Abstract
The clinical usefulness of repeat colonoscopic polypectomy in patients with numerous polyps has not been sufficiently determined. We aimed to analyze the clinical outcomes of colonoscopic polypectomy with surveillance colonoscopies in patients with ≥ 10 polyps. We reviewed the medical records of 152 patients who underwent polypectomy of ≥ 10 polyps at the baseline colonoscopy. We investigated polyp number, polyp size, polypectomy method, procedure time, and adverse events of the baseline colonoscopy. We also investigated the frequency and interval of surveillance colonoscopies and their findings. The mean number of polyps detected at the baseline colonoscopy was 20.0, of which 16.0 polyps were endoscopically resected. The mean size of the largest polyp was 13.4 mm. The mean procedure time was 54.9 min. Post-polypectomy bleeding occurred in 6 (3.9%) patients, all of whom were treated conservatively. No patients developed perforation. With an increasing number of surveillance colonoscopies, the number of detected polyps and the procedure time decreased. Surveillance colonoscopies identified colorectal cancer only in three patients (2.0%), all of which were mucosal cancers that could be curatively treated by polypectomy. Colonoscopic polypectomy with repeat surveillance colonoscopies is a clinically effective, efficient, and safe management option in patients with ≥ 10 polyps.
Subject terms: Cancer screening, Colonoscopy, Gastrointestinal diseases
Introduction
Colorectal cancer is one of the most common cancers worldwide and the third leading cause of cancer-related death1. Since most colorectal cancers occur through the adenoma-carcinoma sequence, early endoscopic detection and removal of precancerous lesions can lower the incidence of and mortality from colorectal cancer2,3. Therefore, repeat regular surveillance colonoscopy after baseline screening colonoscopy is recommended. International guidelines suggest the interval of surveillance colonoscopy should be determined according to the baseline colonoscopy findings, such as the number, size, and histology of detected adenomas4,5.
The consensus update by the U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer in 2020 recommends a 3-year interval for surveillance colonoscopy after endoscopic removal of 5–10 tubular adenomas or ≥ 10 mm because of the increased risk of metachronous advanced neoplasia in patients with multiple adenomas5. In addition, the current guideline by the European Society of Gastrointestinal Endoscopy (ESGE) in 2020 also recommends a 3-year interval for surveillance colonoscopy after endoscopic removal of ≥ 5 adenomas4. Interestingly, while the ESGE guidelines do not specify the surveillance colonoscopy interval for those with removal of > 10 adenomas, the USMSTF recommends a 1-year interval for surveillance colonoscopy after endoscopic removal of > 10 adenomas. However, the strength of the recommendation was weak, and the quality of evidence was very low, indicating the need for further studies on the surveillance strategy after endoscopic removal of > 10 adenomas.
Endoscopic resection is the standard treatment for colon polyps. Depending on the size, shape, and histological diagnosis, various methods can be used, such as cold snare polypectomy (CSP), endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD). The adverse events include bleeding and perforation; however, these methods are generally considered safe. The incidences of delayed bleeding and perforation after CSP and EMR vary from 0.3 to 7.2% and from 0.08 to 1.3%, respectively. The incidences of ESD-associated delayed bleeding and perforation varies from 1 to 10%, depending on the skill of the endoscopist. Post-polypectomy coagulation syndrome occurs in 1.4–3.7% of patients6–10. The risk of adverse polypectomy events increases with the number of polyps. Therefore, caution is required when performing endoscopic resection of many polyps, particularly with repeated resection.
This study investigated the long-term clinical outcomes of repeat endoscopic resection of multiple colorectal polyps in patients with ≥ 10 adenomas on a baseline colonoscopy. In addition, we assessed the effectiveness and safety of repeat endoscopic resection in preventing metachronous advanced neoplasia, suggesting an appropriate surveillance endoscopy strategy for these patients.
Results
Baseline characteristics of patients
The mean age of the 152 patients at the time of baseline colonoscopy was 60.9 years, and 122 (80.3%) patients were men. Twenty-two (14.5% patients) had a family history of colorectal cancer. Only 6 (3.9%) patients underwent genetic testing; 5 showed genetic mutations related to hereditary polyposis syndromes (mutation of APC, EXO1, and STK11). Detailed baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics of patients.
| Characteristics | |
|---|---|
| Age (year) | 60.9 ± 11.2 (median 62.5, range 25–81) |
| Sex | |
| Male | 122 (80.3%) |
| Female | 30 (19.7%) |
| Genetic tests performed | |
| No | 146 (96.1%) |
| Yes | 6 (3.9%) |
| No mutations detected | 1 (0.7%) |
| Relevant mutations detected | 5 (3.2%) |
| APC mutation | 2 (1.3%) |
| EXO1 mutation | 1 (0.6%) |
| STK11 mutation | 2 (1.3%) |
| Smoking status | |
| Current smoker | 41 (27.0%) |
| Ex-smoker | 33 (21.7%) |
| Never-smoker | 78 (51.3%) |
| Alcohol consumption | |
| Yes | 81 (53.3%) |
| No | 71 (46.7%) |
| Body mass index (kg/m2) | 23.6 ± 3.1 (median 23.1, range 18.6–30.4) |
| Family history of colorectal cancer | 22 (14.5%) |
Data are presented as n (%) or mean ± standard deviation with median and range.
APC adenomatous polyposis coli, EXO1 exonuclease 1, STK11 serine/threonine kinase 11.
Baseline colonoscopy findings
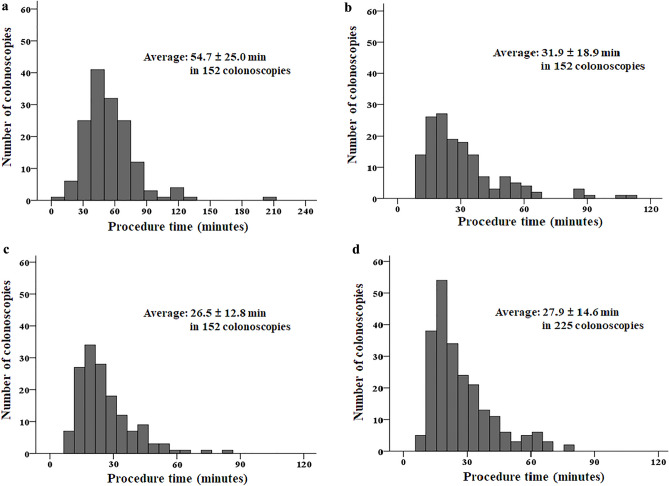
The mean number of polyps detected at baseline colonoscopy was 20.0 ± 22.8 (median 13, range 10–200). According to these, 16.0 ± 12.3 (median 13, range 10–147) were endoscopically resected. The mean size of the largest polyp was 13.4 ± 6.3 mm (median 12.0 mm, range 3.0–40.0 mm). The procedure time ranged from 20 to 210 min (54.9 ± 24.7 min). Only 10 (6.6%) of 152 baseline colonoscopies took > 90 min (Table 2, Fig. 1). EMR was the most frequently performed polypectomy method (1336 polyps, 55.7%), followed by cold forceps polypectomy (CFP) (635 polyps, 26.4%), CSP (411 polyps, 17.1%), endoscopic piecemeal mucosal resection (EPMR) (14 polyps, 0.6%), and ESD (4 polyps, 0.2%). Among the 2370 specimens retrieved and analyzed, 2063 (87.0%) were adenomas, 22 (0.9%) were sessile serrated lesions, 16 (0.7%) were mucosal cancers, and 10 (0.4%) were superficial submucosal cancers with submucosal invasion depth < 1000 µm (Table 2). Delayed bleeding occurred in 6 (3.9%) patients. All cases of delayed bleeding were successfully managed conservatively using endoscopic hemostasis. None of the patients developed perforations.
Table 2.
Baseline colonoscopy findings.
| Characteristics | |
|---|---|
| Number of polyps detected | 20.0 ± 22.8 (median 13, range 10–200) |
| Number of polyps resected | 16.0 ± 12.3 (median 13, range 10–147) |
| Size of the largest polyp, mm | 13.4 ± 6.3 (median 12.0, range 3–40) |
| Colonoscopy procedure time, min | 54.9 ± 24.7 (median 50, range 20–210) |
| < 30 min | 14 (9.2%) |
| 30–59 min | 87 (57.2%) |
| 60–89 min | 41 (27.0%) |
| ≥ 90 min | 10 (6.6%) |
| Colonoscopic polypectomy methods* | |
| CFP | 635 (26.4%) |
| CSP | 411 (17.1%) |
| EMR | 1336 (55.7%) |
| EPMR | 14 (0.6%) |
| ESD | 4 (0.2%) |
| Histological diagnosis‡ | |
| TA/TVA/VA with LGD | 2,032 (85.7%) |
| TA/TVA/VA with HGD | 31 (1.3%) |
| SSL | 22 (0.9%) |
| Mucosal cancer | 16 (0.7%) |
| Submucosal cancer§ | 10 (0.4%) |
| Others (HP and IP) | 259 (10.9%) |
| Complication | |
| Delayed bleeding | 6 (3.9%) |
| Perforation | 0 (0.0%) |
Data are presented as n (%) or mean ± standard deviation with median and range.
CFP cold forceps polypectomy, CSP cold snare polypectomy, EMR endoscopic mucosal resection, EPMR endoscopic piecemeal mucosal resection, ESD endoscopic submucosal dissection, HGD high-grade dysplasia, LGD low-grade dysplasia, SSL sessile serrated lesion, TA tubular adenoma, TVA tubulovillous adenoma, VA villous adenoma, HP hyperplastic polyp, IP inflammatory polyp.
*Colonoscopic polypectomy methods were analyzed in 2400 cases.
‡Histological diagnosis was analyzed in 2370 specimens retrieved after endoscopic resection.
§All submucosal cancers were superficial submucosal cancers with a submucosal invasion depth < 1000 µm from the muscularis mucosa without poor histological features (poorly differentiated adenocarcinoma, lymphovascular invasion, and tumor budding).
Figure 1.
Colonoscopy procedure time (a) baseline colonoscopies, (b) first surveillance colonoscopies, (c) second surveillance colonoscopies, and (d) third and subsequent surveillance colonoscopies.
Surveillance colonoscopy findings
The mean number of surveillance colonoscopies was 3.6 ± 1.8 (median 3, range 2–12) over a follow-up of 64.6 ± 30.1 months. Among patients with complete or near complete removal of all polyps at the baseline colonoscopy, the first surveillance colonoscopy interval was 13.2 ± 5.8 months. In contrast, it was 9.2 ± 5.9 months among patients with incomplete removal of all polyps at the baseline colonoscopy. The mean number of detected polyps at the first surveillance colonoscopy was 12.2 ± 23.4. Of these, 7.5 ± 7.4 polyps were endoscopically resected. The mean size of the largest polyp was 7.7 ± 4.3 mm. The procedure time ranged from 11 to 113 min (31.9 ± 18.9 min). The findings of the first surveillance colonoscopy are summarized in Table 3. Table 3 also shows detailed findings of the second and subsequent surveillance colonoscopies. The number and size of polyps decreased as surveillance colonoscopies were repeated compared to those at baseline colonoscopy (Table 3). In addition, the frequencies of CFP and CSP increased as surveillance endoscopy was repeated, whereas that of EMR decreased. Finally, the surveillance interval became longer as surveillance colonoscopies were repeated.
Table 3.
Surveillance colonoscopy findings.
| The first surveillance colonoscopy | |
| Number of polyps detected | 12.2 ± 23.4 (median 5, range 1–200) |
| Number of polyps resected | 7.5 ± 7.4 (median 5, range 1–49) |
| Size of the largest polyp, mm | 7.7 ± 4.3 (median 7, range 1–30) |
| Colonoscopy procedure time, min | 31.9 ± 18.9 (median 26.5, range 11–113) |
| < 30 min | 91 (59.9%) |
| 30–59 min | 51 (33.5%) |
| 60–89 min | 7 (4.6%) |
| ≥ 90 min | 3 (2.0%) |
| Colonoscopic polypectomy method* | |
| CFP | 446 (41.7%) |
| CSP | 186 (17.4%) |
| EMR | 433 (40.5%) |
| EPMR | 5 (0.4%) |
| ESD | 0 (0.0%) |
| The second surveillance colonoscopy | |
| Number of polyps detected | 10.5 ± 23.1 (median 5, range 0–100) |
| Number of polyps resected | 6.4 ± 6.9 (median 5, range 0–47) |
| Size of the largest polyp, mm | 7.0 ± 4.7 (median 5.5, range 2–35) |
| Colonoscopy procedure time, min | 26.5 ± 12.8 (median 23, range 9–85) |
| < 30 min | 108 (71.0%) |
| 30–59 min | 41 (27.0%) |
| 60–89 min | 3 (2.0%) |
| ≥ 90 min | 0 (0.0%) |
| Colonoscopic polypectomy method | |
| CFP | 490 (53.5%) |
| CSP | 213 (23.2%) |
| EMR | 210 (22.9%) |
| EPMR | 3 (0.3%) |
| ESD | 1 (0.1%) |
| Subsequent surveillance colonoscopies | |
| Number of polyps detected | 11.6 ± 21.2 (median 5, range 0–100) |
| Number of polyps resected | 7.1 ± 7.3 (median 5, range 0–67) |
| Size of the largest polyp, mm | 6.7 ± 4.7 (median 5, range 2–28) |
| Colonoscopy procedure time, min | 27.9 ± 14.6 (median 24, range 10–80) |
| < 30 min | 149 (66.2%) |
| 30–59 min | 64 (28.5%) |
| 60–89 min | 12 (5.3%) |
| ≥ 90 min | 0 (0.0%) |
| Colonoscopic polypectomy method | |
| CFP | 815 (56.9%) |
| CSP | 373 (26.0%) |
| EMR | 233 (16.3%) |
| EPMR | 12 (0.8%) |
| ESD | 0 (0.0%) |
Data are presented as n (%) or mean ± standard deviation with median and range.
CFP cold forceps polypectomy, CSP cold snare polypectomy, EMR endoscopic mucosal resection, EPMR endoscopic piecemeal mucosal resection, ESD endoscopic submucosal dissection.
The cumulative incidence of metachronous advanced neoplasia including cancer, adenoma ≥ 1 cm, and adenoma with high-grade dysplasia and/or villous component, was 6.3% at 1 year, 11.9% at 3 years, and 15.6% at 5 years. Advanced cancer was not detected in any patients during the follow-up period. Early cancers were diagnosed during surveillance colonoscopies in three patients (Table 4). The intervals between prior colonoscopy and early cancer diagnosis were 6, 12, and 21 months, respectively. The size of each cancer was 7, 8, and 12 mm. All three early cancers were completely resected using EMR. Histological examination showed mucosal cancer with clear resection margins in all three cases. Cancer recurrence did not develop during the 18–55-month follow-up after endoscopic resection of early cancer in these patients.
Table 4.
Summary of three patients diagnosed with early cancer during surveillance colonoscopies.
| Sex & age | Prior colonoscopy findings before diagnosis of early cancer during the surveillance colonoscopy | Interval from the previous colonoscopy | Findings of early cancer during the surveillance colonoscopy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of polyps | The largest polyp size | The most advanced histology | Complete removal of all polyps | Location of early cancer | Size of early cancer | Morphology of early cancer | Depth of invasion | Treatment | F/U after treatment | Cancer recurrence | |||
| Patient 1 | M/64 | 4 | 10 | TA with LGD | Yes | 21 months | Transverse colon | 12 mm | LST-NG-FE | Mucosa | EMR | 55 months | No |
| Patient 2 | M/54 | 12 | 16 | TA with LGD | Yes | 12 months | Rectum | 8 mm | Paris type Is | Mucosa | EMR | 32 months | No |
| Patient 3 | M/49 | 26 | 20 | TVA with HGD | No | 6 months | Sigmoid colon | 7 mm | Paris type Is | Mucosa | EMR | 18 months | No |
EMR endoscopic mucosal resection, F/U follow-up, HGD high-grade dysplasia, LGD low-grade dysplasia, TA tubular adenoma, TVA tubulovillous adenoma, LST-NG-FE laterally spreading tumor-non granular-flat elevated.
Delayed bleeding occurred in 2 (0.4%) of the 529 surveillance colonoscopies. All cases of delayed bleeding were successfully managed conservatively using endoscopic hemostasis. No perforation occurred during 529 surveillance colonoscopies.
Surgery was unnecessary for any patients because of detection of endoscopically incurable cancer or colonoscopy-associated adverse events.
Discussion
In our large cohort of patients who underwent repeat colonoscopic polypectomy after resection of ≥ 10 colorectal polyps at the baseline colonoscopy, only 3 of 152 patients developed mucosal cancer. All three lesions were endoscopically resected. No cancer recurrence occurred after the endoscopic resection. Delayed bleeding occurred in 6 (3.9%) of the 152 baseline colonoscopies and in 2 (0.4%) of the 529 surveillance colonoscopies. All delayed bleedings episodes were successfully managed using endoscopic hemostasis. No perforation occurred. Surgery was not required for any reason, including adverse colonoscopy events or the occurrence of endoscopically incurable cancer.
The effectiveness of screening and surveillance colonoscopies can be assessed by achieving their goal, which is reducing colorectal cancer mortality and possible prevention of colorectal cancer by polypectomy2,3. Therefore, we investigated colorectal cancer development and mortality as surrogate markers of the effectiveness of surveillance colonoscopy with repeat polypectomy in patients with ≥ 10 polyps at baseline colonoscopy. In our analyses, colorectal cancer was diagnosed in 3 patients (2.0%) during surveillance colonoscopies. However, all cancers were confined to the mucosal layer (pTis) and could be treated by endoscopic resection without surgery or chemotherapy. No colorectal cancer-related mortality occurred during the follow-up period. Few studies have investigated the effectiveness of repeat polypectomy as a primary interest in patients with ≥ 10 polyps. In a previous study that investigated the usefulness of repeat polypectomy in 90 patients diagnosed with familial adenomatous polyposis, colorectal cancer occurred in 5 (5.6%) during the follow-up period. All the cancers were treatable via endoscopic resection. No recurrence or metastasis was observed11. Based on the results of that study and our experience, we suggest that repeat colonoscopic polypectomy can effectively prevent colorectal cancer and reduce mortality in patients with ≥ 10 polyps.
For widespread adoption of surveillance colonoscopy with repeat polypectomy in patients with ≥ 10 polyps in clinical practice, not only oncological effectiveness but also clinical efficiency should be secured. Therefore, we investigated procedure time, an indicator of time-effectiveness, as a surrogate marker of clinical efficiency. The mean procedure time of the baseline colonoscopy was 54.9 min, and only 10 (6.6%) were ≥ 90 min, which may be an acceptable range of procedure time in clinical practice. The mean procedure time of the first surveillance colonoscopy was 31.9 min, and a procedure time ≥ 90 min was required in only three (2.0%) patients. The procedure time of subsequent surveillance colonoscopies was shorter. These findings suggest that the time-effectiveness improves for surveillance colonoscopies because of the smaller number and size of remaining/recurrent polyps after initial polypectomy primarily for large polyps at the baseline colonoscopy. An interesting point related to procedure time was the resection method. EMR was the most frequently performed resection method during the baseline colonoscopy, accounting for 55.7% of all polypectomies performed. CSP was performed in only 17.1% of polypectomies; however, this study included patients treated beginning in 2004, when CSP was not widely performed. Because of its safety and high complete resection rate12–14, CSP is currently the most commonly used polypectomy method for diminutive and small polyps. Considering its technical simplicity and shorter procedure time compared to EMR, the time-effectiveness of repeat colonoscopy management of patients with ≥ 10 polyps may be even better if CSP is more commonly used. Finally, the proportion of EMR performed decreased with an increasing number of subsequent surveillance colonoscopies. In contrast, the proportions of CSP and CFP increased, suggesting good overall time effectiveness of the strategy of repeat colonoscopy management.
The post-polypectomy bleeding rate was 0.44% in a previous analysis of 15,285 colonoscopies8, and a meta-analysis analyzing 1,966,340 colonoscopies in 21 studies found a post-polypectomy bleeding rate of 0.98% and a perforation rate of 0.08%10. In our study, although perforation did not occur, the delayed bleeding incidence was 3.9% after polypectomy of ≥ 10 polyps at the baseline colonoscopy, which is higher than the 0.44–0.98% reported in previous studies on conventional colonoscopic polypectomy8,10. However, the frequency of clinically significant bleeding after wide-field EMR for large polyps (≥ 20 mm) was 6.7% in a multicenter study15. Other studies analyzing the outcomes of EPMR showed post-polypectomy bleeding rates of 2.3–8.8%16–18. Considering these bleeding frequencies, the delayed bleeding rate of 3.9% after polypectomy of ≥ 10 polyps may be clinically acceptable. In addition, delayed bleeding occurred in only 0.4% of surveillance colonoscopies. Finally, all bleeding episodes were successfully treated without surgery. These findings suggest that repeat colonoscopy may be safe for patients with ≥ 10 polyps.
The cumulative incidence of metachronous advanced neoplasia is an important factor when recommending surveillance colonoscopy intervals. Previous studies including general populations with no focus on patients having ≥ 10 polyps showed that the cumulative incidences of metachronous advanced neoplasia were 3.9–4.7% at 3 years and 4.9–6.3% at 5 years after the removal of 1–2 low-risk adenomas. They were 5.9–6.8% at 3 years and 10–12.2% at 5 years after removal of ≥ 3 low-risk adenomas19–22. The cumulative incidence of metachronous advanced neoplasia at 1 year was 6.3% in our study, which corresponds to an incidence of 5.9–6.8%, the 3-year incidence after removal of ≥ 3 low-risk adenomas in previous studies, for which 3-year surveillance colonoscopy was traditionally recommended. Nonetheless, we suggest a 1-year surveillance colonoscopy after complete or near-complete removal of ≥ 10 polyps would be adequate because multiple polyps at baseline colonoscopy were a risk factor for missed adenomas, an important cause of interval cancer23,24. Therefore, we performed surveillance colonoscopies at 1-year intervals if all polyps were benign and removed at baseline colonoscopy, as recommended by the USMSTF 2020 recommendation25. However, if some low-risk polyps remained in situ after removal of high-risk polyps at baseline colonoscopy, surveillance colonoscopy was performed 6–9 months later to clear the remaining polyps. If malignant polyps were removed at baseline colonoscopy, the first surveillance colonoscopy was performed within 6 months, which is similar to the USMSTF 2016 recommendation of a 3–6-month interval for endoscopy after endoscopic resection of early rectal cancer26. Using this surveillance strategy, we could minimize colorectal cancer development and eliminate colorectal cancer mortality effectively, efficiently, and safely. Therefore, based on the results of our study and previous guidelines, we suggest a 1-year interval for surveillance colonoscopy after removal of ≥ 10 polyps at baseline colonoscopy. Earlier surveillance is recommended if a considerable number of polyps remain or if early cancers are resected. Modification of the subsequent surveillance intervals should be made based on the number of polyps in the first and second surveillance colonoscopies. Usually, a gradual increase in the interval for subsequent surveillance colonoscopies can be recommended based on the decreasing polyp burden because of the prior polypectomy.
This study has several limitations. First, cost-effectiveness analysis was not performed. Because patients with ≥ 10 polyps require long-term follow-up, the cost-effectiveness of repeat colonoscopies should be compared to that of other management options, such as surgery, thereby identifying the best management method from all clinical viewpoints. Second, genetic tests were conducted in only a minority of included patients. Therefore, it was difficult to analyze the usefulness of a repeat colonoscopy strategy along with other surveillance tests for other high-risk organs, such as the stomach, duodenum, and thyroid, where extra-colonic malignancies can develop in patients with genetically confirmed hereditary polyposis syndromes. Third, this study was a retrospective analysis, and the surveillance intervals were not completely consistent across the cohort. Finally, small number (mean 3.6) of surveillance colonoscopies over only 5 years in 152 patients in a single institution may make a confirmative conclusion difficult in this study. Considering the necessity of lifelong management of metachronous polyps, further prospective, large scale, long-term studies adopting a strict surveillance interval strategy are needed for more confirmative analysis.
In conclusion, colonoscopic polypectomy with repeat surveillance colonoscopies is a clinically effective, efficient, and safe management option in patients with ≥ 10 polyps. Repeat colonoscopy can minimize the risk of colorectal cancer development and mortality in these patients.
Methods
Study design
This study was a retrospective review of the medical records of patients in whom multiple colorectal polyps (≥ 10) were removed at a baseline colonoscopy at the Asan Medical Center, Seoul, from January 2004 to December 2019. A review of the colonoscopy and histology database of our institution initially identified 365 patients with ≥ 10 polyps removed in a single colonoscopy. Of these, 213 patients were excluded because of a follow-up period of < 2 years or a diagnosis of colorectal cancer requiring surgery at baseline colonoscopy (Fig. 2). Therefore, 152 patients were included in the final analyses. Medical records, including colonoscopy reports, were also reviewed. Age, sex, and family history of colorectal cancer were also assessed. If genetic tests for hereditary polyposis syndrome were performed, the results were also investigated. The numbers of polyps detected and removed at the baseline and surveillance colonoscopies were assessed. The number of polyps was described as precisely as possible; however, when more than approximately 50 polyps were observed, the number of polyps was described roughly in units of 10. The size of detected polyps and histology of resected polyps at baseline and surveillance colonoscopies were also investigated, as were the total number of surveillance colonoscopies and duration of follow-up. The protocol of this study was approved by the Asan Medical Center Institutional Review Board (IRB 2020–1696). Written informed consent was not obtained from participants because of the retrospective study design. The institutional review board of our institution waived the need for informed consent based on the non-invasive and anonymized nature of this study. This study was performed in accordance with institutional ethical guidelines and the Declaration of Helsinki.
Figure 2.
Flow chart for inclusion of patients.
Colonoscopic polypectomy at the baseline colonoscopy
The general principles of colonoscopic polypectomy at baseline colonoscopy are as follows. First, complete resection of all polyps was attempted if the number of polyps was modest (approximately ≤ 20). Second, if the number of polyps was high, high-risk polyps were removed, and some low-risk polyps were left in situ. High-risk polyps included polyps ≥ 10 mm in size and those with surface features suggesting high-grade dysplasia or cancer based on pit pattern analysis and narrow band imaging analysis. Third, all the resected polyps were retrieved for histological examination. However, diminutive polyps (< 5 mm) assessed as low-risk with high confidence could be discarded after endoscopic resection. The colonoscopy techniques used in this study included CFP, CSP, EMR, EPMR, and ESD (Fig. 3).
Figure 3.
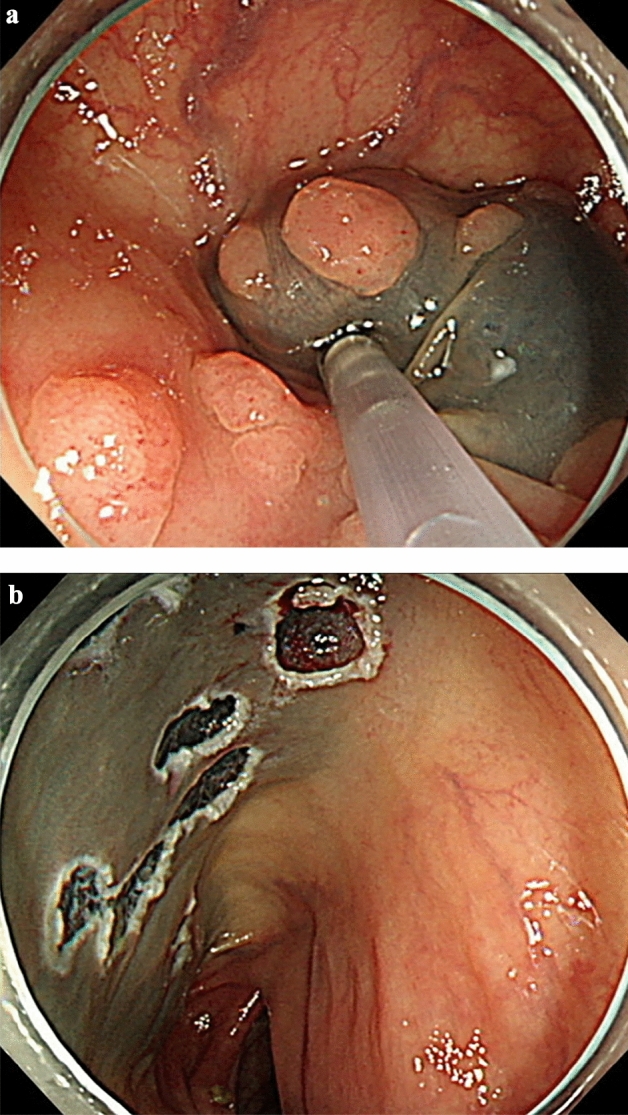
Colonoscopic polypectomy of multiple polyps. (a) Endoscopic mucosal resection (EMR) was performed for multiple polyps. (b) Multiple post-EMR ulcers were noted.
Colonoscopy procedure time and adverse events such as delayed bleeding and perforation were reviewed. Colonoscopy procedure time was defined as the time from the insertion of the colonoscope through the anus to withdrawal of the scope. Delayed bleeding was defined as hematochezia or melena that required endoscopic hemostasis after completion of colonoscopy. The perforation was diagnosed endoscopically or radiologically.
Surveillance colonoscopy
The interval between the baseline colonoscopy and the first surveillance endoscopy was determined based on the size, number, and histology of the polyps and completeness of endoscopic resection at the baseline colonoscopy. In general, surveillance colonoscopy was performed after 1 year if all polyps were removed and were benign. If some low-risk polyps remained in situ after removal of high-risk polyps at the baseline colonoscopy, surveillance colonoscopy was performed after 6–9 months. If malignant polyps were removed at the baseline colonoscopy, the first surveillance colonoscopy was performed within 6 months. The principles of colonoscopic polypectomy of the surveillance colonoscopy are similar to those of baseline colonoscopy.
The intervals between the first and subsequent surveillance colonoscopies were decided according to the findings of the first surveillance colonoscopy. If ≤ 3 polyps < 10 mm were removed, the next surveillance colonoscopy was performed at 3 years. If ≥ 10 polyps were removed, the next surveillance colonoscopy was performed at 1 year. If 4–9 polyps were removed, the next surveillance colonoscopy was performed between 1 and 3 years at the discretion of the endoscopist. Surveillance colonoscopy intervals can be modified if clinically indicated. The surveillance colonoscopy procedure time and adverse events were also reviewed.
Statistical analysis
Categorical and nominal variables are expressed as numbers with percentages, and continuous variables are presented as means ± standard deviations. Analysis of variance, Student’s t-test, and chi-squared test were performed to examine differences among groups. Statistical analyses were performed using Microsoft Office Excel 2010 (Microsoft, Redmond, WA, USA) and IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA).
Author contributions
J.H.P and J.S.B. developed the study concept and designed the study. S.W.H., S.H.P., D.H.Y., B.D.Y., and J.S.B. contributed to the resources. J.H.P. and S.W.H. contributed to the analysis and interpretation of the data. J.H.P. wrote the original draft. S.J.M., S.K.Y., and J.S.B. contributed to supervision. J.S.B. contributed to overall project administration.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy of patients. When this study approved by IRB, data should be discarded without taking it out after use but datasets are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020;70(3):145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013;369(12):1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline: Update 2020. Endoscopy. 2020;52(8):687–700. doi: 10.1055/a-1185-3109. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, et al. Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. 2020;158(4):1131–1153. doi: 10.1053/j.gastro.2019.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest. Endosc. 2012;76(2):255–263. doi: 10.1016/j.gie.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 7.Committee ASoP. Fisher DA, Maple JT, Ben-Menachem T, Cash BD, Decker GA, et al. Complications of colonoscopy. Gastrointest Endosc. 2011;74(4):745–752. doi: 10.1016/j.gie.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Derbyshire E, Hungin P, Nickerson C, Rutter MD. Post-polypectomy bleeding in the english national health service bowel cancer screening programme. Endoscopy. 2017;49(9):899–908. doi: 10.1055/s-0043-113442. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed Y, Othman M. EMR/ESD: Techniques, complications, and evidence. Curr. Gastroenterol. Rep. 2020;22(8):39. doi: 10.1007/s11894-020-00777-z. [DOI] [PubMed] [Google Scholar]
- 10.Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post-colonoscopy complications: A systematic review, time trends, and meta-analysis of population-based studies. Am. J. Gastroenterol. 2016;111(8):1092–1101. doi: 10.1038/ajg.2016.234. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa H, Mutoh M, Iwama T, Suzuki S, Abe T, Takeuchi Y, et al. Endoscopic management of familial adenomatous polyposis in patients refusing colectomy. Endoscopy. 2016;48(1):51–55. doi: 10.1055/s-0034-1392774. [DOI] [PubMed] [Google Scholar]
- 12.Ichise Y, Horiuchi A, Nakayama Y, Tanaka N. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps. Digestion. 2011;84(1):78–81. doi: 10.1159/000323959. [DOI] [PubMed] [Google Scholar]
- 13.Abe Y, Nabeta H, Koyanagi R, Nakamichi T, Hirashima H, Lefor AK, et al. Extended cold snare polypectomy for small colorectal polyps increases the R0 resection rate. Endosc. Int. Open. 2018;6(2):E254–E258. doi: 10.1055/s-0043-125312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schett B, Wallner J, Weingart V, Ayvaz A, Richter U, Stahl J, et al. Efficacy and safety of cold snare resection in preventive screening colonoscopy. Endosc. Int. Open. 2017;5(7):E580–E586. doi: 10.1055/s-0043-105491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahin FF, Rasouli KN, Byth K, Hourigan LF, Singh R, Brown GJ, et al. Prediction of clinically significant bleeding following wide-field endoscopic resection of large sessile and laterally spreading colorectal lesions: A clinical risk score. Am. J. Gastroenterol. 2016;111(8):1115–1122. doi: 10.1038/ajg.2016.235. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg. Endosc. 2013;27(9):3262–3270. doi: 10.1007/s00464-013-2903-x. [DOI] [PubMed] [Google Scholar]
- 17.Luigiano C, Consolo P, Scaffidi MG, Strangio G, Giacobbe G, Alibrandi A, et al. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: A single-center experience with long-term follow-up. Endoscopy. 2009;41(10):829–835. doi: 10.1055/s-0029-1215091. [DOI] [PubMed] [Google Scholar]
- 18.Metz AJ, Bourke MJ, Moss A, Williams SJ, Swan MP, Byth K. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy. 2011;43(6):506–511. doi: 10.1055/s-0030-1256346. [DOI] [PubMed] [Google Scholar]
- 19.Dube C, Yakubu M, McCurdy BR, Lischka A, Kone A, Walker MJ, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: A systematic review and meta-analysis. Am. J. Gastroenterol. 2017;112(12):1790–1801. doi: 10.1038/ajg.2017.360. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman D, Sullivan BA, Hauser ER, Qin X, Musselwhite LW, O'Leary MC, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862–874. doi: 10.1053/j.gastro.2019.07.052. [DOI] [PubMed] [Google Scholar]
- 21.Omata F, Deshpande GA, Suzuki H, Hayashi K, Ishii N, Matoba K, et al. Long-term cumulative incidence of metachronous advanced colorectal neoplasia after colonoscopy and a novel risk factor: A cohort study. Eur. J. Gastroenterol. Hepatol. 2021;33(11):1341–1347. doi: 10.1097/MEG.0000000000002259. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Kim TJ, Baek SY, Ahn S, Kim ER, Hong SN, et al. Risk of metachronous advanced neoplasia in patients with multiple diminutive adenomas. Am. J. Gastroenterol. 2018;113(12):1855–1861. doi: 10.1038/s41395-018-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Park SW, Kim YS, Lee KJ, Sung H, Song PH, et al. Risk factors of missed colorectal lesions after colonoscopy. Medicine (Baltimore) 2017;96(27):e7468. doi: 10.1097/MD.0000000000007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong H, Ren Y, Jiang B. Risk factors associated with missed colorectal lesions in colonoscopy and impact of colonoscopy with anesthesia on miss rate. Scand. J. Gastroenterol. 2021;56(4):484–491. doi: 10.1080/00365521.2021.1879248. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, et al. Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US multi-society task force on colorectal cancer. Gastrointest Endosc. 2020;91(3):463–485. doi: 10.1016/j.gie.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colonoscopy surveillance after colorectal cancer resection: Recommendations of the US multi-society task force on colorectal cancer. Am. J. Gastroenterol. 2016;111(3):337–346. doi: 10.1038/ajg.2016.22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy of patients. When this study approved by IRB, data should be discarded without taking it out after use but datasets are available from the corresponding author on reasonable request.