Abstract
Microalgal biomass has the ability to store huge amount of triacylglycerides as fatty ester methyl esters (FAME) and carotenoids which has made algae as potential candidate for biorefinery approach. Essential fatty acid such as palmitic acid, stearic acid, arachidonic acid and eicospentanoic acid have been produced which are known for their various applications. The present study was aimed to evaluate the influence of different light intensities (120 and 250 μE/m2/s) and photoperiod (16:8h and 13:11h light/dark cycle) on the production of lipid, biomass and lutein. Dunaliella tertiolecta and Nannochloropsis oculata was grown for 23 days in F/2, sea salt media (SSM, Distilled water (DW) and SSM (natural seawater media,NSW) under two different light intensities and photoperiod regimes at 25 ᵒC. SSM (NSW) showed maximum accumulation of lipid in D.tertiolecta (34.56 mg/L/d). SSM (DW)- biomass showed 1.5 times higher lutein productivity of 0.253 mg/L/d under 13:11h light/dark cycle at 250 μE/m2/s compared to same medium under 16:8h light/dark cycles at 120 μE/m2/s. Where as in N.oculata, F/2 biomass showed higher lipid and lutein productivity of 15.69 and 0.279 mg/L/d, respectively The laboratory scale cultivation parameters and related media cost showed the suitability of different culture media adaptation to large scale production.
Keywords: Microalgae, Light intensity, Photoperiod, Lipid, Lutein
1. Introduction
An increasing global concern on healthy foods such as omega-3 fatty acids and carotenoids is a major effort to drive the world's nutraceutical market [1]. In terms of commercialization, pigments from microalgae have a high revenue generation > USD 1 billion (selling price – USD 400/kg) and the global carotenoid market is expected to be USD 1.5 billion in 2019 to USD 2.0 billion by 2022 [2]. Although there still exist a diverse microalgal germplasm to be full explored for carotenoid productions [3]. With increase in demand for healthy foods, their sustainable and cheaper source of production is also one of the major concerns. Microalgae biomass producing various metabolites in high yields which are known for the applications in food and feed are considered as promising feedstock [4] (a).Among the marine algae, species belonging to the class of Eustigmatophycease Nannochloropsis oculata is recognised as fast growing marine microalgae because they have high biomass productivity and lipid yields [5]. Even though, Dunaliella sp. being a halotolerant alga can be resistant to contamination by other species [6] and produce multiple products such as high content of carotenoids and lipid [7]. Furthermore, different light intensity, photoperiods regimes and media composition affects production of lipids and pigments [8,9]. Light intensity and light quality has a major role in impacting the algal cell metabolism including biomass, lipid and protein production [10]. Moreover, culture medium components mainly micronutrients plays a major role in impacting algal growth [11]. The previous research in to the species Nannochloropsis oculata a valuable feedstock for biofuel production accounts for total lipid content from 8% to 50% under different culture conditions such as temperature, CO2 and nitrogen concentration [12]. Researchers have reported ample lipid content of 0.643 g/L/d in the biomass of Nannochloropsis sp. [13].Strains of Dunaliella and Nannochloropsis are known to grow well under different light regimes and high salinity [8].Technical and scientific knowledge about cultivation of D. tertiolecta and N. oculata is also important to enhance the production of biomass in cost effective way with industrial production of multiple products [8]. Numerous studies reported that integrated circular biorefinery approach would provide better cost effectiveness and environmental sustainability for increased microalgal biomass and pigment production [14]. There are several studies with respect to effect of salt conditions or environmental stress conditions on algal biomass productions but limited research has been conducted on the strains D.tertiolecta and N.oculata in terms of its pigment and lipid production under varying light intensity and photoperiods. Several researchers have reported the production of essential omega fatty acids from these two strains. Palmitic, linoleic, oleic, palmitoleic, eicosanoic acids are among the fatty acids produced from Nannocholoropsis sp [4] (b) as well as Dunaliella sp.
Besides their potential for high-added value pigments and lipid content, the current bottlenecks in commercialization lies on production and manufacturing cost. The medium component such as NaNO3 and K2HPO4 accounts for 83.3% and 31% of total cost in medium. Microalgae medium cost US$ 2.6 to 18.3 each for 1000 L in lab-scale cultivation systems [8].To overcome these challenges, replacement of medium components with natural salt component can actually provide improved production of omega-3 fatty acids and other biomolecules [15] in a viable way. Thus, the aim of this study is to explore the effect of culture medium, different light intensities and photoperiod regimes on the production of carotenoids, biomass as well as fatty acid methyl esters in N.oculata and D.tertiolecta. The results of this study may provide an understanding on how the different light intensities and photoperiod regimes helps to improve the enhanced production of metabolites and biomass. The cultivation of N.oculata and D.tertiolecta was also carried out using natural seawater media to test the growth and metabolite production in seawater media.
2. Material and methods
2.1. Sea water sample collection and analysis
Natural seawater (NSW) samples were collected from Gateway of India, sites of Mumbai, India. NSW samples were filtered with 0.22 μm filter paper using Millipore assembly (Millipore Corporation, Billerica, MA, USA). The elemental composition of NSW samples along with Sea salt media (SSM) and F/2 media was performed using Thermofischer scientific atomic absorption spectrophotometer (USA) to determine main macronutrients (Na, Ca, K, Mg, Na, P) and micronutrients, (Co, Cu, F, Fe, Mn, Mo, Zn) involved in algal media and the pH was measured using a pH meter (Hanna, USA). The main characteristics composition of all the culture media were analysed as shown in Table 1.
Table 1.
Elemental composition of F/2 medium, SSM and seawater.
| S. No. | Ions | SSMa (Distilled water) | F/2 (Distilled water) | Seawater |
|---|---|---|---|---|
| 1 | Na (mg/L) | 9276 | 987.5 | 8560 |
| 2 | K (mg/L) | 409 | 120 | 18.18 |
| 3 | Ca (mg/L) | 180.5 | 123.5 | 300 |
| 4 | Co (mg/L) | 0.4 | 0.01 | 0.01 |
| 5 | Cu (mg/L) | 0.1 | 0.013 | 0.29 |
| 6 | Fe (mg/L) | 0.3 | 0.42 | 0.23 |
| 7 | Mg (mg/L) | 143 | 185 | 672 |
| 8 | Cd (mg/L) | 0.1 | 0 | 0.04 |
| 9 | Mn (mg/L) | 0.1 | 0.31 | 0.3 |
| 10 | Pb (mg/L) | 4.5 | ND | 0.13 |
| 11 | Mo | ND | 0.0018 | ND |
| 12 | Zn | 0.008 | 0.005 | ND |
| 13 | TOC (mg/L) | ND | ND | ND |
| 14 | pH | 8 | 8 |
The data in this column are calculated based on the component of SSM. ND: Not detected.
2.2. Strain culture and cultivation
Dunaliella tertiolecta (Accession no. KT860851, TERI, New Delhi) and Nannochloropsis oculata was obtained from the Central Marine Fisheries Research Institute (CMFRI), Kochi. The strains were cultivated photoautotrophically in F/2 medium [16]. To continue with the experimental design, the starter inoculum was cultivated in 500 mL Erlenmeyer flasks containing 200 mL of the selected culture medium with initial cell count of 3 × 106 cells/mL at 25 °C under 16:8h light/dark cycle (120 μE/m2/s) for 7 days.
D.tertiolecta and N.oculata cells was cultivated photoautotrophically in three different media, namely the F/2 medium, SSM (DW) and SSM (NSW) which composed of the same mineral salts as the Seasalt media in natural seawater instead of distilled water. The pH was adjusted to 7.5. The compositions of the different culture media are listed in Table 2. Inoculation was based on the cell count of 3 × 106 cells/mL of the stock culture from previous step after centrifugation and washed three times with water and inoculated in a MC 1000-OD in one common (F/2) and two different media (SSM (DW) and SSM (NSW)).To explore the effect of light intensity and photoperiod, the experiment was carried out during 23 days, illuminated by white LEDs at light intensity of 120 μE/m2/s under 16:8h light/dark cycle, and 13:11 light/dark cycle under 250 μE/m2/s light intensity at 25 ± 1 °C. During all cultivation period, the aeration rate of 0.12 mL/min was provided in all treatments. All the experiments were carried out in triplicates and the values were expressed as mean ± SE.
Table 2.
Nutrient composition of different culture media. F/2 medium (A), SSM (DW) (B), SSM (NSW) (C).
| Media constituent | Medium composition (mM) |
||
|---|---|---|---|
| F/2 (Artificial sea water media) | SSM (Distilled water) | SSM (Natural Seawater) | |
| NaNO3 | 0.88 | – | – |
| NaH2PO4.H2O | 0.036 | – | – |
| Na2SiO3.9H2O | 0.106 | – | – |
| ZnSO4.7H2O | 0.00008 | – | – |
| MnSO4.H2O | 0.0009 | – | – |
| Na2MoO4.2H2O | 0.00003 | – | – |
| CoSO4.7H2O | 0.00005 | – | – |
| CuCl2.2H2O | 0.00004 | 0.469 | 0.469 |
| Fe(NH4)2SO4.6H2O | 0.0117 | – | – |
| Na2EDTA.2H2O | 0.0117 | – | – |
| KNO3 | – | 4.9 | 4.9 |
| KH2PO4 | – | 1.14 | 1.14 |
| FeCl3 | – | 0.009 | 0.009 |
| EDTA | – | 0.0095 | 0.0095 |
| ZnCl2 | – | 0.88 | 0.88 |
| H3Bo3 | – | 0.485 | 0.485 |
| CoCl2.2H2O | – | 0.202 | 0.202 |
| NaCl | 36 g/L | 36 g/L | – |
2.3. Microalgal growth and biomass accumulation
Microalgal growth in both the strains was assessed by calculating values of OD @680 nm every day using OD-viewer software attached to MC 1000-OD to plot the growth curve under different conditions. Growth rate was measured by plotting optical density at the exponential phase to an exponential function with respect to time (number of days) [17]. The dry cell weight of algal biomass was calculated using method of [18]. Biomass productivity (dry biomass, expressed in milligrams per litre of the medium per day) was calculated at 23rd day, their respective dry weight was calculated gravimetrically using Eq. (1).
| (1) |
Where B1 and B2 denotes biomass dry weight at time (T) which is number of days for culture cultivation to harvesting of the culture.
2.4. Lipid extraction and productivity
The total lipids were extracted by previously reported method [19]. 10 mg of dry cell biomass was extracted with chloroform and methanol 2:1(v/v) and vortexed for 2 min to make cell suspension homogenized. The suspension was then centrifuged at 10000 rpm for 15 min. The supernatant was washed with 1 mL water for removal of any impurities. The lower phase of the supernatant was collected in pre weighed vials. The procedure was repeated three times until the cell biomass become colourless. After the extraction was completed, the fractions were dried in an oven at 50ᵒC [20]. The extracted lipid content (%) was determined gravimetrically [21].using eq. (2) and lipid productivity was calculated using eq. (3) below:
| (2) |
| (3) |
2.5. Fatty acid methyl esters (FAME) compositional analysis
FAME was prepared using the method described by Ref. [22]. The extracted lipids were converted to FAME by addition of 400 μL of toluene in dried followed by dropwise additions of acidified methanol (prepared by adding dropwise addition of 1 mL acetyl chloride in 10 mL chilled methanol on ice while continuous stirring for about 1 h).Butylated hydroxyl toluene (BHT) was added to prevent the lipid peroxidation. The FAME were washed further with 5% NaCl followed by addition of hexane. The final layer of hexane was injected in to GC vials for further analysis. Nonadecanoate was used as internal standard. To analyse fatty acid composition Gas chromatography (GC) coupled with mass spectrophotometer (Agilent 122–2332 column, Santa Clara, California, USA) was used. The injection was passed through a column (DB-23) which has 30mm × 0.25 mm dimension with 0.25 μm thickness coupled with mass spectrometer (MS). The oven temperature was maintained at 180 °C for 14 min gradually increasing at 10 °C/min for about 36 min; the oven temperature was again maintained at 220 °C with 4 °C/min increase rate. Helium was used as carrier gas for chromatography. The integration was performed manually using chemstation software. The fatty acid composition was quantified according to the peak area relative to C19 (nonadecanoic) fatty acid internal standard and expressed as a percentage of the total fatty acid content.
2.6. Carotenoids analysis and quantification
The extraction was done using acetone in 25 mg freeze dried biomass followed by vortexing. The samples were then sonicated for 15 min at 20 +-kHz and the samples were centrifuged at 6000 rpm for 15 min at 10 °C. The extraction was repeated till the samples became colorless. The upper phase was used to measure chlorophyll a and b as well as total carotenoids content spectrophotometrically using the equations by Ref. [23].
| (4) |
| (5) |
| (6) |
Where Chla: chlorophyll a; Chlb: chlorophyll; Cart: carotenoid.
The Chla, Chlb and Carotenoid content was calculated using above eqs. (4)–(6)
The ratios of Chla/Chlb, Cart/Chla and Cart/Chlb were also calculated.The fractions were collected each time in separate vial and dried with Nitrogen gas and were dissolved in 1 mL methanol. The acetone fractions were also analysed spectrophotometrically. From this approximately, 20 μL was taken for HPLC analysis.
The HPLC analysis was done using previously described method by A.K. Minhas et al. [56] Pigments were eluted using a 3.0 mm particle sized 250mm × 4.6 mm, 5lm reverse-phase C 18 column connected with a guard column from Phenomenex, Torrance, California, USA. The two mobile phase Solvent system A consisted (Acetonitrile and Methanol) in the ratio 7:1(v/v) and Solvent system B (Acetonitrile, Methanol, Water and Ethyl Acetate) in the ratio 7:0.96:0.04:2 (v/v) with 25 min run time and 1 mL flow rate. The pigments were identified by comparison of retention time against standards and the concentrations of identified pigments were determined using standard calibration curve of respective standards.
2.7. Statistical analysis
All experiments were performed in triplicates. Statistical analysis for the data were analysed by one-way analysis of variance (ANOVA) and Duncan's tests. Data are presented as means of three replications ± standard error.
3. Results and discussion
3.1. Algal cell growth in N. Oculata and D. tertiolecta
The D.tertiolecta and N.oculata are the most encouraging strains of microalgae for biofuel production due to its high adaptability to environmental differences. Sea salt media (SSM) was commonly used for cultivation of D. tertiolecta in-house. The composition of media is described in Table 2. In the present work, natural seawater (NSW) was applied to SSM media lacking sea salt (35 g/L) as a complete alternative for SSM media due to being rich in many inorganic nutrients and trace elements required for algal growth. The ion measurement of seawater (SW) by AAS analysis showed that many elements in NSW can replace the artificial seawater media nutrients (Table 1). Thus, we hypothesized that use of seawater can reduce the requirement of sodium salt present in SSM. In recent year, cultivation of microalgae in seawater is gaining importance for marketable production of metabolites and coproducts due to lower energy and nutrient inputs [24]. In present study, three different culture media as mentioned in Table 2 were used. Duration of light intensity and light availability are two major factors determining the photosynthetic activity of cells [25]. The present study addressed the effect of different culture media under different combination of light intensity and photoperiods (16:8h light/dark cycle, 120 μE/m2/s and 13:11h light/dark cycle, 250 μE/m2/s) in laboratory scale. The 16:8h light/dark cycle, 120 μE/m2/s was selected as it is considered as optimum light and photoperiod for growth of D.tertiolecta and N.oculata. According to [26], 16:8h is optimal photoperiod used for most algal strains. Moreover, 13:11h photoperiods, 250 μE/m2/s combination was selected keeping in mind the outdoor prevailing growing light conditions (13h of day light and 11h of night phase). The biomass of D.tertiolecta and N. oculata cultures was positively associated with the light intensity at 120 and 250 μE/m2/s. However, increasing the light intensity from 120 to 250 μE/m2/s significantly increased the biomass productivity in both the strains, which was ranging from 77.33 ± 1.76–97.33 ± 2.33 mg/L/d in SSM, 62.00 ± 3.46–73.33 ± 3.18 mg/L/d in SSM (NSW) in D.tertiolecta; 47.67 ± 2.60–83.33 ± 2.60 mg/L/d in SSM, 41.00 ± 2.31–68.67 ± 2.03 mg/L/d in SSM (NSW) in N. oculata (Table 3).
Table 3.
Fatty acid composition (wt.%) present in the microalgae Dunaliella tertiolecta and Nannochloropsis oculata under two different light intensity and photoperiod regimes for 23 days.
| Fatty acid (% of total FA) |
Dunaliella tertiolecta (120 μE/m2/s, 25ᵒC, 16:8h light/dark cycle) |
Dunaliella tertiolecta (250 μE/m2/s, 25ᵒC, 13:11h light/dark cycle) |
Nannochloropsis oculata (120 μE/m2/s, 25ᵒC, 16:8h light/dark cycle) |
Nannochloropsis oculata (250 μE/m2/s, 25ᵒC, 13:11h light/dark cycle) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSM (Distilled water) | SSM (natural seawater media) | F/2 | SSM (Distilled water) | SSM (natural seawater media) | F/2 | SSM (Distilled water) | SSM (natural seawater media) | F/2 | SSM (natural seawater media) | SSM (Distilled water) | F/2 | |
| C16:0 | 15.51 ± 0.01 | 22.02 ± 0.03 | 31.95 ± 0.03 | 75.58 ± 0.01 | 38.78 ± 0.011 | 36.00 ± 0.02 | 18.18 ± 0.01 | 17.27 ± 0.04 | 2 8.52 ± 0.09 | 35.43 ± 0.01 | 46.00 ± 0.03 | 28.52 ± 0.02 |
| C 17:0 | 0.39 ± 0.012 | 0.54 ± 0.013 | 1.86 ± 0.06 | 2.10 ± 0.017 | 2.45 ± 0.05 | 4.87 ± 0.016 | 2.08 ± 0.06 | 0.31 ± 0.08 | 0.67 ± 0.09 | 2.08 ± 0.04 | ||
| C18:0 | 6.40 ± 0.07 | 8.64 ± 0.01 | 11.47 ± 0.07 | 19.09 ± 0.025 | 5.74 ± 0.013 | 30.03 ± 0.01 | ||||||
| C20:0 | 2.23 ± 0.04 | 3.01 ± 0.015 | 1.21 ± 0.01 | 0.70 ± 0.05 | ||||||||
| C 21:0 | 0.16 ± 0.025 | |||||||||||
| C 22:0 | 0.19 ± 0.046 | |||||||||||
| subtotal | 24.88 | 34.21 | 33.81 | 87.05 | 57.87 | 38.1 | 20.63 | 22.14 | 30.6 | 42.69 | 77.4 | 30.6 |
| C16:1 | 3.70 ± 0.019 | 2.01 ± 0.01 | 1.66 ± 0.02 | 6.00 ± 0.04 | 6.47 ± 0.02 | 6.04 ± 0.04 | 14.03 ± 0.022 | 4.10 ± 0.01 | ||||
| C18:1 | 5.10 ± 0.011 | 7.94 ± 0.022 | 29.41 ± 0.01 | 2.37 ± 0.05 | 13.00 ± 0.03 | 3.97 ± 0.06 | 6.21 ± 0.013 | 24.89 ± 0.01 | 15.01 ± 0.06 | 3.10 ± 0.014 | 15.07 ± 0.02 | |
| Subtotal | 8.8 | 9.95 | 31.07 | 2.37 | 0 | 19 | 10.44 | 12.25 | 24.89 | 29.04 | 7.2 | 15.07 |
| C 18:2 | 8.30 ± 0.03 | 8.45 ± 0.024 | 16.29 ± 0.04 | 5.99 ± 0.01 | 19.27 ± 0.01 | 12.11 ± 0.021 | 6.54 ± 0.04 | 10.21 ± 0.12 | 15.07 ± 0.02 | 9.98 ± 0.02 | 8.53 ± 0.09 | 21.25 ± 0.03 |
| C16:2 | 6.20 ± 0.05 | 3.88 ± 0.045 | 5.53 ± 0.09 | 7.30 ± 0.010 | 6.23 ± 0.06 | 5.33 ± 0.01 | 4.23 ± 0.04 | |||||
| C 16:3 (n-3) | 0.10 ± 0.021 | 6.54 ± 0.013 | 6.22 ± 0.043 | 2.40 ± 0.05 | 3.46 ± 0.06 | 12.20 ± 0.01 | 7.25 ± 0.08 | 10.48 ± 0.04 | 2.12 ± 0.07 | |||
| C16:4 | 14.40 ± 0.013 | 1.36 ± 0.01 | 1.45 ± 0.012 | 0.70 ± 0.11 | 4.30 ± 0.037 | 1.54 ± 0.012 | ||||||
| C 18:3 | 30.10 ± 0.02 | 23.80 ± 0.022 | 5.54 ± 0.021 | 2.21 ± 0.023 | 18.56 ± 0.03 | 11.30 ± 0.03 | 48.27 ± 0.21 | 34.82 ± 0.06 | 21.24 ± 0.01 | 9.10 ± 0.011 | 6.89 ± 0.04 | 32.49 ± 0.01 |
| C 20:5 | 0.41 ± 0.032 | 0.52 ± 0.02 | 7.81 ± 0.025 | |||||||||
| C 22:6 | 0.27 ± 0.02 | 1.21 ± 0.011 | ||||||||||
| C 20:4 | 0.48 ± 0.01 | 1.42 ± 0.04 | 0.52± | |||||||||
| Subtotal | 59.1 | 44.03 | 35.03 | 10.6 | 41.29 | 42.91 | 68.99 | 65.14 | 44.2 | 19.08 | 15.42 | 53.74 |
SFA: Saturated Fatty Acid; MUFA: Monounsaturated Fatty Acid; PUFA: Polyunsaturated Fatty Acid. ND: Not Detected, Average of cultures in duplicate.
The shift of light/dark cycle and light intensity from 16:8h light/dark cycle, 120 μE/m2/s to 13:11 h L:D, 250 μE/m2/s in both SSM (DW) and SSM (NSW) in both strains yielded higher biomass productivity which was not true when F/2 media (commercial media) was used. This may be due to the presence of high potassium and phosphate salts in both SSM (DW) and SSM (NSW) (Table 1). Light intensity, temperature and cultivation method has a major effect on the biomass productivity [4]. Both the strain produced high biomass productivity of 97.33 ± 2.33 and 73.33 ± 3.18 mg/L/d and 83.33 ± 2.60; 68.67 ± 2.03 mg/L/d in SSM (DW) and SSM (NSW) under 13:11h light/dark cycle, 250 μE/m2/s at 25 °C reaching its maximum productivity at day 23 as compared to biomass obtained from F/2 medium under similar conditions (Fig. 1). Especially, the biomass productivity in F/2 media producing as much biomass of 34.67 ± 2.60 and 36.00 ± 2.31 mg/L/d in both the strains at day 23 under 16:8 h L: D, 120 μE/m2/s and then decline in the productivities were attained under 13:11h light/dark cycle, 250 μE/m2/s respectively compared to SSM (DW) and SSM (NSW).
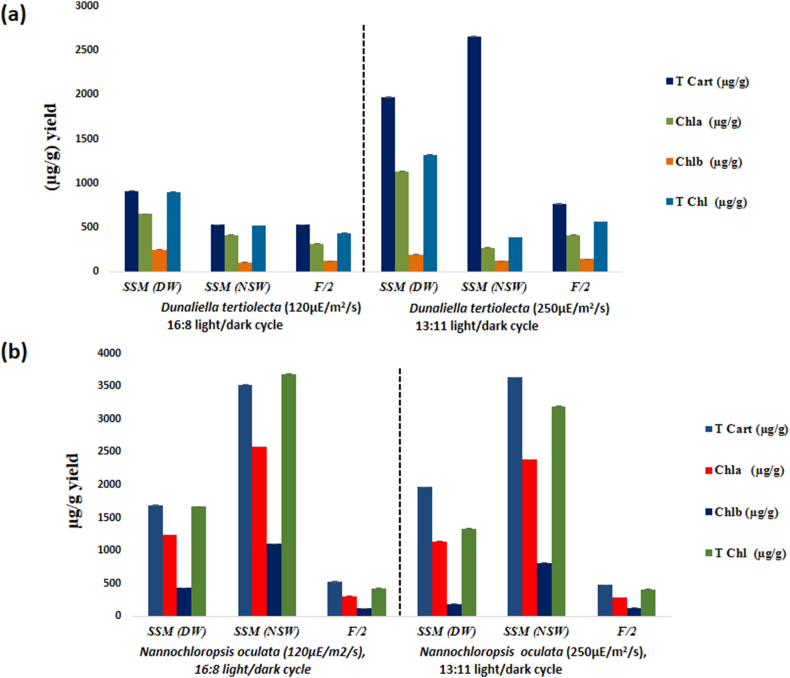
Fig. 1.
Lipid productivity, Lutein and biomass productivity of (a) Dunaliella tertiolecta (b) Nannochloropsis oculata in 23 days old biomass results are presented as mean ± standard error (SE).
This study reveals that cultivation of both the strains in SSM medium could replace the demand of other commercial chemicals being used in F/2 for growth of algae. On other hand, D.tertiolecta and N.oculata also grew well in SSM (NSW) under 13:11h Light/dark cycle, 250 μE/m2/s at day 23 but could not grow well in commercial media F/2. This may be because seawater contains more dissolved ions rich in mg, cl, ca, S [27] compared with F/2 media. Also, further increase in light intensity from 120 to 250 μE/m2/s resulted in decrease in biomass productivity in F/2 which may be due to photo inhibition [28]. While the retardation in microalgal cell biomass was seen in SSM (NSW) in both the strains at day 23 under 16: 8h, 120 μE/m2/s due to lack of duration of light intensity supply used. As reported by Ref. [29]; higher light intensity and longer day length of light: dark cycle resulted in enhanced biomass yields of Tetraselmis chui. In current study, the biomass productivity was increased under the high light intensity at 13:11h light/dark cycle in SSM (DW) and SSM (NSW) (Fig. 1).It is thus suggested, that extending light regimes may impact the production of biomass in positive manner. The present study obtained better biomass productivities results under higher light intensity (250 μE/m2/s) tested. Among the three media tested, SSM (NSW) in both strains showed an inadequate response compared to the sea salt media. Thus, for both the species a light intensity of 250 μE/m2/s was considered ideal for biomass production.
3.2. Effects of light intensity and photoperiod on lipid production
Lipid productivity is considered as one of the important factor in evaluation of strains for biofuel production [30]. In the present study, photoperiod and light intensity in three different media have a considerable effect on lipid productivity as being demonstrated in Table 3. The lipid productivity was measured for both strains in three different media for 23 d under two light regimes (120 μE/m2/s and 250 μE/m2/s) and photoperiods (16:8h and 13:11h light/dark cycle).
The lipid productivity was adversely affected in N.oculata in all three medium tested under 13:11h light/dark cycle at 250 μE/m2/s (Table 3). In D.tertiolecta, with increase in light intensity and prolonged dark duration of 11h enhanced the lipid productivity in alternative medium B compared to SSM and F/2. Moreover, when the light exposure decreased to 13:11h at 250 μE/m2/s in D.tertiolecta, lipid productivity could reach up to 34.56 ± 0.31 mg/L/d in D.tertiolecta in SSM (NSW) at day 23 (Fig. 1a), almost thrice as high as that in F/2 media under similar conditions accounting for 6.84 ± 0.10 mg/L/d of lipid productivity. The response to lipid productivity in D.tertiolecta may be due to fact that strains behave differently under different light regimes. On other hand, N.oculata showed highest lipid productivity of 15.69 ± 0.16 and 13.08 ± 0.08 mg/L/d in F/2 and alternative medium B under 16:8h light/dark cycle photoperiod at 120 μE/m2/s respectively (Fig. 1b). Furthermore, very low lipid productivities were attained in N.oculata under 13:11h light/dark cycle photoperiod at 250 μE/m2/s in all three media, respectively. Thus, the light intensity and exposure of light are main factors driving productivity of photosynthetic algal strains as light/dark cycle delivers energy for transfer of electron from water to NADP thus forming NADPH (nicotinamide adenine dinucleotide phosphate) and produces ATP [31]. The continuous light at high intensity causes photo damages to PSI. Thus, different strain behave differently in response to high light intensity which result in low or high lipid yield [28].This process can be controlled by exposing algal cells to very short cycles of light period and darkness [32]. In present study, the light intensity and the light/dark cycle found to have an influence on lipid productivity in D.tertiolecta and N.oculata.
3.3. Fatty acid composition under different light regimes and light/dark cycle
We analysed the fatty acid composition in both the strains cultivated in three different medium under two light regimes and photoperiod using by gas chromatography (GC) (Agilent 122–2332 column, Santa Clara, California, USA). Light intensity had similar effects on the fatty acid profile in SSM (DW) and SSM (NSW) in both the strains. When grown at 250 μE/m2/s under 13:11h light/dark cycle both the strains had the highest percentage of SFA (75.5%) in D.tertiolecta in SSM. The content of polyunsaturated fatty acids decreased with the increasing light intensity (250 μE/m2/s) under 13:11h light/dark cycle and increased under 16: 8 light/dark cycle at 120 μE/m2/s light intensity in SSM (Distilled water) and SSM (natural seawater media) which was not true in F/2 media (Table 3). The decrease in PUFA percentage was accompanied in the continuous light conditions under 13:11h photoperiod due to photo-oxidative damage [33]. There are studies reporting that the PUFA percentage was also increased under continuous illumination time in N. gaditana biomass [34]. Therefore, the changes may be due to the media composition which resulted in either higher SFA or PUFA [28]. This study clearly indicates that D.tertiolecta and N.oculata algae have an ability for the accumulation of α-linolenic acid (C18:3) as the main acid present under 16:8h light/dark cycle at 120 μE/m2/s light intensity. As shown in this study, extension of the dark period from 8 to 11 h brought an increase in the accumulation of the palmitic acid (C16:0) acid in both the strains under 250 μE/m2/s light intensity. In D.tertiolecta and N.oculata, C 16:0 and C18:3 fatty acid were abundant, at low and high light intensity. The fluctuations in fatty acid composition to light intensity and photoperiods are species specific. It is noteworthy that under high light intensity, the fatty acid composition in Desmodesmus sp. was altered by the dismantling of thylakoid membranes [35]. As expected, N.oculata grown under high light conditions (250 μE/m2/s) under 13:11 h photoperiod showed an increase of 35.4% C16:0 occurred in SSM (NSW) with an associated increase in EPA percentage of 7.81% due to strong relationship between the increased synthesis of neutral lipids and disintegration of the EPA-enriched thylakoid membrane lipids [36]. Nannochloropsis oculata is considered an ideal candidate for EPA production [37]. The highest EPA content of 12% DW was also attained in a Nannochloropsis. The present study has shown that N.oculata produces an essential omega 3 fatty acid i.e eicosapentaenoic acid. With increasing light intensity from 120 to 250 μE/m2/s the percentage of EPA was increased, which is in agreement with the results obtained by Ref. [38] in Nannnochloropsis sp. This clearly indicates that light intensity along with photoperiod plays an important role in altering the fatty acid profile in microalgae. It is remarkable that N.oculata showed no presence of stearic acid (C18:0) under 16:8h photoperiod, 120 μE/m2/s light intensity. Whereas under 13:11h light/dark cycle, 250 μE/m2/s light intensity the percentage of stearic acid (C18:0) was increased in SSM and SSM (NSW) in both the strains. Moreover in both the strains, no presence of stearic acid (C18:0) was observed in commercial media F/2 under 16:8h light/dark cycle, 120 μE/m2/s light intensity.
These results suggest that changes in the fatty acid profile are majorly due to nutrient depletions which are reported in many strains of microalgae [39]. The results are consistent with finding of [40] who reported that increased light intensity from 35 to 420 μE/m2/s had lower the C18:3 content. This clearly indicates that fatty acid chain in different species is dependent on the light intensity and the media for growth. Under stress conditions, chlorophyll molecules gets converted to unstable forms, which further react with dissolved oxygen species. The ROS generated then react with free fatty acids making lipid peroxidase available in inactive form, which further reduces the fatty acid concentration [35].
Besides the FA profile, lipid quality is most important to determine the biodiesel characteristic properties. To produce good quality biodiesel, SFA and C 18:1 are necessary for good stability and the lowest content of PUFA mainly C 18: 3 to improve the oxidative stability [41]. In this study, both strains were found suitable for biodiesel production.
3.4. Quantification of pigments under different light regimes and light/dark cycle photoperiod at end of cultivation periods
The present study explains the fact that increase in light intensity affect the pigment and biomass production due to long duration of time needed for acclimation of cells [25]. The chlorophyll-a, chlorophyll-b, and total carotenoid contents of N.oculata and D.tertiolecta under different light intensities and photoperiod are presented in Fig. 2a & b. The light intensity and photoperiod together affected the total carotenoid and chlorophyll-content in both the strains. Among the strains studied, the total carotenoids content was slightly high in Dunaliella tertiolecta (3635.16 ± 1.59 μg/g) compared to N.oculata (2656.94 ± 8.22 μg/g) under 13:11h light/dark cycle and 250 μE/m2/s light intensity in SSM (NSW). The quantitative distribution of pigment shows that chlorophyll a was the major pigment present in both the strains under 13:11h light/dark cycle and 250 μE/m2/s light intensity; 16:8h light/dark cycle and 120 μE/m2/s light intensity. The chlorophyll a content (32.23 ± 0.53 μg/mL) in alternative medium B under 16:8h light/dark cycle, 120 μE/m2/s light intensity in D.tertiolecta was higher compared to results by Ref. [42] in D.bardawil. The chlorophyll a content was much higher in D.tertiolecta and N.oculata – accounting for 32.23 ± 0.53 μg/mL and 8.18 ± 0.087 μg/mL respectively in SSM and SSM (NSW) compared to Ref. [43] with maximum 2.0 μg/mL of Chl-a without urea.
Fig. 2.
Pigment content in Dunaliella tertiolecta (a) and Nannochloropsis oculata (b) grown under two different light intensities ( 250 and 120 μE/m2/s) and photoperiod regimes (13:11h and 16:8h Light/Dark cycles), Chl:Chlorophyll, Cart: carotenoids; T Chl: Total chlorophyll. All the samples were taken at 23 days. Data results are presented asmeans of triplicate experiments ±SE.
The content of chlorophyll is directly related with the concentration of algal cells [44]. In this study, total carotenoids content was increased under high light intensity in both the strains, however, chlorophyll content was decreased due to the damage concurs in the photosynthetic II reaction centre (PSII) which leads to photo damages of pigments [45]. There are reports justifying that carotenoids content increased by increased light intensity in marine alga [46]. Under high light intensity, algal cells adapt the mechanism for photo protection under stress ) and numerous structural and functional deviations in light-harvesting complexes (LHCs) occurs in many microalgae. These internal membrane antenna system are widely considered to transfer the excitation energy and harvest sunlight for photosynthesis [47]. Thus, the light harvesting antennae of Nannochloropsis spp. belonging to the eustigmatophytes family have a unique property of binding to Chl a particularly. On other hand, different strains of Dunaliella protect themselves from the oxidative stress damages caused by exposure to high light intensity [48,49]. In addition, total carotenoid content of N.oculata was lower than the carotenoid content of D.teriolecta cultured in SSM. The results suggested that D.tertiolecta strain is a potential candidate of chlorophyll-a and total carotenoids. In fact, it has been reported that high light intensity has collective effects on carotenoids content. Besides total carotenoid content, lutein production was determined under two different photoperiod and light intensity in three different media in both the strains. The cells grown in SSM (NSW) showed 2 folds higher accumulation of chlorophylls/lutein content after 23 days of cultivation (Fig. 2a and b). As shown in Fig. 1 lutein productivity rose with an increase in light intensity up to 250 μE/m2/s under 13:11h light/dark cycle in SSM medium and decreased subsequently in SSM (NSW) and F/2. The highest lutein productivity 0.253 ± 0.06 mg/L/d was achieved in D.tertiolecta in SSM at 250 μE/m2/s under 13:11h light/dark cycle and 0.279 ± 0.004 mg/L/d in N.oculata in F/2 at 120 μE/m2/s under 16:8h light/dark cycle. The results of analysis showed that lutein content were ranked in following order: 250 μE/m2/s > 120 μE/m2/s in D. tertiolecta and N.oculata in SSM, SSM (NSW) whereas opposite trend was observed in F/2 medium 120 μE/m2/s > 250 μE/m2/s. The results indicate that the lutein content was less impacted by high increasing the light intensity from 120 to 250 μE/m2/s. Light intensity of 250 μE/m2/s as reported by [50] did not show increment in production of biomass and carotenoids. On other hand [9], also reported that high light intensity could increase the metabolite production up to saturation level beyond that photo inhibition occur. The phenomenon of impact of light intensity is totally strain specific as seen in case of D.tertiolecta and N.oculata. Moreover, photoperiod effect the cell surface and cell diameter of algal cells which together impact the biomass and carotenoid production [9]. Consequently, there are reports justifying the results that at high light intensity, changes occur in the PSI antenna size, lowering the level of ROS thus producing less photodamages to algal cells [47]. Indeed [51], reported that in Nannochloropsis, PSI is quite sensitive under light stress with lack of flavodirron which is essential for protecting the algal cells from photodamages. Reports suggest that presence of hlr1 may be dangerous to marine algal isolate under high light intensity. Moreover, in regions where the sunlight is below the saturation level, hlr1 plays an important role in survival of different algal species in marine environment [47]. Another possibility is that oxidative damage of N. oceanica cells exposed to high light could be mitigated by treatment with SOD and CAT, which allowed scavenging of the ROS superoxide and H2O2. This clearly suggests that ROS molecules, mainly produced at the level of PSI have a major negative impact on Nannochloropsis, when cells are exposed to strong illumination [52]. However, till date no systematic mechanism is available to support the effect of light conditions on carotenoids biosynthesis [53].
4. Cost economics
To further compare the culture media under different light regime and photoperiod, cost analysis was performed with the obtained data (Table 4). The batch considered to calculate was 70 mL culture medium volume in MC-1000OD. Based on the cost analysis of media in laboratory scale production, F/2 medium showed high cost of lutein production in both the strains under 13: 11 light/dark cycle at 250 μE/m2/s (Table 4). On other hand, SSM medium showed lower production cost for lutein under similar conditions accounting for € 71.25/mg and € 113.38/mg, respectively followed by SSM (NSW) (Table 4). F/2 medium showed low lutein cost/mg under 16:8h light/dark cycle at 120 μE/m2/s in both the strains (Table 4) accounting for €67.04 and €49.75. SSM and SSM (NSW) showed low cost of lipid and lutein production per mg under 13: 11 light/dark cycle at 250 μE/m2/s. Whereas F/2 medium showed less lipid and lutein cost per mg under 16:8h light/dark cycle at 120 μE/m2/s in both the strains. This indicates that along with the medium constituent the light intensity and photoperiod plays a major role in impacting the nutraceutical production. In our previous studies, BBM medium showed a cost of lipids and lutein (Rs. 118 and 6560) in two stage cultivation at day 12 [54]. It is worth mentioning that the costly medium components could be replaced with natural seawater nutrients from coastal regions in marine isolates. There are many studies which reported various way to decrease the medium component cost , but few target the pigment cost production. This study presented the important information of target products such as lipid and lutein based on culture medium and cultivation conditions. Table 4 indicates the estimated cost of nutraceutical obtained from F/2, SSM and SSM (NSW) in 70 mL of dry biomass. Brazionis et al., 2008 showed that lutein is important pigment which can protect against the diabetic disorders. It confirms that studies related to low cost media (SSM and SSM (NSW) are important to enhance the biomass and carotenoids production. The lab scale cost economic analysis can provide an estimate to overcome the hurdles in scaling up the algae to large scale production.
Table 4.
Estimated nutraceutical cost evaluation based on the lab scale production.
| Media | Cultivation conditions and operating days | Net Biomass Productivity (mg/70 mL) | Pigments and Lipids | Productivity (mg/70 mL) | Cost (per mg) | Net Factor | Media cost/Litre |
|---|---|---|---|---|---|---|---|
| SSM (DW) | 120 μE/m2/s, 25ᵒ C, 16:8h light/dark cycle | 123.97 | Lipid | 51.29 | € 0.44 | 41.37% | € 0.10 |
| Lutein | 0.25 | € 85.59 | 0.20% | ||||
| SSM (NSW) | 120 μE/m2/s, 25ᵒC, 16:8h light/dark cycle | 99.82 | Lipid | 40.42 | € 0.55 | 40.49% | € 0.13 |
| Lutein | 0.20 | € 118.52 | 0.20% | ||||
| F/2 | 120 μE/m2/s, 25ᵒC, 16:8h light/dark cycle | 55.81 | Lipid | 20.51 | € 1.14 | 36.75% | € 0.54 |
| Lutein | 0.39 | € 67.04 | 0.70% | ||||
| SSM (DW) | 250 μE/m2/s, 25ᵒ C, 13:11h light/dark cycle | 156.70 | Lipid | 41.24 | € 0.57 | 26.32% | € 0.10 |
| Lutein | 0.41 | € 71.25 | 0.26% | ||||
| SSM (NSW) | 250 μE/m2/s, 25ᵒ C, 13:11h light/dark cycle | 118.06 | Lipid | 55.64 | € 0.37 | 47.13% | € 0.13 |
| Lutein | 0.21 | € 124.63 | 0.18% | ||||
| F/2 | 250 μE/m2/s, 25ᵒ C, 13:11h light/dark cycle | 42.47 | Lipid | 11.01 | € 1.26 | 25.92% | € 0.54 |
| Lutein | 0.04 | € 419.72 | 0.09% | ||||
| SSM (DW) | 120 μE/m2/s, 25ᵒ C, 16:8h light/dark cycle | 76.74 | Lipid | 13.85 | € 1.59 | 18.05% | € 0.10 |
| Lutein | 0.06 | € 446.57 | 0.08% | ||||
| SSM (NSW) | 120 μE/m2/s, 25ᵒC, 16:8 h light/dark cycle | 66.01 | Lipid | 21.06 | € 1.02 | 31.90% | € 0.13 |
| Lutein | 0.06 | € 446.59 | 0.09% | ||||
| F/2 | 120 μE/m2/s, 25ᵒC, 16:8h light/dark cycle | 57.96 | Lipid | 25.26 | € 0.87 | 43.58% | € 0.54 |
| Lutein | 0.45 | € 49.75 | 0.78% | ||||
| SSM (DW) | 250 μE/m2/s, 25ᵒ C, 13:11h light/dark cycle | 134.16 | Lipid | 5.88 | € 3.03 | 4.38% | € 0.10 |
| Lutein | 0.11 | € 113.38 | 0.08% | ||||
| SSM (NSW) | 250 μE/m2/s, 25ᵒ C, 13:11h light/dark cycle | 110.55 | Lipid | 8.31 | € 2.02 | 7.52% | € 0.13 |
| Lutein | 0.11 | € 168.06 | 0.10% | ||||
| F/2 | 250 μE/m2/s, 25ᵒ C, 13:11h light/dark cycle | 50.65 | Lipid | 3.40 | € 16.10 | 6.71% | € 0.54 |
| Lutein | 0.04 | € 419.72 | 0.08% |
5. Conclusion
In this study, use of three different media set up under two different light intensities and photoperiod regimes showed enhanced production of biomass, lipid and carotenoids. SSM (NSW) showed higher lipid productivity of 34.56 ± 0.31 mg/L/d in D.tertiolecta. While SSM medium presented 0.253 ± 0.0628 mg/L/d of lutein productivity in D.tertiolecta under 13:11h light/dark cycle at 250 μE/m2/s. D.tertiolecta and N.oculata has the extreme C 16:0 and C18:3 fatty acid accumulation at low and high light intensity. Consequently, use of natural seawater for growth of algae, mainly the major nutrients such as N, P, Ca, and Mg can result in significant production of biomass, lipid and carotenoids with minimum energy cost.
Author contribution
Conceived and designed the experiments: AM and AA, Analysed and interpreted the data: AM and AA, Performed the experiments: SG and AM, Wrote the paper: AM and SG, drafting the article or critically revising its important intellectual content: AA and AM, final approval of the version submitted: AA.
Conflict of interest
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter
The authors declare no conflict of interest in connection with the work submitted.
Acknowledgements
The assistance provided by Ms. Deeprajni for GC–MS and HPLC is duly acknowledged. The assistance provided by Mr. Saksham Gupta is duly acknowledged. The authors are thankful to Dr. Alok Adholeya for reading the paper and analysing the data. The authors were immensely helped by the support provided by the Dr. Vibha Dhawan, Director General (TERI). AM duly acknowledges the financial support provided by DBT funded project on centre of excellence on Integrated Production of Advanced Biofuels and Biocommodities (2018NT17).
References
- 1.Chisti Y. Algae Biotechnology. Springer; Cham: 2016. Large-scale production of algal biomass: raceway ponds; pp. 21–40. [DOI] [Google Scholar]
- 2.McWilliams A. MA; USA: 2018. The Global Market for Carotenoids. BCC Research: Wellesley. [Google Scholar]
- 3.Singh D.P., Khattar J.S., Rajput A., Chaudhary R., Singh R. High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1. 1 under optimized culture conditions. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0221930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhuyar P., Rahim M.H.A., Yusoff M.M., Maniam G.P., Govindan N. A selective microalgae strain for biodiesel production in relation to higher lipid profile. Maejo Int. J. Energy Environ. Commun. 2019;1(1):8–14. [Google Scholar]
- 5.Maglie M., Baldisserotto C., Guerrini A., Sabia A., Ferroni L., Pancaldi S. A co-cultivation process of Nannochloropsis oculata and Tisochrysis lutea induces morpho-physiological and biochemical variations potentially useful for biotechnological purposes. J. Appl. Phycol. 2021:1–16. doi: 10.1007/s10811-021-02511-2. [DOI] [Google Scholar]
- 6.Moura Y.A.S., Viana-Marques D.D.A., Porto A.L.F., Bezerra R.P., Converti A. Pigments production, growth kinetics, and bioenergetic patterns in Dunaliella tertiolecta (Chlorophyta) in response to different culture media. Energies. 2020;13(20):5347. doi: 10.3390/en13205347. [DOI] [Google Scholar]
- 7.Sui Y., Harvey P.J. Effect of light intensity and wavelength on biomass growth and protein and amino acid composition of Dunaliella salina. Foods. 2021;10(5):1018. doi: 10.3390/foods10051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colusse G.A., Mendes C.R.B., Duarte M.E.R., de Carvalho J.C., Noseda M.D. Effects of different culture media on physiological features and laboratory scale production cost of Dunaliella salina. Biotechnol. Reports. 2020;27 doi: 10.1016/j.btre.2020.e00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y., Ibrahim I.M., Harvey P.J. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016;106:305–315. doi: 10.1016/j.plaphy.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorquera O., Kiperstok A., Sales E.A., Embiruçu M., Ghirardi M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010;101(4):1406–1413. doi: 10.1016/j.biortech.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Picardo M.C., de Medeiros J.L., Monteiro J.G.M., Chaloub R.M., Giordano M., Araújo O.D.Q.F. A methodology for screening of microalgae as a decision making tool for energy and green chemical process applications. Clean Technol. Environ. Policy. 2013;15(2):275–291. doi: 10.1007/s10098-012-0508-z. [DOI] [Google Scholar]
- 12.Zakaria B.S., Lin L., Chung T., Dhar B.R. An overview of complementary microbial electrochemical technologies for advancing anaerobic digestion. Adv. Bioener. 2020;5:129–167. doi: 10.1016/bs.aibe.2020.04.004. [DOI] [Google Scholar]
- 13.Paramasivam P., Kanagesan K., Bhuyar P., Govindan N., Maniam G.P. Biomass and lipid production from indigenous Nannochloropsis sp. by employing stress factors for improved biodiesel production. Environ. Dev. Sustain. 2021:1–15. [Google Scholar]
- 14.Banerjee S., Ramaswamy S. Dynamic process model and economic analysis of microalgae cultivation in flat panel photobioreactors. Algal Res. 2019;39 doi: 10.1016/J.ALGAL.2019.101445. [DOI] [Google Scholar]
- 15.Camacho-Rodríguez J., Gallardo-Rodríguez J.J., Cerón-García M.C., García-Camacho F., Molina-Grima E. A new culture medium based on genetic algorithms for Isochrysis galbana production relevant to hatcheries. J. Appl. Phycol. 2021;1:10. doi: 10.1007/s10811-021-02564-3. [DOI] [Google Scholar]
- 16.Guillard R.R., Ryther J.H. Studies of marine planktonic diatoms: I. Cyclotella nana hustedt, and Detonula confervacea (cleve) gran. Can. J. Microbiol. 1962;8(2):229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Min M., Li Y., Chen P., Chen Y., Liu Y., Wang Y., Ruan R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010;162(4):1174–1186. doi: 10.1007/s12010-009-8866-7. [DOI] [PubMed] [Google Scholar]
- 18.Chiu S.Y., Kao C.Y., Tsai M.T., Ong S.C., Chen C.H., Lin C.S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009;100(2):833–838. doi: 10.1016/j.biortech.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 19.Lewis T., Nichols P.D., McMeekin T.A. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J. Microbiol. Methods. 2000;43(2):107–116. doi: 10.1016/S0167-7012(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 20.Idris N.A., Loh S.K., Lau H.L.N., Yau T.C., Mustafa E.M., Vello V., Moi P.S. Palm oil mill effluent as algae cultivation medium for biodiesel production. J. 2018 [Google Scholar]
- 21.Wang X.W., Liang J.R., Luo C.S., Chen C.P., Gao Y.H. Biomass, total lipid production, and fatty acid composition of the marine diatom Chaetoceros muelleri in response to different CO2 levels. Bioresour. Technol. 2014;161:124–130. doi: 10.1016/j.biortech.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Christie W.W., Han X. fourth ed. The Oily Press; Bridgwater, UK: 2010. Lipid Analysis. [DOI] [Google Scholar]
- 23.Lichtenthaler H.K., Buschmann C. Chlorophylls and carotenoids: measurement and characterization by UV‐VIS spectroscopy. Curr. Protoc. Food Analyt. Chem. 2001;1(1):F4. doi: 10.1002/0471142913.faf0403s01. 3. [DOI] [Google Scholar]
- 24.Knoshaug E.P., Dong T., Spiller R., Nagle N., Pienkos P.T. Pre-treatment and fermentation of salt-water grown algal biomass as a feedstock for biofuels and high-value biochemicals. Algal Res. 2018;36:239–248. doi: 10.1016/j.algal.2018.10.024. [DOI] [Google Scholar]
- 25.Reshma R., Devi K.C., Kumar S.D., Santhanam P., Perumal P., Krishnaveni N., Begum A., Pragnya M., Arthikha R., Dhanalakshmi B., Kim M.K. Enhancement of pigments production in the green microalga Dunaliella salina (PSBDU05) under optimized culture condition. Bioresour. Tech. eports. 2021;14 doi: 10.1016/j.biteb.2021.100672. [DOI] [Google Scholar]
- 26.Andersen R.A., editor. Algal Culturing Techniques. Elsevier; 2005. [Google Scholar]
- 27.Millero F.J., Feistel R., Wright D.G., McDougall T.J. The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. Oceanogr. Res. Pap. 2008;55(1):50–72. doi: 10.1016/j.dsr.2007.10.001. [DOI] [Google Scholar]
- 28.Nzayisenga J.C., Farge X., Groll S.L., Sellstedt A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels. 2020;13(1):1–8. doi: 10.1186/s13068-019-1646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meseck S.L., Alix J.H., Wikfors G.H. Photoperiod and light intensity effects on growth and utilization of nutrients by the aquaculture feed microalga, Tetraselmis chui (PLY429) Aquaculture. 2005;246(1–4):393–404. doi: 10.1016/j.aquaculture.2005.02.034. [DOI] [Google Scholar]
- 30.Griffiths M.J., Harrison S.T. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009;21(5):493–507. doi: 10.1007/s10811-008-9392-7. [DOI] [Google Scholar]
- 31.Wahidin S., Idris A., Shaleh S.R.M. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013;129:7–11. doi: 10.1016/j.biortech.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Pulz O. Photobioreactors: production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001;57(3):287–293. doi: 10.1007/s002530100702. [DOI] [PubMed] [Google Scholar]
- 33.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot., Le. 2012 doi: 10.1155/2012/217037. 2012. [DOI] [Google Scholar]
- 34.Matos Â.P., Cavanholi M.G., Moecke E.H.S., Sant'Anna E.S. Effects of different photoperiod and trophic conditions on biomass, protein and lipid production by the marine alga Nannochloropsis gaditana at optimal concentration of desalination concentrate. Bioresour. Technol. 2017;224:490–497. doi: 10.1016/j.biortech.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Gim G.H., Ryu J., Kim M.J., Kim P.I., Kim S.W. Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J. Ind. Microbiol. Biotechnol. 2016;43(5):605–616. doi: 10.1007/s10295-016-1741-y. [DOI] [PubMed] [Google Scholar]
- 36.Solovchenko A., Lukyanov A., Solovchenko O., Didi-Cohen S., Boussiba S., Khozin-Goldberg I. Interactive effects of salinity, high light, and nitrogen starvation on fatty acid and carotenoid profiles in Nannochloropsis oceanica CCALA 804. Eur. J. Lipid Sci. Technol. 2014;116(5):635–644. doi: 10.1002/ejlt.201300456. [DOI] [Google Scholar]
- 37.Hoffmann M., Marxen K., Schulz R., Vanselow K.H. TFA and EPA productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar. Drugs. 2010;8(9):2526–2545. doi: 10.3390/md8092526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal D., Khozin-Goldberg I., Cohen Z., Boussiba S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011;90(4):1429–1441. doi: 10.1007/s00253-011-3170-1. [DOI] [PubMed] [Google Scholar]
- 39.Sharma K.K., Schuhmann H., Schenk P.M. High lipid induction in microalgae for biodiesel production. Energies. 2012;5(5):1532–1553. doi: 10.3390/en5051532. [DOI] [Google Scholar]
- 40.Krzemińska I., Piasecka A., Nosalewicz A., Simionato D., Wawrzykowski J. Alterations of the lipid content and fatty acid profile of Chlorella protothecoides under different light intensities. Bioresour. Technol. 2015;196:72–77. doi: 10.1016/j.biortech.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 41.Murphy R.A., Mourtzakis M., Chu Q.S., Baracos V.E., Reiman T., Mazurak V.C. Supplementation with fish oil increases first‐line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer. 2011;117(16):3774–3780. doi: 10.1002/cncr.25933. [DOI] [PubMed] [Google Scholar]
- 42.Chavoshi Z.Z., Shariati M. Lipid production in Dunaliella bardawil under autotrophic, heterotrophic and mixotrophic conditions. Braz. J. Oceanogr. 2019;67 doi: 10.1590/s1679-87592019024906709. [DOI] [Google Scholar]
- 43.Bhuyar P., Sundararaju S., Rahim M.H.A., Ramaraj R., Maniam G.P., Govindan N. Microalgae cultivation using palm oil mill effluent as growth medium for lipid production with the effect of CO2 supply and light intensity. Biomass Convers. Biorefin. 2021;11(5):1555–1563. [Google Scholar]
- 44.Junior E.N., Kumar M., Pankratz S., Oyedun A.O., Kumar A. Development of life cycle water footprints for the production of fuels and chemicals from algae biomass. Water Res. 2018;140:311–322. doi: 10.1016/j.watres.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 45.Yeesang C., Cheirsilp B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011;102(3):3034–3040. doi: 10.1016/j.biortech.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Xie X., Lu X., Wang L., He L., Wang G. High light intensity increases the concentrations of β-carotene and zeaxanthin in marine red macroalgae. Algal Res. 2020;47 doi: 10.1016/j.algal.2020.101852. [DOI] [Google Scholar]
- 47.Lu Y., Gan Q., Iwai M., Alboresi A., Burlacot A., Dautermann O., Takahashi H., Crisanto T., Lu Y., Peltier G., Morosinotto T., Melis A. Role of an ancient light-harvesting protein of PSI in light absorption and photoprotection. Nat. Commun. 2021;12(1):1–10. doi: 10.1038/s41467-021-20967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulders K.J., Lamers P.P., Martens D.E., Wijffels R.H. Phototrophic pigment production with microalgae: biological constraints and opportunities. J. Phycol. 2014;50(2):229–242. doi: 10.1111/jpy.12173. [DOI] [PubMed] [Google Scholar]
- 49.Park S., Lee Y., Jin E. Comparison of the responses of two Dunaliella strains, Dunaliella salina CCAP 19/18 and Dunaliella bardawil to light intensity with special emphasis on carotenogenesis. ALGAE. 2013;28(2):203–211. doi: 10.4490/algae.2013.28.2.203. [DOI] [Google Scholar]
- 50.Dineshkumar R., Sen R. A sustainable perspective of microalgal biorefinery for co‐production and recovery of high‐value carotenoid and biofuel with CO2 valorization. Biofuels Bioprod. Bioref. 2020;14(4):879–897. doi: 10.1002/bbb.2107. [DOI] [Google Scholar]
- 51.Bellan A., Bucci F., Perin G., Alboresi A., Morosinotto T. Photosynthesis regulation in response to fluctuating light in the secondary endosymbiont alga Nannochloropsis gaditana. Plant Cell Physiol. 2020;61(1):41–52. doi: 10.1093/pcp/pcz174. [DOI] [PubMed] [Google Scholar]
- 52.Sonoike K. Photoinhibition of photosystem I. Physiol. Plantarum. 2011;142(1):56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu C., Liu C., Cheng Y., Du C., Lv T., Guo Y., Han M., Pi F., Zhang W., Qian H. Study on the wall-breaking method of carotenoids producing yeast Sporidiobolus pararoseus and the antioxidant effect of four carotenoids on SK-HEP-1 cells. Prep. Biochem. Biotechnol. 2019;49(8):767–774. doi: 10.1080/10826068.2019.1608448. [DOI] [PubMed] [Google Scholar]
- 54.Minhas A.K., Hodgson P., Barrow C.J., Adholeya A. Two-phase method of cultivating Coelastrella species for increased production of lipids and carotenoids. Bioresour. Tech. eports. 2020;9 doi: 10.1016/j.biteb.2019.100366. [DOI] [Google Scholar]
- 56.Minhas A.K., Hodgson P., Barrow C.J., Sashidhar B., Adholeya A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016;211:556–565. doi: 10.1016/j.biortech.2016.03.121. [DOI] [PubMed] [Google Scholar]