PURPOSE
Germline mutations in DNA repair genes are present in approximately 10% of men with metastatic prostate cancer (mPC), and guidelines recommend genetic germline testing. Notable barriers exist, including access to genetic counseling, insurance coverage, and out-of-pocket costs. The GENTleMEN study was designed to determine the feasibility of an Internet-based, patient-driven germline genetic testing approach for men with mPC.
PATIENTS AND METHODS
In this prospective cohort study, men with mPC provided informed consent via an Internet-based platform and completed a questionnaire including demographics and family cancer history. Supporting medical data were also collected. Genetic testing was performed using the Color Genomics 30-gene targeted panel of cancer predisposition genes on a mailed saliva sample. Men whose test results identified a germline pathogenic or likely pathogenic variant received results by phone or telehealth genetic counseling; other participants received results by email with an option for phone-based or telehealth genetic counseling.
RESULTS
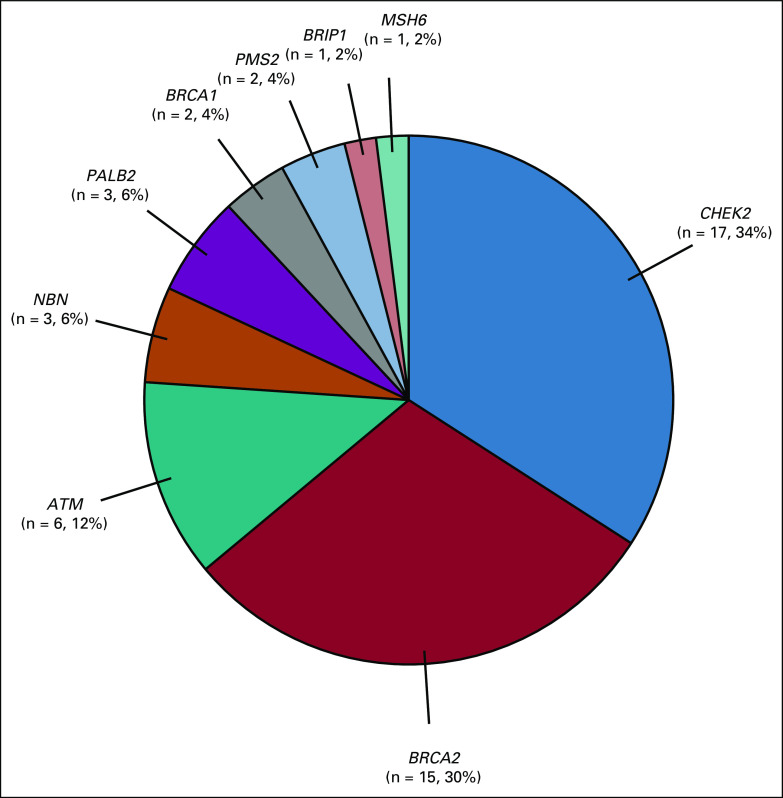
As of August 18, 2021, 816 eligible men were consented, of whom 68% (551) completed genetic testing, and 8.7% (48 of 551) were found to carry a pathogenic or likely pathogenic variant in a germline DNA repair gene: CHEK2 (17), BRCA2 (15), ATM (6), NBN1 (3), BRCA1 (2), PALB2 (2), PMS2 (2), and MSH6 (1). Participants were more likely to complete the testing process if they were non-Hispanic White, married, highly educated, or from a higher-income bracket.
CONCLUSION
Here, we show the feasibility of delivering germline (inherited) genetic testing by a voluntary, patient-driven, Internet-based platform to men with mPC. Preliminary results show rates of germline DNA repair mutations, consistent with other cohorts. Although feasible for some, reduced steps for participation, more dedicated diverse outreach and participant support, and identification and addressing of additional barriers is needed to ensure equitable access and optimization.
INTRODUCTION
Germline DNA repair gene mutations are present in approximately 10% of patients with metastatic prostate cancer (mPC).1,2 Identification of germline DNA repair gene mutations has two important implications for patients with mPC: potential prostate cancer treatment planning and potential family cancer risk. Specifically, olaparib and rucaparib are oral poly adenosine diphosphate-ribose polymerase inhibitors, now approved by the Food and Drug Administration for men with metastatic castration-resistant prostate cancer in association with germline and/or somatic BRCA1 and BRCA2 mutations (rucaparib)1 and BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, and RAD54L (olaparib).2 Finally, immune checkpoint inhibitor pembrolizumab is Food and Drug Administration–approved for patients with mPC whose tumors have evidence of high microsatellite instability, mismatch repair deficiency, or tumor mutational burden ≥ 10 mut/Mb.1-3 The potential family implications are also critically important, with opportunities for genetic testing of relatives and, if relatives carry the same germline genetic variants, options for modified cancer screening and cancer risk reduction. The National Comprehensive Cancer Network (NCCN) guidelines both for Prostate Cancer and for Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic recommend offering germline testing to all men with mPC.4,5 Despite the importance of identifying patients with prostate cancer and germline DNA repair gene mutations, there are important barriers, including limited access to genetics services, insurance coverage, and high out-of-pocket costs.6,7 At the time of study inception, the NCCN guidelines criteria did not yet include consideration for germline genetic testing (2017) or the subsequent recommendation for offering germline genetic testing to all patients with mPC (2019). The overarching goal for the study was in recognition that first, the standard of care was likely to change and second that there was a need and opportunity to consider novel genetic education and testing delivery methods because of the substantial expansion of the number of patients needing genetic counseling and limited genetic counselors. To address these challenges, we conducted the Genetic Testing for Men with Metastatic Prostate Cancer (GENTleMEN) research study to determine whether an Internet-based, patient-driven, cost-free genetic testing program would be feasible for interested patients and could provide clinical genetic testing for patients with mPC by removing barriers of access and cost.
CONTEXT
Key Objective
The GENTleMEN study was designed to determine the feasibility of an Internet-based, patient-driven germline genetic testing approach for men with metastatic prostate cancer.
Knowledge Generated
Preliminary data demonstrate the feasibility of Internet-based, patient-driven, genetic testing with 68% test completion. We found that 8.7% of those completing testing carried pathogenic or likely pathogenic variant in germline DNA repair genes, in the range of other published cohorts. Participants were more likely to complete testing if they were non-Hispanic White, married, highly educated, or from a higher-income bracket.
Relevance
Internet-based, patient-driven genetic testing is a feasible delivery mechanism for a subset of patients. However, our data indicate that further efforts to optimize clinical implementation for a wider cross-section of men with metastatic prostate cancer will be needed, and may include reducing steps for participation, improving educational outreach and further exploring and addressing barriers.
PATIENTS AND METHODS
Design, Setting, and Participants
This prospective, nonpilot feasibility cohort study was approved by the Institutional Review Board of the Fred Hutchinson Cancer Consortium. Informed consent was provided in accordance with the precepts established by the Declaration of Helsinki via a study-specific, Health Insurance Portability and Accountability Act-compliant web application.8
The study opened on September 16, 2016, with the goal of testing up to 2,000 participants. This initial analysis includes participants consenting and completing surveys through August 18, 2021. Approximately two additional months were allowed for the maturation of data, including return of saliva samples, and results reporting. Patients were recruited through a variety of mechanisms: their medical provider, patient support group, prostate cancer newsletters, or social media. Enrollment is patient-driven, which we define as the self-directed patient-participant visits Gentlemen Study website9 (currently on pause, please contact corresponding author for information) or calls the study telephone line. or calls the study telephone line. Research coordinators are available for assistance by phone or email, although participants need to initiate a request for help by phone and/or email, and the study team is not able to actively reach out to participants who do not make requests for help.
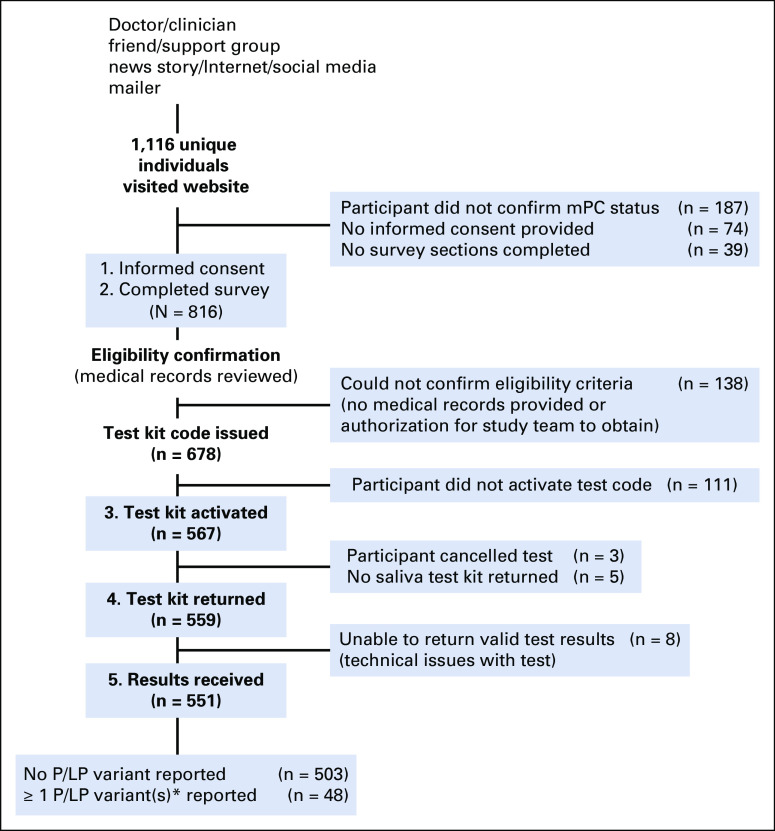
The website provides background information about the study and potential risks and benefits of germline genetic testing before informed consent and participant survey. The website was not designed to be interactive with the participant. Additional optional educational materials and videos are available to participants after test kit activation and provide additional review of the rationale and potential benefits for testing (potential treatment options and family implications), genetic discrimination risks, and Genetic Information Nondiscrimination Act laws. Although the additional materials can be viewed as many times as needed, they are not required for study participation. All study materials are in English and tailored to an 8th grade reading level. An email and telephone number for additional support is provided. Self-directed electronic informed consent is provided via a Health Insurance Portability and Accountability Act-compliant Redcap platform.9 The study flow diagram is shown in Figure 1.
FIG 1.
Study flow diagram. Numbered, bolded boxes contain active steps that participant must complete to proceed with study. *Four genes not specifically associated with prostate cancer risk were excluded: APC, CDKN2A, MITF, and MUTYH. mPC, metastatic prostate cancer; P/LP, pathogenic or likely pathogenic.
Inclusion criteria include individuals age older than 18 years who have documented mPC (as defined by one of the following: radiographic evidence of distant prostate cancer involvement, serum prostate-specific antigen > 100 ng/mL, and/or pathology report indicating prostate cancer); willingness to undergo germline genetic testing; willingness to provide basic demographic information and family cancer history; willingness to complete patient survey at enrollment and at 6-month follow-up; willingness and ability to provide a saliva sample; residing in the United States with a US mailing address; and having a personal email address. Study materials are only available in English, and therefore study participants need to be able to read and write in English to enroll. Exclusion criteria include inability or unwillingness to provide necessary information for eligibility, self-reported history of bone marrow transplant, or hematologic malignancy (because of inability to ensure a valid test result under these conditions).
Survey Instruments
After providing informed consent, participants were directed to complete a survey that included demographics, family cancer history, and six validated instruments: GAD7,10 PHQ9,11 Cancer Distress, Risk Perception, Decision Conflict, and Knowledge of and Concern with Genetic Testing.12,13 Supporting medical records were collected by the research team (with permission from participants) or uploaded directly by participants.
Two versions of the survey were used. Version 2 (V2) had the order of questions rearranged for earlier screening of ineligible individuals. There were 1,423 survey entries in total (V1, n = 23 and V2, n = 1,400). Although eligibility for study required a willingness to complete patient survey at enrollment and at 6-month follow-up, completion of all survey questions was not required. We included participants who returned survey responses to at least one of the six validated instruments.
After filtering out spam and duplicate records, we identified 1,116 unique visitors to the website, of whom 816 participants provided consent and completed the minimum survey response (defined as having completed at least one of six validated survey instruments), had medical records available and reviewed, and were confirmed to be eligible.
After eligibility confirmation, participants were issued a test kit promotional code to receive a test kit. Participants used the promotional code to create an account on the Color Health website14 and to activate their testing kit. After code activation, participants were mailed a saliva collection kit with instructions on sample collection. A study-specific email and phone line are available for questions and/or technical assistance.
Germline Genetic Testing
Germline genetic testing costs were covered by institutional research study funds and by foundational grants. Genetic testing was performed on the saliva sample via the clinical Color Genomics targeted panel of 30 cancer predisposition genes: APC, ATM, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A(p14ARF), CDKN2A (p16INK4a), CHEK2, EPCAM, GREM1, MITF, MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, POLD1, POLE, PTEN, RAD51C, RAD51D, SMAD4, STK11, and TP53.
Statistical Analysis
Patient demographics, decisions to participate, and univariate associations with advancement through stages to complete genetic testing were evaluated using Kruskal-Wallis or Fisher's exact tests. Post hoc multivariate logistic regression examined odds of completing genetic testing for non-Hispanic White participants (v combined Asian and Asian American, African American and Black, Hispanic, Latino and Spanish origin, and mixed and/or other participants) after adjusting for age at diagnosis, marital status, number of biological children, highest education, employment, and income level. All calculations were performed using R version 3.6.3.15 A P value < .05 was considered statistically significant.
RESULTS
Between September 16, 2016, and August 18, 2021, 816 participants were enrolled and included in this analysis. The demographics of the study participants are summarized in Table 1. Eighty-three percent (678 of 816) of participants completed the minimum survey requirements, were confirmed to be eligible after medical records review, and were issued a test kit promotion code. The remaining 16.9% (138 of 816) of participants either did not upload their medical records or did not authorize the study team to do so on their behalf so that eligibility could not be confirmed. Among the 99.4% (811 of 816) of participants for whom the time to complete survey was available, the median time spent for survey completion was 56 minutes. Of the participants who were issued a test kit promotion code, 84% (567 of 678) activated the testing account, of whom 97% (551 of 567) completed the testing process and received results (Fig 1).
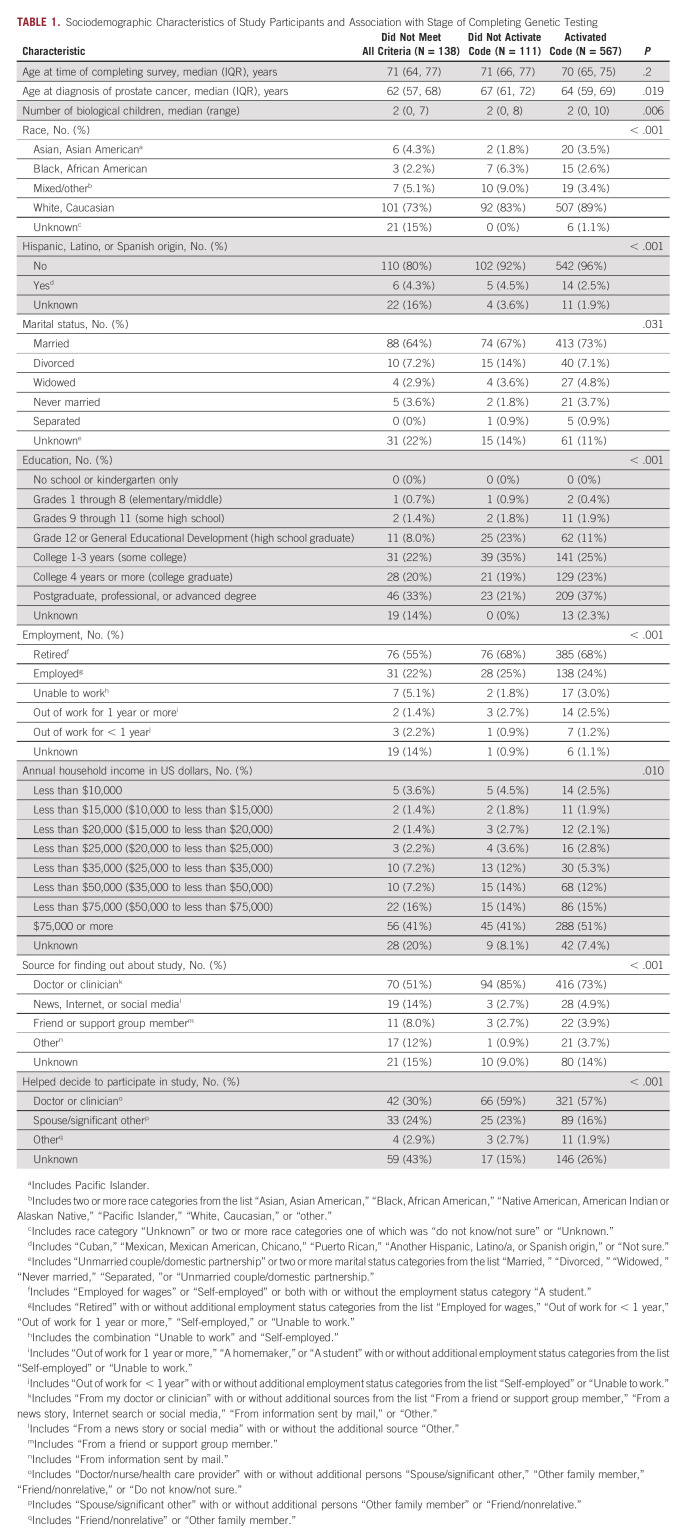
TABLE 1.
Sociodemographic Characteristics of Study Participants and Association with Stage of Completing Genetic Testing
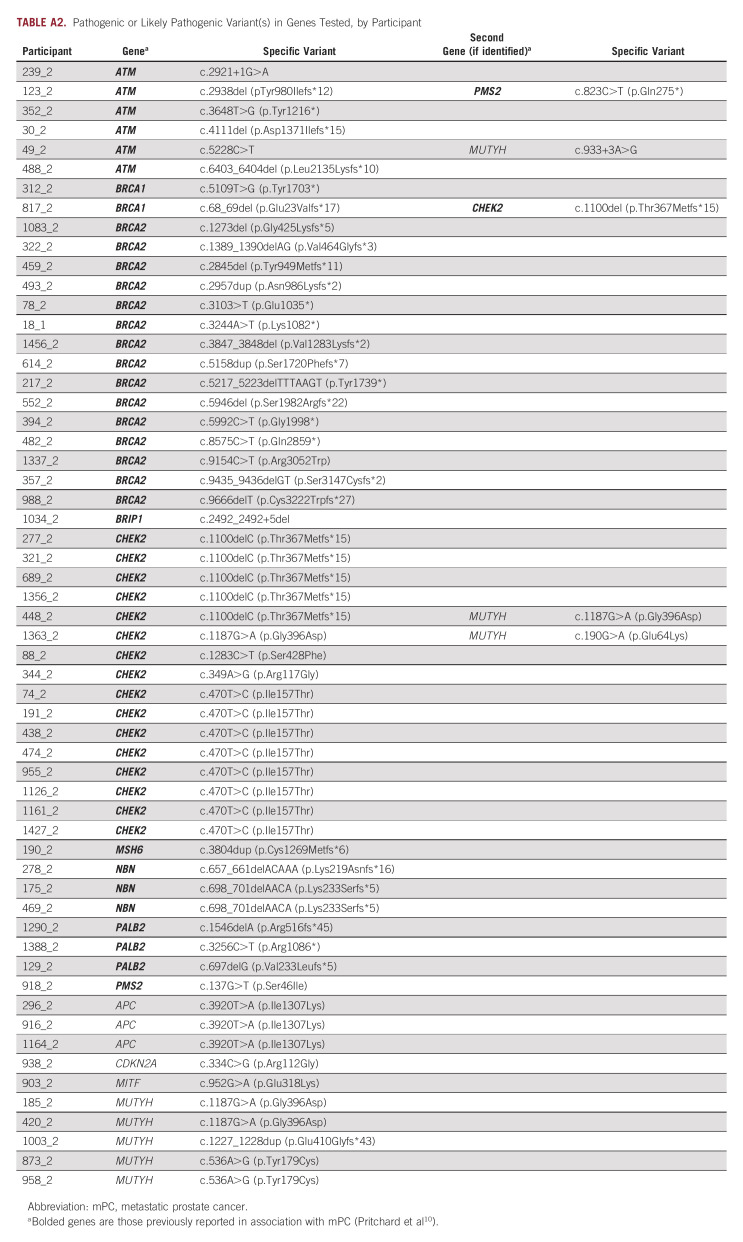
Pathogenic or likely pathogenic variant (P/LPV) in genes previously associated with mPC were identified in 8.7% (48 of 551) of participants who completed testing (Fig 2). Among the 503 participants with no P/LPV in genes not currently associated with prostate cancer, 2% (10 of 503) had P/LPV in genes associated with risk of other nonprostate cancers; 18% (92 of 503) were found to have no P/LPV, but at least one variant of unknown significance (VUS). Two percent (10 of 503) had a P/LPV in a gene not currently associated with prostate cancer and at least one VUS (Appendix Table A1 and Table A2). All participants found to have any P/LPV were required to receive their results through a phone or telehealth genetic counseling visit. Among the 493 men without P/LPV in any of the genes tested, 4% (20 of 493) requested follow-up post-test phone genetic counseling in addition to the results letter, of whom 25% (5 of 20) had a reported VUS.
FIG 2.
Distribution of genes associated with prostate cancer with pathogenic and likely pathogenic variants. Fifty L/PV found among 48 unique patients who underwent genetic testing: One participant found to have L/PV in ATM and PMS2, and one participant found to have L/PV in BRCA1 and CHEK2. L/PV, pathogenic and likely pathogenic variants.
Advancement through the stages of complete testing was associated with race and ethnicity (both P < .001), age at diagnosis (P = .02), marital status (P = .03), number of biological children (P = .006), education (P < .001), employment status (P < .001), and annual household income (P = .01). Most participants completing testing self-identified as non-Hispanic White, married, and reported higher education and annual household income > $75,000 US dollars (Table 1). In multivariate analysis, the odds of completing testing for non-Hispanic White participants was 1.15 times (95% CI, 1.04 to 1.26; P = .005) the odds for other participants. Despite a smaller proportion of Asian and Asian American, African American and Black, Hispanic, Latino and Spanish origin, and mixed or other participants, the dropoff rate at each step was similar across all of these groups (Appendix Fig A1).
DISCUSSION
Germline genetic testing is increasingly relevant to the care of men with mPC and their families, as reflected in various guidelines, including the NCCN Prostate Cancer as well as Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic guidelines.16,17 When the GENTleMEN study was conceptualized and initiated, there was a limited role for germline genetic testing in prostate cancer which was largely reserved for those with a very strong family history of cancer rather than for patients with a personal history of mPC. Thus, the initial study priority in 2016 was to investigate feasibility of a new method (patient-driven, Internet-based) of germline genetic test delivery. In this preliminary report, we observed that the participant follow-through from eligibility verification and test code issuance through completion of genetic testing was approximately two-thirds, demonstrating feasibility of this approach. Moreover, as the COVID-19 pandemic unfolded, participants were able to continue to enroll and proceed through the steps of the study remotely.
The two-thirds completion rate may result from a variety of factors. For example, reliance on patient-driven actions and timing may be suboptimal. Patients may be initiating systemic therapies such as androgen deprivation therapy, oral androgen receptor signaling inhibitors (eg, abiraterone or enzalutamide) and/or docetaxel chemotherapy, and overwhelmed by competing priorities and the participant time burden. The median time to complete survey instruments was unexpectedly long at nearly an hour, and some participants may have simply given up because of time burden. It may also be that in some cases an incomplete understanding of importance of genetic testing or confusion about study process and/or hesitancy or personal decision not to proceed with genetic testing may also have been barriers. Because of the self-driven, multifactorial nature of recruitment for GENTleMEN, we are not able to determine reasons participants do not follow-through if they do not wish to share the feedback.
We believe that removing insurance/cost and access considerations may have helped individuals who were motivated get testing. The baseline knowledge about cancer genetics and validated distress measures will be reported separately and may reveal unique aspects of communication and decision making around genetics in individuals with mPC who opted to participate. We found that the participants in GENTleMEN who completed testing were largely non-Hispanic White, married, highly educated, and from a higher-income bracket. These demographic characteristics may associate with early adoption of new methods along with greater comfort and trust with the Internet and electronic media applications. It may also be the case that these men were more likely to hear about the study because of their medical providers and/or participation in patient support groups may have had further encouragement by married partners to follow-through, and the time and means to complete the survey and testing process. We plan to increase education and outreach to more diverse members of our community to increase awareness of genetic testing and the study. An important limitation is that we are not able to accurately distinguish between participants who changed their mind after consenting and decided not to proceed from those who wished to proceed but ran into technical difficulties and did not reach out for assistance. We acknowledge this will be important to understand further, as the approaches to address each may be different.
The proportion of men with mPC and pathogenic germline mutations in GENTleMEN reported here (approximately 9%) is similar but slightly lower than reported in previous studies (approximately 12%-17%).18-20 This could potentially be due to differences in patient populations, for example, men with the most striking family histories of cancer may be more likely to be referred to clinical genetics and genetic counseling for testing and/or have undergone cascade genetic testing. Thus, participants of the GENTleMEN study may represent individuals without the most striking family cancer histories. On the other hand, some family cancer history may be a motivating factor for participation and follow through. Preliminary analysis did not suggest family history of prostate cancer to be associated with test completion, but further analysis is planned with study completion.
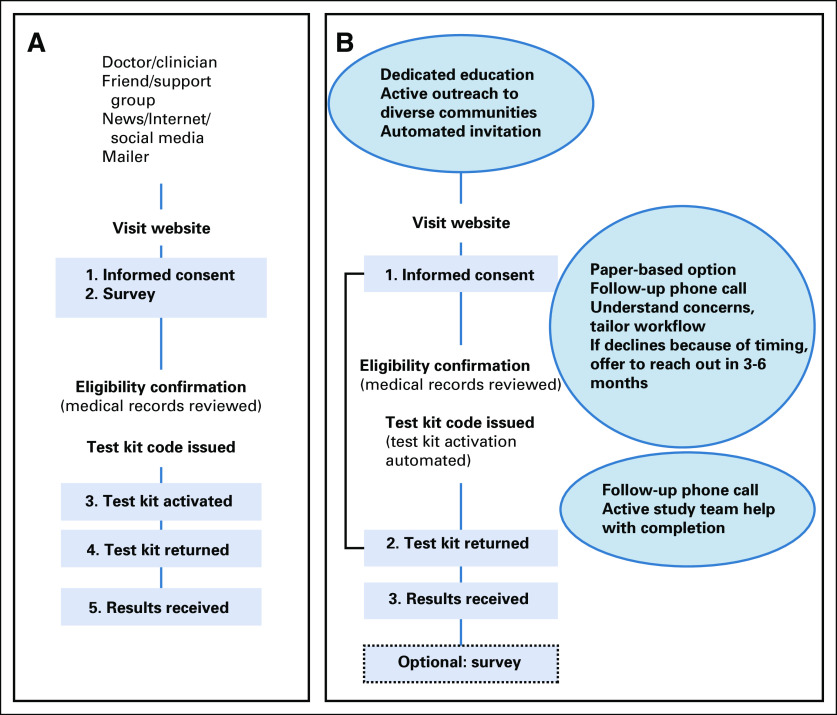
Although our findings demonstrate the feasibility of an Internet-based approach, they also reveal limitations. Completing study surveys and activating testing kit promotional codes represented barriers for participants with significant dropoff at each of these steps. Following these interim findings, the recruitment strategies and workflow are being altered (Fig 3). As discussed above, the GENTleMEN study was not designed to systematically assess reasons participants did not ultimately follow through, but we acknowledge these reasons are important to understand, especially as NCCN guidelines now recommend offering genetic testing, and we anticipate that genetics care access and insurance coverage will improve. For example, other studies have been reported and/or are also investigating novel education and care delivery methods, including mainstreaming21 video education in the PROGEN study (NCT03328091),22 and web-based education in the TARGET study (NCT04447703). These models will help extend and triage genetic counselor services to highest need/impact care points, such as for individuals with pathogenic or likely pathogenic variant, with VUS, and/or in cascade genetic testing. The results from GENTleMEN suggest that a fraction of men with mPC are likely to be able to successfully engage in self-directed, Internet-based genetic testing, similar men in the video-education arm of PROGEN and in the web-based education arm of TARGET.
FIG 3.
Steps to improve completion, inclusiveness, and diversity. (A) Initial study flow (left) and (B) proposed modifications (right). Numbered boxes are required steps for participants. Compared with (A), (B) illustrates reduced participant-required steps from five to three and changes the order of survey instruments. Circles contain additional proposed modifications to support broadened recruitment and more active participant support.
In 2022, germline genetic testing is now standard of care for many individuals with prostate cancer, so insurance coverage may be less of a barrier than in the past. However, there remains the need to minimize disparities observed in the study to date. We observed similar dropoff across all non-White race and ethnicity groups (Asian and Asian American, African American and Black, Hispanic, Latino and Spanish origin, and mixed or other participants). This has been previously reported and warrants urgent attention.23,24 Development of tailored and culturally sensitive and tailored genetic counseling education materials and resources can be codeveloped with appropriate community leader partnerships.
To address some of these issues, we are planning specific modifications to the study flow with dedicated education, active outreach, and engagement within communities that are underrepresented and also plan to minimize or remove study steps that are burdensome to participants (Fig 3). Other steps such as automating and systematizing invitation within a clinic or health care system may also be effective. We plan to offer paper-based survey options, for those more comfortable answering in this format. In addition, more dedicated clinical support resources, through follow-up phone calls, web and in-person options for help (with patient preference and ease of scheduling in mind).
Additional clinical and research efforts are needed to understand and address imbalances that may further exacerbate health disparities. In parallel, we are investigating potential differences observed with recruitment of patients to a germline testing study using cancer registry ascertainment of men with mPC (NCT04254133).25 In addition, further integration with care navigation where possible will also help reduce disparities and promote equitable access to this important aspect of care. This and other studies will be needed to determine whether a patient-driven, Internet-based approach can be further optimized to be broadly feasible and applicable to all patients or whether they are mainly improving convenience to those most likely to be offered and most likely to be enthusiastic in participating in genetic testing. On a patient level, continued attention and clinical partnership with the oncology and genetics teams, dedicated efforts to ensure patient-tailored education, repeated opportunities to engage, and hybrid workflows with additional education, support, and reminders will likely be needed and may require different approaches for different patient needs. From a systems level, multifaceted approaches with collaborative stakeholder involvement, combined with ongoing research and re-evaluation, will be needed to further optimize genetics care access and delivery for all men with prostate cancer meeting criteria for germline genetic testing.
In conclusion, Internet-based, patient-driven delivery of genetic testing is feasible for some patients, and the proportion of pathogenic or likely pathogenic variant in DNA repair genes associated with prostate cancer is consistent with expected rates. Although our approach addresses some barriers to meeting the needs of expanded genetic testing guidelines (access), remaining barriers exist and need further study and optimization to ensure all men with mPC have access to genetic testing and counseling services.
ACKNOWLEDGMENT
We would like to acknowledge our coauthor, D.B., PhD, who passed before the publication of this work. “Deb” was a highly accomplished researcher and mentor to many. Her career's contributions were focused on biobehavioral determinants of diverse forms of health care delivery and health promotion, including cancer prevention and genomic medicine. Her input was foundational to this project, and its impact represents an important part of her enduring legacy.
APPENDIX
FIG A1.
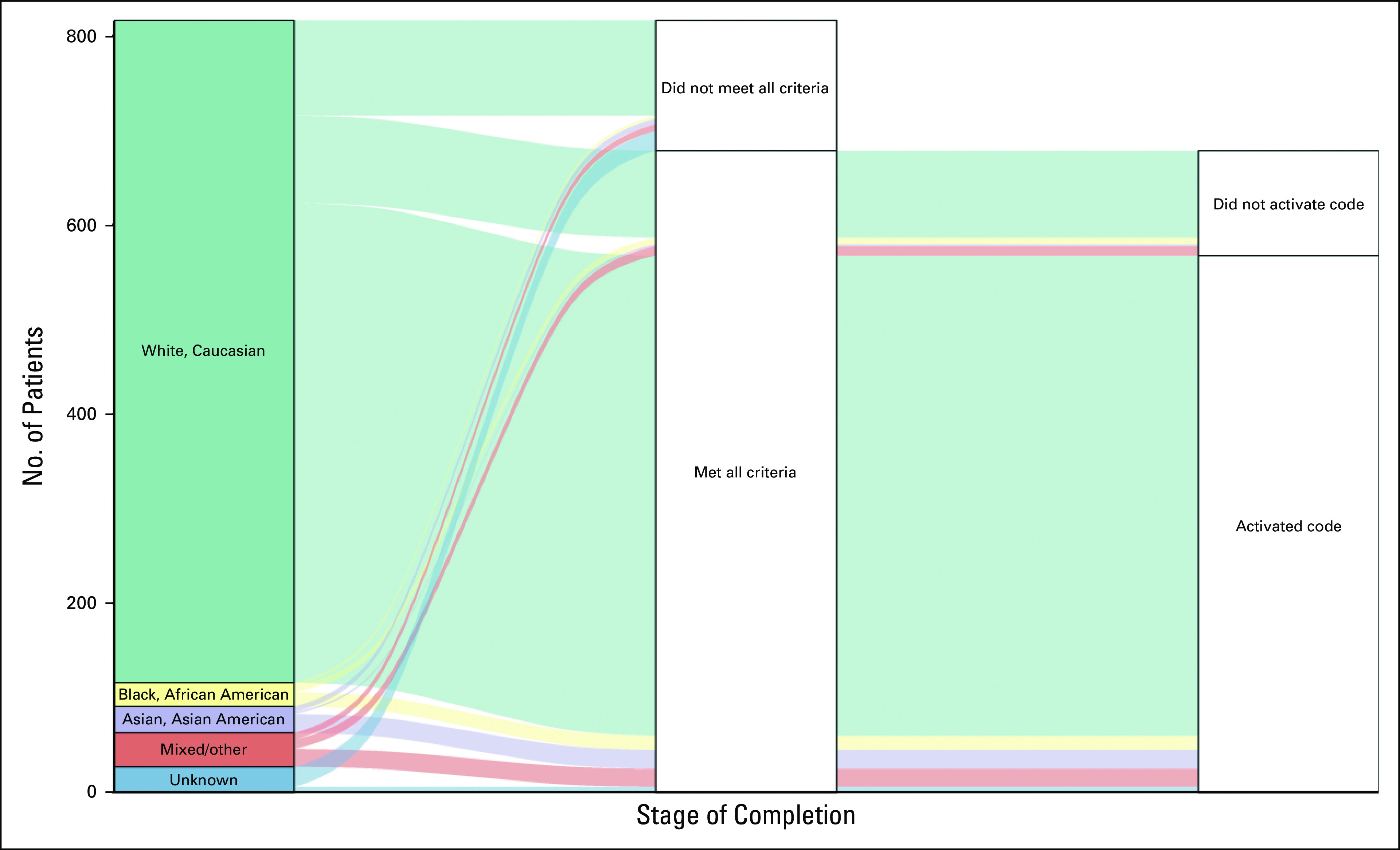
Alluvial plot of stages of completing genetic testing by race category. “Asian, Asian American” includes Pacific Islander; “Mixed/Other” includes two or more race categories from the list “Asian, Asian American,” “Black, African American,” “Native American, American Indian or Alaskan Native,” “Pacific Islander,” “White, Caucasian,” or “Other”; “Unknown” includes race category “Unknown” or two or more race categories one of which was do not know/not sure or “Unknown.”
TABLE A1.
Cross-Tabulation of the Number of P/LPV Associated With Prostate Cancer and the Number of VUS Among Participants Who Completed Testing
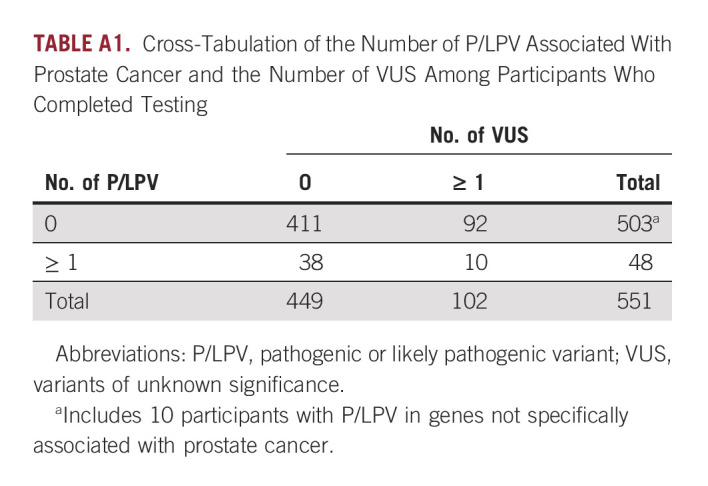
TABLE A2.
Pathogenic or Likely Pathogenic Variant(s) in Genes Tested, by Participant
Heather H. Cheng
Consulting or Advisory Role: AstraZeneca
Research Funding: Sanofi (Inst), Janssen (Inst), Clovis Oncology (Inst), Color Genomics Foundation (Inst), Medivation/Astellas (Inst), Phosplatin Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: Janssen
Roman Gulati
Employment: Linda Fukuda Family Dentistry
Petros Grivas
Consulting or Advisory Role: Merck, Bristol Myers Squibb, AstraZeneca, EMD Serono, Seattle Genetics, Pfizer, Janssen, Mirati Therapeutics, Exelixis, Roche, Genentech, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma, Regeneron, Astellas Pharma, Guardant Health, Urogen Pharma, Gilead Sciences, Silverback Therapeutics, BostonGene, Fresenius Kabi, Lucence, PureTech, G1 Therapeutics
Research Funding: Pfizer (Inst), Clovis Oncology (Inst), Bavarian Nordic (Inst), Bristol Myers Squibb (Inst), Debiopharm Group (Inst), Merck (Inst), QED Therapeutics (Inst), GlaxoSmithKline (Inst), Mirati Therapeutics (Inst), EMD Serono (Inst), G1 Therapeutics (Inst), Gilead Sciences (Inst)
Andrew Hsieh
Honoraria: Hotspot Therapeutics
Research Funding: Denali Therapeutics
Patents, Royalties, Other Intellectual Property: MTOR modulators and uses thereof patent number: 9629843, Use of Translational Profiling to Identify Target Molecules for Therapeutic Treatment Publication number: 20140288097
John K. Lee
Consulting or Advisory Role: Hierax Therapeutics
Speakers' Bureau: Mission Bio
Research Funding: Immunomedics
Patents, Royalties, Other Intellectual Property: Patent related to the development of STEAP1 chimeric antigen receptor T-cell therapy
Michael T. Schweizer
Consulting or Advisory Role: Resverlogix, AstraZeneca, PharmaIn, Sanofi
Research Funding: Janssen (Inst), AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Zenith Epigenetics (Inst), Madison Vaccines, Inc (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Tmunity Therapeutics, Inc (Inst), SignalOne Bio (Inst)
Todd Yezefski
Honoraria: Pfizer/Myovant
Consulting or Advisory Role: Dendreon
Alicia Zhou
Employment: Color Genomics
Stock and Other Ownership Interests: Color Genomics
Travel, Accommodations, Expenses: Color Genomics
Evan Y. Yu
Consulting or Advisory Role: Janssen, Bayer, Merck, Advanced Accelerator Applications, Exelixis, Oncternal Therapeutics
Research Funding: Dendreon (Inst), Merck (Inst), Seattle Genetics (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Blue Earth Diagnostics (Inst), Bayer (Inst), Lantheus Medical Imaging (Inst), Surface Oncology (Inst)
Peter S. Nelson
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Roche/Genentech, Bristol Myers Squibb
Research Funding: Genomic Health (Inst), Janssen Oncology
Expert Testimony: Venable
Travel, Accommodations, Expenses: Janssen Oncology
Bruce Montgomery
Research Funding: AstraZeneca (Inst), Janssen Oncology (Inst), Clovis Oncology (Inst), Astellas Pharma (Inst), Beigene (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by awards from the Institute for Prostate Cancer Research, Pacific Northwest Prostate Cancer SPORE P50 CA097186, Prostate Cancer Foundation, NIH/NCI Cancer Center Support Grant P30 CA015704, T32CA009515, R50 CA221836, DOD PCRP grants: W81XWH-18-1-0354, W81XWH-21-1-0264, and Institute for Translational Health UL1 TR002319.
AUTHOR CONTRIBUTIONS
Conception and design: Heather H. Cheng, Deborah Bowen, Nola Klemfuss, Peter S. Nelson, Bruce Montgomery
Financial support: Roman Gulati, Alicia Zhou, Peter S. Nelson, Bruce Montgomery
Administrative support: Nola Klemfuss, Peter S. Nelson
Provision of study materials or patients: Petros Grivas, Andrew Hsieh, John K. Lee, Michael T. Schweizer, Todd Yezefski, Evan Y. Yu, Bruce Montgomery
Collection and assembly of data: Heather H. Cheng, Nola Klemfuss, Andrew Hsieh, Michael T. Schweizer, Todd Yezefski, Alicia Zhou, Evan Y. Yu
Data analysis and interpretation: Heather H. Cheng, Alexandra O. Sokolova, Roman Gulati, Sarah A. Knerr, Petros Grivas, John K. Lee, Michael T. Schweizer, Todd Yezefski, Alicia Zhou, Bruce Montgomery
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Heather H. Cheng
Consulting or Advisory Role: AstraZeneca
Research Funding: Sanofi (Inst), Janssen (Inst), Clovis Oncology (Inst), Color Genomics Foundation (Inst), Medivation/Astellas (Inst), Phosplatin Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: Janssen
Roman Gulati
Employment: Linda Fukuda Family Dentistry
Petros Grivas
Consulting or Advisory Role: Merck, Bristol Myers Squibb, AstraZeneca, EMD Serono, Seattle Genetics, Pfizer, Janssen, Mirati Therapeutics, Exelixis, Roche, Genentech, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma, Regeneron, Astellas Pharma, Guardant Health, Urogen Pharma, Gilead Sciences, Silverback Therapeutics, BostonGene, Fresenius Kabi, Lucence, PureTech, G1 Therapeutics
Research Funding: Pfizer (Inst), Clovis Oncology (Inst), Bavarian Nordic (Inst), Bristol Myers Squibb (Inst), Debiopharm Group (Inst), Merck (Inst), QED Therapeutics (Inst), GlaxoSmithKline (Inst), Mirati Therapeutics (Inst), EMD Serono (Inst), G1 Therapeutics (Inst), Gilead Sciences (Inst)
Andrew Hsieh
Honoraria: Hotspot Therapeutics
Research Funding: Denali Therapeutics
Patents, Royalties, Other Intellectual Property: MTOR modulators and uses thereof patent number: 9629843, Use of Translational Profiling to Identify Target Molecules for Therapeutic Treatment Publication number: 20140288097
John K. Lee
Consulting or Advisory Role: Hierax Therapeutics
Speakers' Bureau: Mission Bio
Research Funding: Immunomedics
Patents, Royalties, Other Intellectual Property: Patent related to the development of STEAP1 chimeric antigen receptor T-cell therapy
Michael T. Schweizer
Consulting or Advisory Role: Resverlogix, AstraZeneca, PharmaIn, Sanofi
Research Funding: Janssen (Inst), AstraZeneca (Inst), Roche (Inst), Pfizer (Inst), Zenith Epigenetics (Inst), Madison Vaccines, Inc (Inst), Immunomedics (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Tmunity Therapeutics, Inc (Inst), SignalOne Bio (Inst)
Todd Yezefski
Honoraria: Pfizer/Myovant
Consulting or Advisory Role: Dendreon
Alicia Zhou
Employment: Color Genomics
Stock and Other Ownership Interests: Color Genomics
Travel, Accommodations, Expenses: Color Genomics
Evan Y. Yu
Consulting or Advisory Role: Janssen, Bayer, Merck, Advanced Accelerator Applications, Exelixis, Oncternal Therapeutics
Research Funding: Dendreon (Inst), Merck (Inst), Seattle Genetics (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Blue Earth Diagnostics (Inst), Bayer (Inst), Lantheus Medical Imaging (Inst), Surface Oncology (Inst)
Peter S. Nelson
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Roche/Genentech, Bristol Myers Squibb
Research Funding: Genomic Health (Inst), Janssen Oncology
Expert Testimony: Venable
Travel, Accommodations, Expenses: Janssen Oncology
Bruce Montgomery
Research Funding: AstraZeneca (Inst), Janssen Oncology (Inst), Clovis Oncology (Inst), Astellas Pharma (Inst), Beigene (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Abida W, Patnaik A, Campbell D, et al. : Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol 38:3763-3772, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN Guidelines Version 1.2022. Genetic/Familial High-Risk Assessment: Breast and Ovarian. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf.
- 5.NCCN Prostate Cancer Guidelines Version 3.2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 6.Paller CJ, Antonarakis ES, Beer TM, et al. : Germline genetic testing in advanced prostate cancer - practices and barriers: Survey results from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. Clin Genitourin Cancer 17:275-282, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeb S, Li R, Sanchez Nolasco T, et al. : Barriers and facilitators of germline genetic evaluation for prostate cancer. Prostate 81:754-764, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Project REDCAP: Research Electronic Data Capture. http://www.projectredcap.org.
- 9.Gentleman Study. http://www.gentlemanstudy.org.
- 10.Spitzer RL, Kroenke K, Williams JB, et al. : A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 166:1092-1097, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 16:606-613, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinney AY, Butler KM, Schwartz MD, et al. : Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst. 106:dju328, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. : Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 32:618-26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Color Health, Inc. https://www.color.com.
- 15.R version 3.6.3. https://cloud.r-project.org
- 16.Mohler JL, Higano CS, Schaeffer EM, et al. : Current recommendations for prostate cancer genetic testing: NCCN prostate guideline. Can J Urol 26:34-37, 2019. (5 Suppl 2) [PubMed] [Google Scholar]
- 17.Giri VN, Knudsen KE, Kelly WK, et al. : Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus conference 2019. J Clin Oncol 38:2798-2811, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pritchard CC, Mateo J, Walsh MF, et al. : Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 375:443-453, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro E, Romero-Laorden N, Del Pozo A, et al. : PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol 37:490-503, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Yadav S, Hart SN, Hu C, et al. : Contribution of inherited DNA-repair gene mutations to hormone-sensitive and castrate-resistant metastatic prostate cancer and implications for clinical outcome. JCO Precis Oncol 10.1200/PO.19.00067, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheinberg T, Goodwin A, Ip E, et al. : Evaluation of a mainstream model of genetic testing for men with prostate cancer. JCO Oncol Pract 17:e204-e216, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Rana HQ, Stopfer JE, Petrucelli N, et al. : A randomized controlled trial of video-education or in-person genetic counseling for men with prostate cancer (ProGen). J Clin Oncol 38, 2020. (suppl; abstr 1507) [Google Scholar]
- 23.Kwon DH, Borno HT, Cheng HH, et al. : Ethnic disparities among men with prostate cancer undergoing germline testing. Urol Oncol 38:80 e1-80 e7, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Weise N, Shaya J, Javier-Desloges J, et al. : Disparities in germline testing among racial minorities with prostate cancer. Prostate Cancer Prostatic Dis 25:403-410, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan H, Wong RL, Darst B, et al. : Rates of germline genetic testing and DNA damage response mutations found through population-based recruitment of men with incident metastatic prostate cancer. J Clin Oncol 40, 2022. (suppl 16; abstr 10501) [Google Scholar]