093/annonc/mds203 [DOI] [PubMed] [Google Scholar]
- 25.Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Ther Adv Med Oncol. Sep 2014;6(5):229–39. doi: 10.1177/1758834014544121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones APM, McGauran MFG, Jagasia N, Hiscock RJ, Hyde S, Grant P. Efficacy of dexamethasone in the management of malignant small bowel obstruction in advanced epithelial ovarian cancer. Support Care Cancer. Mar 2022;30(3):2821–2827. doi: 10.1007/s00520-021-06694-9 [DOI] [PubMed] [Google Scholar]
- 27.Martinez Castro P, Vargas L, Mancheno A, et al. Malignant Bowel Obstruction in Relapsed Ovarian Cancer With Peritoneal Carcinomatosis: An Occlusive State. Int J Gynecol Cancer. Sep 2017;27(7):1367–1372. doi: 10.1097/IGC.0000000000001049 [DOI] [PubMed] [Google Scholar]
- 28.Pectasides D, Pectasides M, Economopoulos T. Brain metastases from epithelial ovarian cancer: a review of the literature. Oncologist. Mar 2006;11(3):252–60. doi: 10.1634/theoncologist.11-3-252 [DOI] [PubMed] [Google Scholar]
- 29.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. Feb 2012;14(1):48–54. doi: 10.1007/s11912-011-0203-y [DOI] [PubMed] [Google Scholar]
- 30.Stasenko M, Cybulska P, Feit N, et al. Brain metastasis in epithelial ovarian cancer by BRCA1/2 mutation status. Gynecol Oncol. Jul 2019;154(1):144–149. doi: 10.1016/j.ygyno.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. Jul 2018;68(4):284–296. doi: 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol. Apr 2013;129(1):258–64. doi: 10.1016/j.ygyno.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


