Abstract
Objective
Osteoporosis (OP) can be considered a chronic complication of type 2 diabetes mellitus (T2DM). Aberrant activation of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome is associated with the pathogenesis of various inflammation-related diseases, e.g., T2DM and OP. Vitamin D affects the inflammatory pathway and inhibits an excessive inflammatory response. The current study investigated the inter-relationship between vitamin D and inflammasome activation in T2DM.
Method
Hepatocellular carcinoma (HepG2) cells and bone marrow stromal cells (BMSCs) were treated with Conditioned Medium of bone marrow mesenchymal stem cells after VitD treatment (CM-VitD), as well as phosphoinositide 3-kinase (PI3K) specific agonist, 740Y-P, or the PI3K specific inhibitor, LY294002, respectively, or both. 40 Eight-week-old female Sprague Dawley rats were selected and established as a DM model. The rats were injected with CM-VitD, as well as the 740Y-P specific agonist, or the LY294002 inhibitor, respectively, or both. A quantitative reverse transcription polymerase chain reaction and western blotting were conducted to evaluate the expression of messenger ribonucleic acid and protein in the RUX2 gene, alkaline phosphatase (ALP), OsteoPontiN (OPN), peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid-binding protein 4 (FABP4), protein kinase B (AKT), PI3K, NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, interleukin (IL)-1 beta (β), IL-18, and tumor necrosis factor alpha (TNF-α) in the BMSCs and liver tissue of rats. Enzyme-linked immunosorbent assay was used to detect the concentration of inflammatory factors in the cell supernatant and serum of rats.
Results
An isolated co-culture of HepG2/insulin-resistance cells and BMSCs promoted the adipogenic transformation of the latter and inhibited the transformation of BMSCs into osteogenesis. The PI3K specific agonist, 740Y-P, significantly increased the expression of PI3K, AKT, NLRP3, ASC and Caspase-1 while the PI3K specific inhibitor, LY294002, does the opposite. Additionally, CM-VitD reduced the expression of NLRP3, ASC, caspase-1, IL-1β, and IL-18 in BMSCs and rat liver via the PI3K/AKT pathway.
Conclusion
Vitamin D can inhibit the inflammatory response induced by T2DM and promote the osteogenesis of BMSCs, which may play a key role in the treatment of type 2 diabetes patients with OP.
Keywords: Vitamin D, Type 2 diabetes, Osteoporosis, NLRP3 inflammasome
1. Introduction
Type 2 diabetes mellitus (T2DM) and osteoporosis (OP) are two chronic diseases that are more likely to present with age; the former has affected more than 451 million individuals worldwide, and its prevalence is rising due to lifestyle changes [1]. Both of these conditions have become very common worldwide. The prevalence of OP in patients with T2DM was found to be approximately 37.8% [2].
Osteoporosis can be a debilitating disease that is characterized by low bone-mineral density and bone structure destruction, thereby seriously affecting the health of the elderly. Additionally, OP-related fractures increase morbidity and mortality [3,4]. The bone fracture risk of patients with T2DM is higher compared to those without T2DM; this is due to hyperglycemia as well as the excretion of and changes in insulin levels [5]. Furthermore, fracture risk (including spine and femoral fractures) will rise in cases where T2DM has been present long-term [6]. It is thus urgent that a satisfactory and effective treatment for T2DM combined with OP be formulated.
In part, T2DM is a chronic inflammatory disease with a persistently low inflammatory response throughout its progression [7]. Inflammatory cells produce a variety of inflammatory factors via autocrine action, thereby inducing insulin resistance (IR) [8]. IR and chronic inflammation are mutually reinforcing processes. Hyperglycemia and insulin resistance can cause liver damage, causing the liver to overexpress and secrete inflammatory cytokines. Pro-inflammatory cytokines can inhibit the bone regeneration capacity [9]. Existing studies posited the liver, fat, and muscle as the classic organs that play a role in IR; however, bone as a target organ that also serves a purpose in this context has to date not received sufficient attention [[10], [11], [12]]. The specific relationship and mechanism between the activation of inflammatory reactions, IR, and bone loss remain unclear. A better understanding in this regard thus holds great significance for the prevention and treatment of T2DM combined with OP.
The nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome is a cytosolic multiprotein complex that comprises an innate immune receptor protein (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and inflammatory protease caspase-1, which reacts to sign stimuli. The assembled NLRP3 inflammasome activate protease caspase-1 to facilitate the release of interleukin (IL)-1 beta (β) and IL-18 [13].However, aberrant activation of the NLRP3 inflammasome is associated with the pathogenesis of various inflammation-related diseases, e.g., T2DM [14]. The blockade of IL-1β activation improves glycemic control in T2DM [15], which suggests that autoinflammatory mechanisms may contribute to pancreatic injury. Interleukin-1β is a key factor in inflammation and subsequent IR [16]; however, its mechanism in the development of IR requires further research and exploration.
Vitamin D is a type of hormone that promotes the formation of new bone; growing research suggests that it also regulates inflammatory reactions [17]. Vitamin D affects the inflammatory pathway and inhibits excessive downstream inflammatory response; it was also reported to ameliorate hepatic inflammation, steatosis, and IR in mice [18]. However, the effects of vitamin D on the inflammatory response of bone marrow mesenchymal stem cells (BMSCs) in T2DM and its mechanism remain unclear.
In non-infectious inflammatory diseases, such as atherosclerosis and T2DM, the NLRP3 inflammasome plays an important pro-inflammatory role [19,20]. The local macrophages in T2DM produce an excessive inflammatory response and release pro-inflammatory factors in an ongoing manner [21]. With decreased NLRP3 expression in cells, the level of IL-1β and IL-18 is also reduced, and the expression of other effector molecules such as IL-6 is also down-regulated [22]. These results suggest that suppression of the NLRP3 inflammasome in BMSCs may improve an excessive inflammatory response in cases of T2DM.
Bone marrow mesenchymal stem cells overexpress NLRP3, IL-1β, IL-6, and other inflammation-related proteins in high glucose states [23]. Accordingly, the present study used hepatocellular carcinoma (HepG2)/IR cells, a BMSC isolated co-culture cell model, and T2DM rats to observe the effects of a vitamin D-induced BMSC- conditioned medium on the NLRP3 inflammasome, insulin sensitivity, and bone mass to clarify the pathogenesis of IR and bone mass loss in the presence of hyperglycemia.
2. Materials and methods
2.1. Cell culture
The HepG2 cells and BMSCs used in this study were purchased from the China Center for Type Culture Collection. The cells were incubated at 37 °C, with 5% carbon dioxide. The medium was changed every two days. This experiment used cells in the logarithmic growth phase.
2.2. Isolation, culture, proliferation, and the differentiation of bone marrow mesenchymal stem cells
First, the authors sacrificed four-week-old Sprague Dawley (SD) rats by anesthesia and sterilized a local skin area. Then, the bilateral femur and tibia of the rats were removed under sterile conditions. Following sterilization of the removed bones, the bone marrow cavity was exposed and repeatedly washed. Bone marrow fluid was collected and placed in a 50 mL sterile centrifuge tube containing Dulbecco’s modified eagle medium (DMEM), and the bone marrow fluid was pumped and dispersed to make cell suspensions. An individual cell suspension was centrifuged for 20 min, a single cell layer was absorbed, rinsed twice with phosphate-buffered saline (PBS), and centrifuged for 10 min. The supernatant was discarded, leaving behind the cell precipitate. The DMEM medium was added to the cell sediment, blown and inoculated into a 25 cm2 cell culture flask, and cultured in a thermostatic cell incubator.
Cell subcultures were created promptly. The third-generation BMSCs were inoculated into six-well plates and differentiated into chondrocytes using a directed differentiation medium (transforming growth factor beta 1, 20 μg/L; dexamethasone, 10 μmol/L; vitamin C, 10 mmol/L). The BMSCs were isolated and purified by monolayer culture.
2.3. Preparation and concentration of conditioned medium of bone marrow mesenchymal stem cells after vitamin D treatment
The BMSCs were isolated and purified by an adherent culture. After culturing the third-generation BMSCs for 24 h, the culture medium was discarded, the cells were washed twice with PBS, and DMEM was supplemented with 1 × 10−8 mmol/L 1,25-dihydroxyvitamin D3(1,25(OH)2D3). After 48 h, the supernatant was collected (the fusion degree reached 70%–80%). The authors then collected the cell supernatant and centrifuged it at 4 °C (1000 r/min) for 5 min to remove cell debris. The supernatant (with cell debris removed) was then placed in a clean dialysis bag (molecular weight, 3000) and immersed in polyethylene glycol (PEG 20000) to undergo concentration. Meanwhile, urea, uric acid, and other metabolic wastes could be removed from the supernatant via dialysis at a concentration ratio of 1:10.
2.4. Animal treatments
Eight-week-old female SD rats were bought from an animal center and maintained on a standard laboratory diet. All animal experiments were approved by the Animal Care and Use Committee of the Affiliated Hospital of Zunyi Medical University (Zunyi, China). Induction of the DM rats proceeded as follows.
The experimental DM rats were induced by a single intraperitoneal injection of 30 mg/kg freshly prepared streptozotocin (STZ) (Sigma-Aldrich, ST. Louis, MO, USA) in a 0.1 mol/L citrate buffer (pH 4.5) [24]; 72 h following the STZ injection, the DM model was verified by estimation of the rats' fasting blood glucose levels from the animals' tail vein using the Accu-Chek® Compact Plus glucose meter system (Roche Diagnostics, Meylan, France) [25]. Animals with a glucose level >13.9 mmol/L were considered to have DM; 24 h after successful modeling, NC group and DM group did not intervene. DM rats in the DM + CM-VitD group were injected with CM-VitD (1 × 109/L, 2 mL) via the tail vein. Rats in the DM + CM-VitD +740Y-P group were injected with the phosphoinositide 3-kinase (PI3K) specific agonist, 740Y-P (1.0 ng/mL/kg), via the tail vein; following on, the rats were injected with CM-VitD (1 × 109/L, 2 mL). Rats in the DM + CM-VitD + LY294002 group were injected with the PI3K specific inhibitor, LY294002 (2.0 ng/mL/kg), via the tail vein and were subsequently injected with CM-VitD (1 × 109/L, 2 mL).
2.5. Experiment with cells
Insulin resistance of HepG2 cells was induced by insulin (0, 0.1, 0.5, 1.0, and 2.0 μmol/L) and 30 mmol/L glucose to establish the HepG2/IR model.
HepG2/IR cells and BMSCs were isolated and co-cultured, and 1 × 105 HepG2/IR cells were added to the upper chamber and 1 × 105 BMSCs to the lower chamber of Transwell. The volume of upper and lower chamber was increased to 300 μL by adding serum-free medium. The isolate co-culture cells were treated with CM-VitD (0 mmol/L, 10−9 mmol/L, 10−8 mmol/L, and 10−7 mmol/L) for different duration (0,3,6,9,12 h).
HepG2/IR + BMSCs group was not treated. HepG2/IR + BMSCs + CM-VitD group added CM-VitD (10−8 mmol/L, 1000 μL); HepG2/IR + BMSCs + CM-VitD+740Y-P group added PI3K specific agonist 740Y-P (20 μmol/L, 200 μL) and CM-VitD (10−8 mmol/L, 1000 μL); HepG2/IR + BMSCs + CM-VitD + LY294002 group added PI3K specific inhibitor LY294002 (10 μmol/L, 200 μL) and CM-VitD (10−8 mmol/L, 1000 μL).
2.6. Quantitative real-time reverse transcription-polymerase chain reaction
The total ribonucleic acid (RNA) in BMSCs and liver tissue was isolated using a TRIzol™ reagent kit (Invitrogen, Beijing, China) according to the manufacturer’s instructions, with minor alterations. The total RNA concentration was measured using a GeneQuant™ ProRNA/DNA Calculator (Amersham Pharmacia Biotec, UK). The PrimeScript RT Reagent Kit (TakaRa, Dalian, China) was used to reverse transcribe mRNA in BMSCs and liver tissue into complementary deoxyribonucleic acid (cDNA). The 2 SYBR Premix Ex Taq™ II (TakaRa, Dalian, China) reagent was employed to assemble the quantitative reverse transcription polymerase chain reaction (qRT-PCR) system, which was carried out in the Bio-Rad CFX-96 (Bio-Rad, CA, USA) system.
The PCR amplification was performed using 20 μl of reaction system (2 μl of cDNA, 10 μl of the qPCR mix, 1 μl forwarding (FW) primer, 1 μl of reverse (RV) primer, and 6 μl of double-distilled water) at 95 °C for 2 min, 94 °C for 20 s, 60 °C for 20 s, and 72 °C for 30 s (a total of 40 cycles). The PCR primer sequences (FW and RV), and GenBank codes for gene transcripts, were used to assess the mRNA abundance of RUX2, alkaline phosphatase (ALP), OsteoPontiN (OPN), peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid-binding protein 4 (FABP4), protein kinase B (AKT), PI3K, NLRP3, ASC, caspase-1, IL-1β, IL-18, and tumor necrosis factor alpha (TNF-α) in the BMSCs and liver tissue of rats. Amplicon sizes were expressed as the number of base pairs (bp) (Table 1).
Table 1.
Primer sequences of target genes.
| Target gene | Primer sequence (5′-3′) |
|---|---|
| Actin | FW:ACATGCCGCCTGGAGAAA |
| RV:GCCCAGGATGCCCTTTAG | |
| RUX2 | FW:GTGCTCTTGAGATCTCTGG |
| RV:CATCGATCTTCAGAAGTCTC | |
| ALP | FW:ACACCTTGACTGTGGTTACTGCTGA |
| RV:CCTTGTAGCCAGGCCCGTTA | |
| OPN | FW:GAAACTGATGACAACAAACA |
| RV:TGGCGTGAGTTCTTTGGAAA | |
| PPARγ | FW:GTTTGGACCCGCCAGAGGTGA |
| RV:CTTGGTGGGGCTCAGGAGGG | |
| FABP4 | FW:AAACTGGTGGTGGAATGCGT |
| RV:CGCTGGTACTCTCCTCGACT | |
| AKT | FW:GGAATTCATGAGCGACGTGGCTATTGTGAAGG |
| RV:GCTCTAGAGGCCGTGCCGCTGGCCGAGTAGGAG | |
| PI3K | FW:CGAGGTTTTGCTGTTCGGTG |
| RV:CAGGCCAAACCTCTGGCTAA | |
| NLRP3 | FW:TGTTCACTGTTCCTAATC |
| RV:CTGAAACACTGGCTTAAA | |
| ASC | FW:ACAGAAGTGGACGGAGTGCT |
| RV:TCATCTTGTCTTGGCTGGTG | |
| Caspase-1 | FW:TGCCCAGAGCACAAGACTTC |
| RV:TCCTTGTTTCTCTCCACGGC | |
| IL-1β | FW:AGGCACAAGGCACAACAGGCTG |
| RV:GTCCTGGAAGGAGCACTTCATCTGT | |
| IL-18 | FW:AGGAATAAAGATGGCTGCTGAAC |
| RV:GCTCACCACAACCTCTACCTCC | |
| TNF-α | FW:TCTTCTCATTCCTGCTCGTGG |
| RV:TGAGATCCATGCCATTGGC |
2.7. Western blotting
Proteins were isolated from HepG2 cells using a radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions. The tissue stored in the ultra-low temperature refrigerator was added to the (strong) lysis buffer proportionally, incubated at −20 °C for 20 min, ground, and centrifuged at 2000×g for 10 min at 4 °C. The supernatant was collected and the protein concentration was measured using the standard curves.
Next, western blotting was performed. Protein (50 μg) was added to the buffer to prepare the protein specimens, subjected to a water bath for 8 min, and centrifuged at 1000×g for 5 min at 4 °C. The protein was then loaded for electrophoresis, transferred onto a membrane, sealed, incubated with the primary antibody overnight, and again incubated with the secondary antibody for 2 h. Finally, the protein band was scanned and quantified using a protein scanner (Bio-Rad Laboratories).
2.8. Statistical analysis
All of the scientific experiments were repeated at least three times. Homogeneity of variance and normality test were performed before data analysis. Measurement data conforming to normal distribution were represented by mean ± standard deviation, and data with non-normal distribution were analyzed after logarithmic conversion. Comparison between the two groups was performed by t-test. All statistical analyses were conducted using the SPSS Statistics (v.23) (IBM) software program. The images created for this paper were devised using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, United States); P < 0.05 was considered statistically significant.
3. Results
3.1. Establishing the hepatocellular carcinoma/insulin resistance model
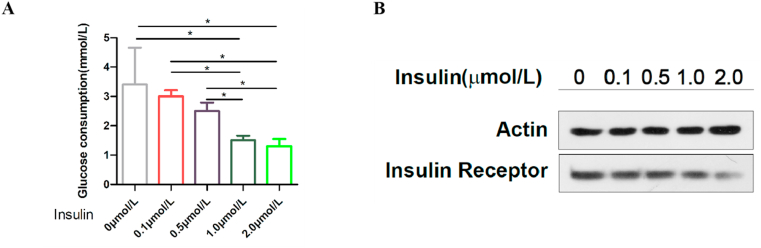
Insulin resistance in HepG2 cells primarily caused a decrease in glucose uptake, which served as a major contributor to hyperglycemia and insulin intervention. The treatment of HepG2 cells with high levels of glucose and a variety of insulin concentrations triggered a significant reduction in insulin receptors [26]. To establish an in vitro IR model of HepG2 cells and evaluate the effects of insulin concentration on glucose metabolism and insulin receptors in the cell model, HepG2 cells were incubated with 30 mmol/L glucose and different concentrations (0, 0.1, 0.5, 1.0, and 2.0 μmol/L) of insulin for 24 h. Glucose consumption and the insulin receptor expression of HepG2 cells were detected to determine whether the HepG2/IR model had been established successfully.
The results showed that the consumption of glucose in the medium-term decreased with an increase in insulin concentration. Glucose consumption in the insulin intervention group was 5.88% (the 0.1 μmol/L group), 17.65% (the 0.5 μmol/L group), 26.47% (the 1.0 μmol/L group), and 27.94% (the 2.0 μmol/L group), respectively. Glucose consumption under insulin stimulation was significantly decreased from the 1.0 μmol/L concentration of insulin, and there was no significant difference between the 1.0 and 2.0 μmol/L groups (Fig. 1(A)). Compared with the control group, protein expression of the insulin receptor in HepG2 cells in the insulin intervention group decreased significantly with an increase in the co-cultured insulin concentration (Fig. 1(B)).
Fig. 1.
The hepatocellular carcinoma (HepG2)/insulin resistance model. (A) The glucose consumption of HepG2 cells was decreased they were treated with different concentrations of insulin (0 μmol/L, 0.1 μmol/L, 0.5 μmol/L, 1.0 μmol/L, and 2.0 μmol/L) for 24 h; *P < 0.05. (B) Protein expression of the insulin receptors was determined by western blotting. The expression of insulin receptors decreased with a gradual increase in the intervention concentration of insulin.
3.2. The co-culture of hepatocellular carcinoma/insulin resistance cells and bone marrow stromal cells promoted the adipogenic transformation of the latter and inhibited their osteogenic transformation
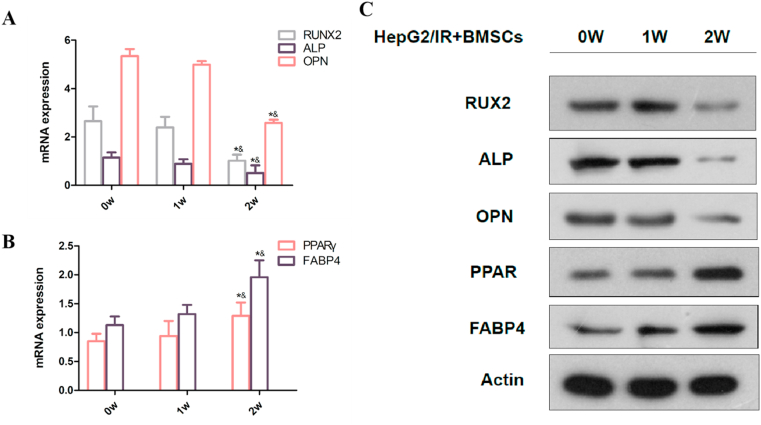
Based on measurements using qRT-PCR and western blotting, the expression of genes related to adipogenic and osteogenic transformation gradually increased and decreased, respectively, during the co-culturing of HepG2/IR and BMSCs. To clarify the effect of HepG2/IR on osteoblastic differentiation, BMSCs were gathered at the beginning of the experiment, during the first and second weeks, respectively. The RUX2 gene, ALP, and OPN were used as osteoblast differentiation markers; these were significantly decreased after two weeks of co-culturing HepG2/IR and BMSCs. Peroxisome proliferator-activated receptor gamma and the FABP4 gene were used as adipogenic differentiation markers; these were significantly increased after the co-culture of HepG2/IR and BMSCs for two weeks [Fig. 2(A–C)]. These results indicated that the co-culture of HepG2/IR cells and BMSCs promoted the transformation of the latter into an adipogenic state and inhibited their transformation into an osteogenic state.
Fig. 2.
Co-culture of hepatocellular carcinoma/insulin-resistance (HepG2/IR) cells and bone marrow stromal cells (BMSCs) promoted the transformation of BMSCs into an adipogenic state and inhibited their transformation to an osteogenic state. (A, B) The messenger ribonucleic (mRNA) expression of the RUX2 gene, alkaline phosphatase (ALP), and OsteoPontiN (OPN) was significantly decreased; the mRNA expression of peroxisome proliferator-activated receptor gamma (PPARγ) and fatty acid-binding protein 4 (FABP4) was significantly increased after two weeks' co-culture of HepG2/IR and BMSCs; *P < 0.05 vs. 0-weeks group; &P < 0.05 vs. 1-week group. (B) Protein expression of the RUX2 gene, ALP, and OPN was significantly decreased; the protein expression of PPARγ and FABP4 was significantly increased after two weeks' co-culture of HepG2/IR and BMSCs.
3.3. The optimal treatment concentration of conditioned medium of bone marrow mesenchymal stem cells after VitD treatment intervention for 9 h was 10−8 mmol/L
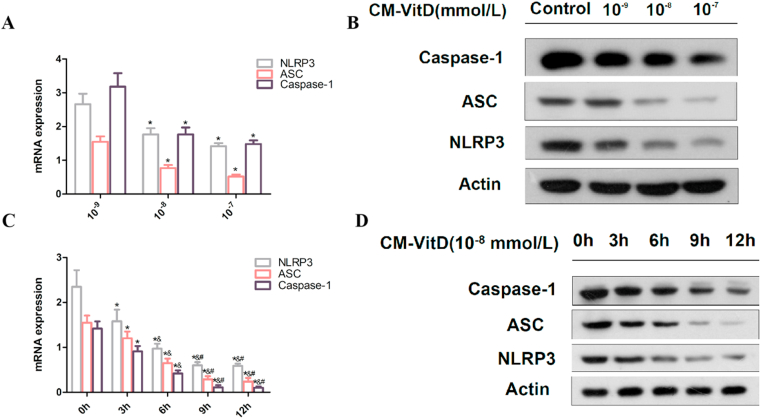
The mRNA and protein expression of NLRP3, ASC, and caspase-1 in BMSCs decreased with an increased concentration of CM-VitD (0 mmol/L, 10−9 mmol/L, 10−8 mmol/L, and 10−7 mmol/L). When the concentration of CM-VitD was 10−8 mmol/L, no statistical difference in mRNA and protein expression was observed compared with the 10−7 mmol/L group. The mRNA and protein expression of NLRP3, ASC, and caspase-1 in BMSCs decreased with an increased CM-VitD intervention time, indicating a time-dependent trend. When the CM-VitD intervention time was 9 h, there was no statistical significance compared with the 12 h group [Fig. 3(A–D)]. This suggested that the optimal intervention concentration of CM-VitD was 10−8 mmol/L, and the optimal intervention time was 9 h; this was followed in follow-on experiments.
Fig. 3.
The optimal treatment concentration and time of Conditioned Medium of bone marrow mesenchymal stem cells after VitD treatment (CM-VitD) are 10−8 mmol/L and 9 h, respectively. (A, B) The messenger ribonucleic acid (mRNA) and the protein expression of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 in bone marrow stromal cells (BMSCs) was decreased with the increased intervention concentration of CM-VitD; *P < 0.05 vs. the 10–9 mmol/L group; &P < 0.05 vs. the 10−8 mmol/L group. (C, D) When 10–8 mmol/L CM-VitD was used to interfere with the BMSCs and hepatocellular carcinoma/insulin-resistance co-culture system, the mRNA and protein expression of NLRP3, ASC, and caspase-1 in BMSCs decreased with an increase in CM-VitD intervention time; *P < 0.05 vs. the 0-h group; &P < 0.05 vs. the 3-h group; #P < 0.05 vs. the 6-h group. @P < 0.05 vs. the 9-h group.
3.4. Overexpression and phosphoinositide 3-kinase knock-down increased and reduced the expression of phosphoinositide 3-kinase, protein kinase B, nucleotide-binding oligomerization domain-like receptor protein 3, apoptosis-associated speck-like protein, and caspase-1
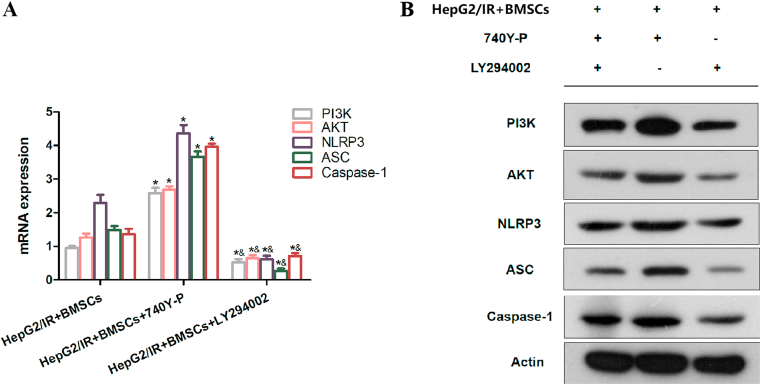
To verify the effects of the PI3K specific agonist and inhibitor, respectively, the authors detected the expression of PI3K, AKT, NLRP3, ASC, and caspase-1 after the co-culture of HepG2/IR and BMSCs. The expression of PI3K, AKT, NLRP3, ASC, and caspase-1 in BMSCs was significantly increased after HepG2/IR and BMSCs were co-cultured with 740Y-P (20 μmol/L, 200 μL). After treatment with LY294002 (10 μmol/L, 200 μL), the expression of PI3K, AKT, NLRP3, ASC, and caspase-1 in BMSCs was significantly decreased, which confirmed the effects of the PI3K activator/inhibitor, as well as the upstream and downstream relationships between PI3K and AKT, NLRP3, ASC, and caspase-1 [Fig. 4(A and B)].
Fig. 4.
The effects of the 740Y-P specific agonist and the LY294002 specific inhibitor. (A, B) The messenger ribonucleic acid (mRNA) and protein expression of phosphoinositide 3-kinase, protein kinase B, nucleotide-binding oligomerization domain-like receptor protein 3, apoptosis-associated speck-like protein containing a caspase recruitment domain, and caspase-1 in bone marrow stromal cells (BMSCs) was increased and decreased when intervened with the 740Y-P specific agonist (20 μmol/L, 200 μL) and the LY294002 specific inhibitor (10 μmol/L, 200 μL), respectively; *P < 0.05 vs. the HepG2/IR + BMSCs group; &P < 0.05 vs. the HepG2/IR + BMSCs +740Y-P group.
3.5. Conditioned medium of bone marrow mesenchymal stem cells after VitD treatment targeted the phosphoinositide 3-kinase/protein kinase B pathway to regulate nucleotide-binding oligomerization domain-like receptor protein 3 activation
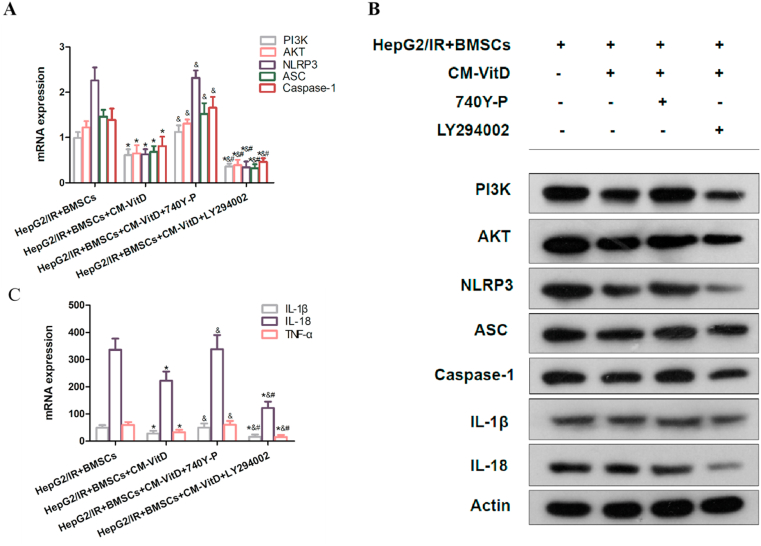
The PI3K/AKT signaling pathway is the backbone of multiple cellular signaling pathways and has particular cross-linking with the mitogen-activated protein kinase signaling pathway [27]. When compared with the HepG2/IR + BMSCs group, the expression of PI3K, AKT, NLRP3, ASC, and caspase-1 in the CM-VitD intervention group was significantly decreased. When 740Y-P was added and co-cultured, the inhibition of PI3K, AKT, NLRP3, ASC, and caspase-1 attributes by CM-VitD was reversed. When LY294002 was added and co-cultured, the mRNA and protein expression of PI3K, AKT, NLRP3, ASC, and caspase-1 was significantly decreased. The levels of inflammatory factors in the CM-VitD intervention group were significantly reduced when compared with the HepG2/IR + BMSCs group. When the 740Y-P was added to the co-culture system, the level of inflammatory factors was increased. When LY294002 was added to the co-culture system, the level of inflammatory factors was further reduced [Fig. 5(A–C)]. This suggested that CM-VitD could regulate the expression and activation of NLRP3 and inflammatory factors via the PI3K/AKT pathway.
Fig. 5.
Conditioned Medium of bone marrow mesenchymal stem cells after VitD treatment reduced the messenger ribonucleic acid (mRNA) and protein expression of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, interleukin (IL)-1 beta (β) and IL-18 through the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathway. (A, B) Compared with the hepatocellular carcinoma/insulin-resistance (HepG2/IR) + bone marrow stromal cells (BMSCs) group, the expression of PI3K, AKT, NLRP3, ASC, caspase-1, IL-1β, IL-18, and tumor necrosis factor alpha in the HepG2/IR + BMSCs + CM-VitD group was significantly decreased. The addition of the 740Y-P specific agonist reversed the inhibition impact of CM-VitD on the PI3K/AKT pathway, and the mRNA and protein expression of these genes was increased. The addition of the LY294002 specific inhibitor significantly decreased the mRNA and protein expression of these genes, compared with the group with the 740Y-P. (C) Compared with the HepG2/IR + BMSCs group, the level of inflammatory factors in the HepG2/IR + BMSCs + CM-VitD group was significantly decreased. Following the 740Y-P intervention, the level of inflammatory factors was increased due to activation of the PI3K/AKT pathway. The addition of LY294002 significantly reduced the level of inflammatory factors; *P < 0.05 vs. the HepG2/IR + BMSCs group; &P < 0.05 vs. the HepG2/IR + BMSCs + CM-VitD group; #P < 0.05 vs. the HepG2/IR + BMSCs + CM-VitD +740Y-P group.
3.6. Conditioned medium of bone marrow mesenchymal stem cells after VitD treatment reduced the expression of nucleotide-binding oligomerization domain-like receptor protein 3, apoptosis-associated speck-like protein, caspase-1, interleukin-1 beta, and interleukin-18 in rat liver
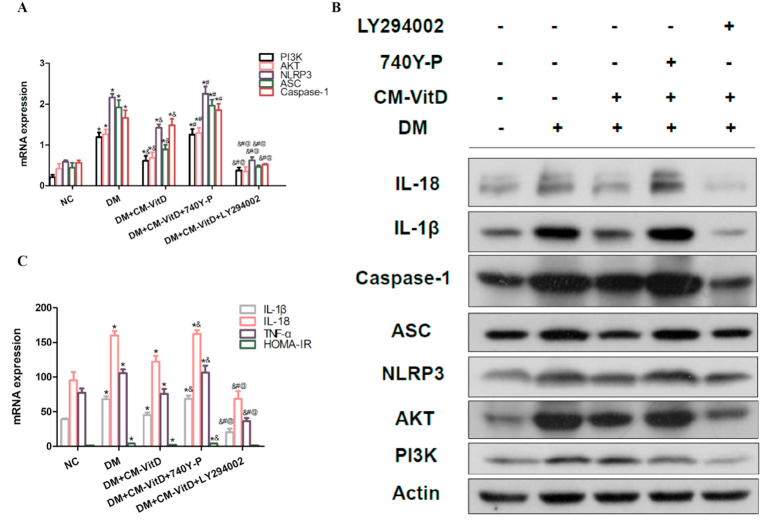
Compared with the negative control (NC) group, the expressions of PI3K, AKT, NLRP3, ASC, and caspase-1 in the DM group were significantly increased; concurrently, Homeostasis model assessment-insulin resistance (HOMA-IR) and the expression of IL-1β, IL-18, and TNF-α were also significantly increased, indicating that IR in DM activated the PI3K/AKT pathway and led to the increased expression of NLRP3, ASC, caspase-1, and inflammatory cytokines downstream. Compared with the DM model group, the expression of PI3K, AKT, NLRP3, ASC, and caspase-1 in the DM + CM-VitD group was significantly decreased, indicating that CM-VitD improved the inflammatory status caused by IR in the DM group. Following the addition of LY294002, the expression of PI3K, AKT, NLRP3, ASC, caspase-1, and inflammatory factors was further decreased [Fig. 6(A–C)]. These results confirmed that CM-VitD reduced IR and inhibited the occurrence of an inflammatory reaction via the PI3K/AKT pathway.
Fig. 6.
(A, B) Compared with the negative control (NC) group, the expression of phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 in the diabetes mellitus (DM) group was significantly increased. Homeostasis model assessment-insulin resistance and the expression of interleukin (IL)-1 beta (β), IL-18, and tumor necrosis factor alpha were also significantly increased. When CM-VitD intervention was added, the expression of PI3K, AKT, NLRP3, ASC, and caspase-1 in rat livers was significantly decreased. However, when the 740Y-P specific agonist was added, the expression of PI3K, AKT, NLRP3, ASC, and caspase-1 was significantly increased. When the LY294002 specific inhibitor was added, the expression of PI3K, AKT, NLRP3, ASC, and caspase-1 was significantly decreased. (C) Compared with the NC group, the levels of inflammatory factors in the rat serum of the DM group were significantly increased. Following CM-VitD injection, the inflammatory factors were decreased. Furthermore, when 740Y-P was added to the DM + CM-VitD group, the level of inflammatory cytokines in the serum was significantly increased. When LY294002 was added to the DM + CM-VitD system, the level of inflammatory cytokines in the serum was significantly decreased; *P < 0.05 vs. the NC group; &P < 0.05 vs. the DM group; #P < 0.05 vs. the DM + CM-VitD group; &P < 0.05 vs. the DM + CM-VitD +740Y-P group; @P < 0.05 vs. the DM + CM-VitD + LY294002 group.
4. Discussion
Type 2 DM and OP are two major healthcare problems worldwide. The former is also considered a risk factor for OP. The high fracture risk among T2DM patients can be induced by hyperglycemia, which plays a vital role in impaired bone metabolism in T2DM patients, leading to reduced bone strength [28]. The growing prevalence among patients with T2DM simultaneously suffering from OP underscores the importance of discussing the pathogenetic mechanisms of both diseases, as well as the need for investigating correlative agents with which to treat them [29].
Vitamin D deficiency is a widespread condition that affects more than a third of the population worldwide. Many individuals have difficulty meeting their daily vitamin D requirements through food intake and being outside in the sun. It is thus important to highlight the need for vitamin D supplementation [30]. Several studies have reported a link between vitamin D deficiency and an increased risk of acquiring several types of inflammatory diseases. In lung inflammation, vitamin D pretreatment decreased TNF-α-induced inflammation via the reduction of mitochondrial fission and mitophagy in A549 cells [31]. Vitamin D supplementation in obese women with mild to moderate depressive symptoms also indicated beneficial impacts on mood and inflammation [32].
The role of vitamin D in bone health and the prevention of OP has been well-documented [33]. Type 2 diabetes is also considered an inflammatory disease, and IR may be affected by regulating inflammation [34]. Research reports Vitamin D is effective in reducing insulin resistance in patients with type 2 diabetes [35], and lower vitamin D is strongly associated with worse diabetic regulation in T2DM subjects [36]. However, in the case of diabetes combined with OP, the mechanism through which vitamin D increases bone mineral density and reduces IR is unclear. In recent years, NLRP3 inflammasome has attracted significant attention for its important role in the pathogenesis of type 2 diabetes; however, its specific mechanism in the development of IR and OP requires further research and exploration.
Phosphoinositide 3-kinase regulates the activation of NLRP3 inflammasome through the AKT pathway [[19], [48]]. Accordingly, the present study conducted experiments to observe whether vitamin D could improve IR and OP in DM by inhibiting the PI3K/AKT/NLRP3 pathways in vitro and in vivo.
The skeletal system undergoes constant lifelong remodeling through functional changes in osteocytes, osteoclasts, and osteoblasts [37]. Once the balance of bone metabolism is disrupted, a variety of bone disorders can develop. Bone marrow stromal cells are derived from bone marrow and possess multiplex potential differentiation abilities [38]. Moreover, bone formation plays a unique and important role in the body after birth, which ultimately increases the expression of bone matrix proteins, primarily by activating important bone-derived transcription factors, e.g., RUX2 [39].
Mesenchymal stem cells can differentiate into different cell lineages upon stimulation. This ability is closely related to achieving a perfect balance between the pluripotency-related genes (which control stem cell proliferation) and genes that can orchestrate the appearance of a specific phenotype [40]. In this regard, adipogenesis and osteoblastogenesis (adipo–osteoblastogenesis) are closely related processes [41].
A HepG2 cell model of insulin resistance induced by hyperinsulinemia was established based on the model in an existing study [42]. The isolated co-culture of HepG2/IR cells and BMSCs promoted the transformation of the latter into an adipogenic state and inhibited their osteogenic transformation. This transformation may have been related to changes in the inflammatory state of the microenvironment following the co-culture with HepG2/IR cells.
Inflammation is a major contributing factor of diabetes and OP [43]. Numerous studies have shown that the NLRP3 inflammasome is formed by the recruitment of the ASC adaptor and caspase-1, resulting in the release of an abundance of inflammatory factors, including IL-1β and IL-18, thereby inducing inflammatory injury [44]. The PI3K/AKT signaling pathway was reported as a vital upstream factor involved in the activation of the NLRP3 inflammasome [45]. This was confirmed by the detection of NLRP3 inflammation mRNA and protein in BMSCs after the intervention of PI3K agonists and inhibitors in the current research. Existing findings also indicated that vitamin D could promote mesenchymal stem cells' differentiation in vitro [46].
In the present study, the authors successfully isolated BMSCs and identified that CM-VitD reversed the inflammatory state induced by co-cultured HepG2/IR cell models and BMSCs. At the cellular level, as well as after the application of CM-VitD in a diabetic rat model, the inflammatory state of liver tissue and IR were decreased. This trend was more obvious after the intervention of the PI3K agonist; contrastingly, the intervention of the PI3K inhibitor was weakened. Vitamin D has been reported to be inversely and independently associated with IR, based on a vitamin D deficiency in both type 2 diabetes and healthy populations worldwide [47].
Altogether, the findings of the present study suggest the possible application of vitamin D for controlling the osteogenic transformation and inhibiting lipogenic transformation of cells by inactivating NLRP3 and downstream inflammatory cytokines. The ability of vitamin D to control transformation and inflammation may represent a novel target for the regulation of osteogenic cell transformation and the treatment of OP. At present, the expression of related factors in the liver tissue of rats has been studied in animal experiments, but the changes in bone mass and bone mineral density of rats have not been detected. Moreover, the mechanism of hyperglycemia causing inflammation, insulin resistance and bone loss has not been thoroughly studied, and more studies are needed to prove it.
Declaration
Ethics approval and consent to participate
This study was conducted with approval from the Ethics Committee of Affiliated Hospital of Zunyi Medical University (No. (2020)2–003). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Author contribution statement
Min Wu: Performed the experiments; Wrote the paper.
Yu-Lan Cai: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yan Yang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Hao-Ming Hu, Yang Yao, Jia Yang: Performed the experiments.
Jia-Jie Deng, Ling Wan: Analyzed and interpreted the data.
Funding statement
Dr. Yan Yang was supported by National Natural Science Foundation of China [82060159], Natural Science Foundation of Guizhou Province [[2020] 1Y314], Science and Technology Program of Guizhou Province [[2020]Y010], Scientific research project of Guizhou Dendrobium Industry Development Research Center [2019003013], Science and Technology Department Planning Project of Guizhou Province [ZK[2022] General 639], Science and Technology Fund Project of Health Commission of Guizhou Province [gzwkj2022-003], Zunyi City Joint Fund project [Zunyi department combine HZ word [2022] No. 332].
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no competing interests.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e13215.
Abbreviations
- AKT/PKB
Protein Kinase B
- ALP
alkaline phosphatase
- ASC
apoptosis-associated speck-like protein containing a CARD
- BMSCs
bone marrow mesenchymal stem cells
- CM-VitD
Conditioned Medium of bone marrow mesenchymal stem cells after VitD treatment
- CO2
carbon dioxide
- DM
diabetes mellitus
- DMEM
Dulbecco’s Modified Eagle Medium
- ELISA
enzyme-linked immuno sorbent assay
- FABP4
Recombinant Fatty Acid Binding Protein 4
- FW
forward
- HepG2
human hepatoma cell 2
- HOMA-IR
Homeostasis model assessment-insulin resistance
- IL-18
interleukin-18
- IL-1β
interleukin-1beta
- IR
insulin resistance
- MAPK
mitogen-activated protein kinase
- NC
Negative control
- NLRP3
nucleotide binding oligomerization domain like receptor protein 3
- OP
osteoporosis
- OPN
OsteoPontiN
- PBS
Phosphate Buffered Saline
- PEG20000
Polyethylene glycol 20000
- PI3K
Phosphatidylinositol 3-kinase
- PPARγ
peroxisome proliferator-activated receptor gamma
- qRT-PCR
Quantitative real-time reverse transcription-polymerase chain reaction
- RIPA
radioimmunoprecipitation assay
- RUX2
Runt-related transcription factor 2
- RV
reverse
- SD
Sprague Dawley
- STZ
streptozotocin
- T2DM
Type 2 diabetes mellitus
- TGF-β1
Transforming growth factor-beta 1
- TNF-α
tumor necrosis factor-alpha
- VitD
Vitamin D
Appendix B. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Cho N.H., Shaw J.E., Karuranga S., et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Si Y., Wang C., Guo Y., Yin H., Ma Y. Prevalence of osteoporosis in patients with type 2 diabetes mellitus in the Chinese mainland: a protocol of systematic review and meta-analysis. Medicine (Baltim.) 2020;99(16) doi: 10.1097/MD.0000000000019762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palacios S. Medical treatment of osteoporosis. Climacteric. 2021:1–7. doi: 10.1080/13697137.2021.1951697. [DOI] [PubMed] [Google Scholar]
- 4.Li S.S., He S.H., Xie P.Y., et al. Recent progresses in the treatment of osteoporosis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.717065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Yang T., Gao X., Xu A. Hybrid deep learning model for risk prediction of fracture in patients with diabetes and osteoporosis. Front. Med. 2022;16(3):496–506. doi: 10.1007/s11684-021-0828-7. [DOI] [PubMed] [Google Scholar]
- 6.Rathmann W., Kostev K. Fracture risk in patients with newly diagnosed type 2 diabetes: a retrospective database analysis in primary care. J. Diabet. Complicat. 2015;29(6):766–770. doi: 10.1016/j.jdiacomp.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Kong M., Xie K., Lv M., et al. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: lessons learned and future promise. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110975. [DOI] [PubMed] [Google Scholar]
- 8.Ren N., Kim E., Li B., et al. Flavonoids alleviating insulin resistance through inhibition of inflammatory signaling. J. Agric. Food Chem. 2019;67(19):5361–5373. doi: 10.1021/acs.jafc.8b05348. [DOI] [PubMed] [Google Scholar]
- 9.Liu H., Li D., Zhang Y., Li M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem. Cell Biol. 2018;149(4):393–404. doi: 10.1007/s00418-018-1643-3. [DOI] [PubMed] [Google Scholar]
- 10.Muzurović E., Mikhailidis D.P., Mantzoros C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism. 2021;119 doi: 10.1016/j.metabol.2021.154770. [DOI] [PubMed] [Google Scholar]
- 11.Caprio S., Perry R., Kursawe R. Adolescent obesity and insulin resistance: roles of ectopic fat accumulation and adipose inflammation. Gastroenterology. 2017;152(7):1638–1646. doi: 10.1053/j.gastro.2016.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert M. Role of skeletal muscle lipids in the pathogenesis of insulin resistance of obesity and type 2 diabetes. J. Diabetes Investig. 2021;12(11):1934–1941. doi: 10.1111/jdi.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding S., Xu S., Ma Y., Liu G., Jang H., Fang J. Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules. 2019;9(12):850. doi: 10.3390/biom9120850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y., Xu W., Zhou R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021;18(9):2114–2127. doi: 10.1038/s41423-021-00740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding S., Xu S., Ma Y., Liu G., Jang H., Fang J. Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules. 2019;9(12):850. doi: 10.3390/biom9120850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105(2):141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Chung C., Silwal P., Kim I., Modlin R.L., Jo E.K. Vitamin D-cathelicidin Axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw. 2020;20(2):e12. doi: 10.4110/in.2020.20.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong B., Zhou Y., Wang W., et al. Vitamin D receptor activation in liver macrophages ameliorates hepatic inflammation, steatosis, and insulin resistance in mice. Hepatology. 2020;71(5):1559–1574. doi: 10.1002/hep.30937. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., Li J., Lin S., Wu Y., He D., Qu P. PI3K regulates the activation of NLRP3 inflammasome in atherosclerosis through part-dependent AKT signaling pathway. Exp. Anim. 2021;70(4):488–497. doi: 10.1538/expanim.21-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gora I.M., Ciechanowska A., Ladyzynski P. NLRP3 inflammasome at the interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells. 2021;10(2):314. doi: 10.3390/cells10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie L., Zhao P., Yue Z., et al. Diabetes induces macrophage dysfunction through cytoplasmic dsDNA/AIM2 associated pyroptosis. J. Leukoc. Biol. 2021;110(3):497–510. doi: 10.1002/JLB.3MA0321-745R. [DOI] [PubMed] [Google Scholar]
- 22.Fouad A.A., Abdel-Aziz A.M., Hamouda A. Diacerein downregulates NLRP3/caspase-1/IL-1β and IL-6/STAT3 pathways of inflammation and apoptosis in a rat model of cadmium testicular toxicity. Biol. Trace Elem. Res. 2020;195(2):499–505. doi: 10.1007/s12011-019-01865-6. [DOI] [PubMed] [Google Scholar]
- 23.Du Q., He D., Zeng H.L., et al. Siwu Paste protects bone marrow hematopoietic function in rats with blood deficiency syndrome by regulating TLR4/NF-κB/NLRP3 signaling pathway. J. Ethnopharmacol. 2020;262 doi: 10.1016/j.jep.2020.113160. [DOI] [PubMed] [Google Scholar]
- 24.Alotaibi M.R., Fatani A.J., Almnaizel A.T., Ahmed M.M., Abuohashish H.M., Al-Rejaie S.S. In vivo assessment of combined effects of glibenclamide and losartan in diabetic rats. Med. Princ. Pract. 2019;28(2):178–185. doi: 10.1159/000496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dogan A., Celik I., Kaya M.S. Antidiabetic properties of lyophilized extract of acorn (Quercus brantii Lindl.) on experimentally STZ-induced diabetic rats. J. Ethnopharmacol. 2015;176:243–251. doi: 10.1016/j.jep.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Zhang J.H., Chen X.Y., et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxidants Redox Signal. 2015;22(10):848–870. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H., Liu J., Zhang Y., Li Q., Wang Q., Gu Z. miR-22 suppresses cell viability and EMT of ovarian cancer cells via NLRP3 and inhibits PI3K/AKT signaling pathway. Clin. Transl. Oncol. 2021;23(2):257–264. doi: 10.1007/s12094-020-02413-8. [DOI] [PubMed] [Google Scholar]
- 28.Napoli N., Chandran M., Pierroz D.D., et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017;13(4):208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 29.Fang P., She Y., Han L., et al. A promising biomarker of elevated galanin level in hypothalamus for osteoporosis risk in type 2 diabetes mellitus. Mech. Ageing Dev. 2021;194 doi: 10.1016/j.mad.2020.111427. [DOI] [PubMed] [Google Scholar]
- 30.Palacios S., Cerdas S., Da Silva R., et al. Vitamin D supplementation: position statement of the iberoamerican society of osteoporosis and mineral metabolism (SIBOMM) Gynecol. Endocrinol. 2021;37(1):10–14. doi: 10.1080/09513590.2020.1858781. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.C., Sung H.C., Chuang T.Y., et al. Vitamin D3 decreases TNF-α-induced inflammation in lung epithelial cells through a reduction in mitochondrial fission and mitophagy. Cell Biol. Toxicol. 2022;38(3):427–450. doi: 10.1007/s10565-021-09629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abiri B., Sarbakhsh P., Vafa M. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutr. Neurosci. 2021;25(10):1–13. doi: 10.1080/1028415X.2021.1945859. [DOI] [PubMed] [Google Scholar]
- 33.Ringe J.D. Plain vitamin D or active vitamin D in the treatment of osteoporosis: where do we stand today. Arch. Osteoporos. 2020;15(1):182. doi: 10.1007/s11657-020-00842-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L., Chen X., Wang H., et al. “djusting internal organs and dredging channel” electroacupuncture ameliorates insulin resistance in type 2 diabetes mellitus by regulating the intestinal Flora and inhibiting inflammation. Diabetes Metab. Syndr. Obes. 2021;14:2595–2607. doi: 10.2147/DMSO.S306861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Liu Y., Zheng Y., Wang P., Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10(3):375. doi: 10.3390/nu10030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erkus E., Aktas G., Kocak M.Z., Duman T.T., Atak B.M., Savli H. Diabetic regulation of subjects with type 2 diabetes mellitus is associated with serum vitamin D levels. Rev. Assoc. Med. Bras.(1992) 2019;65(1):51–55. doi: 10.1590/1806-9282.65.1.51. [DOI] [PubMed] [Google Scholar]
- 37.Kenkre J.S., Bassett J. The bone remodelling cycle. Ann. Clin. Biochem. 2018;55(3):308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Sun Y., Mao W.W., Zhang H., Ni B., Jiang L. Oxidative stress induces downregulation of TP53INP2 and suppresses osteogenic differentiation of BMSCs during osteoporosis through the autophagy degradation pathway. Free Radic. Biol. Med. 2021;166:226–237. doi: 10.1016/j.freeradbiomed.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Xu L., Shen L., Yu X., Li P., Wang Q., Li C. Effects of irisin on osteoblast apoptosis and osteoporosis in postmenopausal osteoporosis rats through upregulating Nrf2 and inhibiting NLRP3 inflammasome. Exp. Ther. Med. 2020;19(2):1084–1090. doi: 10.3892/etm.2019.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balzano F., Garroni G., Cruciani S., et al. Behavioral changes in stem-cell potency by HepG2-exhausted medium. Cells. 2020;9(8):1890. doi: 10.3390/cells9081890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi X., Wu P., Liu J., et al. Candidate kinases for adipogenesis and osteoblastogenesis from human bone marrow mesenchymal stem cells. Mol. Omics. 2021;17(5):790–795. doi: 10.1039/d1mo00160d. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y., Zhang H., Wei Y., et al. Pea-derived peptides, VLP, LLP, VA, and LL, improve insulin resistance in HepG2 cells via activating IRS-1/PI3K/AKT and blocking ROS-mediated p38MAPK signaling. J. Food Biochem. 2020;44(11) doi: 10.1111/jfbc.13454. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L., Wang Q., Su H., Cheng J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J. Biosci. Bioeng. 2021;131(6):671–678. doi: 10.1016/j.jbiosc.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Mulay S.R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019;96(1):58–66. doi: 10.1016/j.kint.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Ives A., Nomura J., Martinon F., et al. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat. Commun. 2015;6:6555. doi: 10.1038/ncomms7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddappa R., Fernandes H., Liu J., van Blitterswijk C., de Boer J. The response of human mesenchymal stem cells to osteogenic signals and its impact on bone tissue engineering. Curr. Stem Cell Res. Ther. 2007;2(3):209–220. doi: 10.2174/157488807781696267. [DOI] [PubMed] [Google Scholar]
- 47.Rafiq S., Jeppesen P.B. Insulin resistance is inversely associated with the status of vitamin D in both diabetic and non-diabetic populations. Nutrients. 2021;13(6):1742. doi: 10.3390/nu13061742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia L., Zhang A., Zheng Q., et al. Quercetin-3-O-β-D-glucuronide inhibits mitochondria pathway-mediated platelet apoptosis via the phosphatidylinositol-3-kinase/ AKT pathway in immunological bone marrow failure. World J. Tradit. Chin. Med. 2022;8(1):115–122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.