PURPOSE
Targeted tyrosine kinase inhibitors (TKIs) and immune-checkpoint inhibitors (ICIs) revolutionized the treatment of metastatic renal cell carcinoma (RCC). Efforts to translate these therapies into the adjuvant setting for local and locoregional RCC have been pursued over the past decade. We sought to provide an updated review of the literature regarding adjuvant therapy in RCC, as well as an analysis of patient characteristics that may portend the most favorable responses.
MATERIALS AND METHODS
Using PubMed, Google Scholar, and Wiley Online Library, we reviewed articles between 2000 and 2022. Search terms included “tyrosine kinase inhibitors,” “adjuvant,” “immunotherapy,” and “renal cell carcinoma.” The articles included were original and published in English. Information on clinical trials was collected from ClinicalTrials.gov, accessed in June 2022.
RESULTS
Landmark trials investigating adjuvant vascular endothelial growth factor (VEGF) inhibitors produced conflicting results, with only a single trial of sunitinib (S-TRAC) resulting in US Food and Drug Administration–approval on the basis of a slightly prolonged progression-free survival (PFS). Subsequent meta-analyses failed to show a benefit for adjuvant VEGF inhibitors. Several trials evaluating ICIs are currently ongoing, with pembrolizumab (KEYNOTE-564) earning US Food and Drug Administration–approval for a prolonged PFS, although overall survival data are not yet mature. Preliminary results from other adjuvant ICI trials have been conflicting.
CONCLUSION
There remains a lack of clear benefit for the use of adjuvant VEGF inhibitors in local and locoregional RCC. Adjuvant ICI investigations are ongoing, with promising results from KEYNOTE-564. It remains to be seen if PFS is an adequate surrogate end point for overall survival. Selection of patients at greatest risk for recurrence, and identification of those at greatest risk of rare but serious adverse events, may improve outcomes.
INTRODUCTION
Renal cell carcinoma (RCC) has been of increasing incidence in the United States and worldwide over the past 4 decades.1,2 Globally, kidney cancer is the 14th most common cancer by incidence.2 In the United States, it represents the 9th most common cancer and is estimated to result in 79,000 new cases and cause 13,920 deaths in 2022.3
CONTEXT
Key Objective
Increasing disease-free survival for patients with renal cell carcinoma (RCC) amendable to resection at intermediate-to-high risk of recurrence has long been a challenge. Treatment of metastatic RCC has been revolutionized with targeted therapies against tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs). Efforts to translate these therapies into the adjuvant setting have been pursued with uneven results. We sought to contextualize efforts to elucidate adjuvant therapies for this population, while also investigating patient characteristics and study design implications in these efforts.
Knowledge Generated
Clinical trials investigating adjuvant TKIs for RCC at high risk for recurrence did not provide strong evidence for their use. Ongoing investigations into ICIs have led to approval of adjuvant pembrolizumab.
Relevance
Adjuvant targeted therapies for RCC at high risk for recurrence represent an unmet need. Pembrolizumab represents a promising ICI option for select patients. Examining specific patient populations within this group can guide future investigations.
Most patients are diagnosed at a stage deemed local or locally advanced and are candidates for surgery that may be considered curative. Among the roughly 70% of patients diagnosed within these early stages, between 20% and 40% will recur.4 When classified into low-, intermediate-, and high-risk using the UCLA Integrated Staging System (UISS), the 5-year recurrence risk was 9.6%, 39.2%, and 58.1%, respectively.5 Much has been done to develop new targets and therapies for adjuvant use, as recurrence will result in greater than half of all patients being considered high-risk.
Translational research has led to therapies directed at cellular targets such as vascular endothelial growth factor (VEGF), which has revolutionized treatment of metastatic RCC (mRCC).6 Following US Food and Drug Administration (FDA)–approval for the first tyrosine kinase inhibitor (TKI) for mRCC in 2005 with sunitinib, a new era of treatment began with development of novel targeted therapies; subsequently, multiple attempts have been made to use TKIs in the adjuvant setting for nonmetastatic disease.7 Despite these efforts, the results from these clinical trials have been conflicting.8 More recently developed immune-checkpoint inhibitors (ICIs) are hoped to portend a more favorable safety profile with positive antitumor responses.9 Clinical trials are underway to assess their use in the adjuvant setting.
In this review, we explore the role of adjuvant targeted therapy in RCC, both historically and in modern practice. We also seek to describe patient populations that may benefit from adjuvant therapy, while weighing risks of adverse effects against potential benefits, and discuss outcomes data imperative to understanding the efficacy of adjuvant therapy.
METHODS
Using PubMed, Google Scholar, and Wiley Online Library, we reviewed articles between 2000 and 2022. Search terms included “tyrosine kinase inhibitors,” “adjuvant,” “immunotherapy,” and “renal cell carcinoma.” Articles were original and published in English. Unpublished works, works not in English, and news articles were not included. Information on clinical trials was collected from ClinicalTrials.gov, accessed in June 2022.
RESULTS
Historical Perspective: Adjuvant Therapy in the Pretargeted Era
In the 1980s, adjuvant immunotherapy agents interferon-alpha and interleukin-2 were pursued, given their favorable results treating metastatic disease. Several trials failed to show improvement in disease-free survival (DFS), both alone and in combination.10 Similarly disappointing results were found investigating adjuvant tumor-derived vaccine therapy.11 Both adjuvant radiotherapy and hormone therapy with medroxyprogesterone acetate were investigated in the latter part of the twentieth century, with neither showing improved DFS.10 As such, further investigation into these modalities was not pursued.
Targeted Era
Background.
Identification of the tumor suppressor gene von Hippel-Lindau (VHL) in the pathogenesis of RCC became the catalyst for targeted therapies. With loss of the suppressing activity of VHL, downstream modulators such as hypoxia-inducible factors (HIFs) persist under inappropriate circumstances, leading to a cascade of molecular events promoting cellular growth.12 Uninhibited, HIF transcription factors go on to regulate several hundred gene products that promote angiogenesis, cell survival, metabolism, and metastasis. One such gene product is VEGF, which contributes to tumorigenesis and is of particular importance in RCC.13
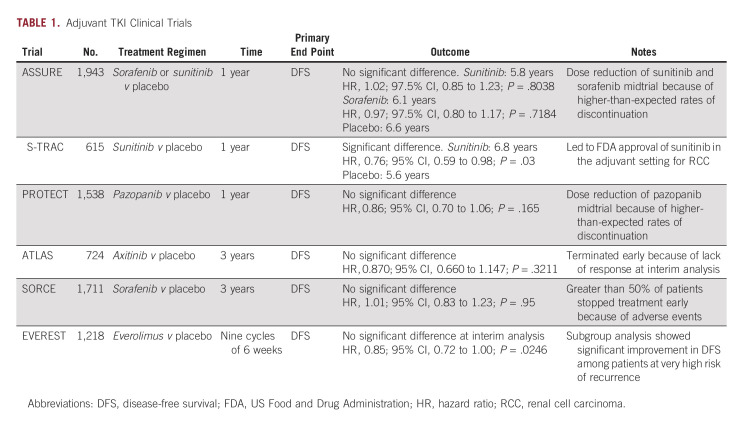
Downstream targets of the VHL-HIF pathway function as receptor tyrosine kinases, which are signaling molecules amenable to pharmacologic targeting to disrupt this dysregulated cascade. Introduction of VEGF-targeting TKIs radically altered mRCC prognosis and became the new standard of care for these patients.9 Following success with TKI therapy in mRCC, efforts were focused on expanding their use into the adjuvant setting for local and locally advanced RCC in five landmark clinical trials (Table 1).
TABLE 1.
Adjuvant TKI Clinical Trials
Sunitinib.
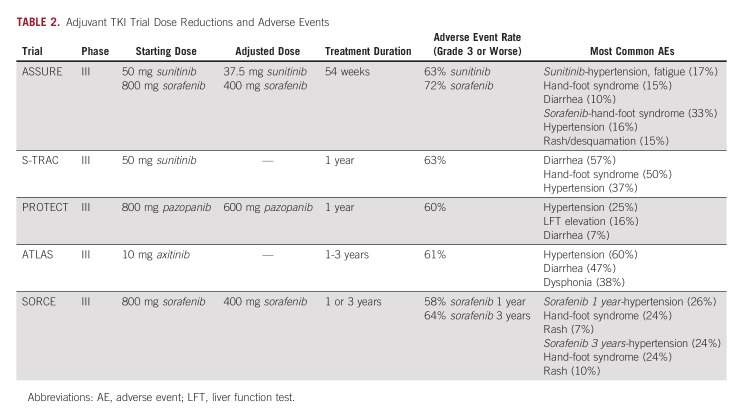
Sunitinib was included in two major TKI trials, ASSURE and S-TRAC. ASSURE investigated adjuvant use of once daily sunitinib, twice daily sorafenib, or placebo in a phase III double-blinded randomized controlled trial (RCT) of patients with high-risk clear-cell or non–clear-cell RCC (ccRCC).14 Patients were randomly assigned and further stratified into intermediate and very high risk of recurrence using the UISS, with the primary outcome of DFS. Both drugs underwent midstudy dose reduction because of high rates of adverse events (AEs; Table 2). ASSURE ultimately found no significant difference in DFS for sunitinib compared with placebo at a median follow-up of 5.8 years, failing to meet the primary end point (median DFS [mDFS] 5.8 years for sunitinib, 6.6 years for placebo [hazard ratio (HR), 1.17; 97.5% CI, 0.90 to 1.52; P = .1762]).14
TABLE 2.
Adjuvant TKI Trial Dose Reductions and Adverse Events
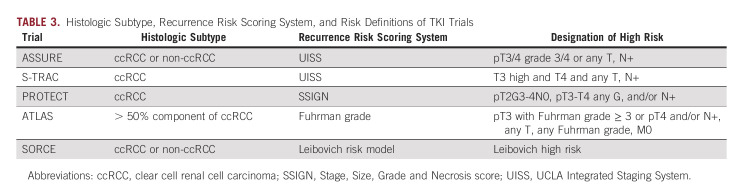
Notably, ASSURE included a variety of RCC histologic subtypes, with just under 80% of patients in the clear-cell group. Although the rationale for TKI use centers on the VHL-HIF-VEGF cascade, its dysregulation is less commonly implicated in tumorigenesis of other subtypes of RCC.15 As such, secondary analysis concerning ccRCC and those considered very-high risk failed to show a significant DFS benefit—5-year DFS was 47.7% in the sunitinib arm and 50.0% in the placebo arm (HR, 0.94; 97.5% CI, 0.74 to 1.19; P = .54).16 With no improvement in DFS in primary or secondary analyses, along with elevated rates of AEs, ASSURE failed to provide evidence to support adjuvant sunitinib in RCC.
S-TRAC included patients with locoregional ccRCC at high risk for recurrence using UISS in a phase III double-blinded RCT evaluating adjuvant sunitinib once daily versus placebo. Unlike ASSURE, patients diagnosed with non-ccRCC were excluded. Primary outcome was DFS, which resulted in a significant difference between the sunitinib group with an mDFS of 6.8 years compared with 5.6 years for placebo (HR, 0.76; 95% CI, 0.59 to 0.98; P = .03).17 Because of AEs, 44% of patients in the sunitinib arm discontinued the drug before the prespecified 1-year regimen (Table 2).
The DFS benefit observed in S-TRAC led to FDA-approval of adjuvant sunitinib for patients with locoregional ccRCC at high risk for recurrence in 2017, although it was denied approval by regulatory officials in Europe.18,19 Current National Comprehensive Cancer Network guidelines designate adjuvant sunitinib for stage III RCC as a category three recommendation, indicating major disagreement that the intervention is appropriate.20 Secondary analysis was unable to demonstrate an overall survival (OS) benefit. However, subgroup analysis showed an mDFS benefit persisted in the highest risk category (T3, no or undetermined nodal involvement, Fuhrman grade ≥ 2, and Eastern Cooperative Oncology Group performance status ≥ 1; or T4 and/or nodal involvement).21
A meta-analysis of adjuvant sunitinib from both studies failed to show a DFS benefit, a result that persisted with non-ccRCC patients from ASSURE excluded.22 With conflicting results from two major clinical trials, adjuvant sunitinib for RCC remains contentious despite FDA approval. Debate surrounding heterogeneity of patient populations, dosing, and the use of DFS as an appropriate metric continues today.23
Sorafenib.
Sorafenib was investigated in ASSURE as previously described. As with sunitinib in this trial, midstudy dose reduction was required because of AEs (Table 2). Primary outcome of DFS with sorafenib was not met, with an mDFS of 73.4 months compared with placebo at 79.6 months (HR, 0.97; 97.5% CI, 0.80 to 1.17; P = .7184).14 Secondary analysis of only ccRCC and those at very high risk of recurrence per UISS failed to demonstrate an mDFS benefit.16 ASSURE failed to provide evidence to support adjuvant sorafenib in RCC, as it did with sunitinib.
The more recent SORCE trial, a phase III double-blinded RCT, investigated adjuvant sorafenib twice daily. Given the results of ASSURE and S-TRAC, both of which used a 1-year treatment duration, SORCE investigated a more prolonged regimen. Patients with ccRCC and non-ccRCC histology at intermediate or high risk of recurrence per the Leibovich risk model were stratified and randomly assigned into 3 years of sorafenib, 1 year of sorafenib +2 years of placebo, or 3 years of placebo. As with ASSURE, midstudy dose reduction was required because of high rates of discontinuation (Table 2). The primary outcome was again DFS, which failed to be met (3 years of sorafenib compared with placebo [HR, 1.01; 95% CI, 0.82 to 1.23; P = .946], 1 year of sorafenib +2 years of placebo compared with placebo, HR, 0.94; 95% CI, 0.77 to 1.14; P = .509). Secondary outcomes included OS, which failed to be met in any of the treatment arms. As with the previously discussed TKI trials, AEs were significant, necessitating dose reductions (Table 2).24 The study failed to provide evidence to support the use of adjuvant sorafenib in RCC at intermediate to high risk of recurrence.
Both ASSURE and SORCE included non-ccRCC with ccRCC and both required midstudy sorafenib dose reduction. A major difference in study design and patient stratification is the use of UISS (ASSURE) versus Leibovich model (SORCE). In using these distinct prediction models, standardization among stratification and definition of recurrence necessarily comes into question, complicating comparison.9
Pazopanib.
The PROTECT trial was a phase III double-blinded RCT evaluating once daily adjuvant pazopanib. As with S-TRAC, patients with non-ccRCC were excluded. Again, midstudy dose reduction was required because of AEs (Table 2).25 The study did not meet its primary end point of DFS (HR, 0.86; 95% CI, 0.70 to 1.06; P = .16); however, a secondary analysis of DFS in patients able to tolerate dose escalation was met (HR, 0.69; 95% CI, 0.51 to 0.94; P = .02).25 Notably, the placebo arm of the reduced-dose group performed significantly better compared with that of the higher-dose arm (64% DFS at 3 years compared with 54%), which may relate to the significant result found. AEs were experienced at rates observed in previous TKI trials (Table 2).25
PROTECT enrolled 84% of patients with T3 or T4 disease, with the remaining 16% T2 disease. Stage, Size, Grade and Necrosis score was used to define risk, as opposed to UISS or Leibovich used in other TKI trials. Because of differences in patient selection and scoring systems, it is difficult to draw comparisons between them. Ultimately, PROTECT failed to provide evidence for adjuvant pazopanib in ccRCC.
Axitinib.
ATLAS enrolled patients with predominant ccRCC in a double-blinded RCT. Patients were to receive twice daily axitinib for at least one but up to 3 years or placebo. The study was terminated early at predetermined interim analysis because of failure to show DFS benefit (HR, 0.87; 95% CI, 0.66 to 1.147; P = .3211). Like PROTECT, included patients had T2 disease (11%), while the remaining 89% were T3, T4, and node-positive patients. Subsequent subgroup analysis of only patients considered high risk (pT3 with FG ≥ 3 or pT4 and/or N+, any T, any FG) was performed once S-TRAC results were published. There was a significant reduction in risk of DFS between the axitinib and placebo arms in the higher-risk group. AEs were seen at rates similar to those described in prior adjuvant TKI trials (Table 2).26 Further analysis was undertaken to evaluate ethnic and treatment duration differences that may have resulted in a DFS benefit; however, neither was supported.27,28
ATLAS failed to meet its primary end point of DFS and was terminated early. However, subgroup analysis supported potential benefits in higher-risk patients with ccRCC. As with prior TKI studies, differences in patient populations and prediction models complicate direct comparison (Table 3).26
TABLE 3.
Histologic Subtype, Recurrence Risk Scoring System, and Risk Definitions of TKI Trials
Meta-analyses.
Following the five landmark trials, meta-analyses were performed to further establish the role of adjuvant TKIs in the face of conflicting individual study results. Early analysis found no benefit to DFS or OS with adjuvant TKI use and a significant increase in AEs.29 However, more recent meta-analyses with stratification of patients into lower and higher risk of recurrence each found improvement in DFS in the higher-risk population, although this benefit did not translate to OS. The elevated risk of AEs remained consistent across analyses.30,31 Elucidating appropriate patients for adjuvant TKI treatment is recommended.
Everolimus.
EVEREST investigates once daily adjuvant mammalian target of rapamycin inhibitor everolimus in a randomized double-blinded RCT (ClinicalTrials.gov identifier: NCT01120249). Participants have ccRCC or non-ccRCC histology. A recently presented analysis reported improvement in DFS in the everolimus group versus placebo (HR, 0.85; 95% CI, 0.72 to 1.00; P = .0246), although it fell short of their prespecified significance value.32 Very high risk subgroup analysis reported improvement in DFS in the everolimus group versus placebo (HR, 0.79; 95% CI, 0.65 to 0.97; P = .011), which may provide support for adjuvant everolimus in this population. As with other adjuvant TKI trials, AEs were common, and resulted in elevated rates of discontinuation. An OS benefit was not demonstrated.32 EVEREST will continue to release data in the coming years, and the peer-reviewed manuscript of the initial abstract presentation is eagerly awaited.
Adjuvant Immunotherapy
Background.
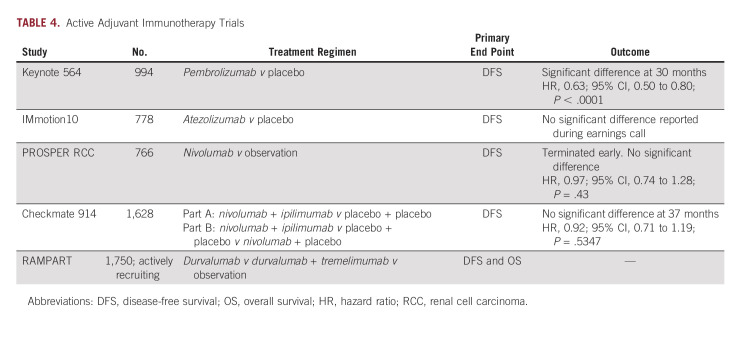
Immunotherapy is a rapidly evolving oncologic treatment option. ICIs have become common first-line agents for treatment of stage IV RCC.33 Positive results from KEYNOTE-564 have led to FDA-approval of pembrolizumab in adults with RCC at increased risk of recurrence following surgery.34 Several other phase III clinical trials are currently underway (Table 4); however, pembrolizumab remains the only ICI recommended by the European Association of Urology.35
TABLE 4.
Active Adjuvant Immunotherapy Trials
Pembrolizumab.
KEYNOTE-564 investigates adjuvant pembrolizumab in patients with ccRCC at high risk for recurrence in a phase III double-blinded RCT.34 Participants had locoregional RCC with a clear-cell component or metastatic disease amenable to resection, and a high risk of recurrence (tumor stage 2 with nuclear grade 4 or sarcomatoid differentiation, tumor stage 3 or higher, regional lymph-node metastasis, or stage M1 with no evidence of disease). Patients receive pembrolizumab or placebo once every 3 weeks for up to 17 cycles. Primary and secondary end points are DFS and OS, respectively.
The median duration of the treatment was 11.1 months for both pembrolizumab and placebo groups. The pembrolizumab group had a significant improvement in DFS (77.3% v 68.1% in placebo group; HR, 0.68; 95% CI, 0.53 to 0.87; P = .002). The estimated percentage of patients who remained alive at 24 months was 96.6% (95% CI, 94.3 to 98.0) in the pembrolizumab group and 93.5% (95% CI, 90.5 to 95.6) in the placebo group.34 Pembrolizumab appeared to be better tolerated (20.7% discontinuation) when compared with TKIs.29 Fatigue represented the most common AE (29.7% v 24.2% placebo).
Thirty-month follow-up data maintained DFS benefit with pembrolizumab (HR, 0.63; 95% CI, 0.50 to 0.80; P < .0001).36 OS at 24 months was 96.2% in the pembrolizumab group, compared with 93.8% for placebo. There has been a total of 66 OS events, with 23 in the treatment arm and 43 in the placebo arm (HR, 0.52; 95% CI, 0.31 to 0.86; P = .0048). The P value did not cross the statistical hypothesis testing boundary and additional follow-up is needed. No additional grade 3-4 AEs occurred.36,37 24-month analysis found significance in the European Union (HR, 0.49; 95% CI, 0.32 to 0.79), while North America and the rest of the world were not independently significant (0.87 [0.53 to 1.41] and 0.81 [0.55 to 1.21], respectively). Regional outcome differences warrant further evaluation.38
Atezolizumab.
Immotion010 evaluates adjuvant atezolizumab (anti–PD-L1) in a double-blind phase III RCT of participants with ccRCC or sarcomatoid histology (ClinicalTrials.gov identifier: NCT03024996). Patients received atezolizumab or placebo once every 3 weeks for 16 cycles or 1 year (whichever occurred first). At a median follow up of 44.7 months (interquartile range, 39.1-51.0 months), treatment group DFS was 57.2 months compared with 49.5 months for placebo (HR, 0.93; 95% CI, 0.75 to 1.15; P = .50). 2-year DFS rates were 67% versus 65%, respectively.39 Negative results from Immotion010 conflict with those of KEYNOTE-564, both of which target the PD-1/PD-L1 pathway, calling into question the similarities between drugs in this class. Subgroup analysis is expected soon.40
Nivolumab.
The PROSPER RCC trial investigates nivolumab (anti–PD-1) in an unblinded phase III RCT (ClinicalTrials.gov identifier: NCT03055013). Patients with ccRCC or non-ccRCC receive nivolumab or standard observation. The treatment group receives neoadjuvant nivolumab once every 2 weeks for two cycles, then every once 2 weeks after surgery for six cycles, and then once every 4 weeks for six more cycles. Notably, trial design incorporates neoadjuvant therapy, likely due in part to findings showing that a similar approach in early-stage non–small-cell lung cancer induced a significant pathologic complete response rate of 45%. Similar data have been demonstrated in patients with muscle-invasive bladder cancer treated with pembrolizumab before cystectomy.41,42 However, the study was terminated early because of futility, as no difference in DFS was observed between the two groups (HR, 0.97; 95% CI, 0.74 to 1.28; P one-sided = .43) and mDFS was not reached. OS data are not yet mature, but were insignificant at the time of data presentation (HR, 1.48; 95% CI, 0.89 to 2.48; P one-sided = .93).43
Ipilimumab.
Checkmate914 (ClinicalTrials.gov identifier: NCT03138512) is a multiarm, quadruple-blinded, phase III RCT investigating nivolumab once every 2 weeks for 6 months and ipilimumab (anti-choline transporter-like protein 4 (anti-CTLA4) once every 6 weeks for 6 months). Participants have predominant clear-cell histology. Part A compares nivolumab + ipilimumab versus placebo, while part B compares nivolumab + ipilimumab versus placebo versus nivolumab + placebo. Recently presented preliminary results demonstrated no improvement in DFS (HR, 0.92; 95% CI, 0.71 to 1.19; P = .5347) at a median follow-up of 37 months. DFS at 24 months was 76.4% and 74% for treatment and placebo groups, respectively.44
Durvalumab and tremelimumab.
RAMPART is a multiarm, open-label, phase III RCT assessing adjuvant durvalumab (anti-CD274) and tremelimumab (anti–CTLA-4) in patients with ccRCC or non-ccRCC (ClinicalTrials.gov identifier: NCT03288532). Study groups include no intervention, durvalumab monotherapy (once every 4 weeks for 1 year), or durvalumab (once every 4 weeks for 1 year) + tremelimumab (on day 1 and week 4 follow-up visit) combined therapy. Primary end points include DFS and OS. Secondary end points include metastasis-free survival and RCC-specific survival time. RAMPART has an estimated primary completion date of July 1, 2024.
Appropriate Patient Selection
Outcomes: physician-assessed and patient-reported.
After appropriate treatment for malignancies of any variety, patients often harbor psychologic stress associated with fear of cancer recurrence or progression.45 This fear can become pathologic and has been associated with decreased quality of life, anxiety, intrusive thoughts, fatigue, and dysfunctional behavior. Fear of cancer recurrence or progression has led to increased health care costs, more frequent emergency department visits, longer hospital stays, and requests for additional doctor visits and imaging studies. Lack of clear guidelines for adjuvant therapy in RCC leaves patients with high levels of anxiety regarding their diagnosis, with many requesting treatment despite potential harms often outweighing benefits.45 Although immunotherapy agents are often seen as having a more favorable side-effect profile than TKIs, they are not entirely benign. Through immunostimulation, side effects can mimic autoimmune disease, resulting in mild to life-threatening symptoms that may endure.46,47
With adjuvant therapy, many patients who will never have had recurrence will be treated, often incurring unnecessary clinical and financial toxicity. Without appropriate studies evaluating OS data in these patients, optimal population selection remains unknown. As with the introduction of many other medications, we must attempt to treat cancer while mitigating risk and avoiding harm. If the treatment does not make patients live longer or better, why give it?48
Risk assessments.
Delineating proper patient selection for adjuvant therapy is a multifactorial challenge. First, patients with poorer outcomes should be considered. Previous data from the National Cancer Data Base showed stage I and II RCC had significantly better survival outcomes than stage III and IV disease—90.4% and 83.4% compared with 66.0% and 9.1%, respectively.49 The results from the SEER database showed 5-year survival rates after nephrectomy of 97.4% (stage I), 89.9% (stage II), 77.9% (stage III), and 26.7% (stage IV).50 With this, stage III and IV RCC are identified as populations that may benefit from adjuvant therapy.
A recent publication compared patients who had stage III RCC with and without lymph node (LN) involvement to patients who had stage IV disease. Using OS as the primary end point, patients with stage III disease without LN involvement (LN−) had a 5-year OS of 61.9%, while patients with stage III disease with LN involvement (LN+) and patients with stage IV disease had an OS of 22.7% and 15.6%, respectively. With overlapping confidence intervals, stage III LN+ and stage IV groups behaved similarly and together may be considered high-risk, potentially benefiting from adjuvant therapy. In addition to nodal involvement, among patients with stage III disease, high-grade tumor histology and non–clear-cell histology were associated with poorer survival.51
Patients with certain forms of variant histology also portend a worse prognosis, making adjuvant therapy a consideration for this population.52 Accompanying a higher 5-year mortality rate, sarcomatoid RCC harbors a significantly higher rate of metastasis at diagnosis, estimated to be 60%-80%. Additionally, there is an 80% likelihood of recurrence after nephrectomy.53-55 Other microscopic features consistently associated with poorer prognosis are tumor necrosis, microvascular invasion, and tumor grade.56 As discussed previously, stage III disease comes in many varieties, each with different predicted OS and DFS dependent upon unique tumor characteristics. With vascular invasion, inferior vena cava involvement portends worsened survival than renal vein involvement, which in turn has worse survival than tumors confined to the kidney.56
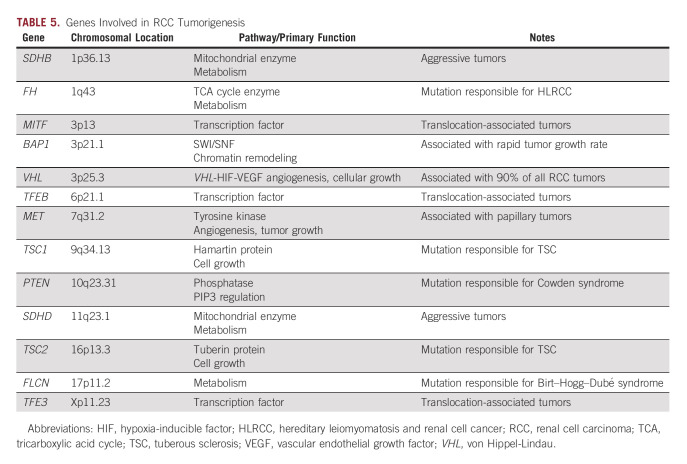
Certain molecular subtypes of RCC may also portend a poorer prognosis. Although VHL remains the most well studied, recently identified genes PBRM1, BAP1, SETD2, and components of the PI3K/AKT pathway are implicated in RCC, and are among those being studied for prognostic significance. Despite identification of many implicated genes, few have been therapeutically targeted successfully (Table 5).57-60 A recent Chinese study investigated immune-related gene signatures interferon-gamma, CTL, the expanded immune gene, and the HLA-A/B molecules as they relate to prognosis in RCC.61 499 samples from patients with localized or advanced RCC were taken from the cancer genome atlas database (TCGA). From the 231 advanced RCC samples, genes were identified that were associated with poorer OS and DFS, including TNFRSF8 and CXCL13.61
TABLE 5.
Genes Involved in RCC Tumorigenesis
In a case-control study in Eastern Europe, investigators identified unique risk factors for the development of ccRCC subtypes A versus B.62 Genes related to hypoxia, angiogenesis, and fatty and organic acid metabolism were associated with ccRCC type A, along with elevated BMI. Conversely, genes responsible for epithelial-to-mesenchymal transition, cell cycle, and wound healing have been associated with RCC type B, along with male sex and exposure to trichloroethylene in this study population.62 Given the robust genetic and environmental risk factors, further investigation is warranted to determine their role in the patient selection algorithm.
End points: DFS versus OS.
One major limitation in adjuvant RCC data is reliance on DFS over OS. The goal of adjuvant therapy is for patients to live longer and/or better. Ideally, trials would report outcomes using OS, but demonstrating significance could take years. Alternatively, DFS has been used as a surrogate. Although the newer immunotherapies are generally thought of as safer than traditional systemic therapy, these medications have the potential to cause significant life-altering side effects. Without a clear OS advantage or significant improvement in quality of life, DFS remains the only metric from which conclusions can be drawn. In a statement from the FDA regarding adjuvant trials, they recommend accounting for the magnitude of improvement in DFS before drug approval.63 Although demonstration of OS benefit is not required for initial approval, it should still be assessed with an interim analysis and show a favorable trend per FDA guidelines. Patients should also be followed to allow a final OS analysis to be conducted.63
DFS as the primary surrogate for benefit in targeted therapy is complicated by several other factors.64 With increased systemic treatments of RCC, patients are living longer, which may make OS susceptible to confounding in ways DFS may not. For instance, in a nonrandomized setting, patients with a higher tumor stage or higher International Metastatic RCC Database Consortium score may be more likely to receive adjuvant treatment. In the randomized setting, although patients may start with one specific treatment, they may ultimately end up receiving systemic treatment. At this point, quantifying the treatment effect on OS becomes challenging and requires complex statistical adjustments and modeling.65
Inevitably, some patients will recur after receiving adjuvant immune-oncologic (IO) monotherapy. In addition to the previously described critiques of the established data on adjuvant IO therapy, lack of data and treatment options for this subset of patients presents a unique challenge for patients and their oncologists. After failing IO monotherapy, should patients then proceed with combination IO dual therapy, IO with a TKI, or something else? This is an important opportunity for future research.
DISCUSSION
In conclusion, to date, elucidating proper adjuvant therapy in locoregional RCC has proven challenging. Despite evidence for various agents, including TKIs, in metastatic disease, these benefits did not translate to landmark clinical trials in the adjuvant setting, aside from S-TRAC. Although DFS improved in certain subgroup analyses, OS was invariably unchanged. As such, use of adjuvant TKIs in RCC remains controversial. With the development of ICIs and their rapid implementation throughout oncology, ongoing trials have shown uneven preliminary results.
Appropriate patient selection and using risk assessment tools represent often-undervalued components of our investigations. Reliance upon metrics such as DFS over OS complicates how we report and qualify data. Greater focus on determining proper end points, meaningful outcomes, and risk assessments will improve adjuvant therapy moving forward.
Eric A. Singer
Consulting or Advisory Role: Merck, Johnson & Johnson/Janssen, Vyriad, Aura Biosciences
Research Funding: Astellas Medivation
No other potential conflicts of interest were reported.
SUPPORT
Supported by a grant from the National Cancer Institute (P30CA072720).
AUTHOR CONTRIBUTIONS
Conception and design: Joseph J. Boyle, Benjamin J. Lichtbroun, Eric A. Singer
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric A. Singer
Consulting or Advisory Role: Merck, Johnson & Johnson/Janssen, Vyriad, Aura Biosciences
Research Funding: Astellas Medivation
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Cancer Institute : Kidney and Renal Pelvis Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2019. National Cancer Institute SEER Program, 2022. https://seer.cancer.gov/statistics-network/explorer/application.html?site=72&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=2 [Google Scholar]
- 2.World Cancer Fund International : Global kidney cancer statistics for the year 2022, July update. https://www.wcrf.org/cancer-trends/kidney-cancer-statistics/ [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 4.Chin AI, Lam JS, Figlin RA, et al. : Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol 8:1-7, 2006 [PMC free article] [PubMed] [Google Scholar]
- 5.Lam JS, Shvarts O, Leppert JT, et al. : Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol 174:466-472, 2005; discussion 472; quiz 801 [DOI] [PubMed] [Google Scholar]
- 6.Barata PC, Rini BI: Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin 67:507-524, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Heng DYC, Kollmannsberger C: Sunitinib. Recent Results Cancer Res 184:71-82, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Gul A, Rini BI: Adjuvant therapy in renal cell carcinoma. Cancer 125:2935-2944, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Larroquette M, Peyraud F, Domblides C, et al. : Adjuvant therapy in renal cell carcinoma: Current knowledges and future perspectives. Cancer Treat Rev 97:102207, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Janowitz T, Welsh SJ, Zaki K, et al. : Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol 40:482-491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jocham D, Richter A, Hoffmann L, et al. : Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: Phase III, randomised controlled trial. Lancet 363:594-599, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Shim BY, Lee S-J, et al. : Loss of von Hippel–Lindau (VHL) tumor suppressor gene function: VHL–HIF pathway and advances in treatments for metastatic renal cell carcinoma (RCC). Int J Mol Sci 22:9795, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto T, Shibasaki F: Hypoxia-inducible factor as an angiogenic master switch. Front Pediatr 3:33, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas NB, Manola J, Uzzo RG, et al. : Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387:2008-2016, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T, Gong J, Maia MC, et al. : Systemic therapy for non–clear cell renal cell carcinoma. Am Soc Clin Oncol Educ Book 37:337-342, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Haas NB, Manola J, Dutcher JP, et al. : Adjuvant treatment for high-risk clear cell renal cancer: Updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol 3:1249-1252, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravaud A, Motzer RJ, Pandha HS, et al. : Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 375:2246-2254, 2016 [DOI] [PubMed] [Google Scholar]
- 18.FDA : FDA Approves Sunitinib Malate for Adjuvant Treatment of Renal Cell Carcinoma. United States Food and Drug Administration, 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sunitinib-malate-adjuvant-treatment-renal-cell-carcinoma [Google Scholar]
- 19.Rothenberg M: Sunitinib Not Granted European Panel Support for Approval in High-Risk RCC. Targeted Oncology, 2018. https://www.targetedonc.com/view/sunitinib-not-granted-european-panel-support-for-approval-in-highrisk-rcc [Google Scholar]
- 20.National Comprehensive Cancer Network . Kidney Cancer (Version 3.2023). https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf [Google Scholar]
- 21.Motzer RJ, Ravaud A, Patard J-J, et al. : Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: Subgroup analyses and updated overall survival results. Eur Urol 73:62-68, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyawali B, Ando Y: Adjuvant sunitinib for high-risk-resected renal cell carcinoma: A meta-analysis of ASSURE and S-TRAC trials. Ann Oncol 28:898-899, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Brown LC, Zhang T, George DJ: The role of targeted therapy in the management of high-risk resected kidney cancer: What have we learned and how will it inform future adjuvant trials. Cancer J 26:376-381, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Eisen T, Frangou E, Oza B, et al. : Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: Results from the SORCE Randomized Phase III Intergroup Trial. J Clin Oncol 38:4064-4075, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Haas NB, Donskov F, et al. : Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol 35:3916-3923, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross-Goupil M, Kwon TG, Eto M, et al. : Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: Results from the phase III, randomized ATLAS trial. Ann Oncol 29:2371-2378, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn DI, Kwon TG, Eto M, et al. : Adjuvant axitinib dose modification in renal cell carcinoma (RCC): Analysis of the ATLAS study. J Clin Oncol 37:4573, 2019. (15_suppl) [Google Scholar]
- 28.Quinn DI, Ng CF, Grande E, et al. : ATLAS trial of adjuvant axitinib in patients with renal cell carcinoma: Subgroup analyses with focus on axitinib dosing and racial groups. ESMO Open 6:100105, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riaz IB, Faridi W, Husnain M, et al. : Adjuvant therapy in high-risk renal cell cancer: A systematic review and meta-analysis. Mayo Clinic Proc 94:1524-1534, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Massari F, Di Nunno V, Mollica V, et al. : Adjuvant tyrosine kinase inhibitors in treatment of renal cell carcinoma: A meta-analysis of available clinical trials. Clin Genitourin Cancer 17:e339-e344, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Laukhtina E, Quhal F, Mori K, et al. : Adjuvant therapy with tyrosine kinase inhibitors for localized and locally advanced renal cell carcinoma: An updated systematic review and meta-analysis. Urol Oncol 39:764-773, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Ryan CW, Tangen C, Heath EI, et al. : EVEREST: Everolimus for renal cancer ensuing surgical therapy—A phase III study (SWOG S0931, NCT01120249). J Clin Oncol 40:LBA4500, 2022. (17_suppl) [Google Scholar]
- 33.George DJ, Lee CH, Heng D: New approaches to first-line treatment of advanced renal cell carcinoma. Ther Adv Med Oncol 13:175883592110347, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choueiri TK, Tomczak P, Park SH, et al. : Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 385:683-694, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Bedke J, Albiges L, Capitanio U, et al. : The 2022 Updated European Association of Urology Guidelines on the use of adjuvant immune checkpoint inhibitor therapy for renal cell carcinoma. Eur Urol 83:10-14, 2023 [DOI] [PubMed] [Google Scholar]
- 36.Choueiri TK, Tomczak P, Park SH, et al. : Pembrolizumab as post nephrectomy adjuvant therapy for patients with renal cell carcinoma: Results from 30-month follow-up of KEYNOTE-564. J Clin Oncol 40:290, 2022. (6_suppl) [Google Scholar]
- 37.Powles T, Tomczak P, Park SH, et al. : Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-Month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23:1133-1144, 2022 [DOI] [PubMed] [Google Scholar]
- 38.Choueiri TK, Tomczak P, Park SH, et al. : Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 385:683-694, 2021 [DOI] [PubMed] [Google Scholar]
- 39.Pal SK, Uzzo R, Karam JA, et al. : Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 400:1103-1116, 2022 [DOI] [PubMed] [Google Scholar]
- 40.Bex A, Uzzo R, Karam JA, et al. : LBA66 IMmotion010: Efficacy and safety from the phase III study of atezolizumab (atezo) vs placebo (pbo) as adjuvant therapy in patients with renal cell carcinoma (RCC) at increased risk of recurrence after resection. Ann Oncol 33:S1431-S1432, 2022 [Google Scholar]
- 41.Necchi A, Raggi D, Gallina A, et al. : Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol 77:439-446, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Forde PM, Chaft JE, Smith KN, et al. : Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 378:1976-1986, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allaf M, Kim SE, Harshman LC, et al. : LBA67 phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. Ann Oncol 33:S1432-S1433, 2022 [Google Scholar]
- 44.Motzer RJ, Russo P, Gruenwald V, et al. : LBA4—Adjuvant nivolumab plus ipilimumab (NIVO+IPI) vs placebo (PBO) for localized renal cell carcinoma (RCC) at high risk of relapse after nephrectomy: Results from the randomized, phase III CheckMate 914 trial. Ann Oncol 33:S808-S869, 2022 [Google Scholar]
- 45.Bergerot CD, Philip EJ, Bergerot PG, et al. : Fear of cancer recurrence or progression: What is it and what can we do about it? Am Soc Clin Oncol Educ Book 42:1-10, 2022 [DOI] [PubMed] [Google Scholar]
- 46.Barquin-Garcia A, Molina-Cerrillo J, Garrido P, et al. : New oncologic emergencies: What is there to know about inmunotherapy and its potential side effects? Eur J Intern Med 66:1-8, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. : Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9:e002435, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmes B: Exploring adjuvant therapies in renal cell carcinoma. Target Ther Oncol 11:28, 2022 [Google Scholar]
- 49.Kane CJ, Mallin K, Ritchey J, et al. : Renal cell cancer stage migration: Analysis of the National Cancer Data Base. Cancer 113:78-83, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Palumbo C, Mistretta FA, Knipper S, et al. : Conditional survival of patients with nonmetastatic renal cell carcinoma: How cancer-specific mortality changes after nephrectomy. J Natl Compr Canc Netw 18:44-51, 2020 [DOI] [PubMed] [Google Scholar]
- 51.Srivastava A, Rivera-Nunez Z, Kim S, et al. : Impact of pathologic lymph node-positive renal cell carcinoma on survival in patients without metastasis: Evidence in support of expanding the definition of stage IV kidney cancer. Cancer 126:2991-3001, 2020 [DOI] [PubMed] [Google Scholar]
- 52.Bakouny Z, Braun DA, Shukla SA, et al. : Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun 12:808, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merrill MM, Wood CG, Tannir NM, et al. : Clinically nonmetastatic renal cell carcinoma with sarcomatoid dedifferentiation: Natural history and outcomes after surgical resection with curative intent. Urol Oncol Semin Original Invest 33:166.e21-166.e29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blum KA, Gupta S, Tickoo SK, et al. : Sarcomatoid renal cell carcinoma: Biology, natural history and management. Nat Rev Urol 17:659-678, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mian BM, Bhadkamkar N, Slaton JW, et al. : Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol 167:65-70, 2002 [PubMed] [Google Scholar]
- 56.Mattila KE, Vainio P, Jaakkola PM: Prognostic factors for localized clear cell renal cell carcinoma and their application in adjuvant therapy. Cancers (Basel) 14:239, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bratslavsky G, Mendhiratta N, Daneshvar M, et al. : Genetic risk assessment for hereditary renal cell carcinoma: Clinical consensus statement. Cancer 127:3957-3966, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ball MW, An JY, Gomella PT, et al. : Growth rates of genetically defined renal tumors: Implications for active surveillance and intervention. J Clin Oncol 38:1146-1153, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linehan WM, Ricketts CJ: The metabolic basis of kidney cancer. Semin Cancer Biol 23:46-55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gene [Internet]: Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004-2022. https://www.ncbi.nlm.nih.gov/gene/ [Google Scholar]
- 61.Cao P, Wu JY, Zhang JD, et al. : A promising prognostic risk model for advanced renal cell carcinoma (RCC) with immune-related genes. BMC Cancer 22:691, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Purdue MP, Rhee J, Moore L, et al. : Differences in risk factors for molecular subtypes of clear cell renal cell carcinoma. Int J Cancer 149:1448-1454, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apolo AB, Msaouel P, Niglio S, et al. : Evolving role of adjuvant systemic therapy for kidney and urothelial cancers. Am Soc Clin Oncol Educ Book 42:1-16, 2022 [DOI] [PubMed] [Google Scholar]
- 64.Rathmell WK, Rumble RB, Van Veldhuizen PJ, et al. : Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol 40:2957-2995, 2022 [DOI] [PubMed] [Google Scholar]
- 65.Msaouel P, Grivas P, Zhang T: Adjuvant systemic therapies for patients with renal cell carcinoma: Choosing treatment based on patient-level characteristics. Eur Urol Oncol 5:265-267, 2022 [DOI] [PubMed] [Google Scholar]