Abstract
Background
Conventional quantitative diffusion-weighted imaging (DWI) is sensitive to changes in tissue microstructure, but its application to evaluating patients with orthopedic hardware has generally been limited due to metallic susceptibility artifacts. The apparent diffusion coefficient (ADC) and T2-values from a multi-spectral imaging (MSI) DWI combined with 2D multi-spectral imaging with a 2D periodically rotated overlapping parallel lines with enhanced reconstruction (2D-MSI PROPELLER DWI) based sequence and a MAVRIC based T2 mapping sequence, respectively, may mitigate the artifact and provide additional quantitative information on synovial reactions in individuals with total hip arthroplasty (THA). The aim of this pilot study is to utilize a 2D-MSI PROPELLER DWI and a MAVRIC-based T2 mapping to evaluate ADC and T2-values of synovial reactions in patients with THA.
Methods
Coronal morphologic MRIs from THA patients underwent evaluation of the synovium and were assigned a synovial classification of ‘normal’, or ‘grouped abnormal’ (consisting of sub-groups ‘infection’, ‘polymeric’, ‘metallosis’, ‘adverse local tissue reaction’ [ALTR], or ‘non-specific’) and type of synovial reaction present (fluid-like, solid-like, or mixed). Regions of interest (ROIs) were placed in synovial reactions for measurement of ADC and T2-values, obtained from the 2D-MSI PROPELLER DWI and T2-MAVRIC sequences, respectively. A one-way analysis of variance (ANOVA) and Kruskal-Wallis rank sum tests were used to compare the differences in ADC and T2-values across the different synovial reaction classifications. A Kruskal-Wallis test was used to compare the ROI areas for the ADC and T2-values. A principal component analysis (PCA) was performed to evaluate the possible effects of ADC values, size of the ADC ROI, T2 values, and size of the T2 ROI with respect to synovial reaction classification.
Results
Differences of ADC and T2 among the individual synovial reactions were not found. A difference of ADC between ‘normal’ and ‘grouped abnormal’ synovial reactions was also not detected even as the ADC area of ‘grouped abnormal’ synovial reactions were significantly larger (p=0.02). The ‘grouped abnormal’ synovial reactions had significantly shorter T2 values than ‘normal’ synovial reactions (p=0.02), and that the T2 area of ‘grouped abnormal’ synovial reactions were significantly larger (p=0.01). A larger ROI area on the T2-maps was observed in the mixed synovial reaction type as compared to the fluid-like reaction type area (p = 0.01). Heterogeneity was noted in calculated ADC and T2 maps. PCA analysis revealed obvious clustering by the ‘normal’ and ‘grouped abnormal’ classifications.
Conclusions:
2D-MSI PROPELLER DWI and MAVRIC-T2 generate quantitative images of periprosthetic tissues within clinically feasible scan times. The combination of derived ADC and T2-values with area of synovial reaction may aid in differentiating normal from abnormal synovial reactions between types of synovial reactions in patients with THA.
Keywords: MAVRIC, T2, ADC, arthroplasty
1. Introduction
Total hip arthroplasty (THA) is the current surgical standard for the treatment of end-stage hip osteoarthritis (OA) with excellent outcomes in reducing joint pain and improving joint function [1]. The future demand for primary and revision THA is likely to increase. Compared to the annual use of THAs in 2014, the projected increase for primary THAs in the U.S. is expected to be 284% by 2040 for both females and males, and across all age groups [2]. This expected rise is caused in part by an aging population, obesity, and rising incidence of OA [3]. Despite the effectiveness of THAs for the treatment of end-stage hip OA, some patients have reported the development of adverse local tissue reactions (ALTR) [4] or prosthetic joint infections (PJI) [5]. Given the rising rates of primary THA, overall revision rates caused by postsurgical complications have also increased, resulting in higher morbidity and operative costs [6], despite advances in implant design and surgical techniques.
Magnetic resonance imaging (MRI) serves as an effective and non-invasive method for monitoring patients with THA for adverse reactions in periprosthetic tissues [7]. Compared to conventional radiography, computerized tomography (CT) and ultrasound, MRI generates images with superior soft-tissue contrast and multiplanar visualization without the need for ionizing radiation. While MRI acquisitions are impacted by significant in-plane and through-plane distortions, pixel pile-up, and signal voids caused by high magnetic susceptibility of implanted metallic orthopaedic components [8], newer three-dimensional (3D) multispectral imaging (MSI) techniques, such as multi-acquisition variable resonance image combination (MAVRIC) can effectively mitigate these artifacts [9]. MAVRIC combines 3D image datasets from distinct frequency bands offset from the central Larmor frequency to overcome signal dropout commonly seen in high-bandwidth fast-spin-echo (FSE) imaging [9]. A total of 24 spectral bins is commonly acquired [9] unless a calibration is used to reduce the number of bins [10]. In previous studies with histologic validation, MAVRIC has been demonstrated to significantly improve periprosthetic soft-tissue visualization of a patient’s synovial response to an implanted hip arthroplasty compared to conventional FSE sequences [11,12].
Beyond morphologic imaging, quantitative MRI (qMRI) methods such as diffusion-weighted imaging (DWI) and T2 mapping have been shown to be sensitive to changes in soft-tissue microstructure, such as articular cartilage, during the development of OA [13,14]. DWI is routinely utilized in stroke evaluation, [15] cancer diagnosis [16] and treatment response [17], as well as musculoskeletal disorders [18,19] with good repeatability [20,21]. Diffusion imaging has previously been demonstrated to detect changes in the spinal cord not detected with conventional T1 or T2 weighted contrasts [22], emphasizing how DWI may complement conventional sequences. DWI also provides means to quantify apparent diffusion coefficient (ADC) values that reflect tissue cellularity and cell membrane integrity. DWI has been previously utilized to non-invasively evaluate and quantify synovitis in knees as an alternative to gadolinium-based contrast agents [23,24]. However, conventional DWI utilizes an echo planar imaging (EPI) readout, which is more susceptible to image distortion than FSE when orthopaedic hardware is present. A multi-spectral DWI technique has been developed which combines 2D multi-spectral imaging with a 2D periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER)-FSE readout and diffusion-encoding to permit calculation of ADC values in the presence of metallic hardware [25]. The application of 2D-MSI PROPELLER DWI has had limited application in the clinical environment to date [26].
Similarly, T2 is sensitive to interactions between water molecules and the surrounding extracellular matrix. While T2 mapping techniques are commonly FSE-based [27], substantial susceptibility artifacts are still generated in the presence of orthopaedic hardware [28]. MAVRIC-based T2 mapping has been shown to be effective in reducing susceptibility artifact near orthopaedic hardware enabling accurate calculation of T2-values [29]. Recently, a large cross-sectional cohort found longer T2-values of ‘fluid-like’ synovial reactions in patients with THA, as well as prolonged T2-values in patients with synovial dehiscence and decompression [30]. As more individuals receive THAs, it would be beneficial to utilize quantitative imaging to complement morphologic imaging, especially for characterizing synovial reactions to implanted arthroplasty devices. Therefore, the purpose of this pilot study was to utilize a 2D-MSI PROPELLER DWI and a MAVRIC-based T2 mapping to evaluate ADC and T2-values of synovial reactions in patients with THA. We hypothesized that DWI ADC and T2-value differences would be found between different morphologic classifications of synovial reactions in patients with THA.
2. Methods
2.1. Study Population
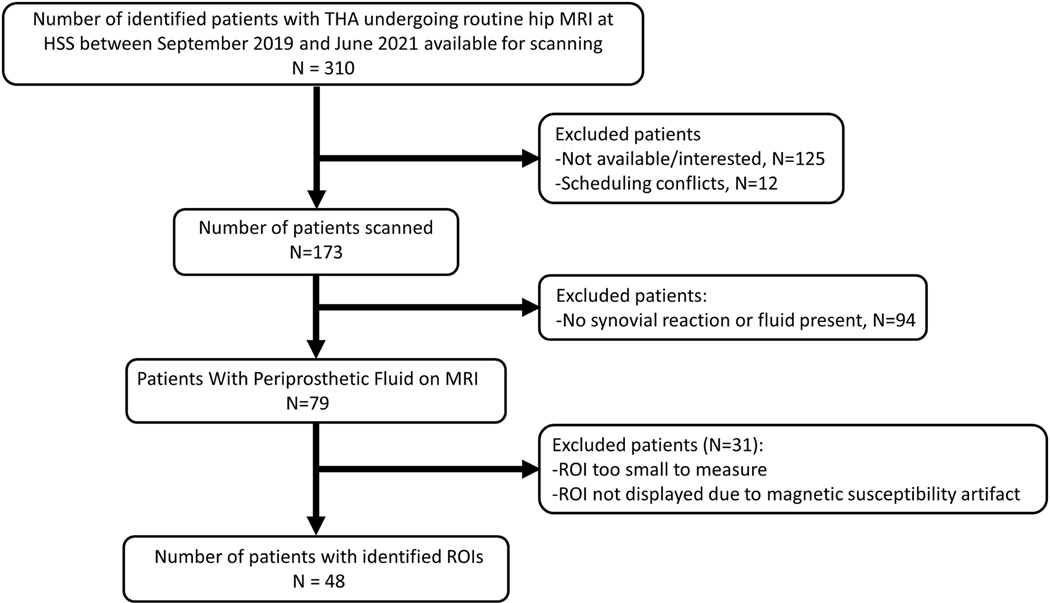
This pilot study was approved by our Institutional Review Board. Patients with THA undergoing routine MRI at our institution were enrolled between September 2019 and June 2021 Written informed consent was obtained from all subjects (Figure 1). A total of 48 subjects were enrolled in this study, as outlined in Figure 1.
Figure 1.
Study enrollment chart
2.2. Image Acquisition
All MR imaging was performed using a 1.5T clinical scanner (MR450, GE Healthcare, Chicago, IL) with an 8-channel cardiac coil. The protocol included conventional axial, sagittal, and coronal 2D-FSE, coronal MAVRIC selective (MAVRIC SL) short-tau inversion recovery (STIR) imaging [12], and an isotropic MAVRIC SL [31] acquisition to assess the presence and morphology of synovial reactions from soft-tissues around the THA. A 2D-MSI PROPELLER DWI [26] and a T2-MAVRIC [31] series were acquired immediately following the morphologic acquisitions, with scan parameters shown in Table 1.
Table 1.
MRI Acquisition Parameters
| Acquisition Parameter | Acquired Series | |||
|---|---|---|---|---|
| MAVRIC-SL | MAVRIC-SL STIR | 2D-MSI PROPELLER DWI | MAVRIC T2 Mapping | |
| Echo Time (ms) | 30 | 40 | 65 | 9.8, 50.8 |
| Repetition Time (ms) | 4000−6000 | 4000−5000 | 2000 | 3500 |
| Field-of-View (cm) | 36−40 | 36−40 | 36−40 | 36−40 |
| Acquisition Matrix | (278−308) x (278−308) | 512 × 256 | 96 × 96 | 512 × 256 |
| Slice Thickness (mm) | 1.3 | 3.6 | 4 | 3.5 |
| Voxel Size (mm3) | 1.3 × 1.3 × 1.3 | (0.70−0.8) x (1.4−1.6) x 3.6 | (3.8−4.2) x (3.8−4.2) x 4 | (0.7−0.8) x (1.4−1.6) x 3.5 |
| Echo Train Length | 48 | 24 | 32 | 48 |
| b-value | - | - | 0, 600 3 gradient directions |
- |
| Number of Excitations | 0.5 | 0.5 | 9 | 0.5 |
| Number of Frequency Bins | 8−16 | 24 | 3 | 14 |
| Frequency Offset (Hz) | 1000 | 1000 | 600 | 1000 |
| RF Pulse Bandwidth (kHz) | 2.25 | 2.25 | 2.25 | 2.25 |
| Receiver Bandwidth (kHz) | ±125 | ±125 | ±31.25 | ±125 |
| Excitation Angle (Deg) | 90 | 90 | 90 | 90 |
| Refocusing Angle (Deg) | 135 | 135 | 180 | 110 |
| Acquisition Time (min) | ∼7 | ∼6 | ∼6 | ∼6 |
Notes: MRI: Magnetic resonance imaging; MAVRIC SL: multi-acquisition variable resonance image combination selective; STIR: short-tau inversion recovery; PROPELLER: periodically rotated overlapping parallel lines with enhanced reconstruction; DWI: diffusion weighted imaging; RF: Radiofrequency. RF Pulse Bandwidth expressed as full width at half maximum (FWHM).
2.3. Image Analysis
The isotropic MAVRIC-SL and MAVRIC STIR images were used to evaluate for the presence of synovitis (yes/no), impression of synovial reaction type (fluid-like, solid-like, or mixed, composed of both fluid and solid components), and synovial classification by a single board-certified radiologist (HGP with 31 years of musculoskeletal MRI experience. The synovial classifications assigned were: 1) ‘normal’: presence of low capsular signal intensity compared to skeletal muscle and thin capsular tissue [32]; 2) ‘ALTR’ - thickened, hyperintense capsule commonly presented with a poor zone of demarcation between neighboring muscle signal intensity [33]; 3) ‘metallosis’: low-signal intensity deposits (LSIDs) found in intra- or extracapsular locations present in all MRI sequences with lower signal intensity than normal capsular tissue [34,35]; 4) ‘polymeric’: intracapsular foci of particulate with intermediate signal intensity debris [32]; 5) ‘infection’: lamellated synovial lining with presence of pericapsular edema [36]; 6) ‘non-specific abnormal’ – reactions that did not fall within any of the above descriptions [12].
The intra-rater repeatability of this synovial classification method has been shown to be substantial to almost perfect (Gwet’s AC1 range: 0.59−0.99) with moderate to almost perfect (Gwet’s AC1 range 0.65−0.97) inter-rater agreement between two musculoskeletal radiologists who independently evaluated 187 subjects while blinded to the implant-bearing surface [12]. The repeatability to define the extent of synovial reaction (i.e., synovial volume) was previously determined to have an intraclass correlation coefficient of 0.99 and a coefficient of repeatability of 1.8 cm3 [35].
Our institutional picture archiving and communication system (PACS) was used to define regions of interest within the synovial reactions on the 2D-MSI PROPELLER DWI images, with simultaneous cross referencing using the isotropic MAVRIC-SL images for to ensure that the regions were aligned. Mean ADC and T2-values were calculated as the mean value within the region of interest (ROI). T2 maps were generated by the scanning software from the T2-MAVRIC software [30] on a pixel-by-pixel basis as T2 = (TE2 − TE1) / [ln(SI1) − ln(SI2)], where TE1 and TE2 are the first and second echo times, and SI1 and SI2 corresponding signal intensities at the corresponding first and second echo times. Similarly, ADC maps were generated from the 2D-MSI PROPELLER DWI acquisition software as: , where S0 is the b-value=0 signal, and Sx, Sy and Sz are diffusivity-encoded signals in all three directions.
2.4. Statistical Analysis
Continuous variables were represented using means and standard deviations. A one-way analysis of variance (ANOVA) was used to compare the differences in ADC and T2-values across the different synovial reaction classifications. A Kruskal-Wallis rank sum test was used to compare the ROI areas for the ADC and T2-values, as the data displayed a non-normal distribution. Post-hoc analysis with Bonferroni correction was performed for multiple comparisons. The 5 classifications of ALTR, Metallosis, Polymeric, Infection and Non-Specific Abnormal were pooled as ‘grouped abnormal’. The Student’s t test was used to compare the difference between normal and grouped abnormal groups. The natural log transformation of the ROI areas for the ADC and T2-values was used to fit the Student’s t test assumptions. A principal component analysis (PCA) was performed to evaluate the possible effects of ADC values, size of the ADC ROI, T2-values, and size of the T2 ROI with respect to synovial reaction classification. A paired samples t-test was used to assess the difference of ADC and T2-values between the repeated ROIs from the same THA across all subjects. Since no significant differences were detected, one pair of ROIs was randomly chosen and included for the analysis. All statistical analyses were performed using R (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria). A p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Enrollment and Synovial Regions of Interest
A total of 48 subjects (age = 68.0±7.8 years [mean ± standard deviation, 26F/22M]) were enrolled, comprising a total of 53 THAs. A total of 37 subjects had one ROI from one THA and 11 subjects had multiple ROIs from unilateral or bilateral THA. For the subjects with more than one ROI for an imaged THA: 3 subjects had one ROI from bilateral THAs producing 6 ROIs in total; 1 subject had two ROIs in the right THA and one ROI in the left THA and each had different synovial classifications; 1 subject had two ROIs from the left THA which had the same synovial classification and one ROI in the right THA; 4 subjects had two ROIs from the same THA and the synovial classifications were the same; 1 subject had two ROIs from the same THA but the synovial classifications were different; 1 subject who three ROIs from the same THA and all ROIs the same synovial classification. In summary, a total of 55 distinct synovial ROIs were included within the 53 THA from 48 subjects.
3.2. ROI Synovial Classification and Type
Over half of the synovial reactions were classified as ‘normal’ (30/55, 54.5%) (Table 2). The other ROIs with synovitis were classified as: ‘ALTR’ (2/55, 3.6%), ‘infection’ (4/55, 7.3%), ‘metallosis’ (7/55, 12.7%), ‘polymeric’ (9/55, 16.4%) or ‘non-specific abnormal’ (3/55, 5.5%).
Table 2.
Morphologic and Quantitative MRI Outcome Measures by Synovial Reaction Classification
| Variables | Normal (n=30) | Non-Specific Abnormal (n=3) | Infection (n=4) | ALTR (n=2) | Polymeric (n=9) | Metallosis (n=7) | p-values§ |
|---|---|---|---|---|---|---|---|
| Fluid-Like Reaction | 30 | 1 | 2 | 0 | 0 | 0 | |
| Mixed Reaction | 0 | 2 | 2 | 2 | 9 | 7 | |
| ADC (μm2/s) | 1561.5±386.9 | 1099.0±206.5 | 2143.5±359.2 | 1554.8±594.9 | 1875.6±523.4 | 1691.4±810.6 | 0.25 |
| Area of ADC ROI (mm2) | 78.9±79.9 | 74.1±46.9 | 143.7±101.1 | 122.5±3.7 | 88.5±73.1 | 143.4±93.4 | 0.10 |
| T2 (ms) | 287.2±104.9 | 86.6±44.3 | 220.4±75.2 | 127±28.4 | 274.1±129.6 | 259.7±168.1 | 0.85 |
| Area of T2 ROI (mm2) | 55.5±61.7 | 46.4±27.1 | 93.2±50.2 | 128±18.1 | 60.5±39.4 | 94.6±50.7 | 0.04 |
Notes: ADC = Apparent diffusion coefficient; ROI = Region of interest; ADC, T2, and area ROI values expressed as mean ± SD.
= Results of comparing variables only for Normal, Polymeric, and Metallosis synovial reactions classifications using an ANOVA or a Kruskal-Wallis rank sum test due to limited sample size of other synovial classifications. Bolded values indicate statistical significance.
For the ROIs evaluated, there was a greater proportion with a fluid-like synovial reaction (33/55, 60%) than ROIs with a mixed synovial reaction (22/55, 40%) (Table 2). All ROIs with a synovial classification of ‘normal’ had a fluid-like synovial reaction, while only mixed synovial reactions were found amongst ROIs with synovial classifications of either ‘ALTR’, ‘metallosis’, or ‘polymeric’. Several mixed synovial reactions were found among synovial classifications of ‘infection’ (2/4, 50%) and ‘non-specific abnormal’ (1/3, 33%) (Table 2).
3.3. ADC and T2-Values
The comparison of ADC and T2-values was conducted only among the three groups of normal, metallosis and polymeric because of the small samples of ALTR, infection and nonspecific abnormal groups. As shown in Table 2 and Figure 2, no significant differences were found in ADC values (p=0.25) and T2 (p=0.85) across the different synovial classifications. The Kruskal-Wallis rank sum test found no difference in the area of ADC ROIs across the different synovial classifications (p=0.10), but a significant difference was found in the in the area of T2 ROIs by synovial classification (p=0.04). The results from multiple comparison showed that T2 area ROI in the metallosis group was significantly larger than in the normal group (P=0.04). A difference of ADC value between ‘normal’ and ‘grouped abnormal’ synovial reactions was not detected (p=0.20), even as the ADC area of ‘grouped abnormal’ synovial reactions were significantly larger (p=0.02). The analysis found that ‘normal’ synovial reactions had significantly longer T2-values than ‘grouped abnormal’ synovial reactions (p=0.02), and that the T2 area of ‘grouped anormal’ synovial reactions were significantly larger (p=0.01) (Table 3, Figure 3).
Figure 2.
Box plots displaying ADC values (A), T2-values (B), and the area of the regions of interest for the corresponding ADC values (C) and T2-values (D) for each synovial classification. For each box, the central mark indicates the median value, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to 1.5 times the interquartile range.
Table 3.
Morphologic and Quantitative MRI Outcome Measures by Grouped Synovial Reaction Classification
| Variables | Normal (n=30) | Grouped Abnormal (n=25) | p-values |
|---|---|---|---|
| ADC (μm2/s) | 1561.5 ± 386.9 | 1748.0 ± 615.6 | 0.20 |
| Area of ADC ROI (mm2) | 78.9 ± 79.9 | 113.7 ± 79.2 | 0.02 |
| T2 (ms) | 287.2 ± 104.9 | 227.2 ± 134.2 | 0.02 |
| Area of T2 ROI (mm2) | 55.5 ± 61.7 | 79.0 ± 46.0 | 0.01 |
Notes: Apparent coefficient; Region of values as mean ± values statistical significance.
ADC = diffusion ROI = interest; All expressed SD. Bolded indicate
Figure 3.
Box plots displaying ADC values (A) and T2-values (B), and the area of the regions of interest for the corresponding ADC values (C) and T2-values (D) for ‘normal’ and ‘grouped abnormal’ synovial classifications. On each box, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to 1.5 times the interquartile range.
Table 4 shows the comparisons between synovial reaction types of ‘fluid-like’ or ‘mixed’. A larger area was observed in the mixed synovial reaction type with the area of the T2 ROI (p=0.01) and ADC area ROI with borderline significance (p = 0.05) compared to the fluid-like reaction type. The ADC and T2-values were not significantly different between the two types.
Table 4:
Morphologic and Quantitative MRI Outcome Measures by Synovial Reaction Type
| Synovial Reaction Type | |||
|---|---|---|---|
| Variables | Fluid-Like (n=33) | Mixed (n=22) | p-values |
| ADC (μm2/s) | 1593.6 ± 426.4 | 1725.4 ± 611.9 | 0.39 |
| Area of ADC ROI (mm2) | 84.0 ± 83.2 | 110.9 ± 75.9 | 0.05 |
| T2 (ms) | 277.8 ± 107.2 | 233.1 ± 139.2 | 0.21 |
| Area of T2 ROI (mm2) | 56.4 ± 60.2 | 80.8 ± 46.3 | 0.01 |
Notes: ADC = Apparent diffusion coefficient; ROI = Region of interest; Values for ADC and T2 expressed as mean ± SD.
The first principal component from PCA accounted for 49.2% of data variance and that the second principal component accounted for 29.3% of data variance, using the ADC and T2-values, and corresponding ROI areas. As shown in Figure 4A and 4B, ROIs assigned a synovial classification of ‘normal’ were separated from the other synovial classifications. The PCA score plot also displays that ROIs assigned as having a fluid-like synovial reaction were separated from ROIs assigned as having a mixed reaction (Fig. 4C). Representative ADC and T2 maps are shown in Figure 5. The maps for ROIs classified as ‘normal’ (Fig. 5A and 5D) tended to have spatially homogenous distributions of ADC and T2-values, respectively, while other classifications tended to display spatially heterogeneous distributions. Some synovial reactions displayed different regions/pools within the ROI which have lower ADC values (5C) or prolonged T2-values (5D), while some reactions had a globally heterogeneous appearance (Fig. 5E).
Figure 4.
Principal component analysis derived from the 4 variables: ADC, T2, area of ADC region of interest, and area of T2 region of interest by synovial reaction classification (A), grouped normal/abnormal synovial reaction classification (B), and synovial reaction type (C). Nearly 50% of the variance was explained by the first principal component for all analyses and the second principal component explained nearly 30% of the variance of the data. The patients with ‘normal’ synovial classification were found grouped on the positive side of PC1, corresponding well with having a ‘fluid-like’ synovial reaction.
Figure 5.
Corresponding coronal ADC maps (A, B, C) and T2 maps (D, E, F) for patients classified as having ROIs of ‘normal’ (A, D), ‘polymeric’ (B, E), and ‘infection’ (C, F) synovial reactions. Note the reduced volume of the ‘normal’ synovial reaction compared to ‘polymeric’ or ‘infection’. Also note the great heterogeneity of the T2 map of the ‘infection’ synovial reaction in comparison to a bi-modal distribution of T2-values for the ‘polymeric’ synovial reaction.
4. Discussion
As the prevalence of THA increases, it may be anticipated that there will be a concomitant increase in the incidence of reactions in soft tissues around THA, such as ALTR and PJI. Therefore, there is a pressing need for non-invasive imaging methods capable of generating images with contrast sensitive to soft tissue reactions to monitor these potential complications. Biomarkers derived from qMRI have been shown to be an effective method in detecting periprosthetic tissue abnormalities [30,37] as previous studies have found that THA complications can be clinically silent [33,38] and may lead to implant failure, resulting in the need for revision with its attendant risk of increasing complications.
This pilot study found significantly lower T2-values and larger reaction areas for ‘grouped abnormal’ synovial reactions. Increased ADC values were generally found for ‘grouped abnormal’ ROIs, but only ADC area was significantly different compared to ‘normal’ synovial reactions. This suggests the involvement of local structures and biological processes in the diffusive capacity within synovial reactions. The increase of ADC values found in pathologic synovial reactions demonstrates increased water mobility that may reflect changes to underlying tissue structure. For instance, histopathological evaluations of periprosthetic tissue retrieved at revision surgery have shown hypersensitivity reactions due to particulate debris and corrosion products from the implanted device that disrupt synovial lining integrity, increase inflammatory cell infiltration, and change tissue organization [37,39]. The utility of heat maps is also beneficial (Fig. 5) as they show how different synovial classifications can display varying degrees of heterogeneity within the defined ROIs. This regional heterogeneity may be attributable to the buildup of large particulate debris and corrosion products, presence of cellular infiltrates, and increased tissue fibrosis within the synovial reaction. The data in this pilot study suggests that both inflammatory and cellularity changes may occur in post-THA complications, such as ALTR, synovitis, osteolysis, and implant loosening.
Given the wide range of ADC and T2-values, and the areal coverage of synovial reactions, our results indicate that mean ADC or T2-values alone may not be sufficiently accurate to capture the variation in ADC or T2-value distribution. The use of PCA aids to further differentiate normal and abnormal synovial reactions while simultaneously accounting for all variables. Over 78% of data variance was explained by the first two principal components, and ROIs with a synovial classification of ‘normal’ tended to have positive values for PC1 as compared to individual synovial reactions or ‘grouped abnormal’ ROIs. These results indicate that using the data reduction technique of PCA sufficiently captures relevant information and may aid in differentiating normal from abnormal synovial reactions for future studies with a larger patient cohort.
While the present study did not find significant differences in T2-values by synovial reaction classification, the results concur with a previous larger cohort study that reported a significant difference between types of synovial reaction type (fluid-like vs. mixed), but not synovial classification [30]. This variation may be attributed to the wide variation of T2-values seen in this study and the limited number of subjects currently enrolled.
Although this pilot study demonstrated the feasibility of DWI of tissues near large prosthetic devices, there were several limitations. First, the current implementation of the 2D-MSI PROPELLER DWI utilizes a 2D acquisition [26], which limited our scan coverage to only 4 image slices to achieve a clinically viable scan time of approximately 6 minutes. As compared to the anatomic coverage of the morphologic MAVRIC-SL and T2 mapping sequences, the 2D-MSI PROPELLER DWI sequence was not able to cover the full anatomical extent of synovial reactions in all subjects. Future development of the pulse sequence would benefit from greater coverage within a clinically feasible scan time as great variability in the synovial reaction volume has been shown to occur in subjects with THA [38]. Second, the 2D-MSI PROPELLER DWI sequence was acquired with 3 diffusion directions but only one b-value and did not incorporate other motion correction strategies to mitigate motion artifacts. Future studies may benefit from using multi-b-value acquisitions with larger b-values to improve sensitivity to lower ADC values [40] as well as the ability to account for any subtle motion by the subject during scanning. Third, the T2 mapping acquisition utilized only 2 echo times of relatively short TEs as compared to the T2-values of water [41]. This limitation, in addition to limited subject enrollment, may have resulted in being less sensitive to differentiating solid- and fluid-like synovial types even as fluid-like synovial reactions were 44ms longer on average. The addition of longer echo times may help differentiate types of synovial reactions at the cost of increased scan time. Fourth, the lack of intraoperative synovial tissue histology prevented comparison of qMRI values with clinical and direct histological validation, which would be required to utilize qMRI as a confirmatory diagnostic in clinical decision-making in subjects with THA [12]. Finally, the variables of THA material and THA length of implantation, were not incorporated in our analysis as much of this information was not available from the subjects. As there was limited sample size in some synovial classifications, this would limit the statistical power and multivariable analysis. We anticipate confounding variables such as age and sex will be considered in future studies.
5. Conclusions
This pilot study presented the initial feasibility evaluation of synovial reactions using 2D-MSI PROPELLER DWI in subjects with implanted THAs. The 2D-MSI PROPELLER DWI and T2-MAVRIC sequences can generate quantitative images near THAs in clinically feasible scan times. The results of this study demonstrate the feasibility of utilizing 2D-MSI PROPELLER DWI and T2-MAVRIC mapping for periprosthetic soft tissue evaluation. Further, trends of ADC and T2-values were found as related to morphologic synovial reaction classifications. This pilot study provides a basis for further work on studying diffusion changes within synovial tissues.
Acknowledgments (role of funding source)
Research reported in this study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01-AR064840 (Koff/Potter) and R21-EB030123 (Koch). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsors had no role in the study design, in the data collection, analysis, and interpretation of data, in the writing of this report, or in the decision to submit the article for publication.
Footnotes
Madeleine A. Gao - Data curation, Investigation, Methodology, Writing - original draft, Writing - review & editing
Ek T. Tan - Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing
John Neri - Data curation, Investigation, Methodology, Visualization, Validation, Writing - review & editing
Qian Li - Formal analysis, Investigation, Visualization, Writing - review & editing
Alissa J. Burge - Investigation, Writing - review & editing
Hollis G. Potter – Conceptualization, Funding acquisition, Supervision, Writing - review & editing
Kevin M. Koch – Conceptualization, Funding acquisition, Resources, Writing - original draft, Writing - review & editing
Matthew F. Koff – Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Visualization, Validation, Writing - original draft, Writing - review & editing,
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: Factors affecting survivorship of acetabular and femoral components. J Bone Jt Surg - Ser A 2002;84:171–7. 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- [2].Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: Future projections to 2020−2040 using the national inpatient sample. J Rheumatol 2019;46:1134–40. 10.3899/jrheum.170990. [DOI] [PubMed] [Google Scholar]
- [3].Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26:355–69. 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, et al. The painful metal-on-metal hip resurfacing. J Bone Jt Surg - Ser B 2009;91:738–44. 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- [5].Galley J, Sutter R, Stern C, Filli L, Rahm S, Pfirrmann CWA. Diagnosis of periprosthetic hip joint infection using MRI with metal artifact reduction at 1.5 T. Radiology 2020;296:98–108. 10.1148/radiol.2020191901. [DOI] [PubMed] [Google Scholar]
- [6].Bozic KJ, Kamath AF, Ong K, Lau E, Kurtz S, Chan V, et al. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin Orthop 2015;473:2131–8. 10.1007/s11999-014-4078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].YM K, MH L, D D, TY T, AA F, HE R. What Is the Natural History of “Asymptomatic” Pseudotumours in Metal-on-Metal Hip Arthroplasty? Minimum 4-Year Metal Artifact Reduction Sequence Magnetic Resonance Imaging Longitudinal Study. J Arthroplasty 2016;31:121–6. 10.1016/J.ARTH.2016.02.070. [DOI] [PubMed] [Google Scholar]
- [8].Koff MF, Shah P, Koch KM, Potter HG. Quantifying image distortion of orthopedic materials in magnetic resonance imaging. J Magn Reson Imaging 2013;38:610–8. 10.1002/JMRI.23991. [DOI] [PubMed] [Google Scholar]
- [9].Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med 2009;61:381–90. 10.1002/mrm.21856. [DOI] [PubMed] [Google Scholar]
- [10].Kaushik SS, Marszalkowski C, Koch KM. External calibration of the spectral coverage for three-dimensional multispectral MRI. Magn Reson Med 2016;76:1494–503. 10.1002/mrm.26065. [DOI] [PubMed] [Google Scholar]
- [11].Hayter CL, Koff MF, Potter HG. Magnetic resonance imaging of the postoperative hip. J Magn Reson Imaging JMRI 2012;35:1013–25. 10.1002/jmri.23523. [DOI] [PubMed] [Google Scholar]
- [12].Koff MF, Esposito C, Shah P, Miranda M, Baral E, Fields K, et al. MRI of THA Correlates with Implant Wear and Tissue Reactions: A Cross-sectional Study. Clin Orthop 2019;477:159–74. 10.1097/CORR.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raya JG. Techniques and applications of in vivo diffusion imaging of articular cartilage. J Magn Reson Imaging JMRI 2015;41:1487–504. 10.1002/jmri.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pedoia V, Majumdar S, Link TM. Segmentation of joint and musculoskeletal tissue in the study of arthritis. Magma N Y N 2016;29:207–21. 10.1007/s10334-016-0532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ. Current Clinical Applications of Diffusion-Tensor Imaging in Neurological Disorders. J Clin Neurol Seoul Korea 2018;14:129–40. 10.3988/JCN.2018.14.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang M, Horvat JV, Bernard-Davila B, Marino MA, Leithner D, Ochoa-Albiztegui RE, et al. Multiparametric MRI model with dynamic contrast-enhanced and diffusion-weighted imaging enables breast cancer diagnosis with high accuracy. J Magn Reson Imaging JMRI 2019;49:864–74. 10.1002/JMRI.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fliedner FP, Engel TB, El-Ali HH, Hansen AE, Kjaer A. Diffusion weighted magnetic resonance imaging (DW-MRI) as a non-invasive, tissue cellularity marker to monitor cancer treatment response. BMC Cancer 2020;20. 10.1186/S12885-020-6617-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Subhawong TK, Jacobs MA, Fayad LM. Diffusion-weighted MR imaging for characterizing musculoskeletal lesions. Radiogr Rev Publ Radiol Soc N Am Inc 2014;34:1163– 77. 10.1148/RG.345140190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oudeman J, Nederveen AJ, Strijkers GJ, Maas M, Luijten PR, Froeling M. Techniques and applications of skeletal muscle diffusion tensor imaging: A review. J Magn Reson Imaging JMRI 2016;43:773–88. 10.1002/jmri.25016. [DOI] [PubMed] [Google Scholar]
- [20].Monte JR, Hooijmans MT, Froeling M, Oudeman J, Tol JL, Maas M, et al. The repeatability of bilateral diffusion tensor imaging (DTI) in the upper leg muscles of healthy adults. Eur Radiol 2020;30:1709–18. 10.1007/s00330-019-06403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Neri JP, Koff MF, Koch KM, Tan ET. Validating the accuracy of multispectral metal artifact suppressed diffusion-weighted imaging. Med Phys 2022. 10.1002/mp.15925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim SY, Shin MJ, Chang JH, Lee CH, Shin YI, Shin YB, et al. Correlation of diffusion tensor imaging and phase-contrast MR with clinical parameters of cervical spinal cord injuries. Spinal Cord 2015;53:608–14. 10.1038/SC.2015.57. [DOI] [PubMed] [Google Scholar]
- [23].Barendregt AM, van Gulik EC, Lavini C, Nusman CM, van den Berg JM, Schonenberg-Meinema D, et al. Diffusion-weighted imaging for assessment of synovial inflammation in juvenile idiopathic arthritis: a promising imaging biomarker as an alternative to gadolinium-based contrast agents. Eur Radiol 2017;27:4889–99. 10.1007/s00330-017-4876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sandford HJC, MacKay JW, Watkins LE, Gold GE, Kogan F, Mazzoli V. Gadolinium-free assessment of synovitis using diffusion tensor imaging. NMR Biomed 2022;35:e4614. 10.1002/nbm.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koch KM, Bhave S, Gaddipati A, Hargreaves BA, Gui D, Peters R, Bedi M, Mannem R, et al. Multispectral diffusion-weighted imaging near metal implants. Magn Reson Med 2018;79:987–93. 10.1002/MRM.26737. [DOI] [PubMed] [Google Scholar]
- [26].Koch KM, Bhave S, Kaushik SS, Nencka AS, Budde MD. Multi-Spectral Diffusion Weighted MRI of the Instrumented Cervical Spinal Cord: A Preliminary Study of 5 Cases. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 2020;29:1071. 10.1007/S00586-019-06239-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging JMRI 2003;17:358–64. 10.1002/JMRI.10263. [DOI] [PubMed] [Google Scholar]
- [28].Verschueren J, Meuffels DE, Bron EE, Klein S, Kleinrensink GJ, Verhaar JAN, et al. Possibility of quantitative T2-mapping MRI of cartilage near metal in high tibial osteotomy: A human cadaver study. J Orthop Res Off Publ Orthop Res Soc 2018;36:1206–12. 10.1002/JOR.23729. [DOI] [PubMed] [Google Scholar]
- [29].Bhave S, Koff MF, Kaushik SS, Potter HG, Koch KM. 3D-multi-spectral T2 mapping near metal implants. Magn Reson Med 2019;82:614–21. 10.1002/mrm.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheung J, Neri JP, Gao MA, Lin B, Burge AJ, Potter HG, et al. Clinical Feasibility of Multi-Acquisition Variable-Resonance Image Combination–Based T2 Mapping near Hip Arthroplasty. HSS Journal® 2021;17:165–73. 10.1177/1556331621994801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zochowski KC, Miranda MA, Cheung J, Argentieri EC, Lin B, Kaushik SS, et al. MRI of Hip Arthroplasties: Comparison of Isotropic Multiacquisition Variable-Resonance Image Combination Selective (MAVRIC SL) Acquisitions With a Conventional MAVRIC SL Acquisition. AJR Am J Roentgenol 2019;213:W277–86. 10.2214/AJR.19.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Potter HG, Nestor BJ, Sofka CM, Ho ST, Peters LE, Salvati EA. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Joint Surg Am 2004;86:1947–54. 10.2106/00004623-200409000-00013. [DOI] [PubMed] [Google Scholar]
- [33].Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI Predicts ALVAL and Tissue Damage in Metal-on-Metal Hip Arthroplasty. Clin Orthop 2014;472:471. 10.1007/S11999-013-2788-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fritz J, Lurie B, Miller TT, Potter HG. MR Imaging of Hip Arthroplasty Implants. Https://DoiOrg/101148/Rg344140010 2014;34. 10.1148/RG.344140010. [DOI] [PubMed] [Google Scholar]
- [35].Hayter CL, Gold SL, Koff MF, Perino G, Nawabi DH, Miller TT, et al. MRI findings in painful metal-on-metal hip arthroplasty. AJR Am J Roentgenol 2012;199:884–93. 10.2214/AJR.11.8203. [DOI] [PubMed] [Google Scholar]
- [36].Plodkowski AJ, Hayter CL, Miller TT, Nguyen JT, Potter HG. Lamellated hyperintense synovitis: potential MR imaging sign of an infected knee arthroplasty. Radiology 2013;266:256– 60. 10.1148/RADIOL.12120042. [DOI] [PubMed] [Google Scholar]
- [37].Sherafati M, Bauer TW, Potter HG, Koff MF, Koch KM. Multivariate use of MRI biomarkers to classify histologically confirmed necrosis in symptomatic total hip arthroplasty. J Orthop Res 2020;38:1506–14. 10.1002/jor.24654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Koff MF, Gao MA, Neri JP, Chiu Y-F, Lin BQ, Burge AJ, et al. Adverse Local Tissue Reactions are Common in Asymptomatic Individuals After Hip Resurfacing Arthroplasty: Interim Report from a Prospective Longitudinal Study. Clin Orthop 2021;479:2633–50. 10.1097/CORR.0000000000001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Campbell P, Ebramzadeh E, Nelson S, Takamura K, Smet KD, Amstutz HC. Histological Features of Pseudotumor-like Tissues From Metal-on-Metal Hips. Clin Orthop 2010;468:2321. 10.1007/S11999-010-1372-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Karki K, Hugo GD, Ford JC, Olsen KM, Saraiya S, Groves R, et al. Estimation of optimal b-value sets for obtaining apparent diffusion coefficient free from perfusion in non-small cell lung cancer. Phys Med Biol 2015;60:7877–91. 10.1088/0031-9155/60/20/7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1−100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys 1984;11:425–48. 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]