PURPOSE
Local consolidative therapy (LCT) for patients with synchronous oligometastatic non–small-cell lung cancer is an evolving treatment strategy, but outcomes following LCT stratified by genetic mutations have not been reported. We sought to identify genomic associations with overall survival (OS) and progression-free survival (PFS) for these patients.
METHODS
We identified all patients presenting between 2000 and 2017 with stage IV non–small-cell lung cancer and ≤ 3 synchronous metastatic sites. Patients were grouped according to mutational statuses. Primary outcomes included OS and PFS following initial diagnosis.
RESULTS
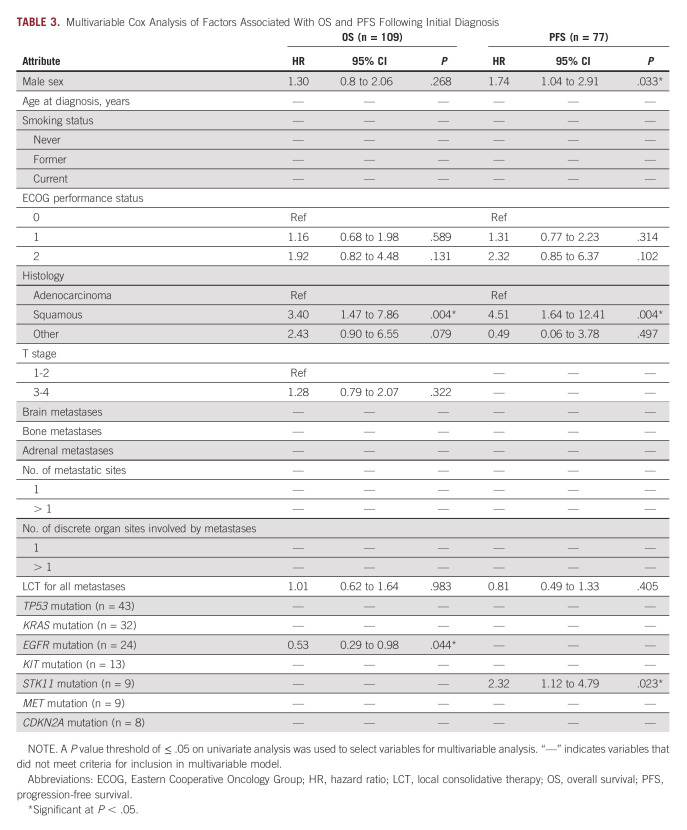
Of 194 included patients, 121 received comprehensive LCT to all sites of disease with either surgery or radiation. TP53 mutations were identified in 40 of 78 (55%), KRAS in 32 of 95 (34%), EGFR in 24 of 109 (22%), and STK11 in nine of 77 (12%). At median follow-up of 96 months, median OS and PFS were 26 (95% CI, 23 to 31) months and 11 (95% CI, 9 to 13) months, respectively. On multivariable analysis, patients with EGFR mutations had lower mortality risk (hazard ratio [HR], 0.53; 95% CI, 0.29 to 0.98; P = .044) compared with wild-type patients, and patients with STK11 mutations had higher risk of progression or mortality (HR, 2.32; 95% CI, 1.12 to 4.79; P = .023) compared with wild-type patients. TP53 and KRAS mutations were not associated with OS or PFS. Among 71 patients with known EGFR mutational status who received comprehensive LCT, EGFR mutations were associated with lower mortality compared with wild-type (HR, 0.45; 95% CI, 0.22 to 0.94; P = .032).
CONCLUSION
When compared with wild-type patients, those with EGFR and STK11 mutations had longer OS and shorter PFS, respectively. EGFR mutations were associated with longer OS among oligometastatic patients treated with comprehensive LCT in addition to systemic therapy.
INTRODUCTION
Roughly half of all patients with non–small-cell lung cancer (NSCLC) initially present with distant metastases, and 30% of these present with a limited number of metastases, a state often termed oligometastatic disease.1 Although the definition of oligometastatic state continues to evolve, it generally refers to patients with one to five discrete metastatic lesions and is associated with a more favorable prognosis versus widely metastatic disease.2,3 For these patients, several recent prospective studies demonstrated that comprehensive treatment with local consolidative therapy to all sites of disease (cLCT) led to significant improvements in both progression-free survival (PFS) and overall survival (OS) compared with treatment with systemic therapy alone.4-7 Furthermore, several retrospective series have also shown that cLCT is associated with prolonged survival among patients presenting with synchronous oligometastatic NSCLC.8-10
CONTEXT
Key Objective
Local consolidative therapy (LCT) for patients with synchronous oligometastatic non–small-cell lung cancer is an evolving treatment strategy, but outcomes following LCT stratified by genetic mutations have not been reported. This retrospective cohort study aimed to identify genomic associations with outcomes for these patients.
Knowledge Generated
In this cohort study of 194 patients, we provide evidence that EGFR mutations are associated with longer overall survival (OS) and that STK11 mutations are associated with shorter progression-free survival. We also demonstrate that patients with EGFR mutations who received both EGFR-targeted therapy and comprehensive LCT had significantly longer overall survival than those with EGFR wild-type tumors (98 v 29 months).
Relevance
These findings suggest that comprehensive LCT, when combined with molecularly targeted therapy for EGFR-mutated patients, may enable long-term survival. As novel molecularly targeted therapies emerge, treatment combining comprehensive LCT with targeted therapy in selected patients warrants further investigation.
Concurrently, there has been growing interest in the development of molecularly targeted agents that can be used in lieu of cytotoxic chemotherapy for patients with advanced disease. Current guidelines recommend testing for molecular biomarkers in NSCLC to guide therapy. Testing for actionable mutations/alterations of EGFR, ALK, ROS1, MET, BRAF, and NTRK has been increasingly performed over the past two decades.11-13 Tyrosine kinase inhibitors (TKIs) targeting these mutations and their downstream effectors have enabled long-term survival.14,15 Despite the parallel efforts of investigation into cLCT and development of targeted therapies in stage IV disease, few studies have characterized the outcomes of patients with NSCLC receiving cLCT on the basis of genetic alterations.16,17 Furthermore, such investigations have been limited to alterations of EGFR without broader consideration of other mutations, for which novel agents are likely to be approved in the coming years.18,19 Therefore, we aimed to understand patterns of molecular alterations in patients presenting with synchronous oligometastatic NSCLC, and to identify associations with outcomes.
METHODS
Patient Selection
After approval by the University of Texas MD Anderson Cancer Center Institutional Review Board (PA16-0061), we identified patients presenting to our institution between January 1, 2000, and December 31, 2017, with stage IV NSCLC and ≤ 3 synchronous (defined as present at the time of initial diagnosis) metastatic lesions. Cohort selection has been previously described.16 We used the MD Anderson Cancer Center GEMINI database, a prospectively collected database including tumor molecular profiles. Discrete metastatic foci within a single organ were counted as separate sites.4 Intrathoracic nodal disease was counted as a single site, regardless of the number of nodes involved, consistent with categorizations previously described.4 Intrathoracic disease stage was assigned using the American Joint Committee on Cancer 8th Edition staging system.20 The radiographic response to initial systemic therapy was assessed using RECIST 1.1 criteria.21 Patients who had local therapy to the primary and all metastatic lesions were considered to have received comprehensive LCT; patients receiving local therapy to some but not all of the sites were considered to have received subcomprehensive LCT; patients not treated with local therapy to any site were considered to have received no LCT. Radiotherapy (RT) treatment details have been previously reported.22
Molecular Profiling
Mutations were characterized for the majority of patients using next-generation sequencing (NGS) analysis using either solid tumor tissue or circulating cell-free DNA (cfDNA). Testing was performed most commonly with the Guardant360 CDx panel (Guardant Health, Palo Alto, CA) consisting of up to 129 genes and associated biomarkers, or with the FoundationOne CDx panel (Foundation Medicine, Cambridge, MA) consisting of up to 324 genes and associated biomarkers.23,24 Additionally, immunohistochemistry and other testing was ordered at the discretion of the treating physician. Particularly for patients who presented before 2014, testing of single genes (eg, EGFR, ALK, and KRAS) was more commonly performed than panel testing.
Statistical Analysis
Fisher exact tests were used to assess the associations between categorical variables, and Wilcoxon rank-sum tests were used to assess associations between continuous variables between the treatment cohorts. The median follow-up time with associated CI was calculated using the reverse Kaplan-Meier method. Time-to-event end points considered latencies following initial diagnosis and were analyzed using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariable Cox analyses were used to determine associations with outcomes. The proportional hazards assumptions for all univariable and multivariable models were evaluated using chi-square tests of Schoenfeld residuals. Tests of the proportional hazards assumptions for death and progression or death using Schoenfeld residuals all yielded P > .05 and thus we failed to reject the null hypotheses that hazards were proportional. A P value threshold of ≤ .05 on univariate analysis was used to select variables for inclusion in each corresponding multivariable model. Statistical analysis was performed with Stata Version 16.1 (StataCorp, College Station, TX).
RESULTS
Baseline Characteristics
A total of 194 patients met inclusion criteria for this study. The patient, disease, and treatment characteristics are displayed in Table 1. A majority of patients were male (57%) and had Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 (91%) at initial diagnosis. The median age at initial diagnosis was 62 years (range, 29-89 years). Most patients (77%) presented with adenocarcinoma histology. The most common sites of metastatic disease at diagnosis were brain (44%), bone (26%), and adrenal glands (19%). Most patients (71%) presented with at least two metastatic sites and most (92%) had disease confined to one discrete organ site.
Table 1.
Baseline Patient, Disease, and Treatment Characteristics
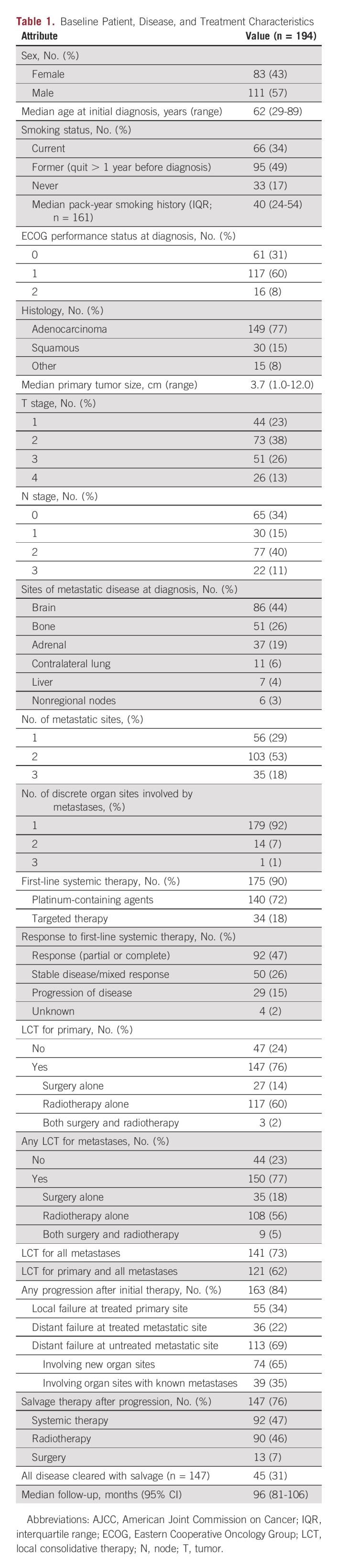
First-line systemic therapy was administered to 90% of patients, with 72% receiving platinum-containing chemotherapy and 18% receiving TKIs with or without chemotherapy. The response to first-line therapy was partial or complete in 47%, stable disease or mixed response in 26%, and progression of disease in 15%; response was unable to be assessed in 12%, given no restaging imaging. LCT was given to the primary site in 145/194 (76%), with 114/145 (79%) of these patients receiving RT alone, 28/145 (19%) receiving surgery alone, and 2/145 (1%) receiving surgery followed by adjuvant RT. LCT was given to metastatic sites in 151/194 (78%) of patients, with 108/151 (72%) of these patients receiving RT alone, 35/151 (23%) receiving surgery alone, and 8/194 (5%) receiving surgery and RT. Comprehensive LCT was provided for 121/194 (62%) patients, subcomprehensive LCT for 52/194 (27%), and no LCT for 21/194 (11%).
Mutational Profiling and Use of Targeted Therapy
Of 194 patients in this series, 112 (58%) had available mutational data. Of these, 69% were tested using an NGS gene panel, whereas 31% were tested specifically for one or more individual genes. The most commonly mutated genes (Data Supplement) were TP53 (43/78; 55%), KRAS (32/95; 34%), and EGFR (24/109; 22%). Of 24 patients with EGFR mutations, five (21%) had a T790M mutation. Only 28 patients in this series received PD-L1 testing; of these, 20 (71%) had a PD-L1 tumor proportion score > 1. The most common co-occurring mutations (Data Supplement) were in TP53 and KRAS (n = 15), TP53 and PD-L1 tumor proportion score > 1 (n = 14), and TP53 and EGFR (n = 10). Among the 24 patients with EGFR mutations, 19 received an EGFR-targeted TKI at some point in their disease course, whereas five did not, and were treated with chemotherapy alone.
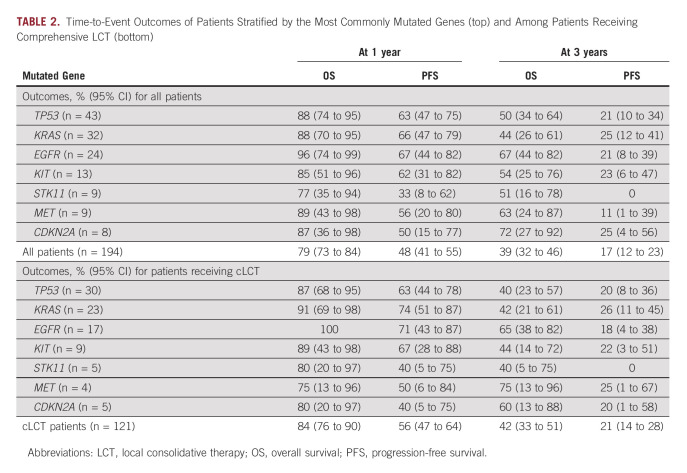
Disease Control and Survival
With an updated median follow-up time of 96 months (95% CI, 81 to 106), the median OS and PFS for this cohort were 26 (95% CI, 23 to 31) months and 11 (95% CI, 9 to 13) months, respectively (Data Supplement). Survival estimates are shown in Table 2, both among all patients and in the subset of patients receiving LCT to all sites of disease. Among all patients, 1- and 3-year OS were estimated to be 79% (95% CI, 73 to 84) and 39% (95% CI, 32 to 46), respectively; 1- and 3-year PFS were estimated to be 48% (95% CI, 41 to 55) and 25% (95% CI, 4 to 56), respectively; survival estimates are similar to those previously reported.8 Concordant results were seen for patients receiving LCT. When stratified by response to initial therapy, patients who had progression of disease with upfront systemic therapy had shorter survival (Data Supplement) compared with patients with stable disease or any response.
TABLE 2.
Time-to-Event Outcomes of Patients Stratified by the Most Commonly Mutated Genes (top) and Among Patients Receiving Comprehensive LCT (bottom)
Univariate analyses of factors associated with OS and PFS are shown in the Data Supplement. Factors associated with shorter OS on univariate analysis included male sex, ECOG 2 performance status, squamous histology, and higher T stage, whereas factors associated with longer OS included LCT for all metastases and EGFR mutation. Factors associated with shorter PFS on univariate analysis included male sex, ECOG 2 performance status, squamous histology, and STK11 mutation, whereas the only factor associated with longer PFS was receipt of cLCT. Multivariable analyses of factors associated with OS and PFS are shown in Table 3. After adjustment for covariates, squamous histology (hazard ratio [HR], 3.40; 95% CI, 1.47 to 7.86; P = .004) was associated with shorter OS and EGFR mutation (HR, 0.53; 95% CI, 0.29 to 0.98; P = .044) was associated with longer OS. After adjustment for covariates, male sex (HR, 1.74; 95% CI, 1.04 to 2.91; P = .033), squamous histology (HR, 4.51; 95% CI, 1.64 to 12.41; P = .004), and STK11 mutation (HR, 2.32; 95% CI, 1.12 to 4.79; P = .023) were associated with shorter PFS.
TABLE 3.
Multivariable Cox Analysis of Factors Associated With OS and PFS Following Initial Diagnosis
Consistent with our prior analysis,8 patients who received LCT to all sites had longer survival than those who did not (Data Supplement), with median OS times of 29 (95% CI, 25 to 42) and 22 (95% CI, 15 to 29) months, respectively. Median 1- and 3-year OS times among patients receiving LCT were estimated to be 84% (95% CI, 76 to 90) and 42% (95% CI, 33 to 51), respectively. However, a positive prognostic association was not reproduced on multivariable analysis when considering EGFR mutational status. On subgroup analysis of 112 patients receiving comprehensive LCT, only EGFR mutation (HR, 0.45; 95% CI, 0.22 to 0.94; P = .032) was found to be associated with longer OS on multivariable analysis (Data Supplement).
PFS stratified by STK11 mutational status is shown in Figure 1A. OS stratified by EGFR mutational status is shown in Figure 1B, and OS stratified by EGFR mutational status for the subset of patients receiving LCT to all sites of disease is shown in Figure 1C. Survival curves stratified by EGFR mutational status and receipt of EGFR-targeted therapy are shown in Figure 1D. Patients with EGFR mutations who received targeted therapy at any point in their disease course had significantly longer survival than those who had EGFR mutations but did not receive targeted therapy or those who did not have EGFR mutations. Estimates for 1- and 3-year OS by mutated gene are provided in Table 2. EGFR-mutated patients had 1- and 3-year OS of 96% (95% CI, 74 to 99) and 67% (95% CI, 44 to 82), respectively. STK11-mutated patients had 1- and 3-year PFS of 33% (95% 8 to 62) and 0%, respectively. Notably, patients with EGFR mutations who received TKIs had a median survival of 87 months (95% CI, 41 to undefined). Among the subset of these patients who also received LCT to all sites of disease, median survival was 98 months (95% CI, 31 to undefined). The presence of an EGFR T790M mutation was not associated with significantly different OS or PFS (Data Supplement). Patient-level outcomes for 112 molecularly characterized patients are provided in Figure 2.
FIG 1.
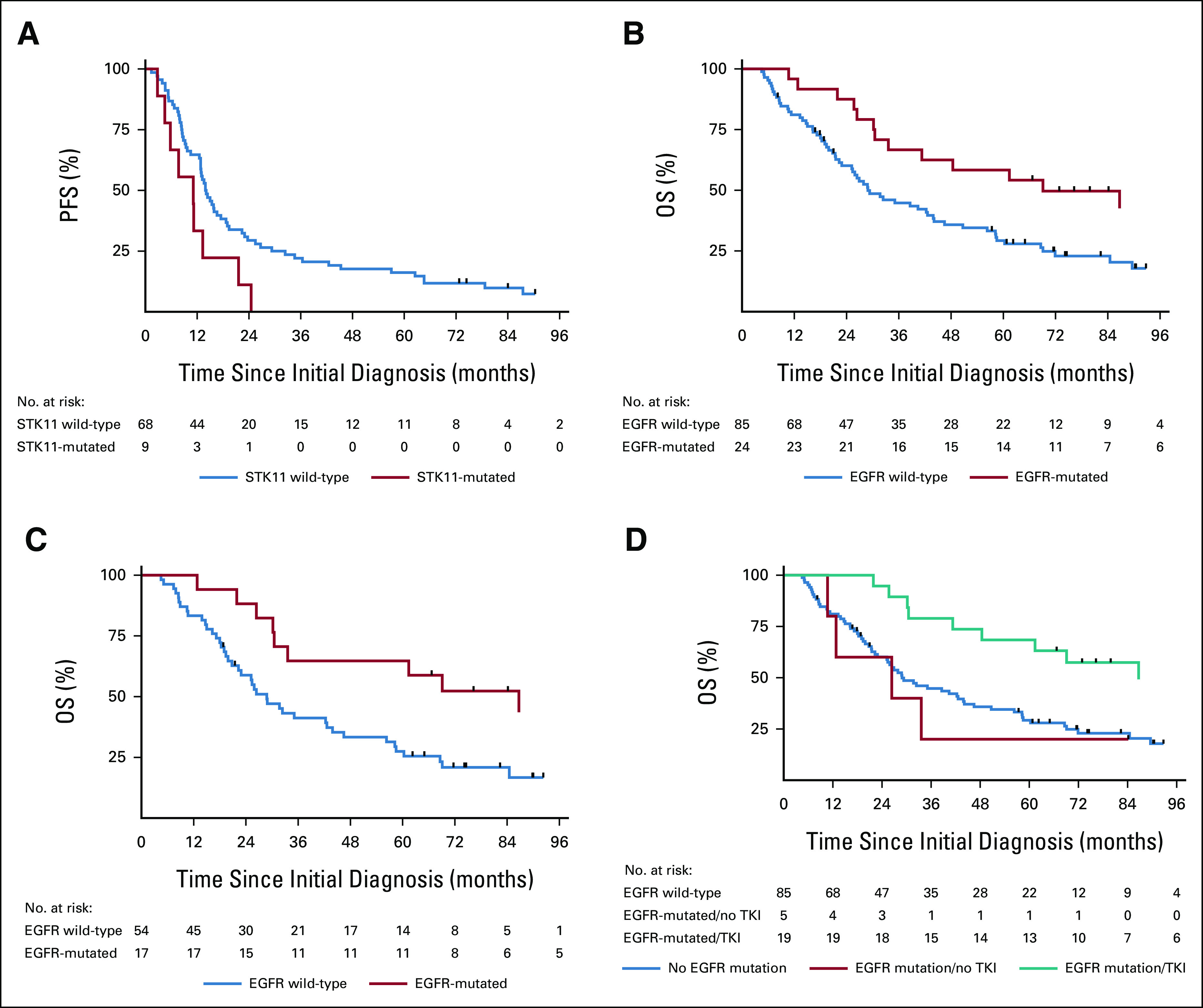
(A) PFS by STK11 mutational status, (B) OS by EGFR mutational status, (C) OS by EGFR mutational status among patients receiving comprehensive LCT, and (D) OS of patients without EGFR mutations, with EGFR mutations without receipt of EGFR-targeted therapy, and with EGFR mutations with receipt of EGFR-targeted therapy. LCT, local consolidative therapy; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
FIG 2.
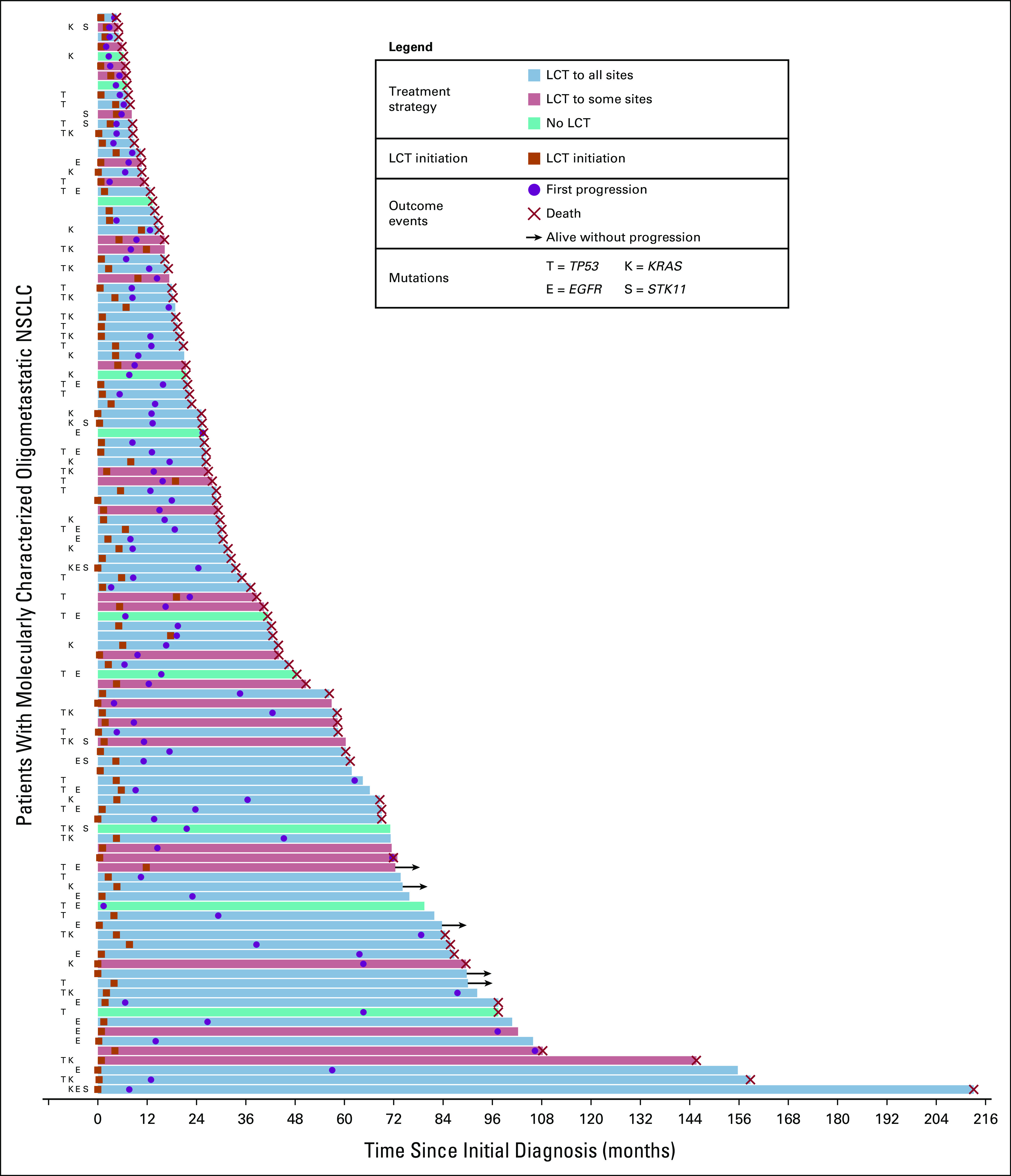
Patient-level outcomes for 112 patients with molecularly characterized oligometastatic NSCLC. The latency between diagnosis and outcome, either last follow-up or death, is represented by the length of each bar shown. Mutation statuses for common mutations are provided. LCT, local consolidative therapy, NSCLC, non–small-cell lung cancer.
After initial therapy, progression occurred in 84% of patients; the most common sites of first progression were distant, untreated sites (69%) and primary disease (34%), followed by distant sites treated by local therapy (22%), accounting for multiple sites of synchronous progression for some patients. Salvage therapy was used in 90% of patients who experienced failure; of these, 63% received systemic therapy, 61% received RT, and 9% received surgery.
DISCUSSION
In this study of 194 patients with synchronous oligometastatic NSCLC, we demonstrate with a median follow-up time of 8 years that cLCT is associated with favorable survival, with a median OS of 29 months with cLCT and 1- and 3-year survival rates of 84% and 42%, respectively. We characterized the mutational landscape of 112 of these 194 patients, showing that TP53, KRAS, EGFR, and KIT mutations were most commonly found on molecular testing. We provide initial benchmark data showing differential outcomes associated with mutation status, including potentially significant associations of EGFR mutation with longer OS and association of STK11 (LKB1) mutations with significantly shorter PFS.
Several large-scale sequencing efforts characterizing the mutational landscape for metastatic NSCLC have been reported, but few efforts have focused on synchronous oligometastatic disease in particular. A recent study of polymetastatic disease identified enrichment of ALK mutations, ALK and ROS1 fusions, and MET copy-number gains in metastatic sites compared with primary tumors.25 Data show that driver alterations derived from NSCLC primary tumors may be shared across metastatic sites. However, metastatic clones may acquire new genetic alterations, highlighting the importance of adjuncts to systemic therapy to overcome genetic divergence, and consideration of repeat NGS testing, particularly if there is disease progression on a prior systemic agent.26 In contrast to polymetastatic NSCLC, oligometastatic disease generally has fewer mutations and may have not yet realized its full metastatic potential, underscoring the importance of LCT to eradicate resistant clones.27,28
The presence an EGFR mutation in a patient with stage IV oligometastatic NSCLC prompts several considerations regarding workup and management. Patients with EGFR mutations are more likely to develop brain metastases,29 yet have longer survival. This is particularly true with receipt of TKI therapy combined with LCT to oligometastatic and oligoprogressive sites, irrespective of type of EGFR mutation or site of metastasis.30,31 Several studies have suggested that comprehensive LCT should be considered for patients who do not show progression on systemic therapy. Our data similarly support that patients who experience progression on upfront systemic therapy may have shorter survival despite use of LCT. Current guidelines also support the use of LCT after stable disease or partial response to first-line systemic therapy, although the ongoing phase III NRG LU002 (ClinicalTrials.gov identifier: NCT03137771), SARON (ClinicalTrials.gov identifier: NCT02417662), LONESTAR (ClinicalTrials.gov identifier: NCT03391869), and OMEGA (ClinicalTrials.gov identifier: NCT03827577) trials will further inform the effectiveness of this strategy.32 There is growing evidence regarding the survival benefit associated with first-line TKI followed by LCT.18,33 We previously reported that patients with EGFR-mutated metastatic NSCLC who received TKI followed by LCT had significantly longer PFS compared with treatment with TKI alone (36 v 14 months; P = .0024). Although our data suggest response to upfront systemic therapy as a potential predictor of benefit from LCT, the optimal use and timing of LCT in patients receiving systemic therapy remains an active area of investigation. An alternative strategy of using upfront RT to first-line TKI therapy for EGFR-mutated NSCLC showed an OS and PFS benefit in the recently published SINDAS (ClinicalTrials.gov identifier: NCT02893332) trial.34
Importantly, we find through this analysis that patients with EGFR mutations receiving both TKIs and comprehensive LCT had the most favorable outcomes, with a median survival of 98 months, which is longer than that observed for any other mutational subset, and remarkably high, considering these patients all presented with stage IV disease at diagnosis. Indeed, this estimate is significantly longer than OS reported in several randomized studies examining the role of LCT,4,6 including that seen in the phase III SINDAS trial, which had a reported median OS of 26 months. Although the optimal timing and sequencing of systemic therapy relative to LCT remains uncertain, it appears that use of LCT and TKI in this subset of patients may confer remarkably long-term survival.
We also found an association of STK11 mutations with shorter PFS in this cohort. The negative prognostic value of STK11 mutations, particularly in combination with KRAS mutations, has been previously reported in several series of polymetastatic NSCLC.35,36 In the present series, the majority of patients with STK11 (six of nine) mutations also had mutation in KRAS. STK11/KRAS comutation is hypothesized to limit responses to traditional systemic agents, along with immunotherapy.37-39 KRAS and TP53 mutations did not correlate with either longer or shorter OS or PFS among patients with oligometastatic NSCLC, likely because of the lack of targeted therapies for these mutations. The United States Food and Drug Administration (FDA) approved sotorasib for patients with KRAS G12C-mutated NSCLC in 2021.40 Investigators from the KRYSTAL study also recently reported favorable outcomes for adagrasib, including encouraging intracranial objective response rates; FDA-accelerated evaluation is pending.41 APR-246, a small-molecule drug that binds to mutant p53 and restores its normal function, has been shown to induce apoptosis in NSCLC cell lines and may synergize with poly-[ADP-ribose] polymerase inhibitors to induce cell death.42 As rationally designed therapies targeting molecular pathways emerge, survival differences between patients of with distinct mutational profiles may attenuate. We hypothesize that the benefit of cLCT may be more pronounced with these more effective systemic agents.
This study has several limitations. Although all patients had oligometastatic disease confirmed both radiographically and on biopsy, the sample is nevertheless heterogeneous with regard to sites of metastatic disease, use of LCT to primary and metastatic sites, and therapies preceding and following LCT. Probable selection bias exists for those patients able to tolerate and benefit most from EGFR-targeted therapies and comprehensive LCT. Molecular profiling in this study was nonuniform; although nearly 70% had comprehensive NGS panel testing, the remainder had testing of single genes, limiting our ability to make robust conclusions about less frequently seen mutations. The majority of patients in this study were treated before widespread use of checkpoint inhibitors, potentially limiting generalizability to patients treated in the present day. Perhaps, the most salient limitation is that it is not possible to understand the contribution of EGFR-targeted therapy versus LCT in prolonging survival; the results of prospective trials such as NORTHSTAR (ClinicalTrials.gov identifier: NCT03410043)43 will be instrumental in better assessing the incremental benefit of LCT.
In conclusion, although the use of systemic therapies, including targeted agents, continues to evolve in the treatment of synchronous oligometastatic NSCLC, comprehensive LCT remains an important tool in selected patients. In this study, we stratified patients with synchronous oligometastatic NSCLC by genetic alterations to provide benchmarks for future comparison. Favorable outcomes were observed for patients with EGFR mutations, the majority of whom received EGFR-targeted agents. We also demonstrated that STK11 mutations may be associated with shorter time to progression, highlighting the role of close surveillance following initial therapy. These data support ongoing trials to elucidate the utility and timing of LCT in patients with oligometastatic NSCLC receiving systemic agents. Further investigation into the prognostic and therapeutic implications of commonly seen mutations is warranted to optimize care for these patients.
Brian De
Honoraria: Sermo
Ethan B. Ludmir
Employment: Alaunos Therapeutics
J. Jack Lee
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: AstraZeneca
Stephen G. Swisher
Honoraria: Armada Health Care, AstraZeneca, Conquer Cancer Foundation, MJH Healthcare Holdings, LLC, HMP Omnimedia, AstraZeneca, Interline Therapeutics
Consulting or Advisory Role: AstraZeneca, Armada Health Care
Research Funding: CairnSurgical, Lilly, Lumicell, OncoNano Inc
Travel, Accommodations, Expenses: AstraZeneca
Don L. Gibbons
Stock and Other Ownership Interests: Exact Sciences, Nektar
Consulting or Advisory Role: Ribon Therapeutics, Mitobridge, Lilly, Menarini, Napa Therapeutics, Onconova Therapeutics, 4D Pharma
Research Funding: Mitobridge, Ribon Therapeutics, Boehringer Ingelheim (Inst), Mirati Therapeutics (Inst), NGM Biopharmaceuticals, AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, BerGenBio, Takeda
Yasir Y. Elamin
Consulting or Advisory Role: Lilly, AstraZeneca, Turning Point Therapeutics, Takeda, Sanofi
Research Funding: Spectrum Pharmaceuticals, AstraZeneca, Takeda, Xcovery, Lilly, Elevation Oncology, Turning Point Therapeutics, Blueprint Medicines, Forward, Precision Therapeutics
Travel, Accommodations, Expenses: Lilly
Daniel R. Gomez
Honoraria: Varian Medical Systems, Merck, Bristol Myers Squibb, AstraZeneca, Reflexion Medical, Vindico Medical Education, US Oncology, GRAIL
Consulting or Advisory Role: Olympus Medical Systems, Medtronic, Johnson & Johnson/Janssen, GRAIL
Research Funding: Merck, Varian Medical Systems, AstraZeneca, Bristol Myers Squibb
Travel, Accommodations, Expenses: Varian Medical Systems, AstraZeneca, Merck, Vindico Medical Education, US Oncology, Driver, Inc
Jianjun Zhang
Honoraria: Roche, Sino-USA Biomedical Platform, Geneplus, Origimed, Innovent Biologics, CancerNet, Zhejiang Cancer Hospital, Suzhou Medical Association, Hengrui Medicine
Consulting or Advisory Role: AstraZeneca, Geneplus, Capital Medical University, Johnson & Johnson/Janssen, Novartis, Hunan Cancer Hospital
Research Funding: Merck, Novartis
Travel, Accommodations, Expenses: Innovent Biologics, Zhejiang Cancer Hospital
Xiuning Le
Consulting or Advisory Role: AstraZeneca, Lilly, EMD Serono, Spectrum Pharmaceuticals, Daiichi Sankyo/Lilly, Novartis, Hengrui Therapeutics, Janssen Oncology, Blueprint Medicines, Sensei Biotherapeutics, AbbVie, Arrivent
Research Funding: Lilly (Inst), Boehringer Ingelheim (Inst), Arrivent (Inst), Teligene (Inst)
Travel, Accommodations, Expenses: Spectrum Pharmaceuticals, EMD Serono
Steven H. Lin
Employment: MD Anderson Cancer Center
Honoraria: AstraZeneca/MedImmune, BeyondSpring Pharmaceuticals
Research Funding: BeyondSpring Pharmaceuticals, Nektar, STCube Pharmaceuticals Inc
Zhongxing Liao
Honoraria: Varian Medical Systems
Consulting or Advisory Role: ReHava Biosciences, AIQ Global, Inc
Speakers' Bureau: Varian Medical Systems
Travel, Accommodations, Expenses: Varian Medical Systems
Joe Y. Chang
Consulting or Advisory Role: Legion Healthcare Partners
Research Funding: Bristol Myers Squibb
Ara Vaporciyan
Travel, Accommodations, Expenses: AstraZeneca
John V. Heymach
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Mirati Therapeutics, Janssen, Nexus Health Systems, Pneuma Respiratory, Lilly (Inst)
Speakers' Bureau: IDEOlogy Health, MJH Life Sciences
Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, Boehringer Ingelheim (Inst), Takeda (Inst)
Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations
Mara B. Antonoff
Consulting or Advisory Role: AstraZeneca
Saumil J. Gandhi
Consulting or Advisory Role: Novocure
Research Funding: Bristol Myers Squibb, Nanobiotix
Travel, Accommodations, Expenses: Novocure
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Preliminary results were presented in part at the 2020 World Conference on Lung Cancer, held virtually on January 28-31, 2021.
SUPPORT
Supported by the generous philanthropic contributions to The University of Texas MD Anderson Lung Moon Shot Program, the GEMINI team, and the National Institutes of Health Cancer Center Support Grant P30 CA016672 to MD Anderson Cancer Center. This work was also supported by the Radiological Society of North America Resident Research Grant (RR2111) to B. De.
B.D. and A.S.F. contributed equally to this work.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request within 1 year of publication.
AUTHOR CONTRIBUTIONS
Conception and design: Brian De, Ahsan S. Farooqi, Kyle G. Mitchell, Ethan B. Ludmir, Daniel R. Gomez, Matthew S. Ning, Zhongxing Liao, Ara A. Vaporciyan, John V. Heymach, Saumil J. Gandhi
Administrative support: Jeff Lewis, Waree Rinsurongkawong, Zhongxing Liao
Provision of study materials or patients: Don L. Gibbons, Daniel R. Gomez, Joe Y. Chang, Ara A. Vaporciyan, John V. Heymach
Collection and assembly of data: Brian De, Ahsan S. Farooqi, Kyle G. Mitchell, Waree Rinsurongkawong, Vadeerat Rinsurongkawong, Yasir Y. Elamin, Mara B. Antonoff, Saumil J. Gandhi
Data analysis and interpretation: Brian De, Ahsan S. Farooqi, Kyle G. Mitchell, Ethan B. Ludmir, Jeff Lewis, J. Jack Lee, Stephen G. Swisher, Don L. Gibbons, Jianjun Zhang, Xiuning Le, Daniel R. Gomez, Matthew S. Ning, Steven H. Lin, Zhongxing Liao, Joe Y. Chang, Mara B. Antonoff, Saumil J. Gandhi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Brian De
Honoraria: Sermo
Ethan B. Ludmir
Employment: Alaunos Therapeutics
J. Jack Lee
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: AstraZeneca
Stephen G. Swisher
Honoraria: Armada Health Care, AstraZeneca, Conquer Cancer Foundation, MJH Healthcare Holdings, LLC, HMP Omnimedia, AstraZeneca, Interline Therapeutics
Consulting or Advisory Role: AstraZeneca, Armada Health Care
Research Funding: CairnSurgical, Lilly, Lumicell, OncoNano Inc
Travel, Accommodations, Expenses: AstraZeneca
Don L. Gibbons
Stock and Other Ownership Interests: Exact Sciences, Nektar
Consulting or Advisory Role: Ribon Therapeutics, Mitobridge, Lilly, Menarini, Napa Therapeutics, Onconova Therapeutics, 4D Pharma
Research Funding: Mitobridge, Ribon Therapeutics, Boehringer Ingelheim (Inst), Mirati Therapeutics (Inst), NGM Biopharmaceuticals, AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, BerGenBio, Takeda
Yasir Y. Elamin
Consulting or Advisory Role: Lilly, AstraZeneca, Turning Point Therapeutics, Takeda, Sanofi
Research Funding: Spectrum Pharmaceuticals, AstraZeneca, Takeda, Xcovery, Lilly, Elevation Oncology, Turning Point Therapeutics, Blueprint Medicines, Forward, Precision Therapeutics
Travel, Accommodations, Expenses: Lilly
Daniel R. Gomez
Honoraria: Varian Medical Systems, Merck, Bristol Myers Squibb, AstraZeneca, Reflexion Medical, Vindico Medical Education, US Oncology, GRAIL
Consulting or Advisory Role: Olympus Medical Systems, Medtronic, Johnson & Johnson/Janssen, GRAIL
Research Funding: Merck, Varian Medical Systems, AstraZeneca, Bristol Myers Squibb
Travel, Accommodations, Expenses: Varian Medical Systems, AstraZeneca, Merck, Vindico Medical Education, US Oncology, Driver, Inc
Jianjun Zhang
Honoraria: Roche, Sino-USA Biomedical Platform, Geneplus, Origimed, Innovent Biologics, CancerNet, Zhejiang Cancer Hospital, Suzhou Medical Association, Hengrui Medicine
Consulting or Advisory Role: AstraZeneca, Geneplus, Capital Medical University, Johnson & Johnson/Janssen, Novartis, Hunan Cancer Hospital
Research Funding: Merck, Novartis
Travel, Accommodations, Expenses: Innovent Biologics, Zhejiang Cancer Hospital
Xiuning Le
Consulting or Advisory Role: AstraZeneca, Lilly, EMD Serono, Spectrum Pharmaceuticals, Daiichi Sankyo/Lilly, Novartis, Hengrui Therapeutics, Janssen Oncology, Blueprint Medicines, Sensei Biotherapeutics, AbbVie, Arrivent
Research Funding: Lilly (Inst), Boehringer Ingelheim (Inst), Arrivent (Inst), Teligene (Inst)
Travel, Accommodations, Expenses: Spectrum Pharmaceuticals, EMD Serono
Steven H. Lin
Employment: MD Anderson Cancer Center
Honoraria: AstraZeneca/MedImmune, BeyondSpring Pharmaceuticals
Research Funding: BeyondSpring Pharmaceuticals, Nektar, STCube Pharmaceuticals Inc
Zhongxing Liao
Honoraria: Varian Medical Systems
Consulting or Advisory Role: ReHava Biosciences, AIQ Global, Inc
Speakers' Bureau: Varian Medical Systems
Travel, Accommodations, Expenses: Varian Medical Systems
Joe Y. Chang
Consulting or Advisory Role: Legion Healthcare Partners
Research Funding: Bristol Myers Squibb
Ara Vaporciyan
Travel, Accommodations, Expenses: AstraZeneca
John V. Heymach
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Mirati Therapeutics, Janssen, Nexus Health Systems, Pneuma Respiratory, Lilly (Inst)
Speakers' Bureau: IDEOlogy Health, MJH Life Sciences
Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, Boehringer Ingelheim (Inst), Takeda (Inst)
Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations
Mara B. Antonoff
Consulting or Advisory Role: AstraZeneca
Saumil J. Gandhi
Consulting or Advisory Role: Novocure
Research Funding: Bristol Myers Squibb, Nanobiotix
Travel, Accommodations, Expenses: Novocure
No other potential conflicts of interest were reported.
REFERENCES
- 1.Loi M, Mazzella A, Mansuet-Lupo A, et al. : Synchronous oligometastatic lung cancer deserves a dedicated management. Ann Thorac Surg 107:1053-1059, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Palma DA, Salama JK, Lo SS, et al. : The oligometastatic state—Separating truth from wishful thinking. Nat Rev Clin Oncol 11:549-557, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Dingemans A-MC, Hendriks LEL, Berghmans T, et al. : Definition of synchronous oligometastatic non–small cell lung cancer—A consensus report. J Thorac Oncol 14:2109-2119, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Gomez DR, Tang C, Zhang J, et al. : Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol 37:1558-1565, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Verma V, Liang F, et al. : Local consolidative therapy versus systemic therapy alone for metastatic non-small cell lung cancer: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 114:635-644, 2022 [DOI] [PubMed] [Google Scholar]
- 6.Iyengar P, Wardak Z, Gerber DE, et al. : Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol 4:e173501, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palma DA, Olson R, Harrow S, et al. : Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 38:2830-2838, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell KG, Farooqi A, Ludmir EB, et al. : Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non–small-cell lung cancer. Clin Lung Cancer 21:37-46.e7, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Jones GD, Lengel HB, Hsu M, et al. : Management of synchronous extrathoracic oligometastatic non-small cell lung cancer. Cancers 13:1893, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashworth AB, Senan S, Palma DA, et al. : An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 15:346-355, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Hanna NH, Robinson AG, Temin S, et al. : Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 39:1040-1091, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Lindeman NI, Cagle PT, Aisner DL, et al. : Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 13:323-358, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Wood DE, Kazerooni EA, Aberle D, et al. : NCCN Guidelines® Insights: Lung cancer screening, version 1.2022: Featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 20:754-764, 2022 [DOI] [PubMed] [Google Scholar]
- 14.Solomon BJ, Mok T, Kim DW, et al. : First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167-2177, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Soria JC, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Elamin YY, Gomez DR, Antonoff MB, et al. : Local consolidation therapy (LCT) after first line tyrosine kinase inhibitor (TKI) for patients with EGFR mutant metastatic non–small-cell lung cancer (NSCLC). Clin Lung Cancer 20:43-47, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Zhou F, Liu H, et al. : Consolidative local ablative therapy Improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol 13:1383-1392, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Lim JU: Management of oligometastasis and oligoprogression in patients with epidermal growth factor receptor mutation-positive NSCLC in the era of third-generation tyrosine kinase inhibitors. Clin Lung Cancer 22:e786-e792, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Manochakian R, James L, et al. : Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol 13:58, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT: The eighth edition lung cancer stage classification. Chest 151:193-203, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Farooqi A, Ludmir EB, Mitchell KG, et al. : Increased biologically effective dose (BED) to the primary tumor is associated with improved survival in patients with oligometastatic NSCLC. Radiother Oncol 163:114-118, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Guardant360 CDx Gene List. Guardant Health. https://guardant360cdx.com/gene-list/ [Google Scholar]
- 24.FoundationOne CDx . Technical Specifications. 2022. https://assets.ctfassets.net/w98cd481qyp0/YqqKHaqQmFeqc5ueQk48w/d12f19680205941ea3fee417f08e9524/F1CDx_Technical_Specifications.pdf [Google Scholar]
- 25.Li D, Huang Y, Cai L, et al. : Genomic landscape of metastatic lung adenocarcinomas from large-scale clinical sequencing. Neoplasia 23:1204-1212, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoś M, Krawczyk P: Genetic clonality as the hallmark driving evolution of non-small cell lung cancer. Cancers (Basel) 14:1813, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang JY, Verma V: Optimize local therapy for oligometastatic and oligoprogressive non-small cell lung cancer to enhance survival. J Natl Compr Cancer Netw 20:531-539, 2022 [DOI] [PubMed] [Google Scholar]
- 28.Blumenthaler AN, Antonoff MB: Classifying oligometastatic non-small cell lung cancer. Cancers 13:4822, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujita Y, Kinoshita M, Ozaki T, et al. : The impact of EGFR mutation status and single brain metastasis on the survival of non-small-cell lung cancer patients with brain metastases. Neurooncol Adv 2:vdaa064, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borghetti P, Bonù ML, Giubbolini R, et al. : Concomitant radiotherapy and TKI in metastatic EGFR- or ALK-mutated non-small cell lung cancer: A multicentric analysis on behalf of AIRO lung cancer study group. Radiol Med 124:662-670, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Weiss J, Kavanagh B, Deal A, et al. : Phase II study of stereotactic radiosurgery for the treatment of patients with oligoprogression on erlotinib. Cancer Treatment and Research Communications 19:100126, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Amini A, Verma V, Simone CB 2nd, et al. : American radium society appropriate use criteria for radiation therapy in oligometastatic or oligoprogressive non-small cell lung cancer. Int J Radiat Oncol Biol Phys 112:361-375, 2022 [DOI] [PubMed] [Google Scholar]
- 33.Hu F, Li C, Xu J, et al. : Additional local consolidative therapy has survival benefit over EGFR tyrosine kinase inhibitors alone in bone oligometastatic lung adenocarcinoma patients. Lung Cancer 135:138-144, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Wang XS, Bai YF, Verma V, et al. : Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated NSCLC. J Natl Cancer Inst 114(5):djac015, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shire NJ, Klein AB, Golozar A, et al. : STK11 (LKB1) mutations in metastatic NSCLC: Prognostic value in the real world. PLoS One 15:e0238358, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosellini P, Amintas S, Caumont C, et al. : Clinical impact of STK11 mutation in advanced-stage non-small cell lung cancer. Eur J Cancer 172:85-95, 2022 [DOI] [PubMed] [Google Scholar]
- 37.Malhotra J, Ryan B, Patel M, et al. : Clinical outcomes and immune phenotypes associated with STK11 co-occurring mutations in non-small cell lung cancer. J Thorac Dis 14:1772-1783, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Li Z, Jian H, et al. : Impact of KRAS mutation subtypes and co-occurring mutations on response and outcome in advanced NSCLC patients following first-line treatment. J Clin Med 11:4003, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farooq H, Bien H, Chang V, et al. : Loss of function STK11 alterations and poor outcomes in non–small-cell lung cancer: Literature and case series of US veterans. Sem Oncol 49:319-325, 2022 [DOI] [PubMed] [Google Scholar]
- 40.Nakajima EC, Drezner N, Li X, et al. : FDA approval summary: Sotorasib for KRAS G12C-mutated metastatic NSCLC. Clin Cancer Res 28:1482-1486, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jänne PA, Riely GJ, Gadgeel SM, et al. : Adagrasib in non–small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med 387:120-131, 2022 [DOI] [PubMed] [Google Scholar]
- 42.Deben C, Lardon F, Wouters A, et al. : APR-246 (PRIMA-1MET) strongly synergizes with AZD2281 (olaparib) induced PARP inhibition to induce apoptosis in non-small cell lung cancer cell lines. Cancer Lett 375:313-322, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Osimertinib Surgery, Radiation Therapy: Treating Patients With Stage IIIB or IV Non-small Cell Lung Cancer With EGFR Mutations, NORTHSTAR Study. https://ClinicalTrials.gov/show/NCT03410043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request within 1 year of publication.