Abstract
Previous study showed that ginsenoside Rg1 (Rg1) and ginsenoside Re (Re) alleviated growth inhibition of broiler chicks with immune stress. The aim of this study was to investigate the effect of Rg1 and Re on inflammatory responses, oxidative stress, and apoptosis in liver of broilers with immune stress induced by lipopolysaccharide (LPS). Forty broiler chicks were randomly divided into 4 groups, each group consisting of 10 chickens. The model group, Rg1 group, and Re group were received continuously interval injection of 250 μg/kg body weight LPS at the age of 12, 14, 33, and 35 days to induce immune stress. Control group was injected with an equivalent amount of sterile saline. Then broilers in Rg1 group and Re group were given 1mg/kg body weight Rg1 and Re intraperitoneally 2 h after the LPS challenge respectively. Blood samples were collected for the detection of hormone levels, inflammatory mediators, and antioxidant parameters. Hepatic tissues were taken for pathological observation. Total RNA was extracted from the liver for real-time quantitative polymerase chain reaction analysis. Our results showed that Rg1 or Re could alleviate histological changes of liver, reduce production of stress-related hormones, inhibit inflammatory responses, and enhance antioxidant capacity in broilers challenged by immune stress. In addition, Rg1 or Re treatment upregulated mRNA expression of antioxidant-related genes and downregulated mRNA expression of inflammation-related factors and apoptosis-related genes in the liver of immune-stressed broilers. The results suggest that the plant extracts containing Rg1 and Re can be used for ameliorating hepatic oxidative stress and inflammation and controlling immune stress in broiler chicks.
Key words: ginsenoside, lipopolysaccharide, inflammatory response, oxidative stress, apoptosis
INTRODUCTION
Lipopolysaccharide (LPS), one of the major constituents of cell wall in Gram-negative bacteria, has long been a stress factor for young broilers and a threat for poultry industry (Han et al., 2020; Shi et al., 2022b; Ye et al., 2022). Immune stress and oxidative damage not only decrease feed intake but also increase nutrients catabolism (Bi et al., 2022b). Continuous interval injection of LPS is generally used to establish model of bacterial infection and immune stress in mammals and birds. LPS triggers Toll-like receptor 4 (TLR4) and activates myeloid differentiation factor 88 (MyD88) (Sangaran et al., 2021). Then nuclear factor-kappa B (NF-κB) translocate to the nucleus and promotes expression of proinflammatory mediators, inflammatory cytokines, and induces oxidative stress in multiple organs such as spleen, intestine, and liver (Aka and Eren, 2019; Tong et al., 2022; Zhang et al., 2022). The maintenance of inflammation changes the partitioning of amino acid and lipid, induces oxidative damage and cell apoptosis, leading to a huge decline of growth performance in broilers (Zheng et al., 2021). Thus, it is urgent to develop strategies to alleviate inflammatory responses and oxidative stress in immune stressed broilers.
An increasing number of studies have demonstrated that herbal extracts are effective to prevent immune stress (Woo et al., 2006; Zhang et al., 2013). Ginsenoside Re and Rg1 are tetracyclic triterpenoid derivatives extracted from the roots of Panax ginseng C. A. Meyer. Both of them have anti-inflammatory, antioxidant, and hepatoprotective effect (Li et al., 2016; Zhao et al., 2021a; Mao et al., 2022; Yang et al., 2022). Our previous study showed that treatment with ginsenoside Rg1 (Rg1) and ginsenoside Re (Re) can reverse growth inhibition (Bi et al., 2022a). However, the effects of Re and Rg1 on regulating the inflammatory responses and oxidative stress in broilers have not been illustrated.
Therefore, in this study, we used a classical immune stress model to investigate the effects of Re and Rg1 on inflammation, oxidative stress in broiler chicks challenged by LPS. We also detected the mRNA expression of related genes in liver to better understand the mechanisms conferring regulating effect of Rg1 and Re. The results may help to screen potential herbs containing ginsenosides Rg1 and Re for preventing immune stress and other inflammation-related diseases in birds and other species of animals.
MATERIALS AND METHODS
Reagents
Ginsenoside Rg1 (purity, 98.92%; molecular formula, C42H72O14; molecular weight, 801.024) and Re (purity, 98%; molecular formula, C48H82O18; molecular weight, 947.166) were purchased from Chengdu Purify Biotechnology Co. Ltd. (Chengdu, China). LPS (O55:B5, E. coli, CAT. No. L2880) was obtained from Sigma–Aldrich (St. Louis, MO). Detection kits for xanthine oxidase (XOD, CAT. No. A002-1-1), catalase (CAT, CAT. No. A007-1-1), total superoxide dismutase (T-SOD, CAT. No. A001-1-2), malondialdehyde (MDA, CAT. No. A003-1-2), reduced glutathione (GSH, CAT. No. A006-2-1), corticosterone (CORT, CAT. No. H205), and adrenocorticotropic hormone (ACTH, CAT. No. H091-1-2) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). ELISA kits for chicken TNF-α (CAT. No. BPE60016), iNOS (CAT. No. BPE60145), IL-1β (CAT. No. BPE60017) and IL-6 (CAT. No. BPE60049) were purchased from Lengdon Bioscienci Co., Ltd. (Shanghai, China).
Animals, Diets, and Experimental Design
One-day-old male Shaver brown broilers were purchased from Sichuan Lihua Poultry Co., Ltd. (Zigong, China) (Bi et al., 2022a). The chickens were housed in 3-layer cages and after 7 days of adaptive feeding, with the room temperature kept at 33 to 35°C for the first week, then dropped by 2 or 3°C per week until maintained at 23°C. Light is adequate, water and food were freely available (Table 1). The animal experiments were performed in accordance with the Animal Care and Use Committee of Southwest University (permit number IACUC-20200701-01). All experimental animals were euthanized at the end of the experiment. Experiments were carried out as follows (Bi et al., 2022a). Forty male broilers were randomly divided into 4 groups with 10 chickens in each group, which were the control group, the model group, the Rg1 group, and the Re group (Table 2). Chickens are housed in single cages, and distributed in the second of 3-layer cages. The model group, the Rg1 group, and the Re group received an intraperitoneal injection of LPS (250 μg/kg body weight, dissolved in sterile saline) at the age of 12, 14, 33, and 35 days to induce immunological stress. The control group was intraperitoneally injected with an equivalent amount of sterile saline. Broilers in the Rg1 group, and the Re group were given 1 mg/kg body weight Rg1 and Re intraperitoneally 2 h after LPS attack, respectively. Both the control group and the model group were injected with an equivalent amount of sterile saline. The LPS dose used was based on our preliminary experiments, and the Rg1 and Re dose was selected according to published studies (Bi et al., 2022a).
Table 1.
Dietary formulation of basal diet.1
| Item | % |
|---|---|
| Composition | |
| Corn | 55.35 |
| Soybean meal | 29.00 |
| Wheat shorts | 2.70 |
| Fish meal | 5.01 |
| Rapeseed oil | 3.17 |
| Salt | 0.27 |
| Dicalcium phosphate | 3.13 |
| Limestone | 0.15 |
| DL-Met | 0.12 |
| Lys | 0.10 |
| Premix2 | 1 |
| Total | 100 |
| Nutrient | |
| Crude protein | 21.10 |
| Crude fiber | 2.24 |
| Ca | 0.92 |
| Total phosphorus | 1.20 |
| Methionine | 0.12 |
| Sodium chloride | 0.27 |
Diet was in pellet form (Φ, 5mm).
Each kilogram of premix compound contained: vitamin A, 7,000 IU; vitamin D3, 2,500 IU; vitamin E, 30 mg; vitamin K3, 1 mg; vitamin B1, 1.5 mg; vitamin B2, 4 mg; vitamin B6, 2 mg; vitamin B12, 0.02 mg; niacin, 30 mg; folic acid, 0.55 mg; pantothenic acid, 10 mg; biotin, 0.16 mg; choline chloride, 400 mg; Cu, 20 mg; Fe, 70 mg; Mn, 100 mg; Zn, 70 mg; I, 0.4 mg, Se, 0.5 mg, and virginiamycin, 20 mg.
Table 2.
Experimental design.
| Group | n | Treatments | Drug |
|---|---|---|---|
| Control | 10 | sterile saline | sterile saline |
| Model | 10 | 250 µg/kg LPS | sterile saline |
| Rg1 | 10 | 250 µg/kg LPS | 1 mg/kg Rg1 |
| Re | 10 | 250 µg/kg LPS | 1 mg/kg Re |
Sample Collection
At wk 2 and 4, ten broilers were selected from each group and blood samples were collected from the jugular vein. Twelve hours after the last LPS injection, 6 chickens in each group were randomly selected and euthanized by cervical dislocation, and the liver was removed by dissection. The serum samples were rested at 37°C for 2 to 3 h and obtained by centrifugation at 3,000 × g for 10 min and then immediately stored at −20°C until further analysis (Shi et al., 2022b). Six liver samples from each group were fixed in 10% formalin. Each liver sample was rinsed with ice-cold sterile saline to remove blood contamination, stored frozen in liquid nitrogen, and then stored at −80°C until all assays were performed.
Measurement of Stress-Related Hormone
The levels of ACTH and CORT in the serum were determined according to the commercial kits’ instructions.
Enzyme-Linked Immunosorbent Assay
The levels of chicken TNF-α, iNOS, IL-1β, and IL-6 in the serum were determined by using the ELISA kits for chicken TNF-α, iNOS, IL-1β, and IL-6, according to the manufacturer's instructions.
Measurement of Oxidative Stress-Related Indicators
The levels of XOD, CAT, T-SOD, MDA, and GSH in the serum were determined by commercial kits and according to the manufacturer's instructions.
Histological Assessment
Liver tissue was fixed in 10% neutral phosphate-buffered formalin for 24 h, dehydrated in a graded ethanol series of 50 to 100%, cleared in xylene, and embedded in paraffin. Tissue sections (5-μm thick) were then prepared and stained with hematoxylin and eosin (H&E) and imaged with light microscopy (Shi et al., 2022a).
RNA Extraction and Quantitative Real-Time PCR Analysis
Total RNA was extracted from the liver using TRIzol reagent (CAT. No. 9101, Takara, Dalian, China) following the manufacturer's guidelines, and a NanoDrop (Thermo Fisher, Waltham, Ma) was used to evaluate the purity and quantity of RNA by spectrophotometric analysis at OD260/280 (Shi et al., 2022a). PrimeScript RT Master Mix (CAT. No. RR036A, Takara) was utilized to convert RNA into cDNA on a T100 thermal cycler (Bio Rad, Hercules, CA). The chicken β-actin was served as the housekeeping gene. RT-qPCR with SYBR Premix Ex Taq II (Tli RNaseH Plus) (CAT. No. RR820A, Takara) on selected genes was performed on a Multiple Real-Time PCR System (Applied Biosystems, Carlsbad, CA) (Bi et al., 2022a). The 2−ΔΔCt method was used to calculate the changes in gene expression levels (Livak and Schmittgen, 2001), and the relative mRNA expression levels of the genes were also normalized to the mRNA expression levels of β-actin. The specific sequences of the primers were shown in Table 3.
Table 3.
Sequences of primers for RT-qPCR.
| Gene name | Gene description | Primers sequence (5′-3′) | Accession number | References | |
|---|---|---|---|---|---|
| Nrf2 | Nuclear factor erythroid 2-related factor 2 | Forward | GAGCCCATGGCCTTTCCTAT | NM_001007858.1 | (Han et al., 2020) |
| Reverse | CACAGAGGCCCTGACTCAAA | ||||
| HO-1 | HO-1 | Forward | AAACTTCGCAGCCACACAAC | NM_205344.1 | (Han et al., 2020) |
| Reverse | GACCAGCTTGAACTCGTGGA | ||||
| SOD1 | Superoxide dismutase 1 | Forward | CCGGCTTGTCTGATGGAGAT | NM_205064.1 | (Han et al., 2020) |
| Reverse | TGCATCTTTTGGTCCACCGT | ||||
| TNF-α | Tumour Necrosis Factor alpha | Forward | CCCCTACCCTGTCCCACAA | NM_204267.1 | (Han et al., 2020) |
| Reverse | TGAGTACTGCGGAGGGTTCAT | ||||
| IL-6 | Interleukin 6 | Forward | CAGCTGCAGGACGAGATGTGCAA | AJ309540 | (Han et al., 2020) |
| Reverse | GCACAGGACTCGACGTTCTGCT | ||||
| TLR4 | Toll-like receptor 4 | Forward | TTCAGAACGGACTCTTGAGTGG | AY064697 | (Han et al., 2020) |
| Reverse | CAACCGAATAGTGGTGACGTTG | ||||
| NF-κB p65 | NF-kappaB subunits p65 | Forward | CAGCCCATCTATGACAACCG | NM_001396038.1 | (Yang et al., 2021) |
| Reverse | CAGCCCAGAAACGAACCTC | ||||
| Bax | Bcl-2-associated X protein | Forward | TCCTCATCGCCATGCTCAT | XM_422067 | |
| Reverse | CCTTGGTCTGGAAGCAGAAGA | ||||
| Bcl-2 | B cell lymphoma-2 | Forward | GATGACCGAGTACCTGAACC | XM_422067 | |
| Reverse | CAGGAGAAATCGAACAAAGGC | ||||
| β-Actin | Beta-actin | Forward | TGCTGTGTTCCCATCTATCG | NM_205518.1 | (Han et al., 2020) |
| Reverse | TTGGTGACAATACCGTGTTCA |
Statistics Analysis
All differences between groups were subjected to one-way ANOVA and Duncan's multiple range test using SPSS version 19.0 software (SPSS, Inc., Chicago, IL). All data were presented as mean ± standard error (SE). Differences between means were regarded as statistically significant at P < 0.05 or P < 0.01.
RESULTS
Stress-Related Hormone Concentrations in Serum
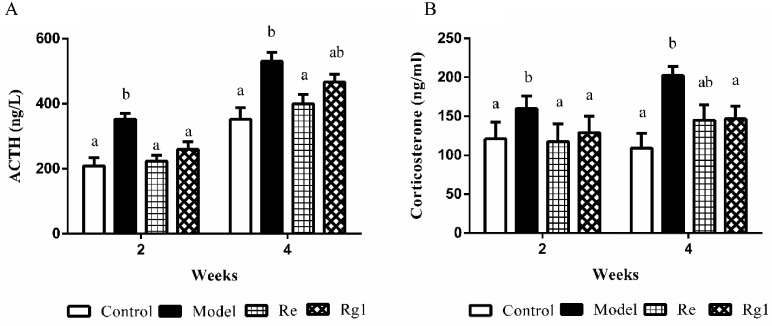
Serum concentrations of ATCH and CORT are shown in Figure 1. Compared with the control group, serum ACTH and CORT concentrations in the model group were significantly higher at wk 2 and 4 (P < 0.05), whereas intraperitoneal injection of Re decreased serum ATCH (2 and 4 wk of age, P < 0.05) and CORT (2 wk of age, P < 0.05) concentrations, and intraperitoneal injection of Rg1 decreased serum ACTH (2 wk of age, P < 0.05) and CORT (2 and 4 wk of age, P < 0.05).
Figure 1.
The levels of adrenocorticotropic hormone (ACTH) (A) and corticosterone (CORT) (B) in serum. The values are represented as means ± SE (n = 10). In each histogram, bars sharing different letters are significantly different (P < 0.05).
Serum Antioxidant Enzyme Activities
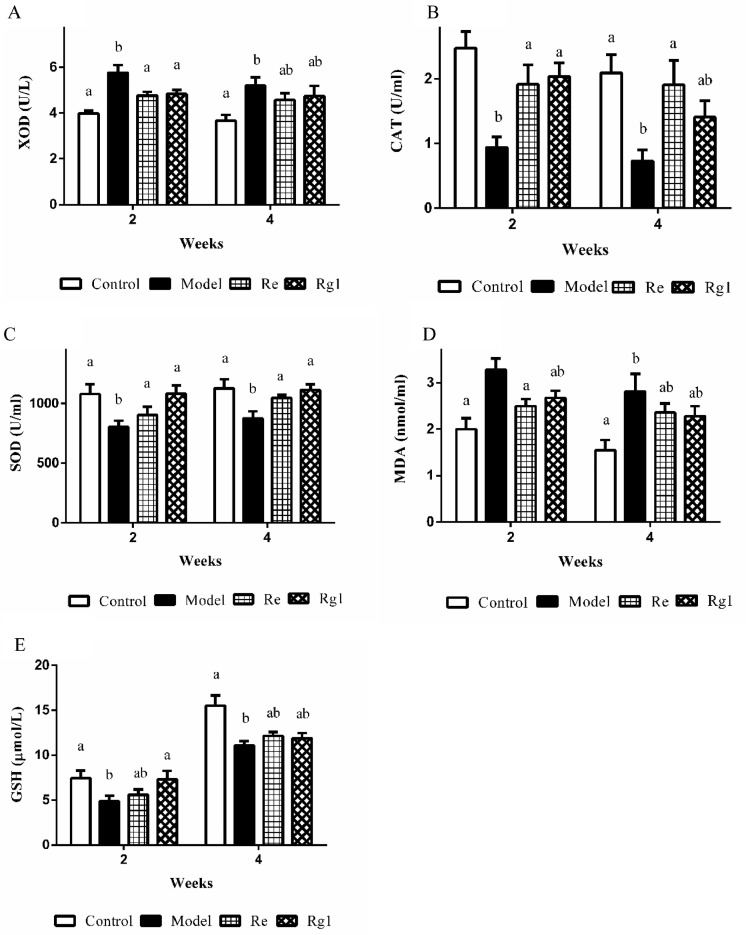
Figure 2 showed the effect of ginsenoside Re or Rg1 on the serum levels of XOD, CAT, T-SOD, GSH, and MDA in broilers with stress. At 2 or 4 wk of age, the enzymatic activities of CAT, T-SOD, and GSH in broiler serum were significantly reduced and the XOD activity and MDA content were significantly increased after LPS injection. Intraperitoneal injection of Re significantly inhibited changes in XOD (2 wk, P < 0.05), CAT (2 and 4 wk of age, P < 0.05), T-SOD (2 and 4 wk of age, P < 0.05), and MDA (2 wk, P < 0. 05), whereas intraperitoneal injection of Rg1 significantly inhibited changes in XOD (2 wk, P < 0.05), CAT (2 wk, P < 0.05), T-SOD (2 and 4 wk of age, P < 0.05), and GSH (2 wk, P < 0.05) changes were significantly inhibited by intraperitoneal injection of Rg1.
Figure 2.
Activities of enzymatic and non-enzymatic antioxidants in serum. Detection for serum xanthine oxidase (XOD) (A), catalase (CAT) (B), total superoxide dismutase (T-SOD) (C), malondialdehyde (MDA) (D), and glutathione (GSH) (E). Values are means ± SE (n = 10). In each histogram, bars sharing different letters are significantly different (P < 0.05).
Contents of Inflammatory Factors in Serum
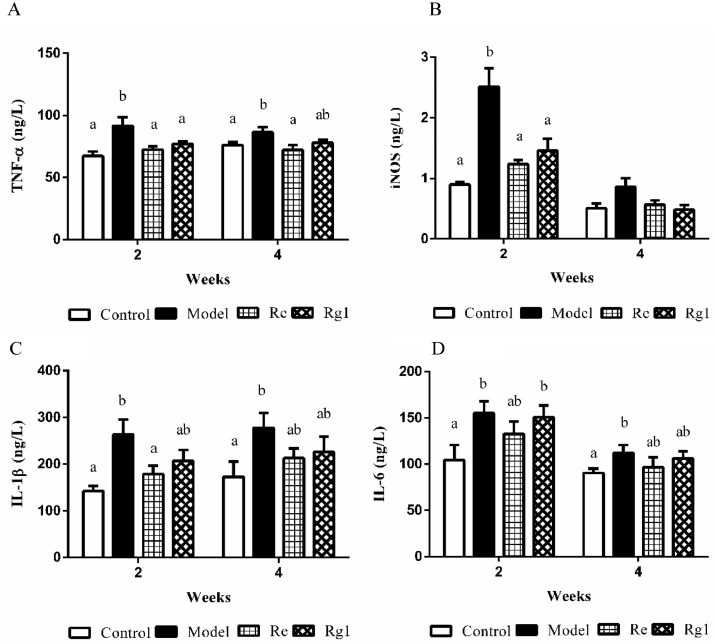
The contents of TNF-α, iNOS, IL-1β, and IL-6 are shown in Figure 3. At 2 wk of age, TNF-α, iNOS, IL-1β, and IL-6 were significantly higher in the model group compared to the control group (P < 0.05), and after treatment with Re, the levels of TNF-α, iNOS, and IL-1β decreased significantly (P < 0.05). TNF-α and iNOS levels were significantly decreased after Rg1 treatment (P < 0.05). At 4 wk of age, TNF-α, IL-1β, and IL-6 were significantly higher in the model group compared to the control group (P < 0.05), and after Re treatment, TNF-α levels was significantly decreased (P < 0.05).
Figure 3.
The levels of adrenocorticotropic hormone (ACTH) (A) and corticosterone (CORT) (B) in serum. The values are represented as means ± SE (n = 10). In each histogram, bars sharing different letters are significantly different (P < 0.05).
Hepatic Inflammatory Gene Expression
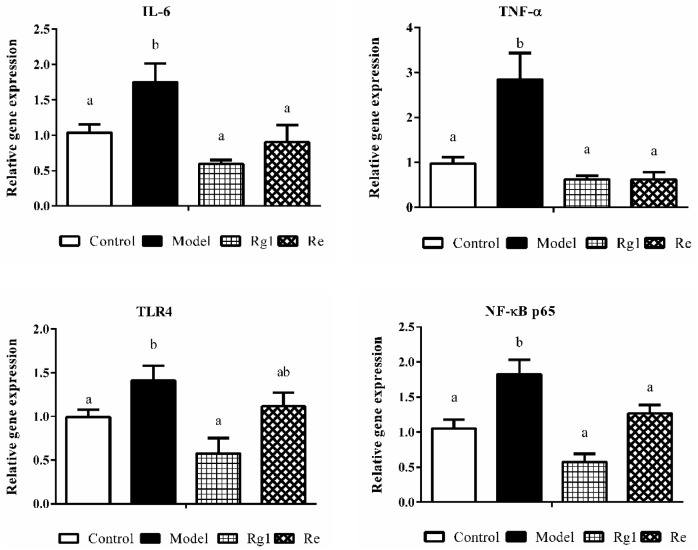
The data of mRNA expression of inflammatory genes in the liver of broiler chickens are shown in Figure 4. Compared with broilers in the control group, LPS injection increased the mRNA expression levels of TNF-α, IL-6, TLR4, and NF-κB p65 in broiler liver (P < 0.05). In contrast, Rg1 treatment decreased the mRNA expression of TNF-α, IL-6, TLR4, and NF-κB p65 (P < 0.05) and Re treatment decreased the mRNA expression of TNF-α, IL-6, and NF-κB p65 (P < 0.05).
Figure 4.
Relative mRNA expression in the liver. The Chicken β-actin was served as the housekeeping gene. Expression of IL-6 (A), TNF-α (B), TLR4 (C), and NF-κB p65 (D) mRNA were assessed by real-time quantitative PCR. A relative quantitative method (2−ΔΔCt) was employed to evaluate the quantitative variation. Data are represented as means ± SE (n = 6). In each histogram, bars sharing different letters are significantly different (P < 0.05).
Hepatic Oxidative Stress-Related Gene Expression
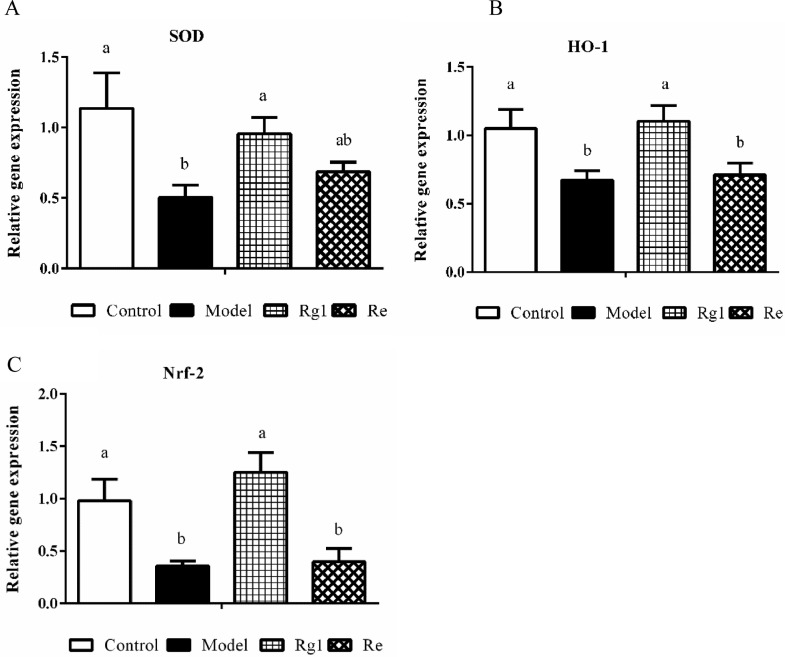
The mRNA expression of antioxidant genes in the liver of broilers are shown in Figure 5. LPS injection declined the mRNA expression levels of SOD, HO-1, and Nrf-2 in broiler liver compared to control chickens (P < 0.05). However, Rg1 treatment reversed the expression of these genes (P < 0.05). Re treatment showed a tendency to increase, although it did not show a significant change (P > 0.05).
Figure 5.
Relative mRNA expression in the liver. The Chicken β-actin was served as the internal control gene. Expression of SOD (A), HO-1 (B), and Nrf-2 (C) mRNA were assessed by real-time quantitative PCR. A relative quantitative method (2−ΔΔCt) was employed to evaluate the quantitative variation. Data are represented as means ± SE (n = 6). In each histogram, bars sharing different letters are significantly different (P < 0.05).
Hepatic Apoptosis Proteins Gene Expression
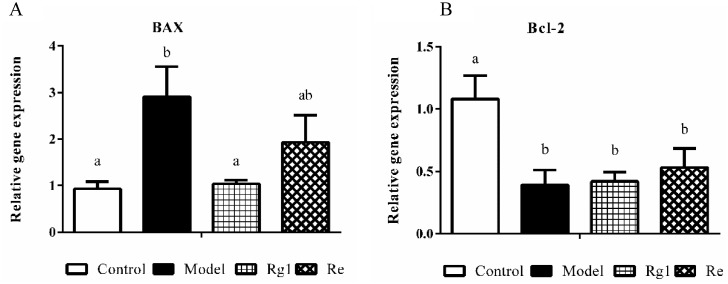
The mRNA expression of apoptosis proteins genes in the liver of broilers are shown in Figure 6. Compared with the control group, Bax mRNA expression levels were significantly higher (P < 0.05) and Bcl-2 mRNA expression levels were significantly lower in the control group. Rg1 treatment prevented the elevation of Bax mRNA expression (P < 0.05).
Figure 6.
Relative mRNA expression in the liver. The Chicken β-actin was served as the internal control gene. Expression of BAX (A) and Bcl-2 (B) mRNA were assessed by real-time quantitative PCR. A relative quantitative method (2−ΔΔCt) was employed to evaluate the quantitative variation. Data are represented as means ± SE (n = 6). In each histogram, bars sharing different letters are significantly different (P < 0.05).
Histological Characterization
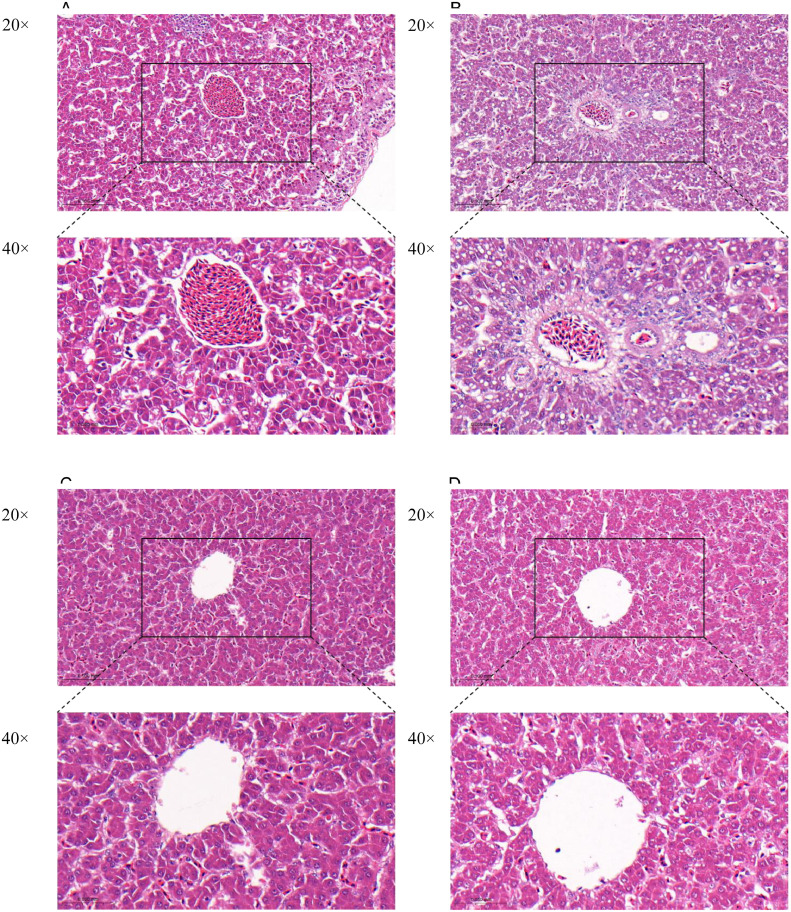
Histological analysis was performed to estimate the extent of liver tissue damage. The results of histological analysis showed that the hepatocytes in the control group were completely intact without histopathological changes (Figure 7A). The cytoplasm within the model group was infiltrated with fat vacuoles of various sizes and shapes (Figure 7B). However, hepatocytes in the Rg1 and Re groups (Figures 7C and 7D) showed less pronounced histopathological changes compared to the model group.
Figure 7.
Histopathological changes of hepatocytes among groups. (HE, on the top, magnification 200×; on the bottom, magnification 400×). (A) Control group; (B) Model group; (C) Rg1 group; (D) Re group.
DISCUSSION
Hepatic inflammation response and oxidative damage are hallmark of immune stress in broilers (Zhao et al., 2021b; Zheng et al., 2021; Bi et al., 2022b). Therefore, regulating inflammatory response and ameliorating oxidative damage are considered effective to prevent immune stress. As one of the major constituents in Panax ginseng C.A. Meyer, ginsenoside Rg3 have anti-inflammatory and antioxidant effects (Liu et al., 2020; Ren et al., 2021; Bi et al., 2022a). In this study, ginsenoside Rg1 and Re reduced the stress-related hormone level, decreased the oxidative damage, inhibited the enhanced inflammatory responses and cell apoptosis, as well as alleviated the pathological changes in liver of broilers challenged by LPS. Due to the protection of liver and regulation of stress-induced catabolism, enhanced body weight gain was observed in chickens in our previous study (Bi et al., 2022a).
Environmental pathogens such as Escherichia coli and its component LPS generally attack chickens and activate inflammatory responses, promoting degradation of amino acids for muscle synthesis and inhibiting growth (Zheng et al., 2021; Bi et al., 2022b). When TLR4 on immune cells recognizes LPS, a series of cascade responses are activated including synthesis and release of TNF-α, IL-1β, IL-6, and iNOS mediating NF-κB signaling pathway (Lu et al., 2008; Lai et al., 2017). The production of proinflammatory cytokines and inducible proinflammatory enzymes released free radicals and induced oxidative stress, causing cell apoptosis, and tissue damage. Antioxidant enzymes (CAT, T-SOD, and GSH) and oxidases (XOD) play an important role in mitigating oxidative stress damage (Hahn et al., 2014; Neha et al., 2019). However, excess oxygen radicals will consume antioxidant enzymes and increase oxidase activity, peroxidizing polyunsaturated fatty acids to lipid peroxides (Tsikas, 2017). Similar to previous reports, challenge with LPS has been demonstrated effective in inducing immune stress in chickens (Rehan et al., 2020; Bai et al., 2022; Wang et al., 2022). The present study showed that LPS treatment increased ACTH and CORT, TNF-α, iNOS, IL-1β and IL-6, XOD and MDA, as well as reduced activities of CAT, T-SOD, and GSH.
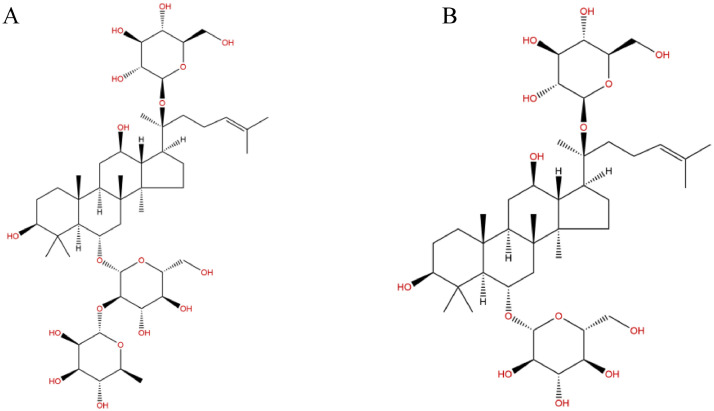
The alleviated effect of ginsenosides Rg1 and Re may attributed to their anti-inflammatory and antioxidant effects. Su et al. reported that Rg1 and Re prevented LPS from binding to TLR4 and blocked LPS-triggered signaling pathways, downregulated the expression and production of multiple proinflammatory mediators, and enhanced the immune response (Su et al., 2012, 2015). Ning et al. (2018) demonstrated that Rg1 also enhanced resistance to oxidative stress and hepatic detoxification. Lee et al. (2020) reported that Re has the potential to suppress lung inflammation by interrupting the inflammatory signaling pathway. Huang et al. (2016) showed that Re protects human umbilical vein endothelial cells from oxidative stress damage by participating in the stress response, antioxidant system, regulation of protein synthesis, transcription and PTM, and repair of mitochondrial function. In the present study, inhibited inflammatory mediators, oxidases, and MDA, as well as elevated antioxidant activities were observed in chickens with immune stress after treatment of ginsenoside Rg1or Re. The results of this study and in our previous studies suggest that herbs containing ginsenosides Rg3, Rg1, and Re could be considered as potential drugs to prevent immune stress in chickens in the future. Though both Rg1 and Re belong to the protopanaxatriol-type saponins (Figure 8), Re has an additional rhamnose at the C-6 position (Bi et al., 2022a). Structural differences between them may explain the discrepancy on antioxidant and anti-inflammation effect.
Figure 8.
Chemical structure of ginsenoside Re (C48H82O18; molecular weight, 947.166) (A); Rg1 (C42H72O14; molecular weight, 801.024) (B).
Nrf-2 is an important transcription factor in the regulation of antioxidant enzymes (Solano-Urrusquieta et al., 2020). Under normal physiological conditions, Nrf-2 binds to Keap1 to form a complex in response to oxidative stress or other pathological stimuli (Bellezza et al., 2018). When Nrf-2 release from the complex and translocated to the nucleus, antioxidant response elements (AREs) are activated, initiating Nrf2-mediated transcriptional process and regulating the expression of a range of antioxidant enzymes such as HO-1, SOD, and glutathione peroxidase (GSH-Px). HO-1 is a phase II antioxidant enzyme regulated by Nrf2 regulated phase II antioxidant enzyme, which is an important inducible stress response protein exhibiting antioxidant properties (Qiao et al., 2014; Raghunath et al., 2018; Hassanein et al., 2020). SOD plays a role in protecting enzymes and proteins from oxygen toxicity in prokaryotes and eukaryotes (Wang et al., 2018; Ali et al., 2020). In this study, Rg1 prevented oxidative stress in liver by upregulating mRNA expression of SOD, HO-1 and Nrf-2. The results were consistent with previous reports that the antioxidant and hepatoprotective effects of ginsenoside Rg1 are mainly achieved through induction of the Keap1-Nrf2-ARE signaling pathway (Gao et al., 2017). Wang et al. also reported that Re protects neurons from oxidative damage at least in part through induction of the Nrf2/HO-1 pathway (Qiao et al., 2022). Therefore, we hypothesized that ginsenosides Rg1 and Re ameliorated LPS-mediated oxidative stress injury in broilers by mediating the Keap1-Nrf2-ARE pathway. However, the exact mechanism of action needs to be verified in the further experiment.
Oxidative stress also increased apoptotic cells in liver as implied by increased expression of Bax and decreased Bcl-2 (Kello et al., 2020; Li et al., 2021). The damage of hepatocytes under oxidative stress was in association with decreased amino acid metabolism and lipid metabolism observed in chickens in our previous study (Bi et al., 2022b). In the present study, ginsenoside Rg1 inhibited the expression of the pro-apoptotic protein Bax but not promoted the expression of anti-apoptotic protein Bcl-2 in liver of broilers challenged by LPS. Therefore, we speculate that Rg1 may reduce LPS-induced apoptosis in hepatocytes by upregulating the expression of Nrf2 and releasing more antioxidant enzymes, as well as inhibiting NF-κB and downstream inflammatory responses.
In conclusion, ginsenoside Rg1 and Re could decreased inflammatory responses and reduced oxidative damage in liver of broiler chicks challenged by LPS. The effect of Rg1 may be attributed to upregulation of Nrf-2, HO-1, SOD-1, and downregulation of TLR4 and NF-κB. The results showed that the Rg1 and Re can be used for ameliorating hepatic oxidative stress and inflammation in broilers and alleviating the immune stress.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32002325), Chongqing Science and Technology Commission (cstc2021jscx-lyjsAX0008), and Fundamental Research Funds for the Central Universities (SWU-KT22011).
Author contributions: Liting Cao and Shicheng Bi conceived and designed the experiments. Weidong Hu, Jianjian Shao and Yiwen Qu performed the experiment. Weidong Hu, Li Zhang, Jun Li and Sihuai Chen contributed to the acquisition, analysis of data. Liting Cao, Weidong Hu, Shicheng Bi and Yue Ma wrote and revised the manuscript. All authors read and agreed to the version of the manuscript.
DISCLOSURES
The authors report no declarations of interest.
REFERENCES
- Aka E., Eren U. Distribution of tlr4 and mhc class ii molecules of the spleen in broiler chicks treated with and without lps in the first 2 weeks of the post-hatch period. Br. Poult. Sci. 2019;60:130–138. doi: 10.1080/00071668.2018.1564238. [DOI] [PubMed] [Google Scholar]
- Ali S.S., Ahsan H., Zia M.K., Siddiqui T., Khan F.H. Understanding oxidants and antioxidants: classical team with new players. J. Food Biochem. 2020;44:e13145. doi: 10.1111/jfbc.13145. [DOI] [PubMed] [Google Scholar]
- Bai D., Liu K., He X., Tan H., Liu Y., Li Y., Zhang Y., Zhen W., Zhang C., Ma Y. Effect of dietary chlorogenic acid on growth performance, antioxidant function, and immune response of broiler breeders under immune stress and stocking density stress. Vet. Sci. 2022;9:582. doi: 10.3390/vetsci9100582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Bi S., Qu Y., Shao J., Zhang J., Li W., Zhang L., Ni J., Cao L. Ginsenoside Rg3 ameliorates stress of broiler chicks induced by Escherichia coli lipopolysaccharide. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.878018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S., Shao J., Qu Y., Hu W., Ma Y., Cao L. Hepatic transcriptomics and metabolomics indicated pathways associated with immune stress of broilers induced by lipopolysaccharide. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Chu S., Zhang Z., Chen N. Hepataprotective effects of ginsenoside rg1 - a review. J. Ethnopharmacol. 2017;206:178–183. doi: 10.1016/j.jep.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Hahn W.S., Kuzmicic J., Burrill J.S., Donoghue M.A., Foncea R., Jensen M.D., Lavandero S., Arriaga E.A., Bernlohr D.A. Proinflammatory cytokines differentially regulate adipocyte mitochondrial metabolism, oxidative stress, and dynamics. Am. J. Physiol.-Endocrinol. Metab. 2014;306:E1033–E1045. doi: 10.1152/ajpendo.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Zhang J., Chen Y., Shen M., Yan E., Wei C., Yu C., Zhang L., Wang T. Dietary taurine supplementation attenuates lipopolysaccharide-induced inflammatory responses and oxidative stress of broiler chickens at an early age. J. Anim. Sci. 2020;98:1–10. doi: 10.1093/jas/skaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanein E.H.M., Sayed A.M., Hussein O.E., Mahmoud A.M. Coumarins as modulators of the keap1/nrf2/are signaling pathway. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/1675957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.D., Zhong X.F., Deng Z.Y., Zeng R. Proteomic analysis of ginsenoside re attenuates hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells. Food Funct. 2016;7:2451–2461. doi: 10.1039/c6fo00123h. [DOI] [PubMed] [Google Scholar]
- Kello M., Takac P., Kubatka P., Kuruc T., Petrova K., Mojzis J. Oxidative stress-induced DNA damage and apoptosis in clove buds-treated mcf-7 cells. Biomolecules. 2020;10:139. doi: 10.3390/biom10010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.L., Liu Y.H., Liu C., Qi M.P., Liu R.N., Zhu X.F., Zhou Q.G., Chen Y.Y., Guo A.Z., Hu C.M. Indirubin inhibits lps-induced inflammation via tlr4 abrogation mediated by the nf-kb and mapk signaling pathways. Inflammation. 2017;40:1–12. doi: 10.1007/s10753-016-0447-7. [DOI] [PubMed] [Google Scholar]
- Lee G.H., Lee W.J., Hur J., Kim E., Lee H.G., Seo H.G. Ginsenoside re mitigates 6-hydroxydopamine-induced oxidative stress through upregulation of gpx4. Molecules. 2020;25:188. doi: 10.3390/molecules25010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li Q., Gao N., Wang Z., Li F., Li J., Shan A. Exopolysaccharides produced by lactobacillus rhamnosus gg alleviate hydrogen peroxide-induced intestinal oxidative damage and apoptosis through the keap1/nrf2 and bax/bcl-2 pathways in vitro. Food Funct. 2021;12:9632–9641. doi: 10.1039/d1fo00277e. [DOI] [PubMed] [Google Scholar]
- Li P., Lv B., Jiang X., Wang T., Ma X., Chang N., Wang X., Gao X. Identification of nf-κb inhibitors following shenfu injection and bioactivity-integrated uplc/q-tof-ms and screening for related anti-inflammatory targets in vitro and in silico. J. Ethnopharmacol. 2016;194:658–667. doi: 10.1016/j.jep.2016.10.052. [DOI] [PubMed] [Google Scholar]
- Liu X., Mi X., Wang Z., Zhang M., Hou J., Jiang S., Wang Y., Chen C., Li W. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis. 2020;11:454. doi: 10.1038/s41419-020-2597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(t)(-delta delta c) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Y.C., Yeh W.C., Ohashi P.S. Lps/tlr4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Mao J., Ma X., Zhu J., Zhang H. Ginsenoside rg1 ameliorates psoriasis-like skin lesions by suppressing proliferation and nlrp3 inflammasomes in keratinocytes. J. Food Biochem. 2022;46:e14053. doi: 10.1111/jfbc.14053. [DOI] [PubMed] [Google Scholar]
- Neha K., Haider M.R., Pathak A., Yar M.S. Medicinal prospects of antioxidants: a review. Eur. J. Med. Chem. 2019;178:687–704. doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- Ning C., Gao X., Wang C., Huo X., Liu Z., Sun H., Yang X., Sun P., Ma X., Meng Q., Liu K. Hepatoprotective effect of ginsenoside rg1 from panax ginseng on carbon tetrachloride-induced acute liver injury by activating nrf2 signaling pathway in mice. Environ. Toxicol. 2018;33:1050–1060. doi: 10.1002/tox.22616. [DOI] [PubMed] [Google Scholar]
- Qiao H., Sai X., Gai L., Huang G., Chen X., Tu X., Ding Z. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a huge review and meta-analysis. Am. J. Epidemiol. 2014;179:1039–1048. doi: 10.1093/aje/kwu024. [DOI] [PubMed] [Google Scholar]
- Qiao J., Zhao Y., Liu Y., Zhang S., Zhao W., Liu S., Liu M. Neuroprotective effect of ginsenoside re against neurotoxin‑induced Parkinson's disease models via induction of nrf2. Mol. Med. Rep. 2022;25:215. doi: 10.3892/mmr.2022.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath A., Sundarraj K., Nagarajan R., Arfuso F., Bian J., Kumar A.P., Sethi G., Perumal E. Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol. 2018;17:297–314. doi: 10.1016/j.redox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan I.F., Youssef M., Abdel-Rahman M.A.M., Fahmy S.G., Ahmed E., Ahmed A.S., Maky M.A., Diab H.M., Shanab O., Alkahtani S., Abdel-Daim M.M., Hassan H., Rehan A.F., Hussien M.A., Eleiwa N.Z., Elnagar A., Abdeen A., Hesham A.E.-L. The impact of probiotics and egg yolk IGY on behavior and blood parameters in a broiler immune stress model. Front. Vet. Sci. 2020;7:145. doi: 10.3389/fvets.2020.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B., Feng J., Yang N., Guo Y., Chen C., Qin Q. Ginsenoside Rg3 attenuates angiotensin ii-induced myocardial hypertrophy through repressing nlrp3 inflammasome and oxidative stress via modulating sirt1/nf-κb pathway. Int. Immunopharmacol. 2021;98 doi: 10.1016/j.intimp.2021.107841. [DOI] [PubMed] [Google Scholar]
- Sangaran P.G., Ibrahim Z.A., Chik Z., Mohamed Z., Ahmadiani A. Lps preconditioning attenuates apoptosis mechanism by inhibiting nf-κb and caspase-3 activity: Tlr4 pre-activation in the signaling pathway of lps-induced neuroprotection. Mol. Neurobiol. 2021;58:2407–2422. doi: 10.1007/s12035-020-02227-3. [DOI] [PubMed] [Google Scholar]
- Shi C., Wang J., Zhang R., Ishfaq M., Li Y., Zhang R., Si C., Li R., Li C., Liu F. Dihydromyricetin alleviates Escherichia coli lipopolysaccharide-induced hepatic injury in chickens by inhibiting the nlrp3 inflammasome. Vet. Res. 2022;53:6. doi: 10.1186/s13567-022-01024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Jin X., Xu Y., Xing Y., Yan S., Guo Y., Cheng Y., Shi B. Effects of total flavonoids of artemisia ordosica on growth performance, oxidative stress, and antioxidant status of lipopolysaccharide-challenged broilers. Antioxidants. 2022;11:1985. doi: 10.3390/antiox11101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Urrusquieta A., Morales-González J.A., Castro-Narro G.E., Cerda-Reyes E., Flores-Rangel P.D., Fierros-Oceguera R. Nrf-2 and nonalcoholic fatty liver disease. Ann. Hepatol. 2020;19:458–465. doi: 10.1016/j.aohep.2019.11.010. [DOI] [PubMed] [Google Scholar]
- Su F., Xue Y., Wang Y., Zhang L., Chen W., Hu S. Protective effect of ginsenosides rg1 and re on lipopolysaccharide-induced sepsis by competitive binding to toll-like receptor 4. Antimicrob. Agents Chemother. 2015;59:5654–5663. doi: 10.1128/AAC.01381-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F., Yuan L., Zhang L., Hu S. Ginsenosides rg1 and re act as adjuvant via tlr4 signaling pathway. Vaccine. 2012;30:4106–4112. doi: 10.1016/j.vaccine.2012.03.052. [DOI] [PubMed] [Google Scholar]
- Tong Y., Yu C., Xie Z., Zhang X., Yang Z., Wang T. Trans-anethole ameliorates lipopolysaccharide-induced acute liver inflammation in broilers via inhibiting nf-kappab signaling pathway. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (mda) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang F., Song Z.W., Shao H.T., Bai D.Y., Ma Y.B., Kong T., Yang F. The influence of immune stress induced by Escherichia coli lipopolysaccharide on the pharmacokinetics of danofloxacin in broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Branicky R., Noë A., Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K.J., Lim J.H., Suh S.I., Kwon Y.K., Shin S.W., Kim S.C., Choi Y.H., Park J.W., Kwon T.K. Differential inhibitory effects of baicalein and baicalin on lps-induced cyclooxygenase-2 expression through inhibition of c/ebpbeta DNA-binding activity. Immunobiology. 2006;211:359–368. doi: 10.1016/j.imbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang L., Zhang C., Guo Y., Li J., Wu C., Jiao J., Zheng H. Ginsenoside rg1 improves alzheimer's disease by regulating oxidative stress, apoptosis, and neuroinflammation through wnt/gsk-3β/β-catenin signaling pathway. Chem. Biol. Drug Des. 2022;99:884–896. doi: 10.1111/cbdd.14041. [DOI] [PubMed] [Google Scholar]
- Yang S., Zhang J., Jiang Y., Xu Y., Jin X., Yan S., Shi B. Effects of artemisia argyi flavonoids on growth performance and immune function in broilers challenged with lipopolysaccharide. Anim Biosci. 2021;34:1169–1180. doi: 10.5713/ab.20.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Zhang C., Fan Q., Lin X., Wang Y., Azzam M., Alhotan R., Alqhtani A., Jiang S. Antrodia cinnamomea polysaccharide improves liver antioxidant, anti-inflammatory capacity, and cecal flora structure of slow-growing broiler breeds challenged with lipopolysaccharide. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.994782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhong Q., Liu N., Song P., Zhu P., Zhang C., Sun Z. Dietary glutamine supplementation alleviated inflammation responses and improved intestinal mucosa barrier of lps-challenged broilers. Animals. 2022;12:1729. doi: 10.3390/ani12131729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao L., Cao F., Ahmad H., Wang G., Wang T. Effects of feeding fermented ginkgo biloba leaves on small intestinal morphology, absorption, and immunomodulation of early lipopolysaccharide-challenged chicks. Poult. Sci. 2013;92:119–130. doi: 10.3382/ps.2012-02645. [DOI] [PubMed] [Google Scholar]
- Zhao J., He B., Zhang S., Huang W., Li X. Ginsenoside rg1 alleviates acute liver injury through the induction of autophagy and suppressing nf-κb/nlrp3 inflammasome signaling pathway. Int. J. Med. Sci. 2021;18:1382–1389. doi: 10.7150/ijms.50919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zhang Q., Xu C., Wang S., Li J., Liu Z., Li S. Methionine selenium antagonizes lps-induced necroptosis in the chicken liver via the mir-155/traf3/mapk axis. J. Cell. Physiol. 2021;236:4024–4035. doi: 10.1002/jcp.30145. [DOI] [PubMed] [Google Scholar]
- Zheng A., Zhang A., Chen Z., Pirzado S.A., Chang W., Cai H., Bryden W.L., Liu G. Molecular mechanisms of growth depression in broiler chickens (gallus gallus domesticus) mediated by immune stress: a hepatic proteome study. J. Anim. Sci. Biotechnol. 2021;12:90. doi: 10.1186/s40104-021-00591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]