Abstract
Facing a warming climate, many tropical species—including the arthropod vectors of several infectious diseases—will be displaced to higher latitudes and elevations. These shifts are frequently projected for the future, but rarely documented in the present day. Here, we use one of the most comprehensive datasets ever compiled by medical entomologists to track the observed range limits of African malaria mosquito vectors (Anopheles spp.) from 1898 to 2016. Using a simple regression approach, we estimate that these species’ ranges gained an average of 6.5 m of elevation per year, and the southern limits of their ranges moved polewards 4.7 km per year. These shifts would be consistent with the local velocity of recent climate change, and might help explain the incursion of malaria transmission into new areas over the past few decades. Confirming that climate change underlies these shifts, and applying similar methods to other disease vectors, are important directions for future research.
Keywords: Anopheles, malaria, geographical range shift, climate change
1. Introduction
In the coming century, most scientific evidence projects that climate change will be responsible for a massive redistribution of global biodiversity [1–3]. Today, the world is already +1.2°C warmer than in the pre-industrial period [4], and this transition is already underway: tropical species are spreading towards the poles, and species everywhere are tracking their thermal niche along elevational gradients. One foundational meta-analysis estimated that, to date, terrestrial species have been moving uphill at a pace of 1.1 m per year, and to higher latitudes at a pace of 1.7 km per year [5].
Among the millions of species on the move are some of the most consequential pathogens, disease vectors and wildlife reservoirs that affect human health and economic development. For example, one study estimated that crop pathogens and agricultural pests were undergoing latitudinal shifts of 3 km per year [6]. Similarly, the North American vector of Lyme disease, the deer tick Ixodes scapularis, has spread over 40 km per year in the northeast [7,8]; the northern and elevational range limits of Ix. ricinus ticks have expanded similarly rapidly in Europe [9–11]. In recent years, mosquito-borne diseases like malaria, dengue and Zika virus have also expanded to new latitudes and elevations [12–14], and will continue to do so in the future, following the thermal limits on transmission set by their ectothermic vectors [15–17]. Some of these expansions have been facilitated by parallel global invasions of Aedes aegypti and Ae. albopictus, which have spread an estimated 250 and 150 km per year, respectively; climate change will allow their spread to continue over the coming century, albeit at a slower pace [18,19].
However, surprisingly little is known about the impacts of climate change on the anopheline vectors of malaria, lymphatic filariasis and O'nyong'nyong virus. Already, warming temperatures could have plausibly permitted expansions into highland east Africa [20]; some Anopheles species have become newly established in high-elevation sites in Latin America [21]; and a groundbreaking study recently found that in the Sahel, these mosquitoes can migrate hundreds of kilometres overnight, transported by wind currents [22]; but no studies have examined whether range shifts are already underway in these species. Here, we revisit a comprehensive dataset describing the geographical distributions of the primary malaria vectors (Anopheles spp.) in sub-Saharan Africa, and test the idea that over the last century, these species have moved southward (away from the equator) and upward (gaining elevation), consistent with hypothesized climate impacts.
2. Methods
(a) . Data
We revisit a recently published compendium of occurrence data for 22 species of Anopheles mosquitoes vectors of malaria in Africa [23]. While some of these data are resolved to finer taxonomy, we used the broadest possible definitions at the species level, treating Anopheles funestus sensu lato and sensu stricto as one species, and all members of the Anopheles gambiae complex—including An. gambiae s.l., s.s., M form, and S form—as another single species. (We elect not to stratify these further, given that some are a nested subset of others, in ways that would be challenging to develop ad hoc rules around.)
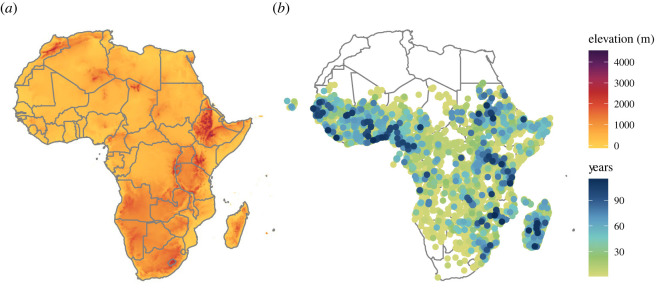
In total, the dataset comprises over a century's (1898–2016) worth of long-term, systematic entomological surveys from malaria programmes, as well as other opportunistic data collected by researchers, gathered from a mix of peer-reviewed publications, technical reports, theses, and archival records. Records span more than 1 year at the majority of sampling sites (61%), covering an average of 8.5 years between the first and last presence record (figure 1b). Parsed into unique spatio-temporal records, the dataset includes a total of 5 04 314 year-locality pairs, with an average of 22 923 records per species. While sampling fluctuates over time and increases during the Global Malaria Eradication Program (GMEP; 1955–1969), the dataset spans the entire century with an incredible level of detail (electronic supplementary material, figure S1).
Figure 1.
(a) The elevational gradient in Africa (averaged to a 10 × 10 fold higher resolution for visual clarity; (b) sites of Anopheles mosquito occurrence in sub-Saharan Africa, where colour represents the maximum temporal span of observations.
For elevational data, we used the GTOPO30 global digital elevation model downloaded as a 30 arc-second resolution grid for Africa from Data Basin (www.databasin.org; figure 1a). We extracted elevation for each distinct occurrence record, using the ‘raster' package in R version 3.3.2. In each year, we extracted the highest-elevation and southernmost records by species. A total of 116 unique locality points, situated on coastlines or islands (e.g. Cape Verde), were beyond the spatial extent of the raster and so were not assigned elevational data.
(b) . Analysis
Studies that analyse evidence for geographical range shifts tend to fall into two categories: (a) simpler study designs that measure change in observed range maxima over time, or (b) more complex designs that use an explicit model of the observation process to estimate the true range boundaries, often using data from throughout the entire geographical range. Our study falls into the first category, building on studies that use ‘pre-post' analyses of range boundaries during multiple discrete intervals [5,24]. These studies generally use a Mann–Whitney test or a similar non-parametric approach to test for significance, and estimate the pace of an observed shift in range boundaries by interpolating between two timepoints. Here, we expand on that approach by using a simple linear regression to both (a) measure the rate at which the observed maxima have changed through continuous time, and (b) apply a built-in measure of significance to the temporal trend. Our approach is only a descriptive tool for summarizing change in the data, and does not estimate true range boundaries or identify causal factors.
For each of 22 species of Anopheles, we used a simple linear regression with the ‘stats’ R package to estimate change in the maximum recorded elevation e and southernmost latitude l for species i, based on values derived from the maximum in each year t since the first record for that species:
| 2.1 |
and
| 2.2 |
The slopes of these regressions β1,i and β3,i were taken as the estimates of range shift velocity for species i. We limited this analysis to cases with at least five or more unique values over time: with this cut-off, we were able to estimate latitudinal trends for 18 species, elevational trends for 20 species, and both for a total of 17 species. Species-level estimates are given in electronic supplementary material, table S1 and other regression summary statistics are given in electronic supplementary material, table S2.
(c) . Limitations
Our dataset includes several kinds of unaddressed heterogeneity, including a century of changes in sampling methodology, species identification (including the rise of molecular methods), and malaria monitoring and control. We anticipate few of these would have a directional effect on our estimates, with the notable exception of an overall trend towards greater sampling intensity. For all modelled species except the rarest in the dataset (An. bwambae), the number of observations has a strong temporal trend (p < 0.001). This could create an artificial bias towards observing false expansions (i.e. higher extreme values may be detected in progressively larger samples drawn from the same underlying observation process).
As a preliminary sensitivity analysis, we ran a second set of models that included the number of observations (by species, per year) as a second predictor
| 2.3 |
and
| 2.4 |
This approach should be interpreted with caution, as it only evaluates whether a temporal trend in range margins is still detectable from a relatively small number of unique data points (approx. 120 or fewer) after accounting for a tightly collinear temporal trend in sampling. Other methods have been proposed that go further, and account for sampling intensity by explicitly estimating ‘true' range boundaries [25,26]. For the most part, these are better suited for smaller, simpler landscapes; a small number of approaches exist that would be able to demarcate species ranges that span the African continent (e.g. [27]), but they are orders of magnitude more computationally intensive, and should be explored in future work.
Our modelling approach also fails to capture any potential nonlinearity in long-term trends. Over the interval of observation, the rates of warming and other environmental changes have been non-stationary, and might not have reached biologically meaningful levels until the latter half of the century [28]. In addition, we expect there might be both lags between climatic changes and range shifts, and additional nonlinearities unrelated to climate in the range shift process, particularly as a result of vector control efforts. Empirical evidence for either pattern is sparse in the broader biological literature, and methodological frameworks to accommodate both are limited. In future work, some of the approaches that better address sampling limitations and climate attribution could also readily improve handling of nonlinear temporal dynamics [27].
3. Results
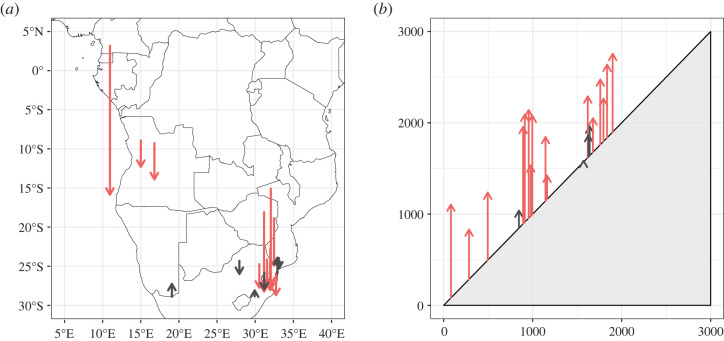
In both elevational and latitudinal maxima, we observed a strong and unambiguous signal consistent with long-term range expansion (figure 2). We found that species’ southern range maxima shifted at an estimated pace of 4.7 km each year (± s.d. of 5.7 km), with 16 of 18 species exhibiting a significant trend (cut-off of p < 0.05). Elevational maxima also shifted rapidly, at an average estimated rate of 6.5 m of altitudinal gain per year (± 3.5 m), with 18 of 20 species exhibiting a significant trend. All estimated elevational trends and most latitudinal trends (15 of 18) were positive, i.e. were consistent with the direction expected from climate-linked geographical range shifts. The correlation between species’ estimated velocity of elevational shifts and estimated velocity of latitudinal shifts (r = 0.34) was insignificant (p = 0.19), suggesting that landscape-level patterns had a stronger influence on the pace of range shifts than variation among species’ intrinsic capacity for dispersal.
Figure 2.
Estimated shifts in Anopheles species’ observed latitudinal and elevational maxima over the twentieth century, where each arrow gives one species’ estimate (see electronic supplementary material, table S1). (a) Species’ estimated southern maxima, where starting points are given at the longitude of the southmost point in the first half of the century (1900–1950), and the arrow shows the estimated latitudinal shift from 1900 to 2000 (chosen as a standardized unit for visualization, rather than the entire observation period, given that some species are sampled over slightly different intervals). (b) Estimated elevational gain, 1900–2000 (y-axis), on a 1:1 elevational ‘gradient' (x-axis gives initial estimated elevational position). Red arrows indicate species for which temporal trends were statistically significant (p < 0.05).
Finally, the sensitivity analysis revealed that observed range maxima were responsive to sampling size, but with different implications for the two variables. Including sampling reduced the rate of observed shifts (though they remained directionally consistent with expectations) to an average latitudinal gain of 2.38 ± 1.66 km per year and an elevational gain of 1.90 ± 0.55 m per year. The effect of sampling was only significant (at p < 0.05) in 12 of 18 models of latitude, and 14 of 18 temporal trends in latitude remained significant. However, for elevation, all 20 models recovered a significant effect of sampling, and only six species still showed a significant trend over time. These results suggest that observed shifts are unlikely to be artefactual, but that sampling effort contributes to observed trends, and may be a more serious confounder over the smaller landscapes that are relevant to elevational gradients.
4. Discussion
We found clear evidence that Anopheles mosquitoes have undergone rapid range shifts over the twentieth century, challenging a long-standing assumption in historical epidemiology that mosquito ranges are mostly stationary over decades or centuries [29,30]. Our findings were consistent with expectations for the direction and pace of climate-linked range shifts, including previous estimates of climate velocity in sub-Saharan Africa [31]. Future work could build on these findings by using more sophisticated methods, such as spatio-temporal occupancy models [27], to formally test the explanatory power of climate change while accounting for sampling bias. If confirmed, the rapid expansion of Anopheles ranges—on average, over 500 km southward and 700 m uphill during the period of observation—would rank among the more consequential climate change impacts on African biodiversity that have been observed to date.
These findings could also suggest a new facet of the complex and contentious relationship between climate change and shifting malaria endemicity in Africa. The thermal limits of the Plasmodium parasite are well established [32,33], and readily superimposed onto climate projections. This mechanistic approach has suggested that malaria will spread into highland east Africa and expand at its southern limits, but transmission will likely decrease as west and central Africa become prohibitively warm [17,34]. Beginning in the early 2000s, several studies have proposed that these impacts might already be observable in east Africa [35–37]. Others have disputed these conclusions, suggesting that they are irreconcilable with long-term progress towards malaria elimination, that trends in the region are better explained by lapsed control programmes and growing drug resistance [38–41], and that climate change is inconsistent with long-term trends at the continental scale [42]. These debates—which remain unresolved—have focused nearly entirely on P. falciparum prevalence or incidence, and have rarely considered direct impacts of climate change on the mosquito vectors of the parasite.
If climate change has allowed Anopheles mosquitoes to invade once-protected colder areas, this might help explain observed changes in the altitudinal limits of malaria transmission [13], without presuming the veracity of a climate-driven, long-term increase in prevalence in these areas. Confirming this chain of causation would be an important step towards resolving one of the longest-standing debates in climate and health research. More broadly, in the coming years, these sorts of direct links between climate, biodiversity change and disease emergence will be increasingly important to quantify in real-time, not just to document a changing world but also to identify and address healthcare needs in newly vulnerable populations.
Data accessibility
No original data are used in this study. The Anopheles dataset is freely available from other previously published research [23].
Electronic supplementary material is available online [43].
Authors' contributions
C.J.C.: conceptualization, data curation, formal analysis, investigation, methodology, supervision, visualization, writing---original draft, writing—review and editing; E.B.: conceptualization, investigation, writing—original draft, writing—review and editing; E.M.: conceptualization, writing—original draft, writing—review and editing; T.N.: conceptualization, writing—original draft, writing—review and editing; S.B.: methodology, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2, 686-690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 2.García Molinos J, et al. 2015. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Chang. 6, 83-88. ( 10.1038/nclimate2769) [DOI] [Google Scholar]
- 3.Lenoir J, Svenning J-C. 2015. Climate-related range shifts—a global multidimensional synthesis and new research directions. Ecography 38, 15-28. ( 10.1111/ecog.00967) [DOI] [Google Scholar]
- 4.Masson-Delmotte Z, Pirani C. 2021. Climate change 2021: the physical science basis. Intergovernmental Panel on Climate Change. See https://report.ipcc.ch/ar6/wg1/IPCC_AR6_WGI_FullReport.pdf. [Google Scholar]
- 5.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024-1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 6.Bebber DP, Ramotowski MAT, Gurr SJ. 2013. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 3, 985-988. ( 10.1038/nclimate1990) [DOI] [Google Scholar]
- 7.Leighton PA, Koffi JK, Pelcat Y, Robbin Lindsay L, Ogden NH. 2012. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 49, 457-464. ( 10.1111/j.1365-2664.2012.02112.x) [DOI] [Google Scholar]
- 8.Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, Jardine CM. 2017. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS ONE 12, e0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindgren E, Tälleklint L, Polfeldt T. 2000. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 108, 119-123. ( 10.1289/ehp.00108119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaenson TGT, Jaenson DGE, Eisen L, Petersson E, Lindgren E. 2012. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 5, 8. ( 10.1186/1756-3305-5-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martello E, Mannelli A, Ragagli C, Ambrogi C, Selmi M, Ceballos LA, Tomassone L. 2014. Range expansion of Ixodes ricinus to higher altitude, and co-infestation of small rodents with Dermacentor marginatus in the Northern Apennines, Italy. Ticks Tick Borne Dis. 5, 970-974. ( 10.1016/j.ttbdis.2014.07.021) [DOI] [PubMed] [Google Scholar]
- 12.Grubaugh ND, et al. 2017. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature 546, 401-405. ( 10.1038/nature22400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Ruiz Carrascal D, Pascual M. 2014. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 343, 1154-1158. ( 10.1126/science.1244325) [DOI] [PubMed] [Google Scholar]
- 14.Rijal KR, et al. 2021. Epidemiology of dengue virus infections in Nepal, 2006–2019. Infectious Diseases Poverty 10, 52. ( 10.1186/s40249-021-00837-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colón-González FJ, Sewe MO, Tompkins AM, Sjödin H, Casallas A, Rocklöv J, Caminade C, Lowe R. 2021. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet Health 5, e404-e414. ( 10.1016/S2542-5196(21)00132-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. 2019. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 13, e0007213. ( 10.1371/journal.pntd.0007213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mordecai EA, Ryan SJ, Caldwell JM, Shah MM, LaBeaud AD. 2020. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet Health 4, e416-e423. ( 10.1016/S2542-5196(20)30178-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer MUG, et al. 2019. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol 4, 854-863. ( 10.1038/s41564-019-0376-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippi CA, Stewart-Ibarra AM, Franklin Bajaña Loor M, Dueñas Zambrano JE, Espinoza Lopez NA, Blackburn JK, Ryan SJ. 2019. Geographic shifts in Aedes aegypti habitat suitability in Ecuador using larval surveillance data and ecological niche modeling: implications of climate change for public health vector control. PLoS Negl. Trop. Diseases 13, e0007322. ( 10.1371/journal.pntd.0007322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni MA, Desrochers RE, Kajeguka DC, Kaaya RD, Tomayer A, Kweka EJ, Protopopoff N, Mosha FW. 2016. 10 years of environmental change on the slopes of Mount Kilimanjaro and its associated shift in malaria vector distributions. Front Public Health 4, 281. ( 10.3389/fpubh.2016.00281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinault LL, Hunter FF. 2011. New highland distribution records of multiple Anopheles species in the Ecuadorian Andes. Malar. J. 10, 236. ( 10.1186/1475-2875-10-236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huestis DL, et al. 2019. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 574, 404-408. ( 10.1038/s41586-019-1622-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyalo D, Amratia P, Mundia CW, Mbogo CM, Coetzee M, Snow RW. 2017. A geo-coded inventory of anophelines in the Afrotropical region south of the Sahara: 1898-2016. Wellcome Open Research. 2, 57. ( 10.12688/wellcomeopenres.12187.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tingley MW, Beissinger SR. 2009. Detecting range shifts from historical species occurrences: new perspectives on old data. Trends Ecol. Evol. 24, 625-633. ( 10.1016/j.tree.2009.05.009) [DOI] [PubMed] [Google Scholar]
- 25.Hassall C, Thompson DJ. 2010. Accounting for recorder effort in the detection of range shifts from historical data. Methods Ecol. Evol. 1, 343-350. ( 10.1111/j.2041-210X.2010.00039.x) [DOI] [Google Scholar]
- 26.Bates AE, et al. 2015. Distinguishing geographical range shifts from artefacts of detectability and sampling effort. Divers. Distrib. 21, 13-22. ( 10.1111/ddi.12263) [DOI] [Google Scholar]
- 27.Rushing CS, Royle JA, Ziolkowski DJ, Pardieck KL. 2019. Modeling spatially and temporally complex range dynamics when detection is imperfect. Sci. Rep. 9, 12805. ( 10.1038/s41598-019-48851-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stott PA, Gillett NP, Hegerl GC, Karoly DJ, Stone DA, Zhang X, Zwiers F. 2010. Detection and attribution of climate change: a regional perspective. Wiley Interdiscip. Rev. Clim. Change 1, 192-211. ( 10.1002/wcc.34) [DOI] [Google Scholar]
- 29.Newfield TP. 2017. Malaria and malaria-like disease in the early Middle Ages. Early Medieval Eur. 25, 251-300. ( 10.1111/emed.12212) [DOI] [Google Scholar]
- 30.Sallares R. 2002. Malaria and Rome: a history of malaria in Ancient Italy. Oxford: OUP. [Google Scholar]
- 31.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462, 1052-1055. ( 10.1038/nature08649) [DOI] [PubMed] [Google Scholar]
- 32.Villena OC, Ryan SJ, Murdock CC, Johnson LR. 2022. Temperature impacts the environmental suitability for malaria transmission by Anopheles gambiae and Anopheles stephensi. Ecology 103, e3685. ( 10.1002/ecy.3685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mordecai EA, et al. 2013. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 16, 22-30. ( 10.1111/ele.12015) [DOI] [PubMed] [Google Scholar]
- 34.Ryan SJ, McNally A, Johnson LR, Mordecai EA, Ben-Horin T, Paaijmans K, Lafferty KD. 2015. Mapping physiological suitability limits for malaria in Africa under climate change. Vector Borne Zoonotic Dis. 15, 718-725. ( 10.1089/vbz.2015.1822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascual M, Ahumada JA, Chaves LF, Rodó X, Bouma M. 2006. Malaria resurgence in the East African highlands: temperature trends revisited. Proc. Natl Acad. Sci. USA 103, 5829-5834. ( 10.1073/pnas.0508929103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsay SW, Martens WJ. 1998. Malaria in the African highlands: past, present and future. Bull. World Health Organ. 76, 33-45. [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso D, Bouma MJ, Pascual M. 2011. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proc. R. Soc. B 278, 1661-1669. ( 10.1098/rspb.2010.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiter P. 2008. Global warming and malaria: knowing the horse before hitching the cart. Malar. J. 7, S3. ( 10.1186/1475-2875-7-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hay SI, Rogers DJ, Randolph SE, Stern DI, Cox J, Shanks GD, Snow RW. 2002. Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol. 18, 530-534. ( 10.1016/S1471-4922(02)02374-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, Myers MF, Snow RW. 2002. Regional warming and malaria resurgence. Nature 420, 628. ( 10.1038/420628a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, Myers MF, Snow RW. 2002. Climate change and the resurgence of malaria in the East African highlands. Nature 415, 905-909. ( 10.1038/415905a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snow RW, Sartorius B, Kyalo D, Maina J, Amratia P, Mundia CW, Bejon P, Noor AM. 2017. The prevalence of Plasmodium falciparum in sub-Saharan Africa since 1900. Nature 550, 515-518. ( 10.1038/nature24059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlson CJ, Bannon E, Mendenhall E, Newfield T, Bansal S. 2023. Rapid range shifts in African Anopheles mosquitoes over the last century. Figshare. ( 10.6084/m9.figshare.c.6425442) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data are used in this study. The Anopheles dataset is freely available from other previously published research [23].
Electronic supplementary material is available online [43].