Introduction
The antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of autoimmune diseases caused by inflammation and necrosis of blood vessels (1). It is a type of small vessel vasculitis characterized by the absence of immune complex deposition. It is sub-clinically divided into the following variants: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic GPA (EGPA). We report a unique case of severe EGPA endocarditis detected on cardiac imaging and confirmed on subsequent rheumatological workup.
Case presentation
A 63-year-old female patient with a past medical history including chronic obstructive pulmonary disease (COPD) and hypothyroidism presented to our hospital complaining of acute shortness of breath. The patient required 2 L nasal cannula (NC) on admission, and the physical exam was remarkable for mild bilateral lower limb edema.
Chest X-ray was ordered showing bilateral pleural effusions. Labs were remarkable for a brain natriuretic peptide (BNP) level of 17 K. The patient was admitted to the intensive care unit for the management of decompensated heart failure. She was started on diuretic therapy with daily weight monitoring.
The following day, an echocardiogram was ordered. It showed severe left ventricular systolic dysfunction with an ejection fraction of 20%. Also, left ventricular dilatation with a small pericardial effusion were seen (Figure 1).
Figure 1.
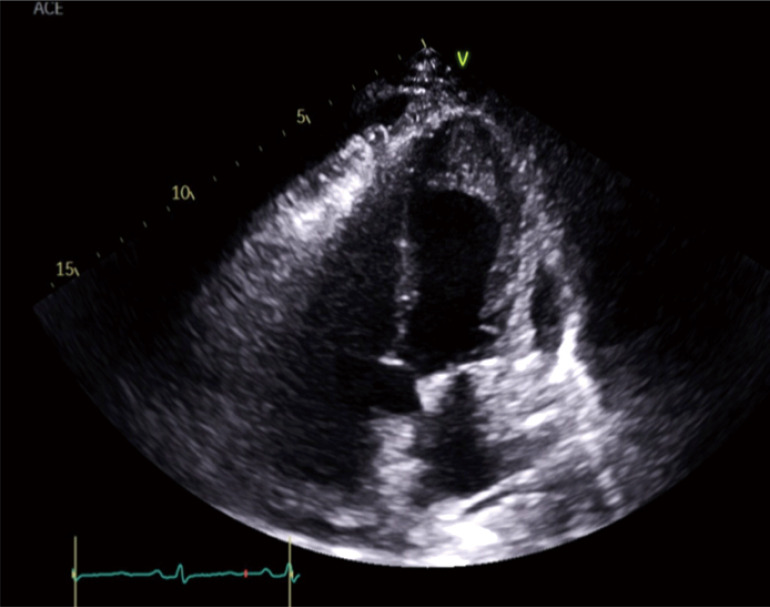
Echocardiogram showing subendocardial enhancement in the left ventricular apex, left ventricular dilatation, severe left ventricular systolic dysfunction, and pericardial effusion.
After being euvolemic, a cardiac magnetic resonance imaging (MRI) showed the appearance of a prominent thrombus within the left ventricular apex, right ventricular apex, and right ventricular outflow tract, with generalized subendocardial enhancement throughout the right and left ventricles. The findings were compatible with eosinophilic endocarditis (Figure 2).
Figure 2.
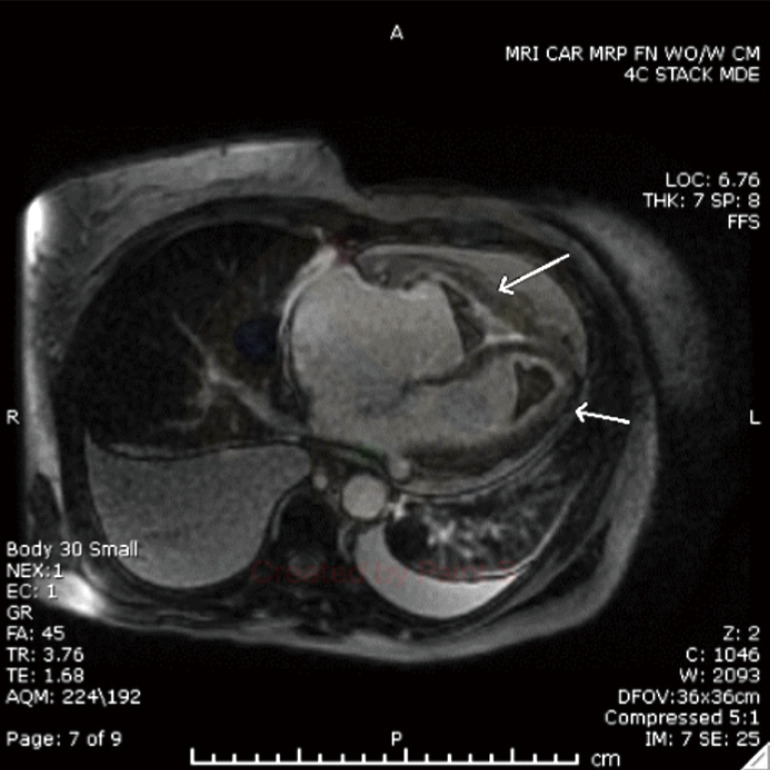
Cardiac MRI with delayed post-contrast 2D showing a thrombus within the left ventricular apex, right ventricular apex, and right ventricular outflow tract, with generalized subendocardial enhancement throughout the right and left ventricles (arrows). MRI, magnetic resonance imaging; 2D, two-dimensional.
The rheumatology team was consulted, and the workup was then ordered. ANCA was positive with a perinuclear ANCA (P-ANCA) titer of 1:160 (negative: <1:20) and negative cytoplasmic ANCA (C-ANCA). Anti-myeloperoxidase was positive of 18.0 level (negative: 0.0–9.0). Cryoglobulins and anti-nuclear antibodies were negative. Complement levels (C3 and C4) were also normal. Labs showed leukocytosis of white blood cell (WBC) count 11.9 with monocytes of 0.1% and eosinophils of 7.7%. Blood cultures (2×) were negative.
Looking more into the history, the patient denied any allergic rhinitis symptoms although she felt that her sinuses were always congested.
Subsequently, the patient underwent an endomyocardial biopsy showing eosinophilic infiltration of the endocardium compatible with EGPA findings.
The patient was started on induction therapy with cyclophosphamide and maintenance therapy with methylprednisolone 80 mg per kg for a total of 6 days. She was then switched to prednisone on the discharge date. With frequent follow-ups with the rheumatology team, the patient reported improvement in her fatigue, shortness of breath, and leg swelling.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
AAV is characterized by the necrosis of small blood vessels such as capillaries, venules, arterioles, and small arteries. It is also associated with ANCA specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA) (2). Screening for the presence of ANCAs is a commonly used diagnostic test for AAV (3). ANCA testing in patients with a high probability of having the disease enhances certainty of its diagnosis (4).
The International Chapel Hill Convention Conference on the Nomenclature of Vasculitides (CHCC 2012) was the first in aiding the classification of AAV based on the etiology and involved vessel (5). EGPA, historically known as Churg-Strauss syndrome, is caused by the necrosis of small vessels. It is characterized by asthma and marked eosinophilia on blood work (6). To confirm its diagnosis, the American College of Rheumatology (ACR) recommended six characteristics including peripheral blood eosinophilia (more than 10% on differential blood cell count), neuropathy, non-fixed pulmonary infiltrates, paranasal sinus abnormalities, and extravascular eosinophilia (7).
The cardiac involvement in EGPA is extremely rare (8). The following study illustrated the incidence of heart pathologies involved in EGPA (9). Cardiovascular involvement in this disease entity is underdiagnosed and not completely understood. In addition, one study has revealed that around 36% of patients with EGPA have also heart pathologies looking at the echocardiography results (10). Neumann et al., who investigated the prevalence and clinical impact of cardiac involvement in EGPA patients in a multicenter, cross-sectional analysis, reported that cardiac involvement occurred in 22 (45%) of 49 patients, and 9 of these (41%) showed pericardial effusions (11).
Cardiac MRI is a technique for detecting heart involvement in GPA with high accuracy (9). Gadolinium enhancement on cardiovascular magnetic resonance (CMR) has been reported to correlate with endomyocardial biopsy evidence of eosinophilic infiltration (12). Delayed-enhancement sequences seem to help identify and categorize eosinophilic endo-myocarditis in the early acute necrotic or late fibrotic stage (13). However, an endomyocardial biopsy remains the gold standard tool for its diagnosis.
The use of daily cyclophosphamide and glucocorticoids have improved the mortality and long-term prognosis of untreated AAV (1). Also, biologics such as B-cell depleting therapy for ANCA-positive and anti-IL-5 treatment for ANCA negative EGPA myocarditis have shown efficiency in controlling the disease activity. Our patient showed a score of 2 using Factor Five Score which indicated severe disease [cardiac involvement and absence of ear, nose, and throat (ENT) involvement]. Therefore, cyclophosphamide was given due to severe disease activity and after declining the use of biologic therapy due to financial reasons. The RAVE trial (14) illustrates more on the management of AAV.
Conclusions
In this case, we illustrate a challenging case of EGPA endocarditis, and we demonstrate the utility of cardiac imaging in its diagnosis. When the diagnosis of this disease entity is suspected and confirmed on imaging, we emphasize the initiation of treatment even if there was no definitive pathological confirmation. Early treatment shows an improved outcome for many of the patients.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-401/coif). The authors have no conflicts of interest to declare.
References
- 1.Yates M, Watts R. ANCA-associated vasculitis. Clin Med (Lond) 2017;17:60-4. 10.7861/clinmedicine.17-1-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1-11. 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 3.Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suárez LF, Guillevin L, et al. Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017;13:683-92. 10.1038/nrrheum.2017.140 [DOI] [PubMed] [Google Scholar]
- 4.Hunter RW, Welsh N, Farrah TE, Gallacher PJ, Dhaun N. ANCA associated vasculitis. BMJ 2020;369:m1070. 10.1136/bmj.m1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain V, Tiwari V. Microscopic Polyangiitis. In: StatPearls. Treasure Island: StatPearls Publishing, 2021. [Google Scholar]
- 6.Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013;65:270-81. 10.1002/art.37721 [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm AB, Pinsky S, Ahmad S, Cunningham A, Chatila K, Boor PJ, Stevenson HL. Endomyocardial biopsy facilitates diagnosis of eosinophilic granulomatosis with polyangiitis (EGPA): a case report. Cardiovasc Pathol 2022;58:107407. 10.1016/j.carpath.2022.107407 [DOI] [PubMed] [Google Scholar]
- 8.Pakbaz M, Pakbaz M. Cardiac Involvement in Eosinophilic Granulomatosis with Polyangiitis: A Meta-Analysis of 62 Case Reports. J Tehran Heart Cent 2020;15:18-26. 10.18502/jthc.v15i1.3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugnet G, Gouya H, Puéchal X, Terrier B, Kahan A, Legmann P, Guillevin L, Vignaux O, French Vasculitis Study Group . Cardiac involvement in granulomatosis with polyangiitis: a magnetic resonance imaging study of 31 consecutive patients. Rheumatology (Oxford) 2017;56:947-56. 10.1093/rheumatology/kew490 [DOI] [PubMed] [Google Scholar]
- 10.Giollo A, Dumitru RB, Swoboda PP, Plein S, Greenwood JP, Buch MH, Andrews J. Cardiac magnetic resonance imaging for the detection of myocardial involvement in granulomatosis with polyangiitis. Int J Cardiovasc Imaging 2021;37:1053-62. 10.1007/s10554-020-02066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann T, Manger B, Schmid M, Kroegel C, Hansch A, Kaiser WA, Reinhardt D, Wolf G, Hein G, Mall G, Schett G, Zwerina J. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine (Baltimore) 2009;88:236-43. 10.1097/MD.0b013e3181af35a5 [DOI] [PubMed] [Google Scholar]
- 12.Baccouche H, Yilmaz A, Alscher D, Klingel K, Val-Bernal JF, Mahrholdt H. Images in cardiovascular medicine. Magnetic resonance assessment and therapy monitoring of cardiac involvement in Churg-Strauss syndrome. Circulation 2008;117:1745-9. 10.1161/CIRCULATIONAHA.107.721738 [DOI] [PubMed] [Google Scholar]
- 13.Baumann S, De Cecco CN, Schoepf UJ, Wince WB, Suranyi P, Spruill LS, Varga-Szemes A. Correlation of cardiac magnetic resonance imaging and histopathology in eosinophilic endomyocarditis. Circ Cardiovasc Imaging 2014;8:e002501. 10.1161/CIRCIMAGING.114.002501 [DOI] [PubMed] [Google Scholar]
- 14.Alam L, Lasam G, Fishberg R. Pericardial effusion with tamponade - an uncommon presentation leading to the diagnosis of eosinophilic granulomatosis polyangiitis: A case report. World J Cardiol 2020;12:460-7. 10.4330/wjc.v12.i9.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


