Abstract
In this study, we report on the electrochemical properties of a solid state lithium ion battery (LIB) using a poly (ethylene glycol) dimethyl ether (PEGDME)-based solid polymer electrolyte (P-SPE). The LIB is prepared using a LiFePO4 (LFP) cathode and graphite anode material with P-SPE, and the kinetic properties of the lithium ions in the P-SPE are investigated. The synthesized P-SPE is shown to be suitable solid polymer electrolyte candidate for LIB applications. LFP and graphite are selected as electrode materials to validate their effectiveness in different battery cells with respect to their high energy density and inherent safety. The five-layer stacked 5 × 6 cm2 pouch-type LIB demonstrates a high capacity of 90 mAh (0.6 mAh/cm2) or more in the initial cycle, and it shows cycle stability with a capacity decrease of 20% over 500 cycles. We test the manufactured pouch-type full cells under extreme conditions (e. g., cutting, crushing and exposure of the battery cell to the atmosphere). LIBs using the developed P-SPE are promising solid polymer electrolyte candidates for wearable LIB as well as high energy LIB applications.
Keywords: Solid polymer electrolyte, Pouch-type, Full cell, N/P ratios, Wearable LIB
Abbreviations: PEGDME, poly (ethylene glycol) dimethyl ether; LFP, LiFePO4; P-SPE, PEGDME-based solid polymer electrolyte; LIB, lithium ion battery; PEO, polyethylene oxide
1. Introduction
The conventional organic carbonate solvents (e.g., ethylene carbonate and ethyl carbonate) that are used in lithium ion batteries (LIBs) pose important safety concerns due to their high flammability and high vapor pressure. Electrolytes that transports charges between battery electrodes with liquid electrolytes have a significant impact on device configuration and efficiency [1]. The difficulties in sealing liquid electrolytes in batteries or solar cells, particularly when associated with this task, and the safety issues posed during device operation are driving the development of solid electrolytes [2].
It has been reported that LIBs can be safely used with polymer electrolytes such as polyethylene oxide (PEO) electrolytes [3,4]. These candidates have the potential to replace conventional liquid electrolytes [5,6]. However, ionic conduction only occurs in the amorphous PEO phase, and the conductivity is two to three times higher than that of the crystalline phase [7]. Accordingly, the conductivity of typical PEO-salt systems is not satisfactory due to the PEO crystallization [8]. Improving conductivity requires the introduction of disorder to prevent crystallization [9], which requires an approach that includes polymer mixing, crosslinking, copolymerization, and the addition of plasticizers [10,11].
Valid alternatives to solid PEO-based electrolytes, which exhibit adequate room temperature conductivity and safety, overcome the safety concerns associated with organic solvents (i.e. eliminating the potential for flammability). However, inexpensive, chemically safe, and non-volatile plasticizers are needed. In this sense, poly (ethylene glycol dimethyl ether) (PEGDME) is a promising option, exhibiting high boiling point (>250 °C), high flash point (220 °C), low vapor pressure (0.01 hPa at 20 °C) and good electrochemical properties [[12], [13], [14]]. According to Hassoun et al., PEGDME Mw 500 g/mol-LiTFSI was reported as a highly viscous and LIB- safe electrolyte [13].
In this study, we present the full cell batteries fabrication and electrochemical properties of an LIB with synthesized P-SPE. The P-SPE is prepared with precise amounts of lithium salt, plasticizer, crosslinking agent, and thermal initiator. Using this P-SPE, half cells and full cells of graphite anode and LFP cathode, which are generally used in LIBs, are fabricated, and their electrochemical properties are investigated for practical application. In addition, the battery is tested under extreme conditions (e.g., packaging, cutting, and exposure to the atmosphere). Finally, high-capacity application scalability is demonstrated by fabricating a five-layer stacked 5 × 6 cm2 pouch cell using a solid electrolyte.
2. Experimental
2.1. Materials
Graphite, LiFePO4(APS, 中天鸿锂清源股份有限公司, China), carbon black (Imerys, Cop., Switzerland), and poly (vinylidene fluoride) (PVDF, Kynar, USA) were dried in a vacuum oven at 80 °C for 12 h, and LiTFSI was dried in a vacuum oven at 120 °C for 1 h before use. The X-ray diffractometer (XRD) and scanning electron microscopy (SEM) data of the purchased LFP are shown in Fig. S1 (data provided by the manufacturer). The density and specific surface area of the LFP were >0.7 g/cm3 and <20 m2/g, respectively. Poly (ethylene-glycol) dimethyl ether (Mn∽500, 99%, Sigma Aldrich) was vacuum distilled until the moisture content was less than 10 ppm. It was then stored in an Ar-filled glove box along with the molecular sieves 4 A (4∽12 mesh). 1-Methyl-2-pyrrolidinone (NMP, anhydrous, 99.5%, Sigma Aldrich), Bisphenol A ethoxylate diacrylate and t-Butyl peroxypivalate (t-BPP, Seka Arkema Co., Japan) were purchased and employed. Bisphenol A ethoxylate diacrylate (BisA), poly (ethylene glycol) dimethyl ether (PEGDME) and t-Butyl peroxypivalate (t-BPP) were used as crosslinking agents, plasticizers and initiators, respectively.
2.2. Preparation of PEGDME-based solid-polymer electrolyte
The liquid PEGDME-based polymer electrolyte was prepared via magnetic stirring in an Ar-filled glove box with the water content at less than 0.1 ppm. A homogeneous precursor solution of lithium salt (LiTFSI), plasticizer (PEGDME), crosslinker (bisphenol A ethoxylate diacrylate) and thermal initiator (t-butyl peroxypivalate (t-BPP)) was prepared. The weight ratio of the plasticizer to the crosslinking agent was 8:2 based on the crosslinking agent. The thermal radical initiator was 1 wt% by weight based on the cross-linker. The [EO]/[Li+] molar ratio was 20. Finally, to obtain a semi-interpenetrating polymer network (semi-IPN) P-SPE, the precursor was crosslinked at room temperature for 30 min. In addition, fluoroethylene carbonate (FEC), which has been reported as being helpful in forming an effective solid electrolyte interphase (SEI) layer on a graphite anode electrode [15], was added to prepare the PEGDME-based polymer electrolyte.
2.3. Fabrication of composite electrode
A composite electrode was prepared by mixing 70 wt% of active material, 22 wt% of polymer electrolyte binder (PVDF; 6.13 wt%, LITFSI; 3.63 wt%, and PEGDME; 12.26 wt%) and 8 wt% of super P in NMP. The polymer electrolyte binder was dissolved in NMP and used. The polymer electrolyte binder and the positive electrode (LFP) or negative electrode (graphite) material were blended in a mixer (Thinky Cop., ARE-310 Thinky mixer, Japan) at 2000 rpm for 20 min. A slurry containing the liquid binder, LFP and graphite was coated on the Al foil (15 μm thick, Welcos Co., Korea) and Cu foil current collector (12 μm thick, Welcos Co., Korea), respectively. The electrodes were dried in a vacuum oven overnight at 100 °C before punching.
2.4. Cell assembly and electrochemical measurements
Coin- and pouch-type solid state cells were assembled in Ar-filled glove box (<0.1 ppm H2O) and a drying room (<- 60 °C dew point), respectively. The LFP/P-SPE/Li and graphite/P-SPE/Li coin-type half cells (CR2032) were assembled, and the lithium storage performance was investigated. The pouch-type assembled solid state (ASS) full cell was manufactured in 3 × 4 cm2 and 5 × 6 cm2 sizes, and the electrode was cut using a Thomson knife-type electrode cutter. The pouch-type five-layer stacked cell was manufactured with a size of 5 × 6 cm2. A schematic diagram of the 5-layer stacked 5 × 6 cm2 size pouch-type full cell is shown in Fig. S2. Fig. S2 consists of two positive and two negative electrodes coated on both-sides. And the single-sided coated anode and cathode electrodes were composed of one each. Five LIBs composed of the 5 × 6 cm2 size electrodes were connected in parallel. In this study, the cell was assembled in a liquid electrolyte (liquid PEGDME-based polymer electrolyte) in which the electrolyte was cross-linked. All assembled cells were prepared via heat treatment in an atmospheric pressure electric furnace at 80 °C for 12 h to solidify the polymer liquid electrolyte.
The electrochemical performance was investigated using a multichannel potentiostatic/galvanostatic coin cell test station (WonaTech, WMPG1000, Korea) and pouch-type cell test station (Toyo, Toscat-3000, Japan) analyzer, respectively. The cells were cycled on a galvanostat over 0.01 ∽ 3.0 V (vs. Li+/Li, graphite/P-SPE/Li coin-type half cells) and 2.4 ∽ 4.2 V (vs. Li+/Li, LFP/P-SPE/Li coin-type half cells and LFP/P-SPE/graphite pouch-type full cells) voltage range.
2.5. Ionic conductivity measurements
The temperature-dependent ionic conductivity was determined in electrical impedance (Hewlett Packard 4192 A LF) using prepared coin cells. A coin cell (CR 2032 type) using stainless steel (SS) as blocking electrodes (SS/P-SPE/SS) was assembled, and the impedance measurements were performed in an Ar-filled glove box. The impedance measurements were made at 303 ∽ 344 K at 10 K intervals. Samples were equilibrated for 15 min at a predetermined temperature prior to each measurement. The ionic conductivity was calculated as follows. The sample radius was 0.6 cm, and the thickness of the electrolyte was measured via SEM (Su70, Hitachi Co., Japan). The ionic conductivity (σ) of the electrolyte was calculated using the value of the bulk resistance (Rb) as follows.
| σ = 1/ρ = l/(Rb A) ---------- | Eq. (1) |
Where l and A are the thickness and effective area of the electrolyte, respectively. Data were collected at a frequency range of 100 mHz–20 MHz with an oscillation voltage of 10 mV.
3. Results and discussion
Fig. 1 shows (a) a photograph (CR 2032 coin size), (b) flammability test and (c) thermogravimetric analysis of the synthesized P-SPE. To access the safety of the P-SPE, a flammability test was carried out as shown in Fig. 1b. According to a previous study [16,17], the EC + EMC liquid mixture burns immediately after ignition. This flammability test demonstrates the strong ability of the P-SPE, which is also confirmed by the TGA results (Fig. 1c). The thermal properties of the prepared P-SPE were evaluated using a thermogravimetric analysis (TGA; SDT Q600 V20.9 Build 20, USA). The sample was heated from room temperature to 500 °C under a flow of nitrogen at a heating rate of 10 °C/min. In the first stage, negligible weight loss was observed below 200 °C, which could be attributed to the volatilization of H2O or the solvent. The main weight loss occurred above approximately 250 °C. This phenomenon demonstrated the strong ability of the polymer matrix in the P-SPE, which was also confirmed by the TGA results, where a clearly improved weight retention was observed with respect to the thermal stability.
Fig. 1.
(a) Photograph, (b) Flammability test, and (c) Thermogravimetric analysis of the solid polymer electrolyte.
Fig. 2a shows the impedance spectrum of the P-SPE as a function of temperature. The impedance spectra of the SS/P-SPE/SS cell resulted in a small compressed semicircle in the high frequency region and a straight line in the low frequency region. The synthesized liquid PEGDME-based polymer electrolyte was a clear solution. The synthesized liquid PEGDME-based polymer electrolyte was heat-treated at 80 °C for 12 h. The heat-treated P-SPE was a white solid polymer (insert bottle image in Fig. 2c). After the impedance test, the coin cell was disassembled and the thickness of the P-SPE was measured using SEM (Fig. 2c). The P-SPE thickness was about 222 μm. The ionic conductivity of the P-SPE was calculated from the impedance data, and it was simply taken as the resistance values corresponding to the intercept of the linear fit of the straight line with the x-axis (i.e., when the imaginary portion is equal to zero) [18]. Then, the ionic conductivity was calculated using Equation (1) (Fig. 2d). At room temperature and 353 K, the ionic conductivities were 2.89 × 10−4 s/cm and 2.47 × 10−3 s/cm, respectively. The ionic conductivity of the P-SPE as a function of temperature showed a nonlinear dependence along the Vogel-Tamman-Fulcher (VTF) behavior [19]. This suggested the movement of Li+ through the amorphous region and plasticizer (PEGDME)-rich zones of the polymer matrix, similar to the behavior of Li+ suggested in the polymer electrolyte. Taking into account the aforementioned results, the P-SPE is a suitable candidate for lithium ion batteries using flexible electrolyte.
Fig. 2.
(a) Impedance spectrum of the P-SPE as a function of temperature, (b) enlarged impedance spectrum of the red square region in Fig. (a), (c) thickness of the P-SPE (SEM image), and (d) ionic conductivity as a function of temperature of the P-SPE calculated using equation (1).
LFP cathode and graphite anode materials were selected as electrode materials to validate the effectiveness in different battery cell systems (at the coin and pouch cell) owing to their high energy density and inherent safety. Two types of solid-state batteries, LFP/P-SPE/Li metal and graphite/P-SPE/Li metal coin-type half cells, were fabricated and their electrochemical performance was investigated using a potentiostatic/galvanostatic coin cell test station analyzer. Fig. 3 shows the rate capability behavior and cycle characteristics according to the loading level of active material in the LFP/P-SPE/Li metal and graphite/P-SPE/Li metal coin cells. The initial three cycles performed at a rate of 0.05C, followed by 0.01C and 0.2C. Fig. 3a shows the cycle characteristics according to the loading level of LFP in the coin-type half cells using the P-SPE. The specific capacity of the LFP/P-SPE/Li metal did not change significantly when the C-rate increased, and very stable cycle characteristics were demonstrated despite the high LFP loading level. After the forty-fifth charge/discharge cycles (0.05C = 3rd cycle, 0.1C = 5th cycle, and 0.2C = 37th cycle), the specific charge capacity of the battery decreased to 145.5 mAh/g, and the capacity retention remained at 97.9%. Fig. 3b shows the cycle characteristics (at 0.05C, 0.1C, and 0.2C) according to the loading level in the graphite/P-SPE/Li metal coin cells. In the graphite anode, the specific capacity decreased rapidly when the loading level of the graphite increased, and it decreased significantly with an increase in the C-rate. The polarization of the cell increased significantly with the increasing C-rate due to the kinetic limitation of the anode, which resulted in a low capacity at a high C-rate (e. g., 446.0 mAh/g at 0.05C, 345.4 mAh/g at 0.1C and 176.5 mAh/g at 0.2C (loading level; 1.9 mg/cm2)). The coulombic efficiency according to the loading level of active material in the LFP/P-SPE/Li metal and graphite/P-SPE/Li metal coin-type half cells was approximately 100%, except during the initial cycle (0.05C rate).
Fig. 3.
The rate capability behavior and cycle characteristics of (a) LFP/PSPE/Li, and (b) graphite/P-SPE/Li coin type half-cell according to loading level.
The charge/discharge profiles with their loading levels of LFP (LFP/P-SPE/Li metal) and graphite (Graphite/P-SPE/Li metal) coin-type half cells shown in the Fig. S3. Fig. 4 shows the second cycles discharge specific capacity and irreversible specific capacity (ISC) loss of the first and second cycles according to loading level of the LFP and graphite coin-type half-cell using P-SPE. The initial charge/discharge specific capacity of the LFP/P-SPE/Li metal half cells showed similar behaviors according to the LFP loading level. As shown in Figs. S3a, S3b, and S3c, the first charge specific capacities of the LFP/P-SPE/Li metal battery were 152.9, 152.7, and 147.1 mAh/g in 6.71, 6.95, and 7.19 mg/cm2 LFP loading level, respectively. The LFP/P-SPE/Li metal battery showed almost reversible charge/discharge behavior at a loading level of about 7 mg/cm2 or less. On the other hand, in the case of the graphite anode half cells (Figs. S3d, S3e, and S3f), the specific discharge capacity decreased as the loading level of graphite increased and showed a high irreversible behavior during the first cycle. The ISC loss of the graphite anode was high (>80 mAh/g) during the first cycle, but low (<20 mAh/g) during the second cycle and beyond. Meanwhile, the ISC loss of the LFP cathode was significantly lower (first cycle: 1.89 ∽ 5.31 mAh/g, second cycle: 0.6 ∽ 3.78 mAh/g) than that of the graphite anode.
Fig. 4.
Second cycles discharge specific capacity and irreversible specific capacity of the first, and second cycles according to the loading level of the (a) LFP/P-SPE/Li, and (b) graphite/P-SPE/Li coin cell.
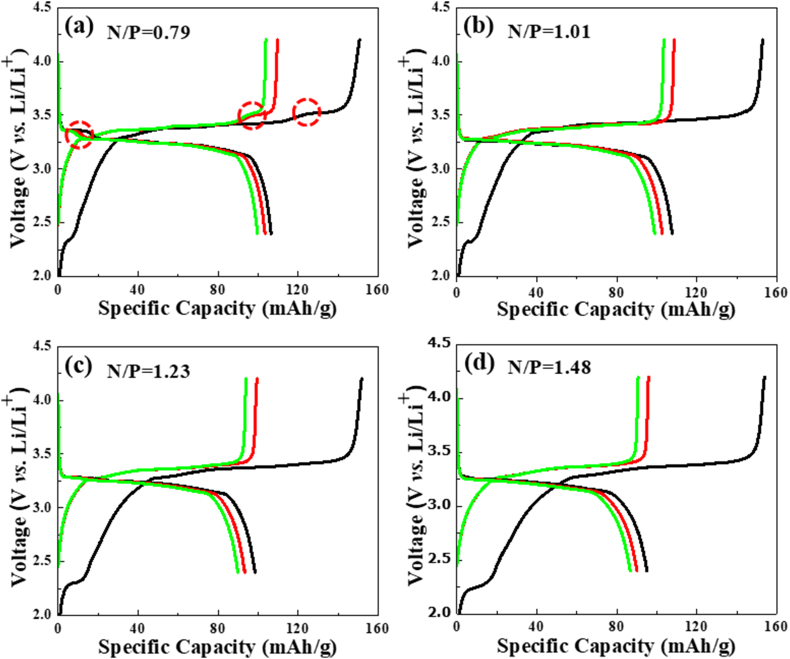
The N/P ratio is the most important factor in designing a high-performance lithium-ion battery for a balanced electrochemical reaction [20,21]. In this study, the anode to cathode area capacity ratio (N/P ratio) is adjusted for a high battery capacity and stable battery behavior without lithium plating in full-cell designs, and it was calculated using a graphite anode specific capacity of 444 mAh/g and an LFP cathode specific capacity of 158 mAh/g of the coin-type half cells test results. The LFP/P-SPE/graphite coin-type full cells were assembled with the N/P ratios of 0.79, 1.01, 1.23, and 1.48. Fig. 5 shows the charge/discharge profiles of the LFP/P-SPE/graphite coin-type full cells with an N/P ratio for the first, second, and third cycles. In the coin-type full cells, the charging specific capacity of the first cycle showed a tendency to slightly increase as the N/P ratio increased. In the charge/discharge profiles of the LFP/P-SPE/graphite coin-type full cells, the ISC loss of the first cycle was large. This behavior was similar to that of the graphite/P-SPE/Li metal coin-type half cells, as shown in Fig. 4. However, the LFP/P-SPE/graphite coin-type full cells showed a low ISC loss after the second cycle or beyond. The specific discharge capacity in the second cycle or beyond showed the highest value in the N/P = 1.01 cell.
Fig. 5.
Charge/discharge profile of the LFP/P-SPE/graphite coin-type full cells with an N/P ratios.
According to a previous study [[22], [23], [24], [25]], the ISC loss during the formation of the solid electrolyte interphase (SEI) has been directly related to the surface area of graphite, regardless of particle size, while the reversible capacity is only affected by the particle size of graphite. The formation and continuous growth of the SEI layer are responsible for the irreversible capacity loss in the initial cycles and following cycles, respectively. In this study, the ISC loss in the first cycle of the coin-type full cells was between 44 and 59 mAh/g, or 29∽38%. Meanwhile, the ISC loss in the second cycle was between 5.8 and 6.4 mAh/g or 5.6∽6.1%. In the LFP/P-SPE/graphite full cell, the first and second cycles were carried out successively at 0.05C to achieve a low ISC loss and good quality SEI layer. In this study, the N/P ratio was designed by adjusting the negative electrode (graphite) loading level based on the positive electrode (LFP) loading level of ≒7.0 mg/cm2 to maximize the capacity and a low irreversible specific capacity of the negative electrode (graphite). The LFP/P-SPE/graphite full cell with a low N/P ratio exhibited a lithium plating phenomenon [[26], [27], [28]] at 3∽3.5 V (Fig. 5a). On the other hand, in the case of a high N/P ratio (>1.01), lithium plating did not occur, but the ISC loss was relatively large in the first cycle. However, the ISC loss in the second cycle was low and almost constant according to the N/P ratio. The larger ISC loss in the initial cycle could be attributed to the SEI layer formation, lithium plating formation at low N/P ratio, and dead layer appearing in the high loading level of graphite as shown in Fig. 3b.
In the pouch-type full cells (3 × 4cm2 size), the lithium plating phenomenon was also observed at a low N/P ratio (Figs. S4 and S5) and effectively suppressed in cells with high N/P ratios (>0.9). In the case of the pouch-type full cells, the charge/discharge specific capacity in the first cycle showed a tendency to decrease as the N/P ratio increased (>N/P ratio; 0.95). However, the ISC loss in the second cycle was low (coin-type≒6.11 mAh/g in N/P = 1.01 and pouch-type 3.2 mAh/g in N/P = 0.95 cell) and tended to stabilize (Fig. S4). In this study, the charge/discharge specific capacity of the full cell of N/P = 0.95 showed the largest specific capacity (charging specific capacity = 149.9, and discharging specific capacity = 119.4 mAh/g: Fig. S4c). The first charge specific capacity showed a value similar to the high loading level of the half cell (Fig. 3, LFP/P-SPE/Li). There was an irreversible specific capacity loss (30.5 mAh/g) in the first cycle. According to a previous study [23,[29], [30], [31]], ISC loss results primarily from the formation of a passivating SEI layer via the decomposition of the electrolyte to form both as an SEI layer and gaseous products on the surface of the graphite during the initial charge/discharge cycles [32]. In the initial cycle of the LFP/P-SPE/graphite full cell, the 0.05C constant current (CC) mode was used to build up a smooth stable solid electrolyte interface (SEI) layer via the slow decomposition of the solid electrolyte on the surface of the graphite [19]. As a result, the SEI layer formed at the interface between the electrolyte and the active material in the first and second cycles. In addition, capacity degradation and self-discharge due to the gas generated by the electrochemical reaction appeared. When lithium-ion cells are packaged in flexible cases (pouch-type cells), these gases are usually removed after SEI layer formation to prevent cell deformation and ensure uniform stack pressure on the electrodes (degassing process) [33,34]. In this experiment, a two-cycle formation process was performed to form a high quality SEI in the anode (graphite). The internal gas was removed via degassing, and sealing was subsequently performed. The two-cycle charge/discharge profile is shown in Fig. S5, which indicates the charge/discharge profile after the formation process and degassing process. The specific charge capacity was 116.7 mAh/g and the irreversible specific capacity was 2.2 mAh/g in the N/P ratio = 0.95 ratio full cell.
Fig. 6a shows the rate capability of the pouch-type (3 × 4 cm2 size) LFP/SPE/graphite full cell (N/P = 0.95), as measured by varying the current density from 0.1 to 2C. Three cycles at 0.05C and five cycles each at 0.1C, 0.2C, 0.5C, 1C, and 2C were performed. At high C-rates ranging from 0.1 to 2C, high and stable discharge specific capacities of 115.8 and 25.6 mAh/g in the LFP/SPE/graphite full cell (N/P = 0.95) were demonstrated as shown in Fig. 6a. Fig. 6b shows the cycle profile of a 3 × 4 cm2 pouch-type LFP/P-SPE/graphite full cell (N/P ratios = 0.9). It exhibited excellent cycle characteristics of about 68% for 300 cycles.
Fig. 6.
(a) C-rate behavior (N/P ratios = 0.95), and (b) cycle performance (N/P ratios = 0.90) of the 3 × 4 cm2 pouch-type LFP/P-SPE/graphite full cells.
The upscaling process is a very important step to validate the feasibility and applicability of developed components in a battery format close to industrial. However, most works related to the development of electrolyte and anode/cathode materials for batteries are only tested at the coin cell level, thus hindering the direct and easy transfer to practical cells and overlooking important aspects of the P-SPEs performance at a practical level [35]. In this study, a five-layer stacked 5 × 6 cm2 pouch-type cell (Fig. S2) was prepared for the experiment to examine the possibility of a large capacity and inherently safe battery using the P-SPE. To minimize the thickness of the cell, the LFP cathode and graphite anode slurry was coated on both sides to prepare the five-layer stacked 5 × 6 cm2 pouch-type battery.
Fig. 7 shows (a) the charge/discharge profile and (b) the cycle characteristics of the five -layer stacked 5 × 6 cm2 pouch-type full cells (N/P = 0.95). Fig. 7a demonstrates the profile for the SEI layer formation of the cell during two cycles of the five-layer stacked 5 × 6 cm2 full cell. Fig. 7b shows the superior cycle performance of the five-layer stacked 5 × 6 cm2 full cell at 0.1C, and the discharge capacity was maintained at 79.5% after the 500th cycle. The 5 × 6 cm2 pouch cells were successfully assembled with good reproducibility, illustrating the scalability and facile process-ability in this study. The safety (crush, cutting, and fully open test) and potential application of the LFP/P-SPE/Graphite 5 × 6 cm2 pouch cells were demonstrated by powering commercial light-emitting diode (LED) lamps and explosive (in the cutting and fully open) of battery. Most strikingly, the pouch cell is still able to keep the LED lamps on even after being laped in cutting and fully open, as shown in Fig. 8. These tests further proved the excellent safety of the developed P-SPE and its potential application in large scale LIBs.
Fig. 7.
(a) Charge/discharge profile, and (b) cycle profile of the five-layer stacked 5 × 6 cm2 size pouch-type full cell.
Fig. 8.
Five-layer stacked 5 × 6 cm2 size pouch-type full cell test. (a) crush, and (b) laped in cutting, and fully open.
4. Conclusion
The ionic conductivity of this P-SPE at room temperature was 2.89 × 10−4 S/cm (303 K) and 2.47 × 10−3 S/cm (343 K). P-SPE applicability expect to the interest building high-performing rechargeable lithium ion batteries owing to the combination of good electrochemical properties and improved safety. High loading level LiFePO4 (6∽7 mg/cm2) lithium ion batteries using P-SPE can deliver a good C-rate response and high capacity (ca. 0.6 mAh/cm2 at 0.1C) with an excellent retention of 79.5% after 500 cycles in fiver layer 5 × 6 cm2 size pouch cell configuration. The scale-up, stability, and applicability of the lithium ion battery using the solid polymer electrolyte developed in this study has been demonstrated, and its application in the industry is expected.
Declarations
Author contribution statement
Injun Jeon: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Won Gi Hong: Conceived and designed the experiments; Analyzed and interpreted the data.
Sol Yoon: Contributed reagents, materials, analysis tools or data.
Yunju Choi: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Hae Jin Kim: Conceived and designed the experiments; Wrote the paper.
Jong-Pil Kim: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
Jong Pil Kim was supported by Korea Basic Science Institute [Grant No. C123000].
Data availability statement
The data that has been used is confidential.
Declaration of interest’s statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e13292.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
FigS1.
FigS2.
FigS3.
FigS4.
FigS5.
References
- 1.Xu K. Non aqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004;104:4303–4418. doi: 10.1021/cr030203g. [DOI] [PubMed] [Google Scholar]
- 2.Meyer W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998;10:439–448. doi: 10.1002/(SICI)1521-4095(199804)10:6<439::AID-ADMA439>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen Tien Manh, Jungdon-Suk, Kang Yongku. Semi-interpenetrating solid polymer electrolyte for LiCoL2-based lithium polymer batteries operated at room temperature. J. Electrochem. Sci. Technol. 2019;10(2):250–255. [Google Scholar]
- 4.Dong Zhou, Shanmukaraj Devaraj, Tkacheva Anastasia, Armand Michel, Wang Guoxiu. Polymer electrolytes for lithium-based batteries. Advances and Prospects, Chem. 2019;5:2326–2352. [Google Scholar]
- 5.He D., Cho S.Y., Kim D.W., Lee C., Kang Y. Enhanced ionic conductivity of semi-IPN solid polymer electrolytes based on star-shaped oligo (ethyleneoxy) cyclotriphosphazenes. Macromolecules. 2012;45(19):7931–7938. [Google Scholar]
- 6.Wang L., Li X., Yang W. Enhancement of electrochemical properties of hot-pressed poly(ethylene oxide)-based nanocomposite polymer electrolyte films for all-solid-state lithium polymer batteries. Electrochem. Acta. 2010;55(6):1895–1899. [Google Scholar]
- 7.Quartarone E., Mustarelli P., Magistris A. PEO-based composite polymer electrolytes. Solid State Ionics. 1998;110:1–14. [Google Scholar]
- 8.Anderman M. Lithium-polymer batteries for electrical behicles: a realistic view. Solid State Ionics. 1994;69:336–342. [Google Scholar]
- 9.Grajales S.T., Dong X., Zheng Y., Baker G.L., Bruening M.L. Effects of monomer composition on CO2-selective polymer brush membranes. Chem. Mater. 2010;22:4026–4033. [Google Scholar]
- 10.Nei de Freitas J., Nogueira A.F., De Paoli M.-A. New insights into dye-sensitized solar cells with polymer electrolytes. J. Mater. Chem. 2009;19:5279. [Google Scholar]
- 11.Nogueira A.F., Longo C., De Paoli Coord M.A. Polymers in dye-sinsitized solar cells: overview and perspectives. Chem. Rev. 2004;248:1455–1468. [Google Scholar]
- 12.Carbone L., Gobet M., Peng J., Devany M., Scrosati B., Greenbaum S., Hassoun J. Polyethylene glycol dimethyl ether (PEGDME)-based electrolyte for lithium metal battery. J. Power Sources. 2015;299(2):460–464. [Google Scholar]
- 13.Bernhard R., Latini A., Panero S., Scrosati B., Hassoun J. Poly (ethylenglycol) dimethylether-lithium bis (trifluoromethanesulfonyl) imide, PEG500DME-LiTFSI, as high viscosity electrolyte for lithium ion batteries. J. Power Sources. 2013;226:329–333. [Google Scholar]
- 14.Di Lecce D., Carbone L., Gancitano V., Hassoun J. Rechargeable lithium battery using non-flammable electrolyte based on tetraethylene glycol dimethyl ether and olivine cathodes. J. Power Sources. 2016;334:146–153. [Google Scholar]
- 15.Kim Hyunjin, Kim Do Youb, Suk Jungdon, Kang Yongku, Lee Jin Bae, Kim Hae Jin, Kim Dong Wook. Stable cycling via absolute intercalation in graphite-based lithium-ion battery incorporated by solidified ether-based polymer electrolyte. Mater. Adv. 2021;2:3898–3905. [Google Scholar]
- 16.Chawla N., Bharti N., Singh S. Recent advances in non-flammable electrolytes for safer. Batteries. 2019;5(1):19. [Google Scholar]
- 17.Julen Castillo, Santiago Alexander, Judez Xabier, Garbayo Inigo, Jose Antonio, Clemente Coca, Carmen Maria, Aitor Morant-Minana, Jose Villaverde, Gonzalez-Marcos Antonio, Zhang Heng, Armand Michel, Li Chunmei. Safe, flexible, and high-performing gel-polymer electrolyte for rechargeable lithium metal batteries. Chem. Mater. 2021;33:8812–8821. [Google Scholar]
- 18.Boulineau S., Courty M., Tarascon J.M., Viallet V. Mechanochemical symthesis of Li argyrodite Li6PS5X(X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ionics. 2012;221:1–5. [Google Scholar]
- 19.Kang Yongku, Seo Yeon-Ho, Lee Changjin. Synethesis and conductivity of PEGME branched poly (ethylene-alt-maleimide) based solid polymer electrolyte. Bull. Kor. Chem. Soc. 2000;21(2):241–244. [Google Scholar]
- 20.Reuter Fluoria, Baasner Anne, Jonas Pampel, Piwko Markus, Dorfler Susanne, Althues Holger, Kaskel Stefan. Importance of capacity balancing on the electrochemical performance of Li[Ni0.8Co0.1Mn0.1]O2 (NCM811)/silicon full cells. J. Electrochem. Soc. 2019;166(14):A3265–A3271. [Google Scholar]
- 21.Chen Zhan, Zhang Lan, Wu Xiangkun, song kaifang, Ren Baozeng, Li Tao, Zhang Suojiang. Effect of N/P ratios on the performance of LiNi0.8Co0.15Al0.05O2//SiOx/Graphite lithium-ion batteries. J. Power Sources. 2019;439:227056–227062. [Google Scholar]
- 22.Shim Joongpyo, Striebed Kathryl A. Effect of electrode density on cycle performance and irreversible capacity loss for natural graphite anode in lithium-ion batteries. J. Power Sources. 2003;119:934–937. [Google Scholar]
- 23.Zaghib K., Nadeau G., Kinoshita K. Effect of graphite particle size on irreversible capacity loss. J. Electrochem. Soc. 2000;147:2110–2115. [Google Scholar]
- 24.Zaghib K., Brochu F., Guerfi A., Kinoshita K. Effect of particle size on lithium intercalation rates in natural graphite. J. Power Sources. 2001;103:140–146. [Google Scholar]
- 25.Tan Shi, Tu Qingsong, Tian Yaosen, Xiao Yihan, Miara Lincoln J., Kononova Olga, Ceder Gerbrand. High active material loading in all-solid state battery electrode via particle size optimization. Adv. Energy Mater. 2020;10:1902881–1902890. [Google Scholar]
- 26.Yang Xiao-Guang, Ge Shanhai, Liu Tent, Leng Yongjun, Wang Cao-Yang. A look into the voltage plateau signal for detection and quantification of lithium plating in lithium-ion cells. J. Power Sources. 2018;395:251–261. [Google Scholar]
- 27.janakiraman Umamaheswari, Garrick Taylor R., Fortier Mary E. Review-lithium plating detection methods in Li-ion batteries. J. Electrochem. Soc. 2020;167:160552–160574. [Google Scholar]
- 28.Campbell Ian D., Marzook Mohamed, Marinescu Monica, Offer Gregory J. How observable is lithium plating differential voltage analysis to identify and quantify lithium plating following fast charging of cold lithium-ion batteries. J. Electrochem. Soc. 2019;166(4):A725–A739. [Google Scholar]
- 29.Aurbach D., Markovsky B., Weissman I., Levi E. Ein-Eli, on the correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim. Acta. 1999;45:67–86. Y. [Google Scholar]
- 30.Chung G.-C., Jun S.-H., Lee K.-Y., Kim M.-H. Effect of surface on the irreversible capacity of various graphitic carbon electrodes. J. Electrochem. Soc. 1999;146:1664–1671. [Google Scholar]
- 31.Joho F., Rykart B., Blome A., Novak P., Wilhelm H., Spahr M.E. Electrochemical characteristics of the negative electrode in lithium-ion batteries: effect of structure and surface properties of the carbon material. J. Power Sources. 2001;78:97–98. [Google Scholar]
- 32.Xiong D.J., Ellis L.D., Nelson K.J., Hynes Toren, Petibon R., R Dahn J. Rapid impedance growth and gas production at the Li-ion cell positive electrode in the absence of a negative electrode. J. Electrochem. Soc. 2016;163(14):A3069–A3077. [Google Scholar]
- 33.Self J., Aiken C.P., Petibon R., Dahn J.R. Survey of gas expansion in Li-ion NMC pouch cells. J. Electrochem. Soc. 2015;162:A796–A802. [Google Scholar]
- 34.Ellis L.D., Allen J.P., Thompson L.M., Harlow J.E., Stone W.J., Hill I.G., Dahn J.R. Quantifying, understanding and evaluating the effects of gas comsumption in lithium-ion cells. J. Electrochem. Soc. 2017;164(14):A3518–A3528. [Google Scholar]
- 35.Dörfler S., Althues H., Härtel P., Abendroth T., Schumm B., Kaskel S. Challenges and key parameters of lithium-sulfur batteries on pouch cell level. Joule. 2020;4(3):539–554. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.