Abstract
Human blood plasma prepared by centrifugation contains not only extracellular vesicles (EVs) but also platelets and erythrocyte ghosts (ery‐ghosts). Here we studied whether analysis of miRNA associated with plasma EVs (EV‐miRNA) is affected by the presence of platelets and ery‐ghosts. EDTA blood was collected from healthy donors (n = 3), and plasma was prepared by the centrifugation protocol recommended by the International Society on Thrombosis and Haemostasis (ISTH), and by a centrifugation protocol from an EV‐miRNA expert lab (non‐ISTH protocol). EVs were isolated from plasma by size‐exclusion chromatography CL‐2B (SEC2B), and concentrations of platelets, activated platelets, ery‐ghosts and EVs (150–1000 nm) were measured by calibrated flow cytometry. Two EV‐associated miRNAs (let7a‐5p and miR‐21‐5p), and one platelet‐associated miRNA (miR‐223‐3p), were measured by qRT‐PCR. Measurements were performed with and without filtration using 0.8 μm track‐etched filters to remove platelets and ery‐ghosts from plasma and EV‐enriched SEC fractions. Plasma prepared by both centrifugation protocols contained platelets and ery‐ghosts, which co‐migrated with EVs into the EV‐enriched SEC2B fractions. Filtration removed platelets and ery‐ghosts (>97%; p ≤ 0.05) and did not affect the EV concentrations (p > 0.17). The miRNA concentrations were 2–4‐fold overestimated due to the presence of platelets but not ery‐ghosts. Thus, filtration of human plasma is expected to improve comparability and reproducibility of quantitative EV‐miRNA studies. Therefore, we recommend to measure and report the plasma concentration of platelets for EV‐miRNA studies, and to filter plasma before downstream analyses or storage in biobanks.
Abbreviations
- APC
Allophycocyanin
- DPBS
Dulbecco's Phosphate‐Buffered Saline
- EDTA
Ethylene Diamine Tetra Acetic acid
- Ery‐ghosts
Erythrocyte ghosts
- EVs
Extracellular vesicles
- EV‐miRNA
Extracellular vesicle‐associated miRNA
- F
Fraction
- FITC
Fluorescein isothiocyanate
- ISTH
International Society on Thrombosis and Haemostasis
- Max.
Maximum
- ns
Not significant
- PE
Phycoerythrin
- PS
Phosphatidylserine
- qRT‐PCR
Quantitative real‐time polymerase chain reaction
- RT
Room temperature
- SEC2B
Size‐exclusion chromatography CL-2B
1. INTRODUCTION
Extracellular vesicles (EVs) are released by cells into body fluids. EVs carry molecular cargo including miRNA, which can be used as a readout of physiological and pathological processes (De Miguel Pérez et al., 2020; He et al., 2021; Van Eijndhoven et al., 2016). Let7a‐5p and miR‐21‐5p are miRNAs that were previously reported to be present in human plasma EV preparations (Van Eijndhoven et al., 2016). Measuring miRNA that is associated with EVs (EV‐miRNA) requires EV isolation. One of the most commonly used EV isolation methods is size‐exclusion chromatography with sepharose CL‐2B (SEC2B), which separates particles (including EVs) with a diameter of about 75 nm and larger from smaller particles and soluble components (Böing et al., 2014). Isolation of EVs is hampered by the presence of non‐EV particles, such as platelets, that may also carry miRNA (Krammer et al., 2020; Tabet , 2014; Vickers et al., 2011). For example, miR‐223‐3p is a platelet‐associated miRNA that is used as a marker for the presence of platelets (Zhang et al., 2020).
To prepare plasma as starting material for SEC2B, blood is centrifuged to prepare ‘platelet‐free plasma’. Multiple centrifugation protocols exist, but the efficacy of these protocols to remove cells is often unknown and depends on pre‐analytical variables (Bettin et al., 2022; Rikkert et al., 2021) . Contrary to what the name suggests, ‘platelet‐free plasma’ still contains platelets (Arraud et al., 2014; Bettin et al., 2022; Cheng et al., 2013; Kim et al., 2022; Mitchell et al., 2016; Muth et al., 2018; Sunderland et al., 2017; Willeit et al., 2013) and erythrocyte ghosts (ery‐ghosts) (Arraud et al., 2014). Both platelets and erythrocytes contain miRNA (Cheng et al., 2013; Mitchell et al., 2016; Muth et al., 2018; Sunderland et al., 2017), although it is unknown whether ery‐ghosts also contain miRNA.
In the present study we demonstrate that human plasma prepared by different centrifugation protocols contains platelets and ery‐ghosts, which co‐migrate with plasma EVs during isolation by SEC2B. Platelets and ery‐ghosts can be efficiently removed by filtration, and the presence of platelets leads to overestimation of the EV‐miRNA concentration.
2. MATERIALS AND METHODS
2.1. Blood collection and plasma preparation
Four tubes of Ethylene Diamine Tetra Acetic acid (EDTA) blood were collected from healthy individuals (n = 3, each on a separate day) in accordance with the guidelines of the Medical Ethical Committee of the Amsterdam Medical Centre, University of Amsterdam (W19_271#19.421). Blood was processed to plasma within 15 min after withdrawal by two centrifugation protocols, see Figure 1. The centrifugation protocol of the ISTH is commonly used, and therefore the results obtained with this protocol are shown. Results obtained with the non‐ISTH centrifugation protocol are in essence similar and confirmatory, and are shown in the Supplementary information. We deliberately included two plasma centrifugation protocols to emphasize that the presence of platelets and ery‐ghosts is a common phenomenon.
FIGURE 1.
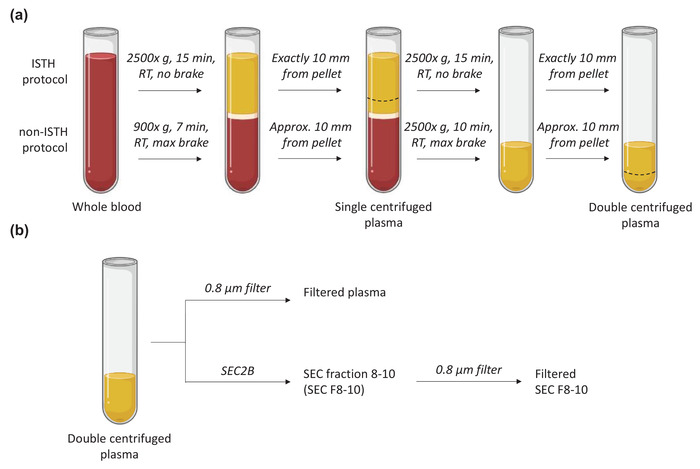
Overview of the sample nomenclature, centrifugation protocols (A) and downstream samples processing (B) in the current study. Approx, approximately; max, maximum; min, minutes; RT, room temperature; SEC2B, size‐exclusion chromatography CL‐2B. This figure was created with BioRender.com
2.2. Removal of platelets and erythrocyte ghosts
For platelet and ery‐ghost removal from plasma and EV‐enriched SEC2B samples, polycarbonate track‐etched membrane filters were used with a pore diameter of 0.8 μm (Bettin et al., 2022). Full details of the methods used in this study can be found in the Supplementary information (Supplementary File 1).
3. RESULTS
3.1. Platelets and ery‐ghosts co‐elute with plasma EVs during SEC2B
Figure 2a shows that plasma prepared according to the ISTH centrifugation protocols contained 3 × 106 platelets/ml and 8 × 105 ery‐ghosts/ml. 1 ml of plasma was used as starting material for SEC2B to isolate EVs. Figure 2b shows that a fraction of both platelets and ery‐ghosts are present in the EV‐enriched fractions (F8‐10) at concentrations of 6 × 105 platelets/ml and 3 × 105 ery‐ghosts/ml. Similar results were found for samples processed according to the non‐ISTH protocol, as shown in Figure S1a,b. Hence, independent of the applied centrifugation protocol, plasma contains platelets and ery‐ghosts that co‐elute with EVs during SEC2B.
FIGURE 2.
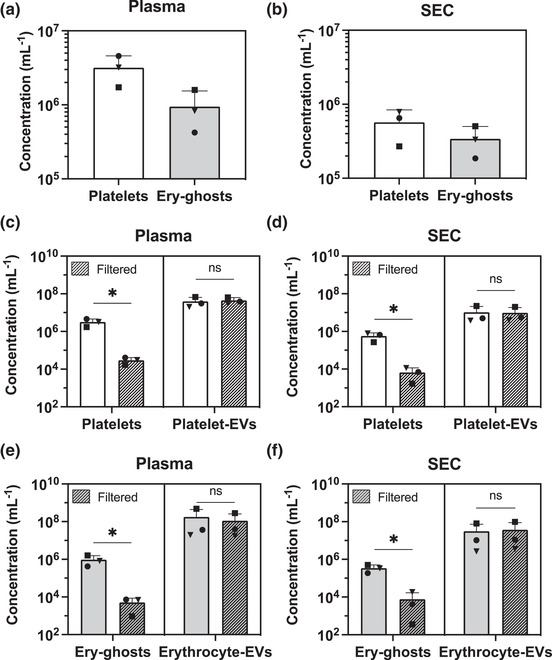
Plasma, prepared using the ISTH centrifugation protocol contains platelets (CD61+, CD41+) and ery‐ghosts (CD235a+) as measured by flow cytometry (A). Platelets and ery‐ghosts are larger than the cut‐off of sepharose CL‐2B, and thus co‐migrate with EVs during SEC2B (B). Filtration (0.8 μm polycarbonate filters) of plasma (C) or the corresponding EV‐enriched SEC2B fractions 8–10 (D) reduces the platelet concentration >97% without affecting the concentration of platelet‐derived EVs (CD61+)**. Filtration also reduces the ery‐ghost concentration >97% (E), whereas the concentration of erythrocyte‐derived EVs (CD235a+)** remains unaffected (F). Experiments were performed using plasma obtained from three healthy controls. A one tailed, paired Student's t‐test was used to test for statistical differences in platelet‐, ery‐ghost‐ and EV concentrations, pre‐ and post‐filtration (panels C–F). A p‐value ≤ 0.05 was considered significant. Ery‐ghost, erythrocyte ghost; EV, extracellular vesicle; ns, not significant; SEC2B, size‐exclusion chromatography CL‐2B; *: p ≤ 0.05; **: EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm). For results of the non‐ISTH centrifugation protocol, see Figure S1
3.2. Filtration removes platelets and ery‐ghosts
Figure 2c,d shows that filtration of plasma and EV‐enriched SEC2B fractions reduced the platelet concentration with >97% (p = 0.03 for both plasma and EV fractions) without affecting the concentration of platelet‐derived EVs (CD61+; p = 0.18 and p = 0.30, respectively). Double staining of platelets using an activation marker (P‐selectin, CD62p) showed that about 1%–2% of the platelets were activated before filtration, and this concentration did not change during and after filtration, as shown in Figure S2. Hence, filtration does not activate platelets but also does not seem to remove activated platelets from plasma. Figure 2e,f shows that filtration also reduced the concentration of ery‐ghosts in plasma and EV‐enriched SEC2B fractions >97% (p = 0.05 and p = 0.04, respectively), whereas the erythrocyte‐derived EV (CD235a+) concentration was unaffected (p = 0.21 and p = 0.19, respectively). The size distributions of EVs (CD235a+/CD45+/CD61+), platelets, and ery‐ghosts before and after filtration of plasma can be found in Figure S3a–c, which confirm that filtration effectively removes (non‐activated) platelets and ery‐ghosts, and has no or minimal effects on the concentration and size distribution of detectable EVs. Figure 1c–f show similar results for the non‐ISTH protocol. Thus, filtration is effective independent of the centrifugation protocol.
3.3. Platelets affect EV‐miRNA quantities
Figure 3a,b shows that filtration of plasma and EV‐enriched SEC2B fractions increased the cycle threshold (Ct) for miRNA detection by 1–2 for let7a‐5p, miR‐21‐5p and miR‐223‐3p. Assuming that the miRNA quantity doubles during each cycle, this increase indicates that 50%–75% of the measured miRNA originates from particles >0.8 μm, including but not limited to platelets and ery‐ghosts, instead of EVs (Goni & Foissac, 2009). Similar results were obtained for the non‐ISTH centrifugation protocol, shown in Figure S4a,b.
FIGURE 3.
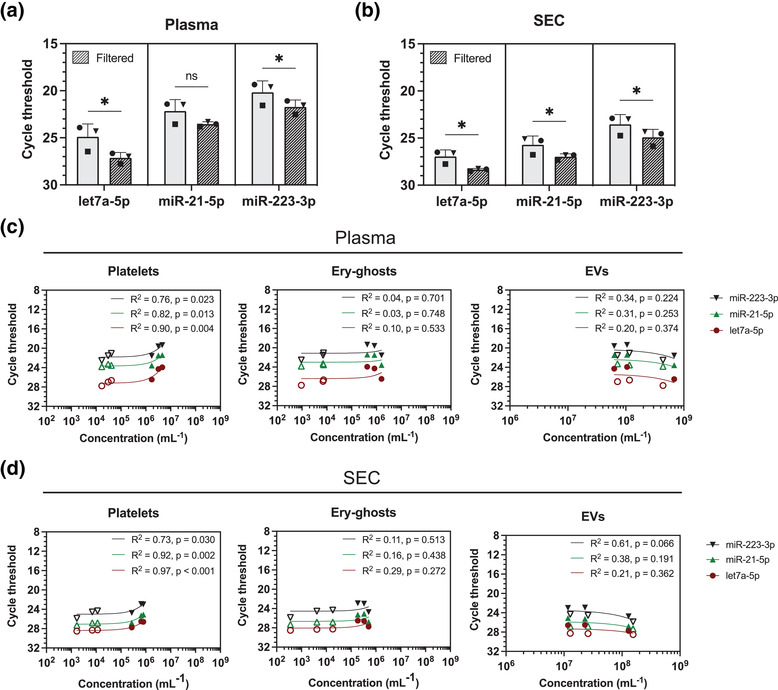
Filtration of plasma, prepared according to the ISTH centrifugation protocol (A) or corresponding EV‐enriched SEC2B fractions (B) leads to a qRT‐PCR cycle threshold increase of 1 to 2, and thus a decrease in miRNA quantity. The quantities of let7a‐5p, miR‐21‐5p and miR‐223‐3p miRNAs detected by qRT‐PCR strongly correlates with the concentration of platelets in plasma (C) especially in unfiltered samples (filled symbols), but not with the concentration of ery‐ghosts or EVs**. For the EV‐enriched SEC2B fractions (D) miRNA quantities also correlate with the platelet concentration, but not with the concentration of ery‐ghosts or EVs**. Experiments were performed using plasma obtained from 3 healthy controls. A one‐tailed, paired Student's t‐test was used to test for statistical differences in miRNA quantities pre‐ and post‐filtration (panels A and B). A linear regression analysis was used to quantify the relationship between the quantity of miRNAs and the concentration of platelets, ery‐ghosts, and EVs (panels C and D). A p‐value ≤ 0.05 was considered significant. Ery‐ghost, erythrocyte ghost; EV, extracellular vesicle; ns, not significant; SEC2B, size‐exclusion chromatography CL‐2B; qRT‐PCR, quantitative real‐time PCR; *, p ≤ 0.05; **, EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–,000 nm). For results of the non‐ISTH protocol see Figure S4).
Next, we performed a regression analysis of the miRNA quantity and the concentration of platelets, ery‐ghosts and EVs in plasma. Figure 3c shows that the quantities of the miRNAs correlate with the platelet concentration in plasma (R 2 = 0.76–0.90, p < 0.05), but not with the concentration of ery‐ghosts (R 2 = 0.03–0.10, p > 0.05) or the total concentration of EVs detectable by our flow cytometer (150–1000 nm; R 2 = 0.20–0.34, p > 0.05). Thus, the quantity of the three analysed miRNAs correlates with the concentration of platelets but not ery‐ghosts or EVs (150–1000 nm) in plasma.
The miRNA quantity correlated also with the platelet concentration in EV‐enriched SEC2B fractions (R 2 = 0.73–0.97, p < 0.05, Figure 3d), and again no correlations were present with the concentration of ery‐ghosts (R 2 = 0.11–0.29, p > 0.05) or the total concentration of detectable EVs (R 2 = 0.21–0.61, p > 0.05). Moreover, neither the concentration of platelet‐derived (CD61+), erythrocyte‐derived (CD235a+) nor leukocyte‐derived (CD45+) EVs correlated with miRNA quantities (R 2 = 0.16–0.66, p > 0.05; Figure S5). Figure S4c,d shows similar results for the non‐ISTH protocol.
4. DISCUSSION
We show that blood plasma prepared by centrifugation contains remaining platelets and ery‐ghosts. The concentration of platelets and ery‐ghosts in plasma depends on the used centrifugation‐protocol, as reflected by higher concentrations of platelets (5.3‐fold) and ery‐ghosts (1.4‐fold) in samples prepared by the non‐ISTH centrifugation protocol compared to the ISTH protocol. Platelets and ery‐ghosts co‐migrate with plasma EVs during SEC2B, and the platelet concentration in both plasma and EV‐enriched SEC2B fractions correlates with the concentrations of let7a‐5p, miR‐21‐5p and miR‐223‐3p. Thus, the presence of platelets affects EV‐miRNA analyses and leads to overestimation of the EV‐associated miRNAs studied. Filtration removes >97% of platelets and ery‐ghosts without affecting the concentration of measured EVs, and the efficacy of filtration is independent from the centrifugation protocol.
Our results confirm that centrifugation does not remove all platelets and ery‐ghosts from human plasma (Arraud et al., 2014; Bettin et al., 2022; Cheng et al., 2013; Kim et al., 2022; Mitchell et al., 2016; Muth et al., 2018; Sunderland et al., 2017; Willeit et al., 2013). Therefore, the term ‘platelet‐free plasma’ is misleading. Contributing to this confusion is the lower limit of detection of haematology analysers. Haematology analysers are used to confirm the absence of platelets in the prepared plasma samples. In the present study the platelet concentrations were determined by flow cytometry, and the platelet concentrations ranged between 106 and 107 platelets/ml. Since the limit of detection of haematology analysers is about 106–107 platelets/ml (Briggs et al., 2007; Slim et al., 2019), part of our plasma samples would have been considered as ‘platelet‐free’. In accordance with previous findings, detectable concentrations of ery‐ghosts were also present in the ‘platelet‐free plasma’ samples (Arraud et al., 2014). In one of our previous studies, we quantified contaminants such as lipoproteins and soluble proteins in SEC2B fractions, but we did not measure the presence of platelets or ery‐ghosts (Böing et al., 2014). In the present study, concentrations of platelets and ery‐ghosts were measured by flow cytometry because this technique can analyse larger plasma volumes than routine haematology analysers (Bettin et al., 2022).
The concentration of platelets and ery‐ghosts in plasma depend on the used centrifugation protocol. Table 1 shows an overview of the plasma preparation protocols that were used during the last 5 years by cancer‐related EV‐miRNA studies (n = 19). We arbitrarily choose cancer‐related EV‐miRNA studies, but our findings likely also hold true for non‐cancer‐related EV‐miRNA studies. From this comparison it becomes clear that plasma preparation protocols are not standardized, and that often no or insufficient details are reported, which hamper comparability and reproducibility of EV‐miRNA results.
TABLE 1.
Overview of plasma preparation protocols in cancer‐related extracellular vesicle‐miRNA studies. ‘–’, Not reported; min, minutes; Ref, reference; RT, room temperature; Temp, temperature; *plasma collection distance from pellet. Search query: ‘plasma’ AND ‘extracellular vesicles’ AND ‘miRNA’ AND (‘PCR’ OR ‘qPCR’ OR ‘qRT‐PCR’) AND ‘cancer’ (accessed 14/03/2022), filtered on last 5 years, humans and English
| Anti‐coagulant | 1st centrifugation | 2nd centrifugation | 3rd centrifugation | Centrifuge acceleration/deceleration | Plasma collection details* | Plasma frozen before platelet removal | Filtration (μm) | Quantified platelets | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Speed (x g) | Time (min) | Temp (°C) | Speed (x g) | Time (min) | Temp (°C) | Speed (x g) | Time (min) | Temp (°C) | ||||||||
| 1 | Heparin | – | – | – | – | – | – | – | – | – | – | – | Yes | – | – | (Lia et al., 2021) |
| 2 | EDTA | 3000 | 15 | 4 | – | – | – | – | – | – | – | – | Yes | 0.8 | – | (Zhang et al., 2022) |
| 3 | EDTA | 2000 | 15 | RT | – | – | – | – | – | – | – | – | Yes | 0.22/0.10 | – | (Xu et al., 2021) |
| 4 | EDTA | 1500 | 15 | – | – | – | – | – | – | – | – | – | Yes | 0.2 | – | (Zabegina et al., 2021) |
| 5 | EDTA | 900 | 7 | – | 2500 | 10 | – | 500 | 10 | – | – | – | Yes | – | – | (Drees et al., 2021) |
| 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | (Panvongsa et al., 2021) |
| 7 | EDTA | 500 | 10 | 4 | 3000 | 30 | 4 | – | – | – | – | – | Yes | 0.22 | – | (Jiang et al., 2021) |
| 8 | EDTA | 4000 | 10 | 4 | – | – | – | – | – | – | – | – | – | – | – | (Chen et al., 2021) |
| 9 | – | – | – | – | – | – | – | – | – | – | – | – | Yes | 0.22 | – | (Chettimada et al., 2020) |
| 10 | EDTA | – | – | – | – | – | – | – | – | – | – | – | Yes | 0.22 | – | (Zabegina et al., 2020) |
| 11 | EDTA/Streck | – | – | – | – | – | – | – | – | – | – | – | Yes | – | – | (Yang et al., 2020) |
| 12 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | (Chen et al., 2020) |
| 13 | EDTA | – | 10 | 4 | – | – | – | – | – | – | – | – | Yes | – | Yes | (Zhang et al., 2020) |
| 14 | EDTA | 1900 | 10 | 4 | 16,000 | 10 | 4 | – | – | – | – | – | Yes | 0.8 | – | (Kloten et al., 2019) |
| 15 | EDTA | 1000 | 10 | 4 | 2000 | 15 | 4 | – | – | – | – | – | Yes | – | – | (Fang et al., 2019) |
| 16 | EDTA | 800–1200 | 10 | 4 | 2500 | 10 | 4 | – | – | – | – | – | Yes | – | – | (Ramayanti et al., 2019) |
| 17 | EDTA | 2000 | 10 | RT | 2000 | 10 | RT | – | – | – | – | – | Yes | – | – | (Endzeliņš et al., 2017) |
| 18 | EDTA | 3000 | 15 | – | 3000 | 15 | – | 15,000 | 40 | 4 | – | – | Yes | 0.22 | – | (Gelibter et al., 2022) |
| 19 | Citrate | 3000 | 15 | – | 3000 | 15 | – | 15,000 | 40 | 4 | – | – | – | 0.22 | – | (Jeppesen et al., 2019) |
Our present results should also raise awareness when looking for validation cohorts in existing biobanks. Similar protocols should be used to compare study results, and preferably the concentration of platelets and ery‐ghosts in plasma samples should be measured and/or they should be removed. In addition, platelets may fragment during freeze‐thaw cycles into particles that are possibly within the size range of EVs, and thus become indistinguishable from real platelet‐derived EVs, which may further hamper true EV‐miRNA analysis (Kim et al., 2022; Mitchell et al., 2016; Sunderland et al., 2017). Therefore, we recommend to filter plasma samples prior to freeze‐thawing to reduce platelet fragmentation.
Recently, we introduced an effective method to remove platelets without affecting detectable concentrations of EVs (Bettin et al., 2022). Filtration, using 0.8 μm polycarbonate track‐etched filters, removes >97% of platelets and ery‐ghosts. The real concentration of platelets and ery‐ghosts in our samples after filtration is probably lower than the reported ∼104 platelets/ml due to background noise and spillover inherent to the used assay. Our results indicate that the concentration of (pre) activated platelets in plasma, which is in accordance with an earlier study (Ahnadi et al., 2003), is unaffected by filtration, meaning that (1) filtration does not cause additional platelet activation, and (2) activated platelets can pass through the filter. Arraud et al. (2014) and Brisson et al. (2017) have described tubular CD41+/phosphatidylserine+ EVs or ‘pseudopods’. These pseudopods are 200–500 nm in diameter and 1–10 μm in length. If orientated correctly, pseudopods will pass through the 0.8 μm filters. Further studies should clarify if the CD62p+ particles that are not removed by filtration are pseudopods, and whether these particles affect downstream miRNA analyses.
Cheng et al. (2013) used 0.22 μm filters to investigate the effect of platelets on miRNA measurements. They used frozen‐thawed samples, however, which may suffer from platelet fragmentation (Bettin et al., 2022; Kim et al., 2022; Mitchell et al., 2016; Muth et al., 2018; Sunderland et al., 2017), and thus it is unclear how effective their methodology is in removal of intact platelets from plasma. In addition, their 0.22 μm polyvinylideenfluoride filters are not produced by a track‐etching technique, meaning that the pore size of the filters is not uniform and particles larger than the cut‐off of the filter may still end up in the filtered samples (Apel, 2001).
The presence of platelets affected the measured concentrations of let7a‐5p, miR‐21‐5p and miR‐223‐3p, indicating that these miRNAs may be partially associated with platelets. These findings confirm results from earlier studies that reported a relationship between the concentration of platelets and quantity of measured miRNA (Cheng et al., 2013; Mitchell et al., 2016; Sunderland et al., 2017). Therefore, in future studies investigating plasma EV‐associated miRNAs, platelets should be removed prior to downstream EV‐miRNA measurements, or filtration experiments should be performed to check if the detection or concentration of the studied miRNAs is affected by platelet removal. The results in our study are meant to raise awareness that remaining platelet and ery‐ghosts may affect downstream assays, and further research is needed to test other filter pore sizes and processing protocols.
In our study no correlation was present between the concentration of EVs and the quantity of measured miRNAs. It is important to note that with the used settings, our flow cytometer can only measure EVs > 150 nm, and the correlation between the analysed miRNAs and EVs < 150 nm was not investigated in this study. Similarly, we measured the most common types of blood cell‐derived EVs that are present in human plasma, but EVs originating from other cell types may be present. In addition, we did not observe any correlation between the concentration of ery‐ghosts and miRNA quantities, suggesting that these miRNAs are not associated with ery‐ghosts. However, further studies should elucidate whether the presence of platelets and ery‐ghosts in human plasma affect the quantity and detection of other miRNAs and other EV profiling analyses, such as proteomics.
In the present study, we focused on the presence and removal of platelets as possible confounder of plasma EV‐miRNA. Plasma also contains (lipo)proteins carrying miRNA (Li et al., 2018). Whereas SEC2B is unable to separate platelets from EVs, SEC2B reduces the concentration of small lipoprotein particles such as HDL by about 100‐fold (Böing et al., 2014). Since the starting concentration of lipoprotein particles in plasma is at least 100,000‐fold higher than the plasma concentration of EVs (Simonsen, 2017), there is still a 1000‐fold higher concentration of small lipoprotein particles compared to EVs after SEC2B. In addition, chylomicrons and (V)LDL particles have similar size ranges to EVs, and it is known that SEC2B does not separate chylomicrons and (V)LDL particles from EVs (Van Deun et al., 2020). This is illustrated in the transmission electron microscopy images shown in Figure S6. These lipoprotein particles will also not be removed by the 0.8 μm filters, and since these lipoproteins may be carriers of miRNAs (Li et al., 2018), we cannot exclude that part of the miRNA signal in our samples is due to lipoprotein contamination. At present, we are testing and comparing multiple SEC resins and combinations thereof for their ability to isolate EVs and concurrently (further) reduce the concentration of lipoproteins compared to SEC2B. In addition, although SEC2B leads to a 300–600 fold enrichment in EVs compared to soluble proteins (Arroyo et al., 2011), we cannot exclude that our samples contain miRNA that is associated with soluble proteins.
5. CONCLUSIONS
Plasma prepared by centrifugation contains platelets and ery‐ghosts, which co‐isolate with EVs. The presence of platelets results in overestimation of the studied EV‐associated miRNAs, and likely also affects other downstream EV analyses. Therefore, we recommend to measure and report the concentration of both platelets and ery‐ghosts, and to remove platelets and ery‐ghosts using 0.8 μm polycarbonate track‐etched filters before freezing plasma samples. Filtration will yield samples with negligible concentrations of platelets, which will improve comparability and reproducibility of plasma EV‐miRNA studies.
AUTHOR CONTRIBUTIONS
Jillian W.P. Bracht: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft; Writing—review & editing. Mandy Los: Data curation; Investigation; Methodology; Writing—original draft; Writing—review & editing. Monique A. J. van Eijndhoven: Data curation; Investigation; Methodology; Writing—review & editing. Britta Bettin: Conceptualization; Methodology; Writing—original draft; Writing—review & editing. Edwin van der Pol: Conceptualization; Data curation; Funding acquisition; Methodology; Writing—original draft; Writing—review & editing. Rienk Nieuwland: Conceptualization; Formal analysis; Funding acquisition; Investigation; Writing—original draft; Writing—review & editing
CONFLICT OF INTEREST
Edwin van der Pol is co‐founder and shareholder of Exometry BV (Amsterdam, the Netherlands), a company focused on standardization of flow cytometry measurements. D. Michiel Pegtel is co‐founder and CSO of Exbiome BV, a company focused on the development of diagnostic tests to detect cancer from blood samples. All other authors declare no conflict of interest.
Supporting information
Supplementary Figure 1. Plasma, prepared using the non‐ISTH centrifugation protocol, still contains platelets (CD61+, CD41+) and ery‐ghosts (CD235a+) as measured by flow cytometry (A). Platelets and ery‐ghosts are larger than the cut‐off of Sepharose CL‐2B, and thus co‐migrate with EVs during SEC2B (B). Filtration (0.8 μm polycarbonate filters) of plasma (C), or corresponding EV‐enriched SEC2B fractions 8–10 (D), reduces the platelet concentration >97%, without affecting the concentration of platelet‐derived EVs (CD61+)*. Filtration also reduces the ery‐ghost concentration >97% (E), while the concentration of erythrocyte‐derived EVs (CD235a+)* remains unaffected (F). Experiments were performed using plasma obtained from three healthy controls. A one‐tailed, paired Student's t‐test was used to test for statistical differences in platelet‐, ery‐ghost and EV concentrations, pre‐ and post‐filtration (panels C–F). A p‐value ≤0.05 was considered significant. Ery‐ghost, erythrocyte ghost; EV, extracellular vesicle; ns, not significant; SEC2B, size‐exclusion chromatography CL‐2B; *, EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 2. Plasma prepared using the ISTH centrifugation protocol contains activated platelets (CD61+, CD62p+) as measured by flow cytometry. The concentration of activated platelets did not change upon filtration of the plasma, indicating that filtration did not cause additional platelet activation, but also did not remove the activated platelets from plasma. Experiments were performed using plasma obtained from two healthy controls. A one‐tailed, paired Student's t‐test was used to test for statistical differences in activated platelet concentrations, pre‐ and post‐filtration. A p‐value ≤ 0.05 was considered significant. F‐plasma, filtered plasma; ns, not significant.
Supplementary Figure 3. The size distribution of EVs* (CD235a+, CD45+ and CD61+) (A) platelets (CD61+, CD41+) (B) and ery‐ghosts (CD235a+) (C) before (left) and after (right) filtration of plasma prepared according to the ISTH centrifugation protocol, as measured by flow cytometry. Experiments were performed using plasma obtained from three healthy controls. Ery‐ghost: erythrocyte ghost; EV: extracellular vesicle; *: EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 4. Filtering out remaining cells from plasma prepared using the non‐ISTH centrifugation protocol (A) or corresponding EV‐enriched SEC2B fractions (B) leads to a qRT‐PCR cycle threshold increase of 1–2, and thus in a decrease of miRNA quantity. The quantity of let7a‐5p, miR‐21‐5p and miR‐223‐3p miRNAs detected by qRT‐PCR correlates with the concentration of platelets in plasma (C) especially in unfiltered samples (filled symbols), but not with the concentration of ery‐ghosts or EVs**. For the EV‐enriched SEC2B fractions (D) miRNA quantities also correlate with the concentration of platelets, but not with the concentration of ery‐ghosts or EVs**. Experiments were performed using plasma obtained from 3 healthy controls. A one‐tailed, paired Student's t‐test was used to test for statistical differences in miRNA quantities pre‐ and post‐filtration (panels A and B). A linear regression analysis was used to quantify the relationship between the quantity of miRNAs and the concentration of platelets, ery‐ghosts, and EVs** (panels C and D). A p‐value ≤ 0.05 was considered significant. Ery‐ghost, erythrocyte ghost; EV, extracellular vesicle; ns, not significant; SEC2B, size‐exclusion chromatography CL‐2B; qRT‐PCR, quantitative real‐time PCR; *, p ≤ 0.05; **: EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 5. The quantity of let7a‐5p, miR‐21‐5p and miR‐223‐3p miRNAs detected by qRT‐PCR does not correlate with the concentration of measured platelet‐derived (CD61+), erythrocyte‐derived (CD235a+), or leukocyte‐derived (CD45+) EVs* in the EV‐enriched SEC2B fractions (obtained from plasma prepared using the ISTH protocol). Experiments were performed using plasma obtained from three healthy controls. A linear regression analysis was used to quantify the relationship between the quantity of miRNAs and the concentration of EVs. EV: extracellular vesicle; SEC2B: size‐exclusion chromatography CL‐2B; qRT‐PCR: quantitative real‐time PCR; *: EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 6. TEM images of unfiltered (left) and 0.8 μm filtered (right) EV‐enriched SEC2B fractions. EV‐ and lipoprotein particles pre‐ and post‐filtration have a similar size range, indicating that while particles s>0.8 μm are removed, particles s<0.8 μm (including EVs and lipoprotein particles) remain unaffected. The shown images are representative examples. Scale bars are 200 nm.
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGEMENTS
The authors would like to acknowledge C.M. Hau (Laboratory of Experimental Clinical Chemistry, Amsterdam University Medical Centers, the Netherlands) for excellent technical support. This work was funded by a TPP‐TKI grant from Health Holland (AQrate project).
Bracht, J. W. P. , Los, M. , van Eijndhoven, M. A. J. , Bettin, B. , van der Pol, E. , Pegtel, D. M. , & Nieuwland, R. (2023). Platelet removal from human blood plasma improves detection of extracellular vesicle‐associated miRNA. Journal of Extracellular Vesicles, 12, e12302. 10.1002/jev2.12302
DATA AVAILABILITY STATEMENT
The data supporting the main findings of this study are available through: https://doi.org/10.6084/m9.figshare.c.6126783.v1 and https://doi.org/10.6084/m9.figshare.c.6126768.v1
REFERENCES
- Ahnadi, C. E. , Sabrinah Chapman, E. , Lépine, M. , Okrongly, D. , Pujol‐Moix, N. , Hernández, A. , Boughrassa, F. , & Grant, A. M. (2003). Assessment of platelet activation in several different anticoagulants by the Advia 120 Hematology System, fluorescence flow cytometry, and electron microscopy. Thrombosis and Haemostasis, 90(5), 940–948. [DOI] [PubMed] [Google Scholar]
- Apel, P. (2001). Track etching technique in membrane technology. Radiation Measurements, 34(1–6), 559–566. [Google Scholar]
- Arraud, N. , Linares, R. , Tan, S. , Gounou, C. , Pasquet, J. ‐ M. , Mornet, S. , & Brisson, A. R. (2014). Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. Journal of Thrombosis and Haemostasis, 12(5), 614–627. [DOI] [PubMed] [Google Scholar]
- Arroyo, J. D. , Chevillet, J. R. , Kroh, E. M. , Ruf, I. K. , Pritchard, C. C. , Gibson, D. F. , Mitchell, P. S. , Bennett, C. F. , Pogosova‐Agadjanyan, E. L. , Stirewalt, D. L. , Tait, J. F. , & Tewari, M. (2011). Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America, 108(12), 5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettin, B. , Gasecka, A. , Li, B. , Dhondt, B. , Hendrix, A. , Nieuwland, R. , & Van Der Pol, E. (2022). Removal of platelets from blood plasma to improve the quality of extracellular vesicle research. Journal of Thrombosis and Haemostasis, 20, 2679–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böing, A. N. , Van Der Pol, E. , Grootemaat, A. E. , Coumans, F. A. W. , Sturk, A. , & Nieuwland, R. (2014). Single‐step isolation of extracellular vesicles by size‐exclusion chromatography. Journal of Extracellular Vesicles, 3, 23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, C. , Harrison, P. , & Machin, S. J. (2007). Continuing developments with the automated platelet count. International Journal of Laboratory Hematology, 29(2), 77–91. [DOI] [PubMed] [Google Scholar]
- Brisson, A. R. , Tan, S. , Linares, R. , Gounou, C. , & Arraud, N. (2017). Extracellular vesicles from activated platelets: A semiquantitative cryo‐electron microscopy and immuno‐gold labeling study. Platelets, 28(3), 263–271. [DOI] [PubMed] [Google Scholar]
- Chen, F. , Xu, B. , Li, J. , Yang, X. , Gu, J. , Yao, X. , & Sun, X. (2021). Hypoxic tumour cell‐derived exosomal miR‐340‐5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. Journal of Experimental & Clinical Cancer Research, 40(1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Quan, Y. , Fan, S. , Wang, H. , Liang, J. , Huang, L. , Chen, L. , Liu, Q. , He, P. , & Ye, Y. (2020). Exosome‐transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Letters, 475, 119–128. [DOI] [PubMed] [Google Scholar]
- Cheng, H. H. , Yi, H. S. , Kim, Y. , Kroh, E. M. , Chien, J. W. , Eaton, K. D. , Goodman, M. T. , Tait, J. F. , Tewari, M. , & Pritchard, C. C. (2013). Plasma processing conditions substantially influence circulating microRNA biomarker levels. PLoS ONE, 8(6), e64795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chettimada, S. , Lorenz, D R. , Misra, V. , Wolinsky, S M. , & Gabuzda, D. (2020). Small RNA sequencing of extracellular vesicles identifies circulating miRNAs related to inflammation and oxidative stress in HIV patients. BMC Immunology [Electronic Resource], 21(1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel Pérez, D. , Rodriguez Martínez, A. , Ortigosa Palomo, A. , Delgado Ureña, M. , Garcia Puche, J. L. , Robles Remacho, A. , Exposito Hernandez, J. , Lorente Acosta, J. A. , Ortega Sánchez, F. G. , & Serrano, M. J. (2020). Extracellular vesicle‐miRNAs as liquid biopsy biomarkers for disease identification and prognosis in metastatic colorectal cancer patients. Scientific Reports, 10(1), 3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees, E. E. E. , Roemer, M. G. M. , Groenewegen, N. J. , Perez‐Boza, J. , Eijndhoven, M. A. J. , Prins, L. I. , Verkuijlen, S. A. W. M. , Tran, X. ‐. M. , Driessen, J. , Zwezerijnen, G. J. C. , Stathi, P. , Mol, K. , Karregat, J. J. J. P. , Kalantidou, A. , Vallés‐Martí, A. , Molenaar, T. J. , Aparicio‐Puerta, E. , Dijk, E. , Ylstra, B. , … Pegtel, D. M. (2021). Extracellular vesicle miRNA predict FDG‐PET status in patients with classical Hodgkin Lymphoma. Journal of Extracellular Vesicles, 10(9), e12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endzeliņš, E. , Berger, A. , Melne, V. , Bajo‐Santos, C. , Soboļevska, K. , Ābols, A. , Rodriguez, M. , Šantare, D. , Rudņickiha, A. , Lietuvietis, V. , Llorente, A. , & Linē, A. (2017). Detection of circulating miRNAs: comparative analysis of extracellular vesicle‐incorporated miRNAs and cell‐free miRNAs in whole plasma of prostate cancer patients. BMC cancer, 17(1), 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, H. , Liu, Y. , He, Y. , Jiang, Y. , Wei, Y. , Liu, H. , Gong, Y. , & An, G. (2019). Extracellular vesicledelivered miR5055p, as a diagnostic biomarker of early lung adenocarcinoma, inhibits cell apoptosis by targeting TP53AIP1. International Journal of Oncology, 54(5), 1821–1832. [DOI] [PubMed] [Google Scholar]
- Gelibter, S. , Marostica, G. , Mandelli, A. , Siciliani, S. , Podini, P. , Finardi, A. , & Furlan, R. (2022). The impact of storage on extracellular vesicles: A systematic study. Journal of Extracellular Vesicles, 11(2), e12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni, R. G. , & P, Foissac, S. (2009). The qPCR data statistical analysis. Integromics SL White Paper .
- He, X. , Park, S. , Chen, Y. , & Lee, H. (2021). Extracellular Vesicle‐Associated miRNAs as a Biomarker for Lung Cancer in Liquid Biopsy. Frontiers in Molecular Biosciences, 8, 630718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen, D. K. , Fenix, A. M. , Franklin, J. L. , Higginbotham, J. N. , Zhang, Q. , Zimmerman, L. J. , Liebler, D. C. , Ping, J. , Liu, Q. , Evans, R. , Fissell, W. H. , Patton, J. G. , Rome, L. H. , Burnette, D. T. , & Coffey, R. J. (2019). Reassessment of Exosome Composition. Cell, 177(2), 428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L. , Zhang, Y. , Li, B. , Kang, M. , Yang, Z. , Lin, C. , Hu, K. , Wei, Z. , Xu, M. , Mi, J. , Wang, R. , & Wu, F. (2021). miRNAs derived from circulating small extracellular vesicles as diagnostic biomarkers for nasopharyngeal carcinoma. Cancer Science, 112(6), 2393–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J. , Rames, M. J. , Tassi Yunga, S. , Armstrong, R. , Morita, M. , Ngo, A. T. P. , Mccarty, O. J. T. , Civitci, F. , Morgan, T. K. , & Ngo, T. T. M. (2022). Irreversible alteration of extracellular vesicle and cell‐free messenger RNA profiles in human plasma associated with blood processing and storage. Scientific Reports, 12(1), 2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloten, V. , Neumann, M. H. D. , Di Pasquale, F. , Sprenger‐Haussels, M. , Shaffer, J. M. , Schlumpberger, M. , Herdean, A. , Betsou, F. , Ammerlaan, W. , Af Hällström, T. , Serkkola, E. , Forsman, T. , Lianidou, E. , Sjöback, R. , Kubista, M. , Bender, S. , Lampignano, R. , Krahn, T. , & Schlange, T. (2019). Multicenter Evaluation of Circulating Plasma MicroRNA Extraction Technologies for the Development of Clinically Feasible Reverse Transcription Quantitative PCR and Next‐Generation Sequencing Analytical Work Flows. Clinical Chemistry, 65(9), 1132–1140. [DOI] [PubMed] [Google Scholar]
- Krammer, T. L. , Mayr, M. , & Hackl, M. (2020). microRNAs as promising biomarkers of platelet activity in antiplatelet therapy monitoring. International Journal of Molecular Sciences, 21(10), 3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. , Wong, D. K. , Luk, F. S. , Kim, R. Y. , & Raffai, R. L. (2018). Isolation of Plasma Lipoproteins as a Source of Extracellular RNA. Methods in Molecular Biology, 1740, 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia, G. , Di Vito, C. , Bruno, S. , Tapparo, M. , Brunello, L. , Santoro, A. , Mariotti, J. , Bramanti, S. , Zaghi, E. , Calvi, M. , Comba, L. , Fascì, M. , Giaccone, L. , Camussi, G. , Boyle, E. M. , Castagna, L. , Evangelista, A. , Mavilio, D. , & Bruno, B. (2021). Extracellular Vesicles as Biomarkers of Acute Graft‐vs.‐Host Disease After Haploidentical Stem Cell Transplantation and Post‐Transplant Cyclophosphamide. Frontiers in immunology, 12, 816231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, A. J. , Gray, W. D. , Hayek, S. S. , Ko, Y.‐A. , Thomas, S. , Rooney, K. , Awad, M. , Roback, J. D. , Quyyumi, A. , & Searles, C. D. (2016). Platelets confound the measurement of extracellular miRNA in archived plasma. Scientific Reports, 6, 32651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth, D C. , Powell, B H. , Zhao, Z. , & Witwer, K W. (2018). miRNAs in platelet‐poor blood plasma and purified RNA are highly stable: a confirmatory study. BMC Research Notes, 11(1), 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panvongsa, W. , Siripoon, T. , Worakitchanon, W. , Arsa, L. , Trachu, N. , Jinawath, N. , Ngamphaiboon, N. , & Chairoungdua, A. (2021). Plasma extracellular vesicle microRNA‐491‐5p as diagnostic and prognostic marker for head and neck squamous cell carcinoma. Cancer Science, 112(10), 4257–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramayanti, O. , Verkuijlen, S. A. W. M. , Novianti, P. , Scheepbouwer, C. , Misovic, B. , Koppers‐Lalic, D. , Van Weering, J. , Beckers, L. , Adham, M. , Martorelli, D. , Middeldorp, J. M. , & Pegtel, D. M. (2019). Vesicle‐bound EBV‐BART13‐3p miRNA in circulation distinguishes nasopharyngeal from other head and neck cancer and asymptomatic EBV‐infections. International Journal of Cancer, 144(10), 2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikkert, L. G. , Coumans, F. A. W. , Hau, C. M. , Terstappen, L. W. M. M. , & Nieuwland, R. (2021). Platelet removal by single‐step centrifugation. Platelets, 32(4), 440–443. [DOI] [PubMed] [Google Scholar]
- Simonsen, J. B. (2017). What Are We Looking At? Extracellular Vesicles, Lipoproteins, or Both? Circulation Research, 121(8), 920–922. [DOI] [PubMed] [Google Scholar]
- Slim, C. L. , Wevers, B. A. , Demmers, M. W. H. J. , Lakos, G. , Hoffmann, J. J. M. L. , Adriaansen, H. J. , Kooren, J. A. , & Storm, H. (2019). Multicenter performance evaluation of the Abbott Alinity hq hematology analyzer. Clinical Chemistry and Laboratory Medicine, 57(12), 1988–1998. [DOI] [PubMed] [Google Scholar]
- Sunderland, N. , Skroblin, P. , Barwari, T. , Huntley, R P. , Lu, R. , Joshi, A. , Lovering, R C. , & Mayr, M. (2017). MicroRNA Biomarkers and Platelet Reactivity: The Clot Thickens. Circulation Research, 120(2), 418–435. [DOI] [PubMed] [Google Scholar]
- Tabet, F. , Vickers, K. C. , Cuesta Torres, L. F. , Wiese, C. B. , Shoucri, B. M. , Lambert, G. , Catherinet, C. , Prado‐Lourenco, L. , Levin, M. G. , Thacker, S. , Sethupathy, P. , Barter, P. J. , Remaley, A. T. , & Rye, K.‐A. (2014). HDL‐transferred microRNA‐223 regulates ICAM‐1 expression in endothelial cells. Nature Communications, 5, 3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun, J. , Jo, A. , Li, H. , Lin, H. Y. , Weissleder, R. , Im, H. , & Lee, H. (2020). Integrated Dual‐Mode Chromatography to Enrich Extracellular Vesicles from Plasma. Advanced biosystems, 4(12), 1900310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijndhoven, M. A. J. , Zijlstra, J. M. , Groenewegen, N. J. , Drees, E. E. E. , Van Niele, S. , Baglio, S. R. , Koppers‐Lalic, D. , Van Der Voorn, H. , Libregts, S. F. W. M. , Wauben, M. H. M. , De Menezes, R. X. , Van Weering, J. R. T. , Nieuwland, R. , Visser, L. , Van Den Berg, A. , De Jong, D. , & Pegtel, D. M. (2016). Plasma vesicle miRNAs for therapy response monitoring in Hodgkin lymphoma patients. JCI Insight, 1(19), e89631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers, K C. , Palmisano, B T. , Shoucri, B M. , Shamburek, R D. , & Remaley, A T. (2011). MicroRNAs are transported in plasma and delivered to recipient cells by high‐density lipoproteins. Nature Cell Biology, 13(4), 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit, P. , Zampetaki, A. , Dudek, K. , Kaudewitz, D. , King, A. , Kirkby, N. S. , Crosby‐Nwaobi, R. , Prokopi, M. , Drozdov, I. , Langley, S. R. , Sivaprasad, S. , Markus, H. S. , Mitchell, J. A. , Warner, T. D. , Kiechl, S. , & Mayr, M. (2013). Circulating microRNAs as novel biomarkers for platelet activation. Circulation Research, 112(4), 595–600. [DOI] [PubMed] [Google Scholar]
- Xu, Y.‐F. , Xu, X. , Bhandari, K. , Gin, A. , Rao, C. V. , Morris, K. T. , Hannafon, B. N. , & Ding, W.‐Q. (2021). Isolation of extra‐cellular vesicles in the context of pancreatic adenocarcinomas: Addition of one stringent filtration step improves recovery of specific microRNAs. PLoS ONE, 16(11), e0259563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Lariviere, M. J. , Ko, J. , Till, J. E. , Christensen, T. , Yee, S. S. , Black, T. A. , Tien, K. , Lin, A. , Shen, H. , Bhagwat, N. , Herman, D. , Adallah, A. , O'hara, M. H. , Vollmer, C. M. , Katona, B. W. , Stanger, B. Z. , Issadore, D. , & Carpenter, E. L. (2020). A Multianalyte Panel Consisting of Extracellular Vesicle miRNAs and mRNAs, cfDNA, and CA19‐9 Shows Utility for Diagnosis and Staging of Pancreatic Ductal Adenocarcinoma. Clinical Cancer Research, 26(13), 3248–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabegina, L. , Nazarova, I. , Knyazeva, M. , Nikiforova, N. , Slyusarenko, M. , Titov, S. , Vasilyev, D. , Sleptsov, I. , & Malek, A. (2020). MiRNA let‐7 from TPO(+) Extracellular Vesicles is a Potential Marker for a Differential Diagnosis of Follicular Thyroid Nodules. Cells, 9(8), 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabegina, L. , Nazarova, I. , Nikiforova, N. , Slyusarenko, M. , Sidina, E. , Knyazeva, M. , Tsyrlina, E. , Novikov, S. , Reva, S. , & Malek, A. (2021). A New Approach for Prostate Cancer Diagnosis by miRNA Profiling of Prostate‐Derived Plasma Small Extracellular Vesicles. Cells, 10(9), 2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Yang, J. , Chen, Y. , Jiang, F. , Liao, H. , Liu, X. , Wang, Y. , Kong, G. , Zhang, X. , Li, J. , Gao, J. , & Shen, L. (2022). miRNAs derived from plasma small extracellular vesicles predict organo‐tropic metastasis of gastric cancer. Gastric Cancer, 25(2), 360–374. [DOI] [PubMed] [Google Scholar]
- Zhang, K. , Dong, C. , Chen, M. , Yang, T. , Wang, X. , Gao, Y. , Wang, L. , Wen, Y. , Chen, G. , Wang, X. , Yu, X. , Zhang, Y. , Wang, P. , Shang, M. , Han, K. , & Zhou, Y. (2020). Extracellular vesicle‐mediated delivery of miR‐101 inhibits lung metastasis in osteosarcoma. Theranostics, 10(1), 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M.‐W. , Shen, Y.‐J. , Shi, J. , & Yu, J.‐G. (2020). MiR‐223‐3p in Cardiovascular Diseases: A Biomarker and Potential Therapeutic Target. Frontiers in Cardiovascular Medicine, 7, 610561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Plasma, prepared using the non‐ISTH centrifugation protocol, still contains platelets (CD61+, CD41+) and ery‐ghosts (CD235a+) as measured by flow cytometry (A). Platelets and ery‐ghosts are larger than the cut‐off of Sepharose CL‐2B, and thus co‐migrate with EVs during SEC2B (B). Filtration (0.8 μm polycarbonate filters) of plasma (C), or corresponding EV‐enriched SEC2B fractions 8–10 (D), reduces the platelet concentration >97%, without affecting the concentration of platelet‐derived EVs (CD61+)*. Filtration also reduces the ery‐ghost concentration >97% (E), while the concentration of erythrocyte‐derived EVs (CD235a+)* remains unaffected (F). Experiments were performed using plasma obtained from three healthy controls. A one‐tailed, paired Student's t‐test was used to test for statistical differences in platelet‐, ery‐ghost and EV concentrations, pre‐ and post‐filtration (panels C–F). A p‐value ≤0.05 was considered significant. Ery‐ghost, erythrocyte ghost; EV, extracellular vesicle; ns, not significant; SEC2B, size‐exclusion chromatography CL‐2B; *, EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 2. Plasma prepared using the ISTH centrifugation protocol contains activated platelets (CD61+, CD62p+) as measured by flow cytometry. The concentration of activated platelets did not change upon filtration of the plasma, indicating that filtration did not cause additional platelet activation, but also did not remove the activated platelets from plasma. Experiments were performed using plasma obtained from two healthy controls. A one‐tailed, paired Student's t‐test was used to test for statistical differences in activated platelet concentrations, pre‐ and post‐filtration. A p‐value ≤ 0.05 was considered significant. F‐plasma, filtered plasma; ns, not significant.
Supplementary Figure 3. The size distribution of EVs* (CD235a+, CD45+ and CD61+) (A) platelets (CD61+, CD41+) (B) and ery‐ghosts (CD235a+) (C) before (left) and after (right) filtration of plasma prepared according to the ISTH centrifugation protocol, as measured by flow cytometry. Experiments were performed using plasma obtained from three healthy controls. Ery‐ghost: erythrocyte ghost; EV: extracellular vesicle; *: EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 4. Filtering out remaining cells from plasma prepared using the non‐ISTH centrifugation protocol (A) or corresponding EV‐enriched SEC2B fractions (B) leads to a qRT‐PCR cycle threshold increase of 1–2, and thus in a decrease of miRNA quantity. The quantity of let7a‐5p, miR‐21‐5p and miR‐223‐3p miRNAs detected by qRT‐PCR correlates with the concentration of platelets in plasma (C) especially in unfiltered samples (filled symbols), but not with the concentration of ery‐ghosts or EVs**. For the EV‐enriched SEC2B fractions (D) miRNA quantities also correlate with the concentration of platelets, but not with the concentration of ery‐ghosts or EVs**. Experiments were performed using plasma obtained from 3 healthy controls. A one‐tailed, paired Student's t‐test was used to test for statistical differences in miRNA quantities pre‐ and post‐filtration (panels A and B). A linear regression analysis was used to quantify the relationship between the quantity of miRNAs and the concentration of platelets, ery‐ghosts, and EVs** (panels C and D). A p‐value ≤ 0.05 was considered significant. Ery‐ghost, erythrocyte ghost; EV, extracellular vesicle; ns, not significant; SEC2B, size‐exclusion chromatography CL‐2B; qRT‐PCR, quantitative real‐time PCR; *, p ≤ 0.05; **: EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 5. The quantity of let7a‐5p, miR‐21‐5p and miR‐223‐3p miRNAs detected by qRT‐PCR does not correlate with the concentration of measured platelet‐derived (CD61+), erythrocyte‐derived (CD235a+), or leukocyte‐derived (CD45+) EVs* in the EV‐enriched SEC2B fractions (obtained from plasma prepared using the ISTH protocol). Experiments were performed using plasma obtained from three healthy controls. A linear regression analysis was used to quantify the relationship between the quantity of miRNAs and the concentration of EVs. EV: extracellular vesicle; SEC2B: size‐exclusion chromatography CL‐2B; qRT‐PCR: quantitative real‐time PCR; *: EVs were measured using flow cytometry (Apogee A60) with a size detection range of 150–1000 nm).
Supplementary Figure 6. TEM images of unfiltered (left) and 0.8 μm filtered (right) EV‐enriched SEC2B fractions. EV‐ and lipoprotein particles pre‐ and post‐filtration have a similar size range, indicating that while particles s>0.8 μm are removed, particles s<0.8 μm (including EVs and lipoprotein particles) remain unaffected. The shown images are representative examples. Scale bars are 200 nm.
Supplementary information
Supplementary information
Supplementary information
Data Availability Statement
The data supporting the main findings of this study are available through: https://doi.org/10.6084/m9.figshare.c.6126783.v1 and https://doi.org/10.6084/m9.figshare.c.6126768.v1


