Abstract
CAC measures subclinical atherosclerosis and improves risk stratification. CAC characteristics—including vessel(s) involved, number of vessels, volume, and density —have been shown to differentially impact risk. We assessed how dispersion—either the number of calcified vessels or CAC phenotype (diffuse, normal, and concentrated)—impacted cause-specific mortality. The CAC Consortium is a retrospective cohort of 66,636 participants without CHD who underwent CAC scoring. This study included individuals with CAC>0 (N=28,147). CAC area, CAC density, and CAC phenotypes [derived from the index of diffusion (ID=1-(CAC in most concentrated vessel/total Agatston score) were calculated. Associations between CAC characteristics and cause-specific mortality was assessed. Participant details included (N=28,147): mean age 58.3 years, 25% female, 89.6% white, and 66% had 2+ calcified vessels. Diabetes, hypertension, and hyperlipidemia were predictors of multivessel involvement (p<0.001). After controlling for overall CAC score, those with 4-vessel CAC involvement had more CAC area and less dense calcifications compared to those with 1-vessel. There was a graded increase in all-cause, CVD- and CHD-specific mortality as the number of calcified vessels increased. Among those with 2 or more vessels involved (N=18,516), a diffuse phenotype was associated with a higher CVD-specific mortality and had a trend towards higher all-cause and CHD-specific mortality compared with a concentrated CAC phenotype. Diffuse CAC involvement was characterized by less dense calcification, more CAC area, multiple coronary vessel involvement, and presence of certain traditional risk factors. There is a graded increase in all-cause, CVD- and CHD-specific mortality with increasing CAC dispersion.
Keywords: Coronary Artery Calcium, Index of Diffusion, Computed Tomography, Coronary Heart Disease
1. INTRODUCTION
The burden of CAC—a strong correlate of total coronary atherosclerotic plaque1—is associated with the risk of coronary heart disease (CHD) and cardiovascular disease (CVD)2–4. As such, CAC scoring is now incorporated in clinical practice guidelines given its demonstrated use as a powerful adjunct to both traditional risk factors5–7 and risk-prediction models for helping to characterize risk8, 9. The original and widely used Agatston score is derived by summing the scores of all individual lesions (determined by multiplying the total lesion area by a weighting factor accounting for maximum density in Hounsfield units)10. Contrary to the initial assumptions of the Agatston score, CAC density has since been shown to be inversely related to CHD and CVD events11 with mean density outperforming peak density12. Lesion characteristics differentially impact risk—Compared to “spotty microcalcifications”, which are pathologic of a more vulnerable plaque, calcified nodules are far less likely to cause CHD13. Statin therapy increases CAC, likely by creating more dense plaques, which can be a sign of stabilization14. Other potential characteristics that may improve upon the Agatston score’s predictive ability—but not yet accounted for— include the number and identity of vessels affected15, the overall CAC distribution16, and the number of calcified plaques.17–19 Given these considerations, we sought to understand how measures of CAC dispersion—considered as either the number of calcified vessels involved or by CAC phenotype, using an index of diffusion (ID), which apportions how much each vessel contributes to the total Agatston score—are associated with all-cause and cause-specific mortality.
2. METHODS
The complete details and rationale behind the CAC Consortium have been published elsewhere20. Briefly, the CAC Consortium is a retrospective cohort study including 66,636 participants from four institutions—Cedars-Sinai Medical Center in Los Angeles, CA; PrevaHealth Wellness Diagnostic Center in Columbus, OH; Harbor-UCLA Medical Center in Torrance, CA; and the Minneapolis Heart Institute in Minneapolis, MN20. Eligible participants were at least 18 years of age, were asymptomatic and without clinical evidence of CHD, and underwent CAC testing after a clinically indicated physician referral20. For these analyses, only participants with CAC>0 were included (N=28,147). Analyses for ID were further restricted to only those with two or more calcified vessels (N=18,516). Consent was obtained at participating institutions at time of CAC scanning20.
CAC scans were obtained by using either electron beam tomography (~93% of scans) or multi-detector computed tomography (CT) (~7% of scans)20. Each site performed these non-contrast, cardiac-gated scans using common protocols20 and calcium score was quantified using the Agatston method10. Mean vessel area that contained calcium was calculated. Directly measured CAC density data was confined to the Minneapolis site and was available for 20,052 (30%) of participants in the CAC Consortium20, or 10,374 for the purposes of this analysis of patients with prevalent CAC. Mean density, rather than calculated peak density, was used for the purpose of density-based analyses as this was measured directly at the patient level. In order to determine the relationship between calcified vessels involved to mean density and mean area, we performed linear regression separately for mean density (modeled as a continuous variable) and mean area (log-transformed and modeled as a continuous variable) with adjustments for age, sex, study site, and CAC score.
For any given calcium score, vessel involvement was categorized from 1 to 4 vessels. For those with ≥2 vessel CAC involvement, diffuseness was described using ID. Consistent with prior literature, this was calculated by taking each vessel’s Agatston score and dividing it by the total Agatston score, i.e. the proportion that each vessel contributes to the total Agatston score (ID = 1 - (CAC in most concentrated vessel/total Agatston score)16. A higher ID indicates more diffuse calcified coronary disease, and a lower ID indicates a less diffuse phenotype. For each grouping of number of vessels (i.e., 2-, 3-, or 4-vessel involvement), we identified the 25th & 75th percentile for ID. Consistent with prior literature, a “diffuse” phenotype was defined when the ID was greater than the 75th percentile for the respective number of vessel category. Similarly, the phenotype was categorized as “concentrated” if the ID was less than the 25th percentile. Given that the Agatston score takes area into consideration, we have performed sensitivity analyses using an area-based ID, which considers the area of each calcified vessel as a proportion of the total calcified area.
Clinical risk factors and laboratory-based data were collected either at the visit when CAC testing was ordered or at the time of CAC testing20. Hypertension was defined as having a prior diagnosis of hypertension or being treated with anti-hypertensives; dyslipidemia was defined as having a prior diagnosis of hyperlipidemia or dyslipidemia, being treated with lipid-lowering therapies, or having low-density lipoprotein cholesterol (LDL-C) >160 mg/dL, high-density lipoprotein cholesterol (HDL-C) <40 mg/dL in men and <50 mg/dL in women, or fasting triglycerides >150 mg/dL; Diabetes was defined as a prior diagnosis of diabetes or being treated with oral hypoglycemics or insulin20. Family history of CHD was defined as having a first-degree relative with a presence of CHD; the Columbus, OH site used a definition of premature family history, i.e., age <55 years old in men or age <65 years in women20. Smoking was defined as either a never smoker, a current smoker, or a former smoker20.
Mortality data was ascertained by linking participant data to the Social Security Death Index Death Master File (SSDIDMF) using a validated algorithm21. This algorithm uses a hierarchical matching process of patient identifiers to link to the SSDIDMF database20. Mortality data was obtained through June 1st, 201420. Cause of death data was obtained through the National Death Index and were reported using ICD coding and then categorized as “CVD, cancer, pulmonary disease, gastrointestinal disease, nervous system disorders, endocrine and metabolic disease, injury and poisoning, or other”20. CVD includes CHD, stroke, congestive heart failure, and other circulatory disorders.
Continuous data are presented as mean (standard deviation) and categorical data as total number (percentage). Data are presented by the number of vessels that have calcium involvement and/or ID phenotype. Using ordinal logistic regression, the marginal predicted probability of number of vessels involved was determined by risk factor status. Predicted probabilities were based on a model which included the CAC score and traditional cardiovascular risk factors. Increased probability of multi-vessel involvement was expressed as a marginal percentage increase for each individual risk factor.
Absolute event rates of all-cause and cause-specific mortality are presented per 1,000 person-years. Kaplan-Meier curves were used to plot all-cause and cause-specific mortality by diffusivity group (concentrated, normal, and diffuse), and used the log rank test to determine whether survival differed among diffuse groups. Multivariable-adjusted Cox proportional hazards models were used to determine the relative hazard of all-cause and cause specific death according to number of vessels involved.
Data was analyzed using Stata/SE 15. A p-value of <0.05 was considered statistically significant.
3. RESULTS
Among the 66,636 participants in the CAC Consortium, 28,147 (58.3 years old (SD 10.3), 25% female, and 89.6% white) met inclusion criteria. The percentage of participants with calcium in only a single vessel was 34.2%, in 2 vessels was 27.1%, and in 3 or 4-vessels was 38.7%. Participants with multivessel involvement were more likely to be older, male, and non-white. With increasing CAC strata, the proportion of participants with 3- or 4-vessel involvement also increased (55.4% of participants with CAC ≥300 vs. 18.1% of participants with CAC=1–99). Additionally, there were significant differences in number of vessels involved based on the presence of diabetes mellitus (p<0.001), hypertension (p<0.001), hyperlipidemia (p<0.001), and current smoking (p<0.001) (Table 1).
Table 1.
Baseline Characteristics of Study Cohort*
| Characteristic | Number of Vessels with CAC | ||||
|---|---|---|---|---|---|
| 1 Artery (N= 9,631) | 2 Arteries (N= 7,622) | 3 Arteries (N= 7,434) | 4 Arteries (N= 3,460) | All (N=28,147) | |
| Age, mean (SD)‡ | 54.6 (9.5) | 57.6 (10.0) | 60.9 (10.0) | 64.2 (10.0) | 58.3 (10.3) |
| Sex‡ | |||||
| Female | 3,112 (32.3) | 1,947 (25.5) | 1,457 (19.6) | 526 (15.2) | 7,042 (25.0) |
| Male | 6,519 (67.7) | 5,675 (74.5) | 5,977 (80.4) | 2,934 (84.8) | 21,105 (75.0) |
| Race (N=23,039)† | |||||
| Black | 167 (2.1) | 135 (2.2) | 158 (2.6) | 66 (2.3) | 526 (2.3) |
| White | 7,206 (90.8) | 5,523 (89.5) | 5,328 (88.6) | 2,590 (89.0) | 20,647 (89.6) |
| All Others | 565 (7.1) | 516 (8.3) | 531 (8.8) | 254 (8.7) | 1,866 (8.1) |
| Current Smoking‡ | 893 (9.3) | 781 (10.3) | 839 (11.3) | 370 (10.7) | 2,883 (10.2) |
| Diabetes‡ | 538 (5.6) | 579 (7.6) | 864 (11.6) | 540 (15.6) | 2,521 (9.0) |
| Family History of CHD | 4,746 (49.3) | 3,699 (48.5) | 3,648 (49.1) | 1,705 (49.3) | 13,798 (49.0) |
| Hypertension‡ | 2,814 (29.2) | 2,804 (36.8) | 3,278 (44.1) | 1,758 (50.8) | 10,654 (37.9) |
| Hyperlipidemia‡ | 5,616 (58.3) | 4,824 (63.3) | 4,975 (66.9) | 2,439 (70.5) | 17,854 (63.4) |
| Baseline Statin Use (N=16,236) | 1,212 (20.4) | 1,184 (28.0) | 1,481 (37.0) | 896 (43.3) | 4,773 (29.4) |
| CAC Score Group‡ | |||||
| CAC = 1–99 | 8,845 (91.8) | 4,861 (63.8) | 1,745 (23.5) | 228 (6.6) | 15,679 (55.7) |
| CAC = 100–299 | 717 (7.4) | 1,963 (25.8) | 2,255 (30.3) | 634 (18.3) | 5,569 (19.8) |
| CAC = 300+ | 69 (0.72) | 798 (10.5) | 3,434 (46.2) | 2,598 (75.1) | 6,899 (24.5) |
| Index of Diffusion‡ | |||||
| Concentrated | 9,631 (100) | 3,170 (41.6) | 1,624 (21.9) | 339 (9.8) | 14,764 (52.5) |
| Normal | 0 (0) | 2,128 (27.9) | 3,305 (44.5) | 1,725 (49.9) | 7,158 (25.4) |
| Diffuse | 0 (0) | 2,324 (30.5) | 2,505 (33.7) | 1,396 (40.4) | 6,225 (22.1) |
Data are presented as No. (%) unless otherwise indicated
p<0.05,
p<0.001
In unadjusted analyses, there was a graded decrease in mean calcium density with each additional vessel involved for those at intermediate CAC scores (100–299) (Figure 1). After adjustment, there was a graded decrease in mean density in all CAC groups. For example, in the CAC scores ≥300 group, compared to single vessel involvement, the mean density was lower in 2-, 3-, and 4-vessels (−23.0 HU, −38.7 HU, and −42.9 HU, respectively) (all p<0.001) (Table 2). Full results for mean CAC density and mean CAC area as a function of number of vessels with CAC abnormality are shown in Table 2. A sensitivity analysis was conducted stratifying by area tertiles rather than CAC groupings and was consistent with these results (Appendix Table 2A). Among those with CAC scores greater than 300, there was a 4 unit increase in area among 2-vessels, 6 unit increase in area among 3-vessels, and 6 unit increase in area among 4-vessels, compared to single vessel involvement. A sensitivity analysis was performed excluding MDCT scans and was consistent with these findings.
Figure 1.
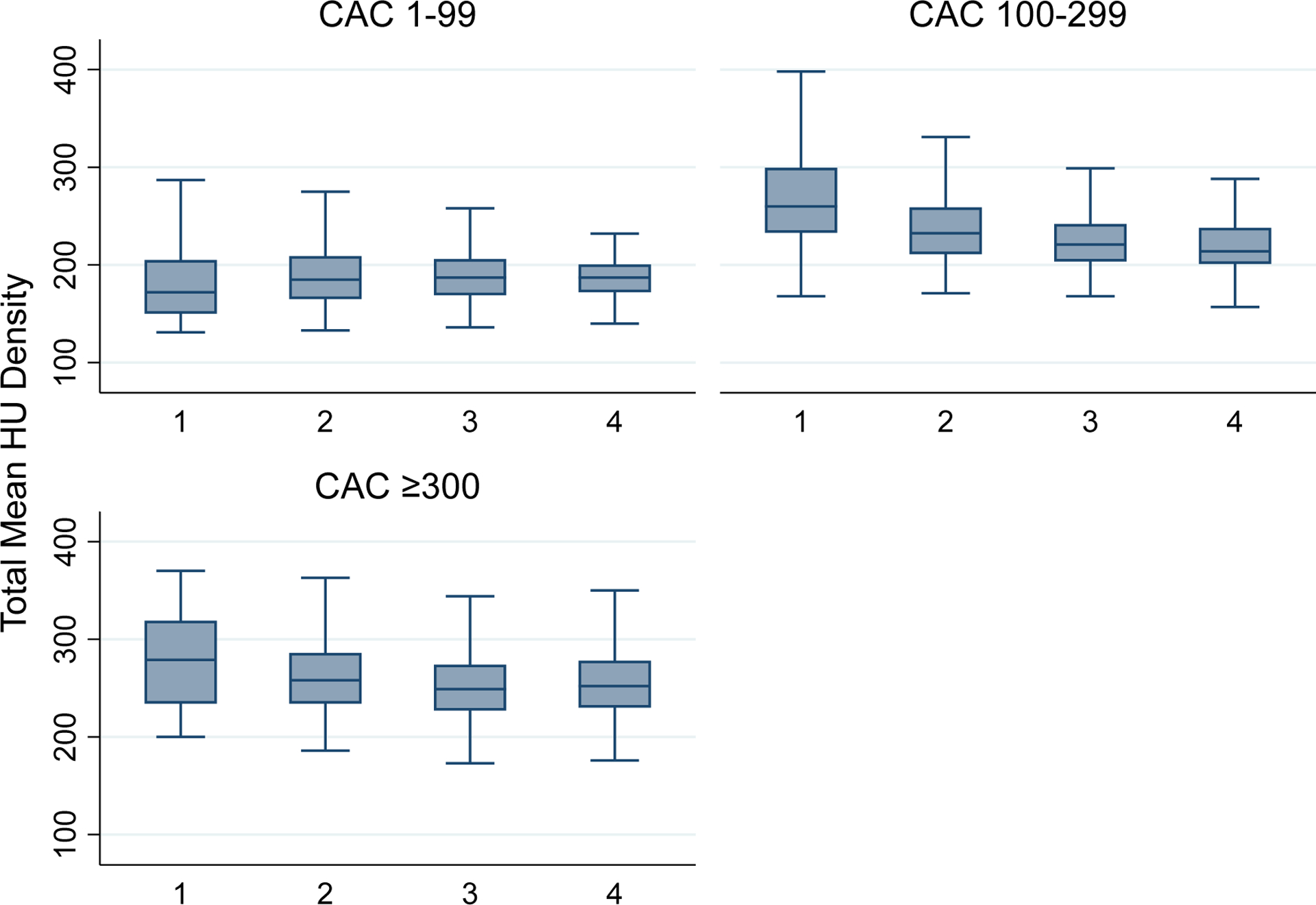
Density of Calcium per Vessel Stratified by CAC Groupings
Table 2.
Mean CAC Density and Mean CAC Area by Number of Vessels with CAC
| Number of Vessels | |||||
|---|---|---|---|---|---|
| 1 Artery | 2 Arteries | 3 Arteries | 4 Arteries | ||
| Mean Density * ,‡ | CAC Score Group | ||||
| CAC = 1–99 | 1 (ref) | −19.4 (−20.9 to −17.9) |
−33.0 (−35.4 to −30.6) |
−40.6 (−46.6 to −34.4) |
|
| CAC = 100–299 | 1 (ref) | −29.9 (−34.9 to −24.8) |
−43.8 (−48.9 to −38.8) |
−51.6 (−57.9 to −45.4) |
|
| CAC = 300+ | 1 (ref) | −23.0 (−36.3 to −9.7) |
−38.7 (−51.6 to −25.9) |
−42.9 (−55.9 to −30.0) |
|
| Area * | CAC Score Group | ||||
| CAC = 1–99 | 1 (ref) | 1.10 (1.08 to 1.11) |
1.11 (1.09 to 1.14) |
1.09 (1.03 to 1.15) |
|
| CAC = 100–299 | 1 (ref) | 1.02 (1.01 to 1.03) |
1.05 (1.04 to 1.06) |
1.07 (1.06 to 1.09) |
|
| CAC = 300+ | 1 (ref) | 1.04 (1.02 to 1.06) |
1.06 (1.04 to 1.08) |
1.06 (1.04 to 1.08) |
|
Density is measured in HU, Area is measured in mm2
Model includes age (as a continuous variable), sex, calcium score, and study site.
p<0.05,
p<0.001
Of the 18,516 participants with ≥2 vessels with CAC involvement there was substantial heterogeneity in CAC phenotype. Among our cohort, 5,133 (27.7%) had a concentrated phenotype, 7,158 (38.7%) had a normal phenotype, and 6,225 (33.6%) had a diffuse phenotype. Those with a concentrated, normal and diffuse phenotype had an ID of 10.3 (SD 6.1), 32.7 (SD 8.3) and 49.3 (SD 8.1), respectively.
Through ordered logistic regression diabetes mellitus (p<0.001), hypertension (p<0.001), and hyperlipidemia (p<0.001) were predictive of increased vessel involvement. The presence of diabetes, hypertension, and hyperlipidemia were associated with an increase of 11.1%, 9.9%, and 7.5%, respectively, in the likelihood of having 3- or 4-vessel disease compared to the absence of each risk factor, respectively.
There was a graded increase in all-cause mortality and cause-specific mortality based on the number of vessels involved. For all-cause mortality, there was a 4-fold increase comparing 4-vessel involvement to 1-vessel involvement; for CVD-specific mortality, there was a 7-fold increase comparing 4-vessel involvement to 1-vessel involvement; and for CHD-specific mortality, there was 10-fold increase comparing 4-vessel involvement to 1-vessel involvement (Table 3). When analyzing only those with ≥2 vessel involvement in ID groupings, comparing a diffuse phenotype to a concentrated phenotype, the effect size was more modest—1.7-fold increase for all-cause mortality, 1.9-fold increase for CVD-specific mortality, and 2.2-fold increase for CHD-specific mortality (Table 3). Absolute event rates using an area-based ID demonstrated similar trends with a stepwise increase from concentrated to diffuse for all-cause, CVD-specific, and CHD-specific (Appendix Table 3A).
Table 3.
Absolute Event Rates per 1000 patient-years by Vessel Number and Diffusivity
| All-cause Mortality | CVD* Mortality | CHD Mortality | ||
|---|---|---|---|---|
| Number of Vessels | 1-Vessel | 3.3 (3.0 to 3.7) | 0.7 (0.6 to 0.9) | 0.3 (0.2 to 0.4) |
| 2-Vessel | 4.9 (4.4 to 5.3) | 1.5 (1.3 to 1.8) | 0.8 (0.6 to 1.0) | |
| 3-Vessel | 8.2 (7.6 to 8.9) | 3.2 (2.8 to 3.6) | 1.8 (1.6 to 2.2) | |
| 4-Vessel | 12.9 (11.8 to 14.2) | 5.1 (4.4 to 5.9) | 3.0 (2.5 to 3.7) | |
| Index of Diffusion | Concentrated | 5.3 (4.8 to 6.0) | 1.8 (1.5 to 2.2) | 0.9 (0.7 to 1.2) |
| Normal | 8.2 (7.6 to 8.8) | 3.1 (2.7 to 3.5) | 1.7 (1.4 to 2.0) | |
| Diffuse | 8.9 (8.2 to 9.6) | 3.4 (2.9 to 3.8) | 2.0 (1.7 to 2.4) |
In both a minimally adjusted model, there was a significant increase in All-Cause mortality for those with 3- or 4-vessel disease compared to those with 1-vessel disease (1.3 [95% CI 1.1 to 1.5] and 1.4 [95% CI 1.1 to 1.7], respectively). For both CVD-specific and CHD-specific, there was a stepwise increase with each successive vessel included (Table 4). After further adjustment with clinical risk factors, such as hypertension, diabetes, and dyslipidemia, there was a 2-fold higher risk of CHD-specific mortality with 2-vessel disease (1.9 [95% CI 1.1 to 3.4]), and roughly a 2.5-fold higher increase for 3-vessel and 4-vessel disease (2.6 [95% CI 1.5 to 4.5] and 2.5 [95% CI 1.4 to 4.5], respectively) (Table 4).
Table 4.
Hazard Ratios of All-cause and cause-specific mortality by number of vessels and Index of Diffusion (ID)
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| All-Cause Mortality | CVD-specific Mortality | CHD-specific Mortality | All-Cause Mortality | CVD-specific Mortality | CHD-specific Mortality | |
| Vessel Number | ||||||
| 1-Vessel | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 2-Vessel | 1.1 (0.9 to 1.4) |
1.4 (0.9 to 1.9) |
2.0
(1.1 to 3.6) |
1.1 (0.9 to 1.3) |
1.3 (0.9 to 1.9) |
1.9
(1.1 to 3.4) |
| 3-Vessel |
1.3
(1.1 to 1.5) |
1.8
(1.3 to 2.5) |
2.7
(1.6 to 4.7) |
1.2
(1.04 to 1.5) |
1.7
(1.2 to 2.4) |
2.6
(1.5 to 4.5) |
| 4-Vessel |
1.4
(1.1 to 1.7) |
2.0
(1.3 to 2.8) |
2.7
(1.5 to 4.9) |
1.3
(1.1 to 1.6) |
1.8
(1.2 to 2.6) |
2.5
(1.4 to 4.5) |
| Index of Diffusion (Only ≥ 2 vessels) | ||||||
| Concentrated | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Normal | 1.04 (0.9 to 1.2) |
1.2 (0.9 to 1.6) |
0.9 (0.6 to 1.2) |
1.02 (0.9 to 1.2) |
1.2 (0.9 to 1.6) |
0.9 (0.6 to 1.3) |
| Diffuse | 1.2 (0.97 to 1.4) |
1.5
(1.1 to 2.1) |
1.3 (0.8 to 2.0) |
1.2 (0.95 to 1.4) |
1.4
(1.02 to 2.0) |
1.2 (0.8 to 19) |
Model 1 includes age (as a continuous variable), sex, study site, baseline statin use, and Agatston CAC score (continuous).
Model 2 includes Model 1 + hypertension, dyslipidemia, and diabetes
CVD mortality includes the following: other circulatory disorder, CHD, stroke, and congestive heart failure.
In unadjusted survival analyses stratified by ID phenotype, there were significant differences in all-cause mortality (p<0.001) and a trend towards significance for CVD-specific mortality (p=0.06). After adjustment, there were no differences in all-cause mortality (compared to Concentrated, p=0.813 for Normal and p=0.152 for Diffuse) or CHD-specific mortality by ID phenotype (compared to Concentrated, p=0.485 for Normal and p=0.371 for Diffuse). For CVD-specific mortality, there was a significantly higher HR for the Diffuse ID phenotype compared to the Concentrated phenotype of 1.43 (95% CI 1.02–1.99, p=0.035). There was no difference comparing the Normal phenotype to the Concentrated phenotype (p=0.331). Full results are reported in Table 4 and Figure 2.
Figure 2.
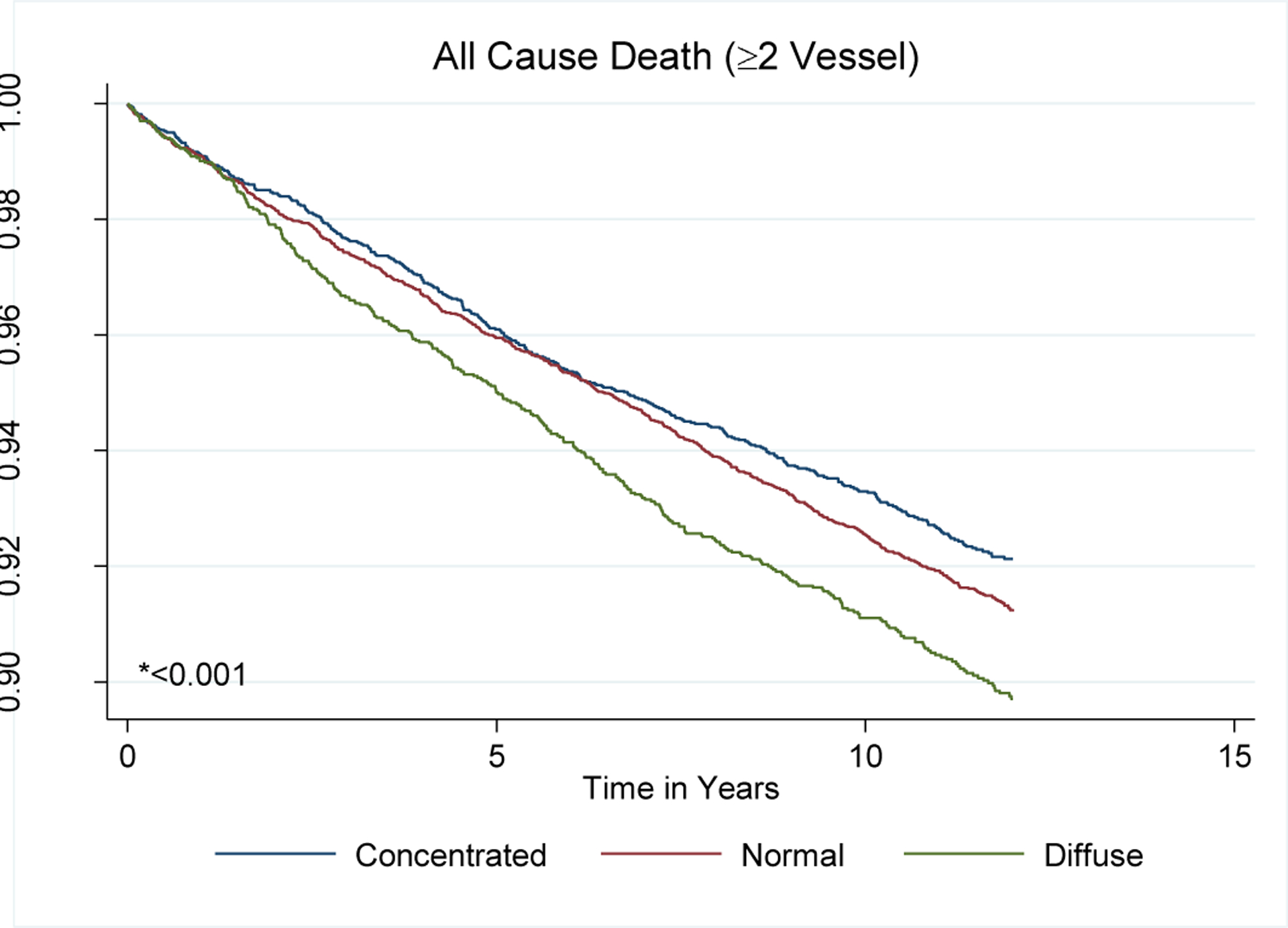
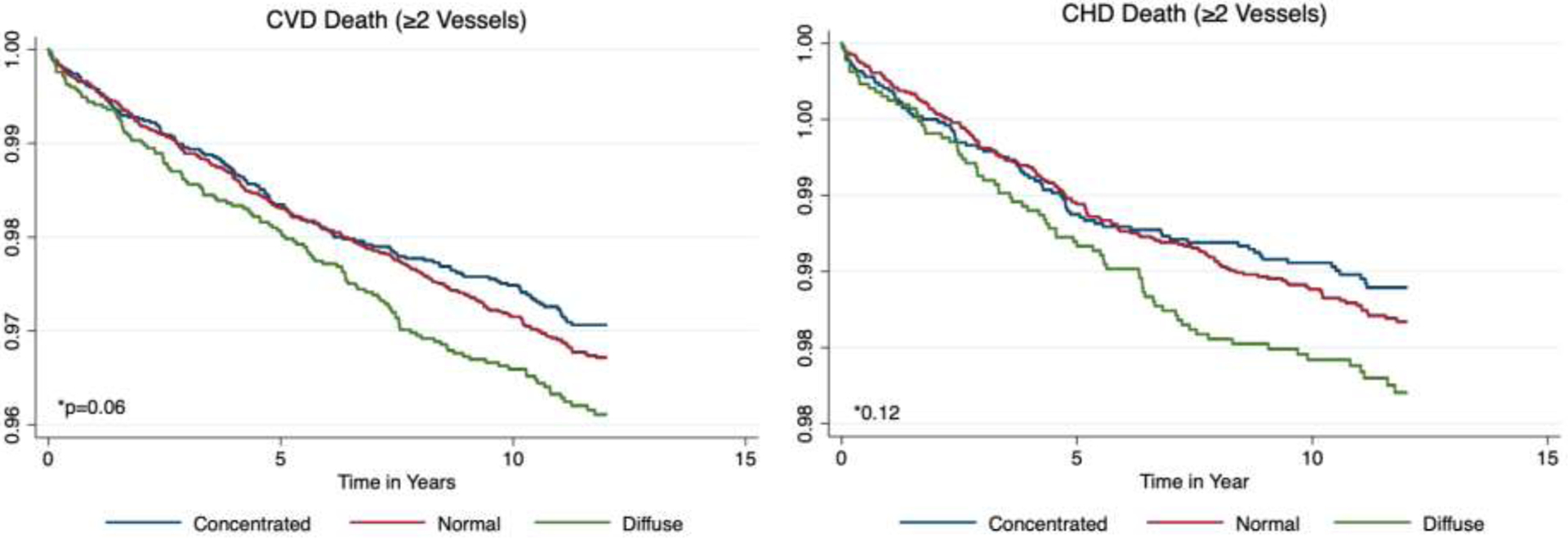
All-cause and cause-specific mortality Kaplan-Meier Curves by Index of Diffusion
A stratified analysis by baseline statin use was performed and identified a potential protective effect of a Normal phenotype compared to a Concentrated phenotype for all-cause and cause-specific mortality and a more pronounced hazard of the Diffuse phenotype compared to a Concentrated phenotype for all-cause and cause-specific mortality (Table 4a). A sensitivity analyses was performed to see the impact of scanner type on cause-specific mortality and did not demonstrate significant differences from the main model estimates.
4. DISCUSSION
In this retrospective analysis of participants with any CAC, we explored CAC dispersion—defined either by vessel number of by ID phenotypes—and its association with cause-specific mortality. Diffuse disease, determined by vessel number. was associated with increased mortality from CVD, CHD, or any cause. When restricting to individuals with 2 or more calcified vessels, there was a similar all-cause and CHD-specific mortality trend comparing the diffuse phenotype to a concentrated phenotype, though this did not reach significance. A diffuse phenotype, however, was associated with a higher risk of CVD-specific mortality compared to the concentrated phenotype. Stratified analyses of baseline statin use and mortality outcomes by ID phenotype suffered from a large amount of missing data leading to large confidence intervals and likely reflects residual confounding due to hidden patterns of missing data.
We found significant differences in the prevalence of diffuse disease based on sex, race, and CAC score groupings. Traditional risk factors such as diabetes, hypertension, and hyperlipidemia were significant predictors of more diffuse 3- or 4-vessel involvement. Previous studies using other testing modalities have found that diabetes22–26, hypertension26, and dyslipidemia26 are associated with more extensive disease. Autopsy data (n=2,029) has shown high-grade multivessel atherosclerotic disease in 58% of diabetic patients compared to 41% non-diabetic patients (p<0.001)24. In decedents who did not have clinical signs of atherosclerosis, 50% of diabetic individuals were found to have multivessel disease compared to 31% of non-diabetic individuals24, raising the specter of diabetes being an important marker of subclinical multivessel disease. Angiographically, in a study of over 600 participants, individuals 3-vessel disease were more like to have diabetes compared to those without diabetes (47.1% vs 27.6%, p<0.001)27. Valsania et al. similarly found that not only was diabetes associated with more severe 3-vessel disease (47% with >70% stenosis compared to 6%), but was also associated with more distal coronary involvement compared to nondiabetic individuals28.
Prior studies involving coronary computed tomography angiography (CCTA) have since demonstrated the power of non-invasive detection of multivessel disease among patients with diabetes, hyperlipidemia, or hypertension23, 26. Blanke et al. found that diabetic participants had 2-vessel or 3-vessel/left main disease at almost 50% higher rates of their non-diabetic counterparts and that this was associated with a 2.6 higher hazard ratio of all-cause mortality23. Tomizawa and colleagues described similar findings among participants with hypertension or hyperlipidemia26. In their study of 1,161 participants, they found much higher segmental severity scores in participants with hypertension, dyslipidemia, and diabetes—2.7 ± 4.3 segments involved for hypertension, 4.0 ± 4.8 for diabetes, and 2.0 ± 3.7 for hyperlipidemia—compared to no risk factors (0.8 ± 1.8 segments, all p<0.0005 compared to none)26. The authors also found that these risk factors were associated with higher CAC scores but did not describe the distribution of CAC disease26. Other studies have demonstrated associations between CAC score in subgroups of participants with diabetes, hypertension, and dyslipidemia, but do not describe the extent of disease or vessel involvement29. To the best of our knowledge, our study is the first to demonstrate these risk factors can predict more diffuse involvement on CAC testing.
The ability to non-invasively characterize, quantify, and describe the phenotypes of calcific disease is an important new paradigm in risk characterization. The CCTA literature provides evidence for this—Bittencourt et al., in a study of over 3,000 individuals without a prior history of coronary artery disease, provide evidence that those with extensive disease (defined as multiple segments involved) had higher rates of CVD mortality and myocardial infarctions30. Building on this, they found that a risk prediction model that included plaque distribution—defined by segment involvement score —in addition to disease severity (obstructive vs. nonobstructive) and traditional clinical variables—performed best30. More recently, Reynolds et al. found that anatomic extent of disease was more predictive of composite cardiovascular events than ischemia severity by functional testing in the ISCHEMIA trial31. These findings have helped introduce a new paradigm of risk prediction—that total plaque burden and diffuse involvement may present a more precise assessment of risk, which, in turn, can provide more targeted, aggressive therapeutics.
Tota-Maharaj and colleagues, in a study of almost 1,000 individuals, reported significant heterogeneity in CAC distribution, particularly among those with intermediate CAC scores (CAC 1–400)32. For instance, 54% of individuals with CAC>400 had 4-vessel disease compared to 20% and 2% of individuals with CAC = 101–400 and CAC 1–100, respectively32. They also demonstrated that multivessel CAC involvement better approximated increases in segmental involvement score on CTA32. In a subsequent study of 23,058 participants, Tota-Maharaj et al. found a stepwise increase in mortality rates per 1000 person-years for each additional calcified vessel33. Our findings echo these and highlight that increasing vessel involvement is a poor prognostic marker among those with calcified coronary vessels.
Building on these earlier observations, the Society of Cardiovascular Computed Tomography (SCCT), developed a way of incorporating vessel number into CAC reports—the CAC-Data Reporting System (CAC DRS)34. The CAC DRS proposes a standardized approach to reporting that incorporates the Agatston score or visual assessment with the vessel number34. As this would help to more accurately stratify individuals, increasing CAC DRS scores could lead to more intensive therapy and risk factor modification34. Subsequent analyses that have specifically incorporated the CAC DRS score have confirmed its performance in stratifying ASCVD events35.
Our findings provide additional evidence for the importance of incorporating markers of CAC dispersion into both reporting and practice. It stands to reason that incorporating CAC distribution, in addition to total CAC score, allows for a better approximation of total plaque burden, which is a more precise predictor of subsequent cardiovascular death. Blaha et al. confirmed this in a study from the Multi-Ethnic Study of Atherosclerosis cohort, which demonstrated that diffuse disease within CAC groupings portends a significantly poorer prognosis16 and by including these regional findings to CAC score the discriminatory capabilities improve16. As a result, a working group proposed that the next iteration of CAC scoring might incorporate these findings—regional distribution, diffusivity, lesion-specific characteristics, and location of calcified disease—and might be particularly important for those with CAC scores in an intermediate range36. Our findings provide evidence of incorporating these methods into clinical practice.
Our study has a few limitations to note—first, those who were referred for CAC testing were done so at physician discretion for the purpose of ASCVD risk-stratification, and as such, may not be representative of individuals who undergo testing for other reasons. Second, this study is also a retrospective, observational design study and suffers from the possibility of additional unmeasured confounding despite our best attempt to control for these. Third, our description of dispersion does not approximate other types of diffuse phenotypic definitions, such as proximal versus distal disease and overall number of plaques. Lastly, measurements of mean CAC density were calculated at the patient level rather than the lesion-specific level, and it remains uncertain whether lesion-specific CAC features meaningfully add to risk prediction.
In summary, we have shown that specific traditional risk factors are associated with a more diffuse CAC distribution and that increasing vessel number is associated all-cause and cause-specific mortality. Further restricting to individuals with 2 or more calcified vessels using the ID demonstrated a significant difference in CVD-specific mortality and a trend of increasing all-cause and CHD-specific mortality. Incorporating details of CAC distributions is important to consider in future CAC score algorithms and in guiding clinical management towards intensification of preventive measures.
Supplementary Material
ACKNOWLEDGMENTS
The authors are greatly indebted to all those who contributed data to the CAC Consortium.
Grant Support:
NIH/NHLBI L30 HL110027
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the authors report conflicts of interest related to this project
Declaration of interest
None of the authors have any relevant financial relationships to disclose.
REFERENCES
- 1.Ardehali R, Nasir K, Kolandaivelu A, Budoff MJ and Blumenthal RS. Screening patients for subclinical atherosclerosis with non-contrast cardiac CT. Atherosclerosis. 2007;192:235–42. [DOI] [PubMed] [Google Scholar]
- 2.Brown ER, Kronmal RA, Bluemke DA, Guerci AD, Carr JJ, Goldin J and Detrano R. Coronary calcium coverage score: determination, correlates, and predictive accuracy in the Multi-Ethnic Study of Atherosclerosis. Radiology. 2008;247:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA and Blumenthal RS. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2009;158:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson AO, Blaha MJ, Arnan MK, Sacco RL, Szklo M, Herrington DM and Yeboah J. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc Imaging. 2014;7:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw LJ, Raggi P, Schisterman E, Berman DS and Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–33. [DOI] [PubMed] [Google Scholar]
- 6.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD and Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasir K, Rubin J, Blaha MJ, Shaw LJ, Blankstein R, Rivera JJ, Khan AN, Berman D, Raggi P, Callister T, Rumberger JA, Min J, Jones SR, Blumenthal RS and Budoff MJ. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–73. [DOI] [PubMed] [Google Scholar]
- 8.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P and Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenland P, LaBree L, Azen SP, Doherty TM and Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–5. [DOI] [PubMed] [Google Scholar]
- 10.Agatston Arthur S. WRJ, Hildner Frank J., Zusmer Noel R., Viamonte Manuel Jr. and Detrano Robert. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 11.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ and Allison MA. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular EventsCoronary Artery Plaque and Cardiovascular EventsCoronary Artery Plaque and Cardiovascular Events. JAMA. 2014;311:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzaye O, Razavi AC, Dardari ZA, Berman DS, Budoff MJ, Miedema MD, Obisesan OH, Boakye E, Nasir K, Rozanski A, Rumberger JA, Shaw LJ, Mortensen MB, Whelton SP and Blaha MJ. Mean Versus Peak Coronary Calcium Density on Non-Contrast CT: Calcium Scoring and ASCVD Risk Prediction. JACC Cardiovasc Imaging. 2022;15:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos-Gallego CG, Picatoste B and Badimon JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep. 2014;16:401. [DOI] [PubMed] [Google Scholar]
- 14.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM and Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. [DOI] [PubMed] [Google Scholar]
- 15.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, Bairey Merz CN, Al-Mallah MH, Budoff MJ and Blaha MJ. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaha MJ, Budoff MJ, Tota-Maharaj R, Dardari ZA, Wong ND, Kronmal RA, Eng J, Post WS, Blumenthal RS and Nasir K. Improving the CAC Score by Addition of Regional Measures of Calcium Distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahti SJ, Feldman DI, Dardari Z, Mirbolouk M, Orimoloye OA, Osei AD, Graham G, Rumberger J, Shaw L, Budoff MJ, Rozanski A, Miedema MD, Al-Mallah MH, Berman D, Nasir K and Blaha MJ. The association between left main coronary artery calcium and cardiovascular-specific and total mortality: The Coronary Artery Calcium Consortium. Atherosclerosis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnson Y, Rozanski A, Gransar H, Friedman JD, Hayes SW, Thomson LE, Tamarappoo B, Slomka P, Wang F, Germano G, Dey D and Berman DS. Comparison of the Coronary Artery Calcium Score and Number of Calcified Coronary Plaques for Predicting Patient Mortality Risk. Am J Cardiol. 2017;120:2154–2159. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Rozanski A, Dey D, Arnson Y, Gransar H, Friedman J, Hayes SW, Thomson LEJ, Tamarappoo B, Shaw LJ, Min JK, Rumberger JA, Budoff MJ, Miedema MD, Blaha MJ and Berman DS. Age- and gender-adjusted percentiles for number of calcified plaques in coronary artery calcium scanning. J Cardiovasc Comput Tomogr. 2019;13:319–324. [DOI] [PubMed] [Google Scholar]
- 20.Blaha MJ, Whelton SP, Al Rifai M, Dardari ZA, Shaw LJ, Al-Mallah MH, Matsushita K, Rumberger JA, Berman DS, Budoff MJ, Miedema MD and Nasir K. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Mallah MH, Keteyian SJ, Brawner CA, Whelton S and Blaha MJ. Rationale and design of the Henry Ford Exercise Testing Project (the FIT project). Clin Cardiol. 2014;37:456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu ZG, Yang ZG, Dong ZH, Zhu ZY, Peng LQ, Shao H, He C, Deng W, Tang SS and Chen J. Characteristics of coronary artery disease in symptomatic type 2 diabetic patients: evaluation with CT angiography. Cardiovasc Diabetol. 2010;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanke P, Naoum C, Ahmadi A, Cheruvu C, Soon J, Arepalli C, Gransar H, Achenbach S, Berman DS, Budoff MJ, Callister TQ, Al-Mallah MH, Cademartiri F, Chinnaiyan K, Rubinshtein R, Marquez H, DeLago A, Villines TC, Hadamitzky M, Hausleiter J, Shaw LJ, Kaufmann PA, Cury RC, Feuchtner G, Kim YJ, Maffei E, Raff G, Pontone G, Andreini D, Chang HJ, Chow BW, Min J and Leipsic J. Long-Term Prognostic Utility of Coronary CT Angiography in Stable Patients With Diabetes Mellitus. JACC Cardiovasc Imaging. 2016;9:1280–1288. [DOI] [PubMed] [Google Scholar]
- 24.Goraya TY, Leibson CL, Palumbo PJ, Weston SA, Killian JM, Pfeifer EA, Jacobsen SJ, Frye RL and Roger VL. Coronary atherosclerosis in diabetes mellitus: a population-based autopsy study. J Am Coll Cardiol. 2002;40:946–53. [DOI] [PubMed] [Google Scholar]
- 25.Pundziute G, Schuijf JD, Jukema JW, Boersma E, Scholte AJ, Kroft LJ, van der Wall EE and Bax JJ. Noninvasive assessment of plaque characteristics with multislice computed tomography coronary angiography in symptomatic diabetic patients. Diabetes Care. 2007;30:1113–9. [DOI] [PubMed] [Google Scholar]
- 26.Tomizawa N, Nojo T, Inoh S and Nakamura S. Difference of coronary artery disease severity, extent and plaque characteristics between patients with hypertension, diabetes mellitus or dyslipidemia. Int J Cardiovasc Imaging. 2015;31:205–12. [DOI] [PubMed] [Google Scholar]
- 27.Melidonis A, Dimopoulos V, Lempidakis E, Hatzissavas J, Kouvaras G, Stefanidis A and Foussas S. Angiographic study of coronary artery disease in diabetic patients in comparison with nondiabetic patients. Angiology. 1999;50:997–1006. [DOI] [PubMed] [Google Scholar]
- 28.Valsania P, Zarich SW, Kowalchuk GJ, Kosinski E, Warram JH and Krolewski AS. Severity of coronary artery disease in young patients with insulin-dependent diabetes mellitus. Am Heart J. 1991;122:695–700. [DOI] [PubMed] [Google Scholar]
- 29.Youssef G and Budoff MJ. Coronary artery calcium scoring, what is answered and what questions remain. Cardiovasc Diagn Ther. 2012;2:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, Nasir K, Gowdak LH, Hainer J, Brady TJ, Di Carli MF, Hoffmann U, Abbara S and Blankstein R. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–91. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds HR, Shaw LJ, Min JK, Page CB, Berman DS, Chaitman BR, Picard MH, Kwong RY, O’Brien SM, Huang Z, Mark DB, Nath RK, Dwivedi SK, Smanio PEP, Stone PH, Held C, Keltai M, Bangalore S, Newman JD, Spertus JA, Stone GW, Maron DJ and Hochman JS. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation. 2021;144:1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tota-Maharaj R, Al-Mallah MH, Nasir K, Qureshi WT, Blumenthal RS and Blaha MJ. Improving the relationship between coronary artery calcium score and coronary plaque burden: addition of regional measures of coronary artery calcium distribution. Atherosclerosis. 2015;238:126–31. [DOI] [PubMed] [Google Scholar]
- 33.Tota-Maharaj R, Joshi PH, Budoff MJ, Whelton S, Zeb I, Rumberger J, Al-Mallah M, Blumenthal RS, Nasir K and Blaha MJ. Usefulness of regional distribution of coronary artery calcium to improve the prediction of all-cause mortality. Am J Cardiol. 2015;115:1229–34. [DOI] [PubMed] [Google Scholar]
- 34.Hecht HS, Blaha MJ, Kazerooni EA, Cury RC, Budoff M, Leipsic J and Shaw L. CAC-DRS: Coronary Artery Calcium Data and Reporting System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12:185–191. [DOI] [PubMed] [Google Scholar]
- 35.Dzaye O, Dudum R, Mirbolouk M, Orimoloye OA, Osei AD, Dardari ZA, Berman DS, Miedema MD, Shaw L, Rozanski A, Holdhoff M, Nasir K, Rumberger JA, Budoff MJ, Al-Mallah MH, Blankstein R and Blaha MJ. Validation of the Coronary Artery Calcium Data and Reporting System (CAC-DRS): Dual importance of CAC score and CAC distribution from the Coronary Artery Calcium (CAC) consortium. J Cardiovasc Comput Tomogr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R and Cainzos-Achirica M. Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc Imaging. 2017;10:923–937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


