Abstract
Aims/hypothesis
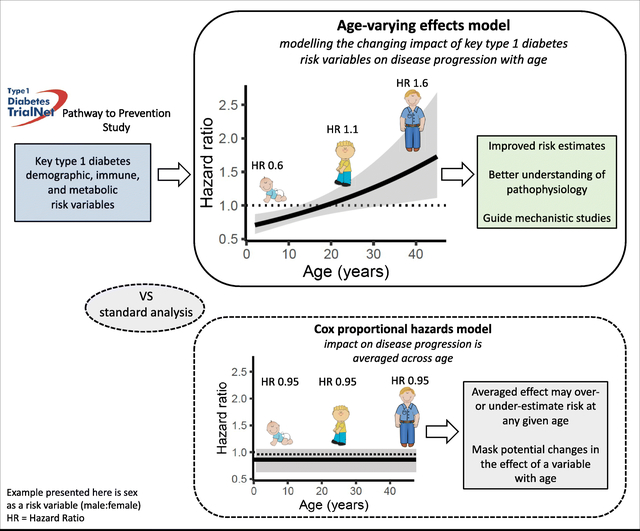
Age is known to be one of the most important stratifiers of disease progression in type 1 diabetes. However, what drives the difference in rate of progression between adults and children is poorly understood. Evidence suggests that many type 1 diabetes disease predictors do not have the same effect across the age spectrum. Without a comprehensive analysis describing the varying risk profiles of predictors over the age continuum, researchers and clinicians are susceptible to inappropriate assessment of risk when examining populations of differing ages. We aimed to systematically assess and characterise how the effect of key type 1 diabetes risk predictors changes with age.
Methods
Using longitudinal data from single- and multiple-autoantibody-positive at-risk individuals recruited between the ages of 1 and 45 years in TrialNet’s Pathway to Prevention Study, we assessed and visually characterised the age-varying effect of key demographic, immune and metabolic predictors of type 1 diabetes by employing a flexible spline model. Two progression outcomes were defined: participants with single autoantibodies (n=4893) were analysed for progression to multiple autoantibodies or type 1 diabetes, and participants with multiple autoantibodies were analysed (n=3856) for progression to type 1 diabetes.
Results
Several predictors exhibited significant age-varying effects on disease progression. Amongst single-autoantibody participants, HLA-DR3 (p=0.007), GAD65 autoantibody positivity (p=0.008), elevated BMI (p=0.007) and HOMA-IR (p=0.002) showed a significant increase in effect on disease progression with increasing age. Insulin autoantibody positivity had a diminishing effect with older age in single-autoantibody-positive participants (p<0.001). Amongst multiple-autoantibody-positive participants, male sex (p=0.002) was associated with an increase in risk for progression, and HLA DR3/4 (p=0.05) showed a decreased effect on disease progression with older age. In both single- and multiple-autoantibody-positive individuals, significant changes in HR with age were seen for multiple measures of islet function. Risk estimation using prediction risk score Index60 was found to be better at a younger age for both single- and multiple-autoantibody-positive individuals (p=0.007 and p<0.001, respectively). No age-varying effect was seen for prediction risk score DPTRS (p=0.861 and p=0.178, respectively). Multivariable analyses suggested that incorporating the age-varying effect of the individual components of these validated risk scores has the potential to enhance the risk estimate.
Conclusions/interpretation
Analysing the age-varying effect of disease predictors improves understanding and prediction of type 1 diabetes disease progression, and should be leveraged to refine prediction models and guide mechanistic studies.
Keywords: Age, Disease progression, Prediction, Risk factors, Type 1 diabetes
Graphical Abstract
Introduction
The impact of age on the rate of progression of type 1 diabetes is now irrefutable. Observations of prospective birth cohorts have repeatedly demonstrated an inverse relationship between age of seroconversion and speed of progression to clinical disease [1, 2]. In addition, the incidence of risk variables has also been demonstrated to vary with age [3, 4]. In particular, autoantibodies have shown age-specific incidence patterns that have themselves been associated with particular rates of disease progression [5]. In fact, where the relative importance of risk variables has differed between studies, the age of the study cohorts can frequently be implicated as the key difference [6, 7]. At-risk cohorts are commonly analysed for progression to type 1 diabetes using survival analysis techniques, including Kaplan–Meier plots and Cox proportional hazards models [8]. Fundamentally, these models rely on the assumption that the variables being investigated have a constant impact on risk over time, i.e. that the hazard is proportional. Without a comprehensive analysis describing the varying risk profiles of predictors over the age continuum, researchers and clinicians are susceptible to inappropriate assessment of risk when examining populations of differing ages.
As a step towards determining the age-varying effect of known risk variables, we used a flexible spline model to test the validity of the proportional hazards assumption and describe the nature of the variation of hazards over the age spectrum. By uncovering age-dependent patterns of risk, this modelling approach allows a more granular understanding of the influence of predictors on disease progression, which has the potential to deepen our understanding of the differences between paediatric and adult-onset disease, improve prediction models, and inform mechanistic studies designed to understand disease heterogeneity.
Methods
Study population
TrialNet’s Pathway to Prevention (PTP) study (ClinicalTrials.gov identifier: NCT00097292) recruits at-risk first-degree relatives (ages 1–45 years) and second-or third-degree relatives (ages 1–20 years) of individuals with type 1 diabetes, and prospectively follows these individuals for the development of islet autoantibodies and/or progression to clinical disease; it is conducted across sites in USA, Canada, UK, Germany, Italy, Australia and New Zealand [9]. Open access to the data from TrialNet’s PTP study is available at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) repository (https://repository.niddk.nih.gov/studies/tn01-nh/?query=TrialNet). Participants were included in this analysis if they completed at least two follow-up visits and had confirmed single or multiple autoantibody positivity. Informed consent was obtained from all study participants and the study was approved by the responsible ethics committee. From March 2004 to April 2019, the PTP study screened 219,718 individuals, of whom 49,617 participants had at least two visits, and 8176 of these individuals had at least one confirmed positive islet autoantibody.
Study protocol
The PTP study protocol initially tested each participant for autoantibodies to insulin (MIAA), GAD65 (GADA) and insulinoma-associated antigen (IA-2A). If at least one of these autoantibodies was considered positive on the first test, tests for islet cell antibodies (ICA) and zinc transporter 8 antibodies (ZnT8A; available from 2012 onwards) were subsequently performed on the same sample. After confirmed autoantibody positivity, participants had 6–12-monthly assessments that included HbA1c, OGTTs and repeat autoantibody testing.
Laboratory measures
Metabolic measures
An OGTT was performed after an overnight fast. Glucose and C-peptide measurements were obtained at baseline, 30, 60, 90 and 120 min after the glucose load (oral glucose dose 1.75g/kg, maximum 75g). Calculations made from these variables included AUC for C-peptide using the trapezoid method; early C-peptide response (defined as the difference between 30 min and baseline C-peptide values); and HOMA-IR, which is the product of fasting insulin (mU/l) and fasting glucose (mmol/l), divided by 22.5. Index60 [10] and the Diabetes Prevention Trial Type 1 Risk Score (DPTRS) [11] are type 1 diabetes risk scores developed from Diabetes Prevention Trial Type 1 data (see electronic supplementary material [ESM] Methods).
Autoantibody assay
ICA was measured by indirect immunofluorescence at the University of Florida, Gainesville, FL (cut-off for positive ≥10 JDF units) [12]. GADA, MIAA, IA-2A and ZnT8A were measured by RIA at the Barbara Davis Center for Childhood Diabetes [13, 14]. ZnT8A testing was introduced to TrialNet’s PTP study in 2012. From June 2010, GADA and IA-2A harmonised assays for NIDDK Consortia were used [15, 16]. Cut-off values to define a positive result for the standard assays performed at the Barbara Davis Center were GADA >0.032, IA-2A >0.049, MIAA >0.01 and ZnT8A >0.020, and those for the harmonised assays were GADA >20 NIDDK units/ml and IA-2A >5 NIDDK units/ml. If results from both the standard and the harmonised assays were available at a particular time point, results from the harmonised assay were used.
HLA typing
HLA genotyping for class II DRB1, DQA1 and DQB1 alleles was performed at the Barbara Davis Center using DNA-based typing with oligonucleotide probes, as previously described [17].
Diagnosis of diabetes
TrialNet protocol definitions to diagnose type 1 diabetes were used, which encompass the ADA criteria [18]. Clinical (stage 3) diabetes was defined as having unequivocal symptoms of hyperglycaemia, including diabetic ketoacidosis, with at least one of random or 2 hour glucose ≥11.1mmol/l, or fasting glucose ≥7.0mmol/l. If symptoms were not present, two separate consecutive glucose measures were required from separate days within one year.
Statistical methods
Outcome events were classified as the occurrence of multiple autoantibodies or a diagnosis of type 1 diabetes within single-autoantibody-positive individuals, or a diagnosis of type 1 diabetes within multiple-autoantibody-positive individuals. Baseline descriptive summary statistics for variables use counts and percentages for categorical variables, and means and standard deviations for continuous variables. These summaries were produced for the overall population and for each outcome group.
Risk is measured by the hazard rate, and modelled using a particular structure of the binary logistic regression model [19], implemented in the casebase R package [20]. The logistic regression model was fitted using the mgcv R package [21, 22], which allows the incorporation of spline terms that employ automatic smoothness selection. Each risk predictor of interest is modelled individually, and select groups of variables are combined in multivariable models. Variable effects are decomposed into constant mean proportional hazards terms and potentially time-varying smooth effects, and the significance of these time-varying effects are interpreted. Where the time-varying effect (HRt) p value was ≤0.05, the variable was considered to have a significant age-dependent variation in risk. Encounters during the first year of life were excluded due to potential confounding maternal autoantibodies. Model-based estimates after 45 years of age are not presented due to a paucity of observations (ESM Fig. 1). The standard Cox proportional hazards model was fitted to the same data to provide a comparator with the mean effect from the time-varying effect model. Further details on the statistical methods are given in ESM Methods. The analysis code for this study is available upon request via email to the primary author.
Results
Time-dependent analysis applied to TrialNet Pathway to Prevention
From the 8176 relatives with confirmed autoantibodies on at least two occasions, 47,029 participant encounters were available for analysis. Demographic and baseline variables are detailed in ESM Table 1. Two progression outcomes were considered: (1) a single autoantibody to a composite endpoint of multiple autoantibodies or type 1 diabetes, and (2) multiple autoantibodies to type 1 diabetes. Of the 4893 single-autoantibody-positive individuals, 743 met the composite endpoint of multiple autoantibodies or type 1 diabetes; of the 3856 individuals with multiple autoantibodies, 896 developed type 1 diabetes. The mean HR and the estimated significance of the variation of hazard as a function of age for each of these risk variables (HRt p value) are summarised in Table 1.
Table 1.
Mean HR for progression and the estimated significance of the variation of hazard as a function of age (HRt) for type 1 diabetes risk variables
| Single to multiple/type 1 diabetes | Multiple to type 1 diabetes | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Group | HR (95% CI)a | p value HRt | HR (95% CI)a | p value HRt | ||
| Genetic data | |||||||
| Sex | Male vs female | 1.30 (1.10, 1.53) | 0.414 | 0.95 (0.83, 1.09) | 0.002 | ||
| Relationship to proband | 2nd vs 1st | 0.54 (0.28, 1.02) | 0.707 | 0.81 (0.51, 1.30) | 0.536 | ||
| 3rd vs 1st | 0.01 (0.00, 47.9) | 0.423 | 0.55 (0.24, 1.24) | 0.062 | |||
| HLA | DR3+/DR4− vs DR3−/DR4− | 2.18 (1.63, 2.92) | 0.007 | 1.27 (0.99, 1.64) | 0.738 | ||
| DR3−/DR4+ vs DR3−/DR4− | 2.57 (1.94, 3.41) | 0.704 | 1.35 (1.07, 1.69) | 0.373 | |||
| DR3+/DR4+ vs DR3−/DR4− | 3.22 (2.33, 4.45) | 0.379 | 1.31 (1.01, 1.70) | 0.05 | |||
| Autoantibodies | |||||||
| GADA | Positive vs negative | 2.71 (2.17, 3.37) | 0.008 | 0.54 (0.43, 0.68) | 0.112 | ||
| IA-2A | Positive vs negative | 2.41 (1.77, 3.28) | 0.492 | 2.64 (2.26, 3.09) | 0.677 | ||
| MIAA | Positive vs negative | 0.49 (0.35, 0.68) | < 0.001 | 1.06 (0.91, 1.23) | 0.014 | ||
| ZNT8b | Positive vs negative | 3.96 (2.71, 5.80) | 1.73 (1.47, 2.04) | 0.728 | |||
| ICA | Positive vs negative | 2.17 (1.62, 2.89) | 0.645 | 1.70 (1.44, 2.00) | 0.316 | ||
| Metabolic variables | |||||||
| BMI (z score) | 1.16 (1.02, 1.32) | 0.007 | 1.2 (1.11, 1.31) | 0.405 | |||
| HbA1c (mmol/mol or %) | 1.42 (1.26, 1.60) | 0.451 | 2.54 (2.30, 2.82) | 0.198 | |||
| log2 fasting glucose (mmol/l) | 1.20 (1.10, 1.31) | < 0.001 | 1.62 (1.48, 1.78) | < 0.001 | |||
| log2 fasting insulin (pmol/l) | 1.09 (0.96, 1.24) | 0.003 | 1.02 (0.93, 1.12) | 0.402 | |||
| log2 HOMA-IR | 1.12 (0.99, 1.28) | 0.002 | 1.10 (1.00, 1.21) | 0.465 | |||
| log2 C-peptide AUC (nmol/l) | 0.79 (0.68, 0.91) | 0.083 | 0.65 (0.59, 0.72) | 0.003 | |||
| log2 total C-peptide (nmol/l) | 0.83 (0.73, 0.96) | 0.006 | 0.71 (0.64, 0.78) | 0.013 | |||
| Early C-peptide secretion (nmol/l) | 0.86 (0.81, 0.90) | 0.113 | 0.47 (0.41, 0.53) | 0.027 | |||
| Risk scores | |||||||
| Index60 | 2.15 (1.84, 2.51) | 0.007 | 3.89 (3.49, 4.33) | < 0.001 | |||
| DPTRS | 2.35 (2.07, 2.67) | 0.861 | 3.64 (3.34, 3.97) | 0.178 | |||
For continuous variables, HR represents one SD increase. See ESM Table 1 for the SD for each variable
Small numbers of single-ZnT8A-positive individuals limited the inferences that could be made regarding the age-varying effect
Sex and time-dependent effect on disease progression
The Cox proportional hazard model suggested that male sex was not strongly associated with an increased risk of progression to type 1 diabetes in single-autoantibody-positive individuals (HR 1.25, 95% CI 1.08, 1.45) and multiple-autoantibody-positive individuals (HR 0.90, 95% CI 0.78, 1.02) (ESM Table 2). Additionally, there was no age-varying effect of male sex on risk of disease progression for those with a single autoantibody (p=0.414). However, in multiple-autoantibody-positive individuals, male sex was associated with an increase in risk for progression to type 1 diabetes as age increased (p=0.002) (Fig. 1a). The effect of male sex was relatively protective in younger multiple-autoantibody-positive children, with the HR rising to a point estimate > 1.5 in adults with multiple autoantibodies. Hazard rates plotted by sex as a function of age in multiple-autoantibody-positive individuals showed that the increase in HR with male sex was due to a decreasing hazard rate for female participants with increasing age, compared with a relatively stable hazard rate in male participants (Fig. 1b).
Fig 1.
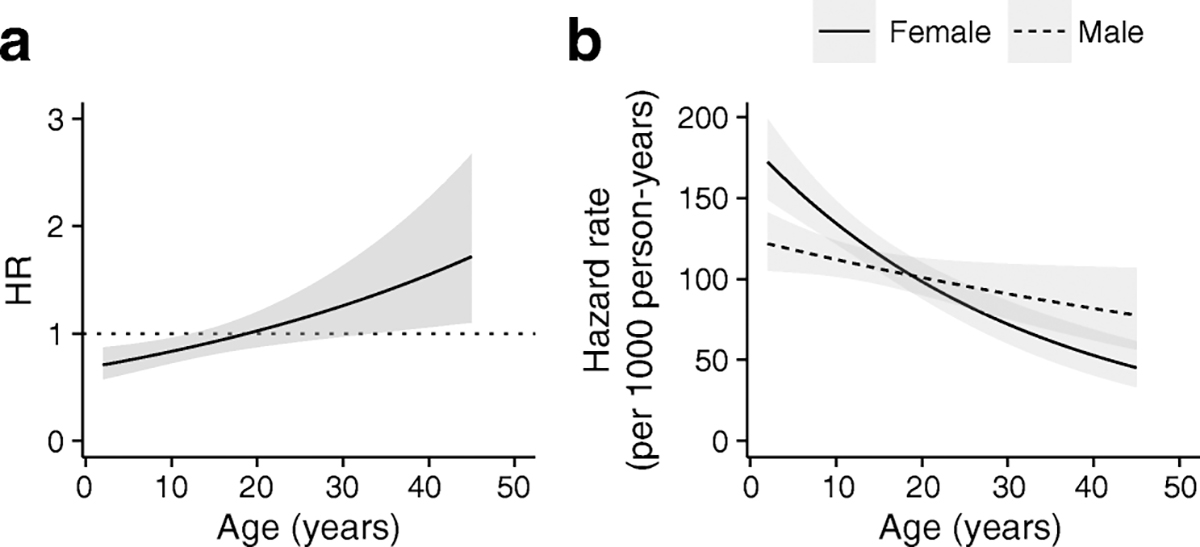
Model-estimated age-dependent effect of sex on progression from multiple-autoantibody to type 1 diabetes. (a) Age-dependent HR associated with being male for progression from multiple autoantibody positivity to type 1 diabetes (p=0.002). (b) Estimated hazard rate for progression from multiple autoantibody positivity to type 1 diabetes by sex and age. Grey shading indicates 95% CI
High-risk HLA alleles and time-dependent effect on disease progression
The Cox proportional hazard model found that single-autoantibody-positive individuals with high-risk HLA alleles had a greater risk of disease progression (ESM Table 2). However, the age-varying effect model found that the effect of HLA-DR3+/DR4− on disease progression was not constant with age in single-autoantibody-positive individuals, with a relatively small effect in children rising to a slightly increased hazard beyond adolescent years (p=0.007) (ESM Fig. 2a). HLA-DR3−/DR4+ and HLA-DR3+/DR4+ did not show an age-varying effect in single-autoantibody-positive individuals (p=0.704 and p=0.379, respectively) (Table 1). For those with multiple autoantibodies, the Cox proportional hazard model demonstrated a mild increase in risk with HLA-DR3+/DR4− (HR 1.30; CI 1.01–1.67), HLA-DR3−/DR4+ (HR 1.38; CI 1.10–1.73) and HLA-DR3+/DR4+ (HR 1.49; CI 1.18–1.90) (ESM Table 2). In contrast to single-autoantibody-positive individuals, HLA-DR3+/DR4+ demonstrated an age-varying effect, with an increased hazard for progression in children that declined with increasing age (p=0.05) (ESM Fig. 2b). No age-varying effect on disease progression was found for HLA-DR3+/DR4− or HLA-DR3−/DR4+ in multiple-autoantibody-positive individuals (p=0.738 and p=0.373, respectively) (Table 1). GADA is often reported to be present at higher rates in older individuals with HLA-DR3, and MIAA is often reported to be present in very young individuals with HLA-DR4 [23, 24]. Therefore, to determine whether these specific autoantibodies contributed to the age-dependent effects seen with HLA, the age-varying effect of HLA-DR3 was adjusted for GADA positivity and that of HLA-DR4 was adjusted for MIAA positivity. There was no significant change to the age-dependent effect of these high-risk HLA alleles in either cohort (data not shown).
Islet autoantibodies and time-dependent effect on disease progression
Amongst single-autoantibody-positive individuals, all the islet autoantibodies (GADA, ZnT8A, IA-2A and ICA) except MIAA were associated with an increased risk of disease progression using the Cox proportional hazard model (ESM Table 2). Using the age-varying effect model, the effect of GADA and MIAA, but not IA-2A or ICA, was found to vary significantly with age in single-autoantibody-positive individuals (Table 1). The effect of GADA on disease progression increased with increasing age, with the point estimate at 45 years of age being over four times that seen in children (p=0.008) (Fig. 2a). The HR of MIAA, on the other hand, decreased with increasing age; the presence of MIAA had a protective effect on progression in adults (p<0.001) (Fig. 2b). The small number of single-ZnT8A-positive individuals limited the inferences that could be drawn regarding the age-varying effect. Amongst multiple-autoantibody-positive individuals, using the Cox proportional hazards model, MIAA had no effect on progression (HR 1.00; 95% CI 0.87, 1.15), GADA had a protective effect (HR 0.48; 95% CI 0.40, 0.56) and the remaining islet autoantibodies (IA-2A, ZnT8A and ICA) were associated with an increased risk of disease progression (ESM Table 2). No age-dependent risk for progression from multiple autoantibody positivity was found for any of the autoantibodies except MIAA (Table 1). However, the slight increase in the effect of MIAA with increasing age was not significant when adjusted for the number of autoantibodies.
Fig 2.

Model-estimated age-dependent effect of islet autoantibodies on progression from single autoantibody positivity to multiple autoantibodies/type 1 diabetes. (a) Age-dependent HR associated with being GADA-positive for progression from single autoantibody positivity to multiple autoantibodies/type 1 diabetes (p=0.008). (b) Age-dependent HR for being MIAA-positive for progression from single autoantibody positivity to multiple autoantibodies/type 1 diabetes (p<0.001). Grey shading indicates 95% CI; the dotted line indicates HR of 1
Metabolic variables and time-dependent effect on disease progression
BMI z score and measures of blood glucose levels and insulin resistance were positively associated, and measures of beta cell secretory capacity were negatively associated with disease progression using the Cox proportional hazards model for single- and multiple-autoantibody-positive individuals (ESM Table 2). Using the age-varying effect model, BMI z score had an age-dependent increase in risk of progression in single-autoantibody-positive adults compared with children (p=0.007) (Fig. 3a). A similar increase in the effect on risk of progression with increasing age was seen for HOMA-IR (p=0.002) (Fig. 3b). To explore this relationship, a multivariable model with BMI z score and HOMA-IR was used, and it was found that the age-dependent risk seen with BMI z score was no longer significant when adjusted for HOMA-IR (p=0.271) (Fig. 3c). However, the age-dependent risk seen with HOMA-IR was unaffected by adjusting for BMI z score. An age-varying effect was not seen for BMI z score and HOMA-IR in multiple-autoantibody-positive individuals (Table 1).
Fig 3.
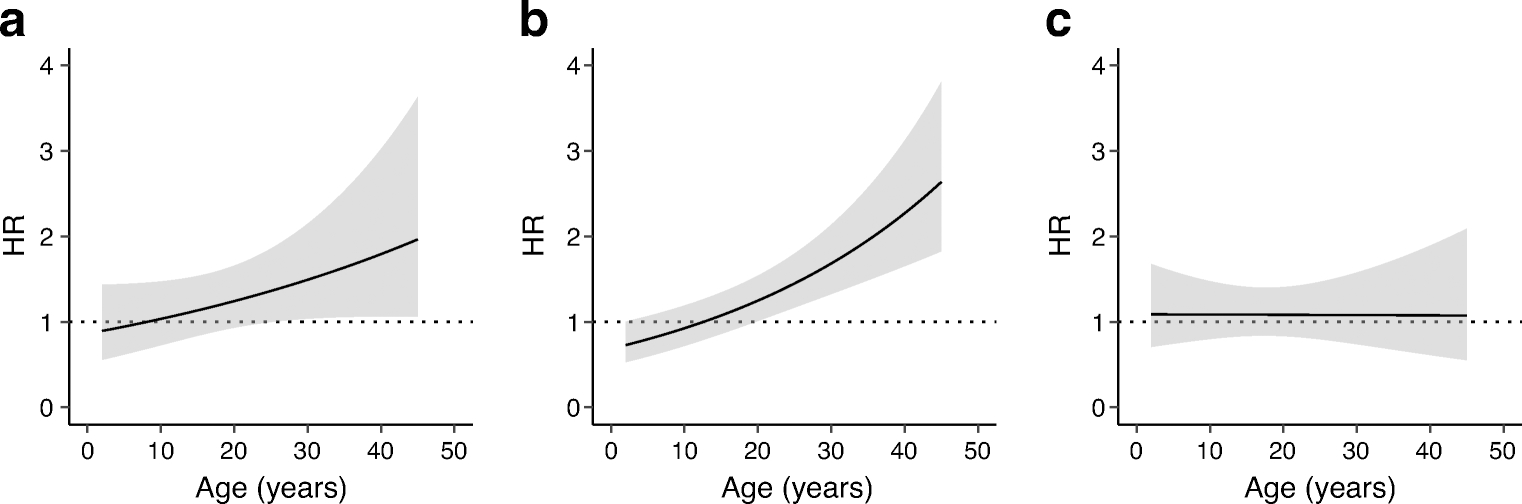
Model-estimated age-dependent effect of BMI (z score) and HOMA-IR on progression from single autoantibody positivity to multiple autoantibodies/type 1 diabetes. HR represents one SD increase. (a) Age-dependent HR for BMI (z score) for progression from single autoantibody positivity to multiple autoantibodies/type 1 diabetes (p=0.007). (b) Age-dependent HR for HOMA-IR (log2) for progression from single autoantibody positivity to multiple autoantibodies/type 1 diabetes (p=0.002). (c) Age-dependent HR for BMI (z score) adjusted for log2(HOMA-IR) for progression from single autoantibody positivity to multiple autoantibodies/type 1 diabetes (p=0.271). Grey shading indicates 95% CI
HOMA-IR is a product of fasting glucose and fasting insulin. In single-autoantibody-positive individuals, the effect of fasting glucose and fasting insulin on disease progression was found to increase with increasing age (p<0.001 and p=0.003, respectively) (ESM Fig. 3a, b). Fasting glucose had an even more pronounced effect on progression with age in multiple-autoantibody-positive individuals compared with single-autoantibody-positive individuals, increasing by close to threefold by 45 years of age (p<0.001) (ESM Fig. 3c). In contrast, fasting insulin did not demonstrate an age-dependent change in hazard in multiple-autoantibody-positive individuals. Early C-peptide secretion and C-peptide AUC demonstrated no age-varying effect on disease progression in single-autoantibody-positive individuals (p=0.113 and p=0.083, respectively). However, in multiple-autoantibody-positive individuals, a pattern of a greater protective effect on disease progression in adults compared with children was seen for both higher early C-peptide secretion and C-peptide AUC (p=0.027 and p=0.003, respectively) (ESM Fig. 4a,b).
Testing disease prediction risk estimates for an age-varying effect
As we found a significant variation of effect with age for a number of variables that contribute to established prediction risk models (Index60 and DPTRS), we sought to understand whether incorporating the age-dependent effects of the individual components would enhance these risk scores. Index60 is calculated using measures of fasting C-peptide, 60 min glucose and 60 min C-peptide. It was found to have a diminishing predictive capacity with increasing age for both single- and multiple-autoantibody-positive individuals (p=0.007 and p<0.001, respectively) (ESM Fig. 5a,b). Using a multivariable model, we tested the age-varying effects of the individual components of Index60 to determine whether they improved performance of risk prediction beyond that attributed through Index60 alone. It was found that the age-dependent effect of 60 min C-peptide contributed significantly to risk even after accounting for Index60 in single-autoantibody-positive individuals (p < 0.001). This was not found to be the case for fasting C-peptide or 60 min glucose. On the other hand, fasting C-peptide and 60 min glucose had a significant contribution to risk after accounting for Index60 amongst multiple-autoantibody-positive individuals (p=0.01 and p<0.001, respectively). This suggests that the predictive capacity of Index60 has the potential to be enhanced by incorporating into the risk estimate the age-varying effect of 60 min C-peptide amongst single-autoantibody-positive individuals, and fasting C-peptide and 60 min glucose amongst multiple-autoantibody-positive individuals.
DPTRS comprises BMI, age, total glucose, fasting C-peptide and total C-peptide, where total glucose (sum of 30–120 min) and total C-peptide (sum of 30–120 min) are used as surrogates for glucose AUC and C-peptide AUC, respectively. The risk predicted by DPTRS did not vary significantly with age in either single- or multiple-autoantibody-positive individuals (p=0.861 and p=0.178, respectively) (ESM Fig. 5c,d). Of the individual components, the time-dependent effects of fasting C-peptide and total glucose were found to contribute significantly to risk of progression after accounting for DPTRS in single-autoantibody-positive individuals (p=0.002 and p<0.001, respectively). For multiple-autoantibody-positive individuals, only the time-varying effect of BMI was found to have the potential to enhance risk prediction additional to the DPTRS (p<0.001).
Discussion
The effects of disease predictors are often assumed to be proportional across the age spectrum, despite age being a key stratifier for disease progression. Here, we systematically tested the proportional hazards assumption in type 1 diabetes prediction modelling, and demonstrated that multiple disease predictors have age-dependent effects. In particular, the effects of sex, HLA, and a number of islet autoantibodies and metabolic variables change across the age spectrum in both single- and multiple-autoantibody-positive individuals.
Sex
Epidemiological studies in children have consistently reported a male-to-female ratio of 1 for the incidence of type 1 diabetes [25]. However, less appreciated is the higher incidence of disease in men that has been repeatedly demonstrated amongst older individuals [26]. Our results suggest that this difference in incidence of type 1 diabetes between men and women in older individuals begins early in the disease process and is due to a declining hazard rate in women compared with a constant rate in men. Differences between sexes have long been observed in the most common type 1 diabetes animal model, the non-obese diabetic (NOD) mouse. Autoimmune diabetes disproportionately affects female NOD mice, with more rapid immune infiltration of the islets in the female mice [27]. Our results may be supportive of a more aggressive, female-predominant endotype, as modelled by the NOD mouse, driving paediatric type 1 diabetes. While interesting work in NOD mice suggests an impact of sex hormones on disease progression [28], the mechanisms underlying the age-varying effect of sex is unknown, and human studies are clearly warranted.
Islet autoantibodies and high-risk HLA alleles
GADA and MIAA have been identified as key primary autoantibodies in the early stages of type 1 diabetes. GADA has been associated with increased risk of disease progression predominantly in older cohorts [15, 29], and a slower rate amongst younger cohorts [30, 31]. Using age-varying effect modelling, we demonstrated an increasing HR with increasing age for single-GADA-positive individuals, which accelerated amongst older adults to greater than four times that of the youngest children. In contrast, although MIAA has been identified as a key predictor of progression in prospective birth cohort studies [30, 31], independent risk has not been demonstrated in cross-sectionally recruited cohorts [29, 32]. By modelling risk across the age spectrum, we have shown an increased hazard of progression from single autoantibody positivity in the MIAA-positive paediatric population that is lost with increasing age. This granularity is typically obscured when proportional hazards are used, explaining the conflicting results in studies with differing age ranges. It is interesting to note that none of the islet autoantibodies demonstrated an age-varying effect for progression from multiple autoantibody positivity to clinical disease. This suggests that once multiple autoantibodies have developed, the drivers of the age-varying effect of GADA and MIAA seen in single-autoantibody-positive individuals are no longer relevant to disease progression.
We found that the risk of disease progression conferred by HLA alleles varies with age, independent of which autoantibody is present. HLA-DR3 was found to have a relatively greater effect on disease progression in adults with a single autoantibody compared with children; notably this was independent of GADA positivity. We also found that HLA-DR3/4, independent of MIAA positivity, has a relatively greater effect on disease progression in multiple-autoantibody-positive children compared with multiple-autoantibody-positive adults. Not only does this hold relevance for prediction models, but it points to the possibility of age-varying effects of other type 1 diabetes risk genes.
Metabolic variables
Increased insulin resistance has long been implicated in accelerating beta cell stress and promoting diabetes onset [33–35]. However, studies have not been consistent [36–39]. Of note, studies often did not model single- and multiple-autoantibody-positive individuals separately, and/or only used peri-diagnostic BMI. Here, we found that BMI and the insulin resistance surrogate, HOMA-IR, have an increasing effect on disease progression with increasing age only in single-autoantibody-positive but not multiple-autoantibody-positive individuals. Furthermore, we found the age-varying effect of BMI was no longer significant when HOMA-IR was accounted for. Conversely, the age-varying effect of HOMA-IR remained significant when adjusting for BMI. Although HOMA-IR is not validated for assessing insulin resistance in preclinical type 1 diabetes, these results suggest that BMI alone cannot simply be used as a surrogate for insulin resistance. Hence, the diverse conclusions regarding the role of BMI on disease progression may be confounded by both its age-varying effect in single-autoantibody-positive individuals and the differences in the degree of correlation between BMI and insulin resistance amongst study cohorts.
Interestingly, fasting glucose, a major component of the HOMA-IR calculation, had a large increase in effect with increasing age in both single- and multiple-autoantibody-positive individuals. This is consistent with a study showing an association between higher fasting glucose at diagnosis and older age of type 1 diabetes diagnosis [40]. C-peptide AUC also demonstrated a significant age-varying effect for both single- and multiple-autoantibody-positive individuals. It is worth noting there is still minimal data available regarding what constitutes ‘normal’ beta cell function across the age spectrum and how this compares with autoantibody-positive relatives. What is clear from this data is that glucose and surrogate measures for beta cell function derived from the OGTT should not be interpreted with the same weight across children and adults.
Risk prediction scores
Both Index60 and DPTRS have been shown to be excellent predictors of disease progression [41, 42]. However, we found that both scores had the potential to be enhanced by incorporating the age-varying effect of particular covariates. This demonstrates that, even when age is a component of a calculated score, as is the case with the DPTRS, it does not encapsulate the changing hazard that its component covariates may have with age. In particular, the age-varying effect of measures of C-peptide was found to have the potential to enhance prediction in single-autoantibody-positive individuals for both Index60 and DPTRS. These results suggest the possibility that, when developing future risk scores, investigators may improve prediction by incorporating the age-varying effects of covariates, particularly those covariates that demonstrate a significant change of hazard across the ages.
Limitations
This study was restricted to relatives of people with type 1 diabetes, and may not represent the general population. However, population studies suggest that once autoimmunity is established, the progression rate to clinical disease is comparable to that for genetically at-risk cohorts [43]. The recruitment design of the PTP study enabled a wide capture of ages, a feature that is important for assessing age-varying effects; a similarly wide age range would require decades of follow-up in studies that begin recruitment from birth. However, the recruitment strategy limited the data available from time of seroconversion to current autoantibody status and the contribution that this data may have had to risk, as well as the number of children under 2 years of age. In addition, there was a paucity of individuals between 20 to 30 years of age – a common clinical trial accrual pattern [44]. These limitations may have led to an underestimation of the age-varying effects seen. The time-varying effects modelling used the assumption common to survival analyses that, during ages where follow-up is not available for a given individual, individuals with similar covariates are representative. A composite endpoint of multiple autoantibody positivity and clinical disease was used for progression of single-autoantibody-positive individuals to maximise the number of events available to study. However, when clinical disease alone was used as the endpoint, the age-varying effects seen did not change significantly. As with all retrospective analyses of data, the statistical significance of the results needs to be interpreted in context, and clinically relevant time-varying effects may have been overlooked.
Concluding remarks
Analysing type 1 diabetes predictors using an age-varying effect model provides a novel approach for understanding disease progression. Accounting for the age-varying effect of disease predictors has the potential to improve the accuracy of prediction models, even for well-established estimates. Moreover, this approach could be applied to any disease predictor to shed light on how age may contribute to conflicting conclusions regarding its role in disease risk. In particular, the age-varying effects of sex, HLA, islet autoantibodies and glycaemic measures of islet function on disease progression observed in this study provide new insights into the heterogeneity of type 1 diabetes, and demand further investigation for understanding biological mechanisms. Identifying risk variables with age-varying effects may be used to guide studies that take advantage of the different effects seen at different ages, and uncover the causal mechanisms behind these differences, leading to greater insights into disease aetiology. Finally, building risk estimates using the age-varying effects of covariates will allow disease prediction to be guided by the risk factors that are most relevant at that individual’s age. This will improve the accuracy of disease prediction, ensure that intervention trials target the most rapidly progressing individuals, and by better defining an individual’s projected rate of progression, help determine intervention efficacy.
Supplementary Material
Research in context.
What is already known about this subject?
Age is one of the most important stratifiers of disease progression in type 1 diabetes
The estimated impact of several type 1 diabetes risk variables on disease progression have been inconsistent where the age of the cohorts investigated differed, suggesting that some type 1 diabetes risk variables may have age-specific risk patterns
Currently, at-risk cohorts for type 1 diabetes are typically analysed with the assumption that risk variables have a proportional hazard over time, potentially diminishing the ability to predict risk in cohorts of differing ages
What is the key question?
Which key type 1 diabetes risk variables have age-specific patterns of risk and what do these patterns look like?
What are the new findings?
This is the first comprehensive analysis and visual representation of the age-varying effects of key type 1 diabetes risk factors
Risk variables, within all categories explored, demonstrated significant age-varying effects, including sex, HLA, autoantibodies. BMI and measures of islet function and glucose tolerance
Multivariable analyses suggested that incorporation of the age-varying effect of the individual components of risk scores has the potential to enhance their risk estimation
How might this impact on clinical practice in the foreseeable future?
Incorporating the age-varying effect of risk is key to improving our understanding and prediction of type 1 diabetes at different ages, refining prediction models, and guiding mechanistic studies
Acknowledgements
The authors thank the participants and their families, as well as the many TrialNet clinical teams who conducted study visits and recruited participants.
Funding
This work was funded by NIDDK (R03DK127475 to CS), JDRF (1-SRA-2020-900 to JMW), and the Victorian State Government Operational Infrastructure Support and Australian National Health and Medical Research Council Research Institute Infrastructure Support Scheme (JMW). We acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided data for this study. The Type 1 Diabetes TrialNet Study Group is a clinical trials network currently funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, JDRF and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085461, U01 DK085465, U01 DK085466, U01 DK085476, U01 DK085499, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993 and UC4DK117009.
Abbreviations
- DPTRS
Diabetes Prevention Trial Type 1 Risk Score
- GADA
GAD65 autoantibody
- IA-2A
Insulinoma-associated protein 2 autoantibody
- ICA
Islet cell autoantibody
- MIAA
Insulin autoantibody
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NOD
Non-obese diabetic
- PTP
Pathway to Prevention
- ZnT8A
Zinc transporter-8 autoantibodies
Footnotes
Authors’ relationships and activities The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data availability
Data used in this study were derived from the TrialNet PTP study. Data from the TrialNet PTP Study are publicly available via the NIDDK data repository website. The analysis code for this study is available upon request via email to the corresponding author.
References
- [1].Ziegler AG, Bonifacio E, the BABYDIAB-BABYDIET Study Group (2012) Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 55(7): 1937–1943. 10.1007/s00125-012-2472-x [DOI] [PubMed] [Google Scholar]
- [2].Krischer JP, Liu X, Lernmark A, et al. (2017) The Influence of Type 1 Diabetes Genetic Susceptibility Regions, Age, Sex, and Family History on the Progression From Multiple Autoantibodies to Type 1 Diabetes: A TEDDY Study Report. Diabetes 66(12): 3122–3129. 10.2337/db17-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Inshaw JRJ, Walker NM, Wallace C, Bottolo L, Todd JA (2018) The chromosome 6q22.33 region is associated with age at diagnosis of type 1 diabetes and disease risk in those diagnosed under 5 years of age. Diabetologia 61(1): 147–157. 10.1007/s00125-017-4440-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bosi E, Boulware DC, Becker DJ, et al. (2017) Impact of Age and Antibody Type on Progression From Single to Multiple Autoantibodies in Type 1 Diabetes Relatives. J Clin Endocrinol Metab 102(8): 2881–2886. 10.1210/jc.2017-00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Endesfelder D, Zu Castell W, Bonifacio E, et al. (2019) Time-Resolved Autoantibody Profiling Facilitates Stratification of Preclinical Type 1 Diabetes in Children. Diabetes 68(1): 119–130. 10.2337/db18-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Redondo MJ, Sosenko J, Libman I, et al. (2020) Single Islet Autoantibody at Diagnosis of Clinical Type 1 Diabetes is Associated With Older Age and Insulin Resistance. J Clin Endocrinol Metab 105(5):1629–1640. 10.1210/clinem/dgz296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].So M, Speake C, Steck AK, et al. (2021) Advances in Type 1 Diabetes Prediction using Islet Autoantibodies: Beyond a Simple Count. Endocr Rev. 42(5):584–604. 10.1210/endrev/bnab013 [DOI] [PubMed] [Google Scholar]
- [8].Cox D (1972) Regression Models and Life-Tables. Journal of the Royal Statistical Society 34: 187–220 [Google Scholar]
- [9].Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. (2009) The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 10(2): 97–104. 10.1111/j.1399-5448.2008.00464.x [DOI] [PubMed] [Google Scholar]
- [10].Sosenko JM, Skyler JS, DiMeglio LA, et al. (2015) A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 38(2): 271–276. 10.2337/dc14-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sosenko JM, Krischer JP, Palmer JP, et al. (2008) A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care 31(3): 528–533. 10.2337/dc07-1459 [DOI] [PubMed] [Google Scholar]
- [12].Greenbaum CJ, Schatz DA, Cuthbertson D, Zeidler A, Eisenbarth GS, Krischer JP (2000) Islet cell antibody-positive relatives with human leukocyte antigen DQA1*0102, DQB1*0602: identification by the Diabetes Prevention Trial-type 1. J Clin Endocrinol Metab 85(3): 1255–1260. 10.1210/jcem.85.3.6459 [DOI] [PubMed] [Google Scholar]
- [13].Yu L, Boulware DC, Beam CA, et al. (2012) Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care 35(6): 1213–1218. 10.2337/dc11-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yu L, Rewers M, Gianani R, et al. (1996) Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 81(12): 4264–4267. 10.1210/jcem.81.12.8954025 [DOI] [PubMed] [Google Scholar]
- [15].Bingley PJ, Boulware DC, Krischer JP, et al. (2016) The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia 59(3): 542–549. 10.1007/s00125-015-3830-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bonifacio E, Yu L, Williams AK, et al. (2010) Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 95(7): 3360–3367. 10.1210/jc.2010-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mychaleckyj JC, Noble JA, Moonsamy PV, et al. (2010) HLA genotyping in the international Type 1 Diabetes Genetics Consortium. Clin Trials 7(1 Suppl): S75–87. 10.1177/1740774510373494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].American Diabetes Association (2018) 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 41(Suppl 1): S13–S27. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- [19].Hanley JA, Miettinen OS (2009) Fitting Smooth-in-Time Prognostic Risk Functions via Logistic Regression. The International Journal of Biostatistics 5: 1–2520231866 [Google Scholar]
- [20].Bhatnagar S, Turgeon M, Islam J, Saarela O, Hanley J (2020) Flexible Smooth-in-Time Hazards and Risk Functions via Logistic and Multinomial. http://www.maths.bristol.ac.uk/R/web/packages/casebase/casebase.pdf. Accessed 15 August 2021.
- [21].Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 73(1): 3–36 [Google Scholar]
- [22].Wood SN (2003) Thin plate regression splines. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 65(1): 95–114 [Google Scholar]
- [23].Giannopoulou EZ, Winkler C, Chmiel R, et al. (2015) Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia 58(10): 2317–2323. 10.1007/s00125-015-3672-y [DOI] [PubMed] [Google Scholar]
- [24].Krischer JP, Lynch KF, Lernmark A, et al. (2017) Genetic and Environmental Interactions Modify the Risk of Diabetes-Related Autoimmunity by 6 Years of Age: The TEDDY Study. Diabetes Care 40(9): 1194–1202. 10.2337/dc17-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soltesz G, Patterson CC, Dahlquist G, et al. (2007) Worldwide childhood type 1 diabetes incidence--what can we learn from epidemiology? Pediatr Diabetes 8 Suppl 6: 6–14. 10.1111/j.1399-5448.2007.00280.x [DOI] [PubMed] [Google Scholar]
- [26].Diaz-Valencia PA, Bougneres P, Valleron AJ (2015) Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health 15: 255. 10.1186/s12889-015-1591-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Young EF, Hess PR, Arnold LW, Tisch R, Frelinger JA (2009) Islet lymphocyte subsets in male and female NOD mice are qualitatively similar but quantitatively distinct. Autoimmunity 42(8): 678–691. 10.3109/08916930903213993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fitzpatrick F, Lepault F, Homo-Delarche F, Bach JF, Dardenne M (1991) Influence of castration, alone or combined with thymectomy, on the development of diabetes in the nonobese diabetic mouse. Endocrinology 129(3): 1382–1390. 10.1210/endo-129-3-1382 [DOI] [PubMed] [Google Scholar]
- [29].Orban T, Sosenko JM, Cuthbertson D, et al. (2009) Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 32(12): 2269–2274. 10.2337/dc09-0934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Endesfelder D, Hagen M, Winkler C, et al. (2016) A novel approach for the analysis of longitudinal profiles reveals delayed progression to type 1 diabetes in a subgroup of multiple-islet-autoantibody-positive children. Diabetologia 59(10): 2172–2180. 10.1007/s00125-016-4050-0 [DOI] [PubMed] [Google Scholar]
- [31].Siljander HT, Simell S, Hekkala A, et al. (2009) Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes 58(12): 2835–2842. 10.2337/db08-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jacobsen LM, Bocchino L, Evans-Molina C, et al. (2020) The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia 63(3): 588–596. 10.1007/s00125-019-05047-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wilkin TJ (2001) The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia 44(7): 914–922. 10.1007/s001250100548 [DOI] [PubMed] [Google Scholar]
- [34].Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC (2004) Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 47(10): 1661–1667. 10.1007/s00125-004-1507-3 [DOI] [PubMed] [Google Scholar]
- [35].Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP, Diabetes Prevention Trial-Type 1 Study Group (2007) Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care 30(9): 2314–2320. 10.2337/dc06-2389 [DOI] [PubMed] [Google Scholar]
- [36].Lauria A, Barker A, Schloot N, et al. (2015) BMI is an important driver of beta-cell loss in type 1 diabetes upon diagnosis in 10 to 18-year-old children. Eur J Endocrinol 172(2): 107–113. 10.1530/EJE-14-0522 [DOI] [PubMed] [Google Scholar]
- [37].Ferrara CT, Geyer SM, Evans-Molina C, et al. (2017) The Role of Age and Excess Body Mass Index in Progression to Type 1 Diabetes in At-Risk Adults. J Clin Endocrinol Metab 102(12): 4596–4603. 10.1210/jc.2017-01490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Betts P, Mulligan J, Ward P, Smith B, Wilkin T (2005) Increasing body weight predicts the earlier onset of insulin-dependant diabetes in childhood: testing the ‘accelerator hypothesis’ (2). Diabet Med 22(2): 144–151. 10.1111/j.1464-5491.2004.01368.x [DOI] [PubMed] [Google Scholar]
- [39].Kuchlbauer V, Vogel M, Gausche R, et al. (2014) High birth weights but not excessive weight gain prior to manifestation are related to earlier onset of diabetes in childhood: ‘accelerator hypothesis’ revisited. Pediatr Diabetes 15(6): 428–435. 10.1111/pedi.12107 [DOI] [PubMed] [Google Scholar]
- [40].Greenbaum CJ, Cuthbertson D, Krischer JP, et al. (2001) Type I diabetes manifested solely by 2-h oral glucose tolerance test criteria. Diabetes 50(2): 470–476. 10.2337/diabetes.50.2.470 [DOI] [PubMed] [Google Scholar]
- [41].Nathan BM, Boulware D, Geyer S, et al. (2017) Dysglycemia and Index60 as Prediagnostic End Points for Type 1 Diabetes Prevention Trials. Diabetes Care 40(11): 1494–1499. 10.2337/dc17-0916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sosenko JM, Skyler JS, Palmer JP, et al. (2015) The development, validation, and utility of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS). Curr Diab Rep 15(8): 49. 10.1007/s11892-015-0626-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ziegler AG, Kick K, Bonifacio E, et al. (2020) Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany. JAMA 323(4): 339–351. 10.1001/jama.2019.21565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ferrari A, Montello M, Budd T, Bleyer A (2008) The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer 50(5 Suppl): 1101–1104. 10.1002/pbc.21459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study were derived from the TrialNet PTP study. Data from the TrialNet PTP Study are publicly available via the NIDDK data repository website. The analysis code for this study is available upon request via email to the corresponding author.


