Summary
Background
Reducing socioeconomic inequalities in cancer is a priority for the public health agenda. A systematic assessment and benchmarking of socioeconomic inequalities in cancer across many countries and over time in Europe is not yet available.
Methods
Census-linked, whole-of-population cancer-specific mortality data by socioeconomic position, as measured by education level, and sex were collected, harmonized, analysed, and compared across 18 countries during 1990–2015, in adults aged 40–79. We computed absolute and relative educational inequalities; temporal trends using estimated-annual-percentage-changes; the share of cancer mortality linked to educational inequalities.
Findings
Everywhere in Europe, lower-educated individuals have higher mortality rates for nearly all cancer-types relative to their more highly-educated counterparts, particularly for tobacco/infection-related cancers [relative risk of lung cancer mortality for lower- versus higher-educated = 2.4 (95% confidence intervals: 2.1–2.8) among men; = 1.8 (95% confidence intervals: 1.5–2.1) among women]. However, the magnitude of inequalities varies greatly by country and over time, predominantly due to differences in cancer mortality among lower-educated groups, as for many cancer-types higher-educated have more similar (and lower) rates, irrespective of the country. Inequalities were generally greater in Baltic/Central/East-Europe and smaller in South-Europe, although among women large and rising inequalities were found in North–Europe (relative risk of all cancer mortality for lower- versus higher-educated ≥1.4 in Denmark, Norway, Sweden, Finland and the England/Wales). Among men, rate differences (per 100,000 person-years) in total-cancer mortality for lower-vs-higher-educated groups ranged from 110 (Sweden) to 559 (Czech Republic); among women from approximately null (Slovenia, Italy, Spain) to 176 (Denmark). Lung cancer was the largest contributor to inequalities in total-cancer mortality (between-country range: men, 29–61%; women, 10–56%). 32% of cancer deaths in men and 16% in women (but up to 46% and 24%, respectively in Baltic/Central/East-Europe) were associated with educational inequalities.
Interpretation
Cancer mortality in Europe is largely driven by levels and trends of cancer mortality rates in lower-education groups. Even Nordic-countries, with a long-established tradition of equitable welfare and social justice policies, witness increases in cancer inequalities among women. These results call for a systematic measurement, monitoring and action upon the remarkable socioeconomic inequalities in cancer existing in Europe.
Funding
This study was done as part of the LIFEPATH project, which has received financial support from the European Commission (Horizon 2020 grant number 633666), and the DEMETRIQ project, which received support from the European Commission (grant numbers FP7-CP-FP and 278511). SV and WN were supported by the French Institut National du Cancer (INCa) (Grant number 2018-116). PM was supported by the Academy of Finland (#308247, # 345219) and the European Research Council under the European Union's Horizon 2020 research and innovation programme (grant agreement No 101019329). The work by Mall Leinsalu was supported by the Estonian Research Council (grant PRG722).
Keywords: Socioeconomic inequalities, Cancer mortality, Between- and within countries cancer inequalities, Cancer disparities, Social gradient
Research in context.
Evidence before this study
The assessment, monitoring and reduction of socioeconomic inequalities in cancer has become one of the most important priorities for public health policy, globally and in Europe. We searched PubMed for previously published studies addressing socioeconomic inequalities in cancer for both incidence and mortality. We focused particularly on international studies that could compare socioeconomic inequalities in cancer across countries. An example search strategy used includes the following: “((((socioeconomic inequalities OR educational inequalities) AND (cancer OR neoplasm OR carcinoma OR neoplasms [MeSH Terms]) AND (incidence OR mortality))) AND English [Language] AND (“1980”[Date - Publication]: “2022”[Date - Publication]))”. We also searched and reviewed references from retrieved articles to identify additional studies. Despite the increase in research activities on the topic, a study that could systematically assess and compare socioeconomic inequalities in cancer across many countries in Europe and over time is not yet available.
Added value of this study
The present study represents the most comprehensive comparative assessment of the magnitude and temporal trends of socioeconomic inequalities in cancer in Europe. Cancer-specific mortality data by socioeconomic status, as measured by educational level, were collected and harmonized across 18 countries in Europe and for multiple points in time over the period 1990–2015. We have assessed absolute and relative educational inequalities in cancer mortality, trends by education-level, sex, country, and cancer-type; and the share of cancer mortality linked with less-than-higher educational levels.
Implications of all the available evidence
Socioeconomic inequalities in mortality exist for most forms of cancer everywhere in Europe, with higher mortality rates for individuals at the lower ends of the social hierarchy. Nevertheless, the magnitude of these inequalities varies greatly by country and over time. A substantial fraction (about 32% in men and 16% in women, but up to 46% and 24%, respectively in Baltic/Central/East-Europe) of cancer deaths in Europe were associated with educational inequalities. Whereas the most advantaged in society are relatively protected against cancer mortality independently of where they live in the continent, for the least advantaged the country of residence is of great importance with respect to cancer mortality. This also implies that reducing cancer mortality among the most disadvantaged is crucial to lower national average rates of cancer mortality. Socioeconomic inequalities in cancer mortality are increasing rapidly among women, particularly for lung cancer and even in countries with a long-established tradition of equitable welfare and social justice policies, such as the Nordic countries. The present study calls for a systematic measurement, monitoring and action upon the substantial socioeconomic inequalities in cancer in Europe.
Introduction
For many decades, national and international bodies have committed extensive resources into measuring of the burden of cancer at the population level. While there remain significant gaps in the knowledge of cancer incidence, prevalence, survival, and mortality, it is now possible to draw a general picture of the cancer problem at the national level for many countries.1,2 Over time, another challenge has overwhelmingly emerged: by measuring only the average values at national or regional levels, a critical component of the cancer dynamics has gone largely unchecked, namely the diversity in the scale, profile and trends of cancer according to socioeconomic position.3 Wherever data are available, individuals with lower socioeconomic position—whether measured by education, occupational class, income or other indicators—are at disproportionally higher risk of dying from the most common forms of cancer, compared to their more advantaged fellow citizens.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The social gradient may nevertheless vary across countries, over time and for different cancer types.
The need to reduce socioeconomic inequalities in health and cancer is receiving greater attention today than ever before,3,15, 16, 17 and it has been recognized as a matter of social justice and human rights, as well as beneficial from the economic perspective,18 and consequently prioritized in the public health Agenda,19 and in the Sustainable Development Goals process.20 One of the priorities of the European Union (EU) in the area of health is “Europe's Beating Cancer Plan”, which aims to “identify trends, disparities and inequalities between Member States and regions” and establish a Cancer Inequalities Registry as a means of “reducing cancer inequalities across the EU”.21 Recently, a Lancet Commission on women and cancer has been launched to focus on the nexus of “gender, power, and cancer”, taking an intersectional feminist approach to cancer inequalities.22
The awareness that socioeconomic inequalities in health are both real and largely avoidable has immediate implications for policy recommendations and resource allocation for prevention and control at continental, national and regional level, within and outside the health system. Such decisions need to be supported by a rigorous measurement and long-term monitoring of cancer inequalities. Some studies have documented inequalities in cancer incidence and survival using aggregated data.23,24 But in general, the routine surveillance systems are not yet designed to capture the profound socioeconomic differences in cancer outcomes. Therefore, despite the increasing research activities on the topic, studies that could systematically assess and compare socioeconomic inequalities in cancer in Europe across several countries and over time at the individual level are not yet available. To overcome this unavailability of data, the OECD recommends using standardised approaches, preferably based on a longitudinal design that links mortality data with census, and using education as socioeconomic indicator.25 In support of all these actions, we conducted a comprehensive and comparative assessment of the magnitude and temporal trends of educational inequalities in cancer mortality using linked, whole-of-population data from 18 European countries.
Methods
We collected and harmonized mortality data by cause of death, over extended periods of time, for 18 European countries spanning across regions of the continent [North: Norway, Sweden, Finland, Denmark, England/Wales; West/South: Belgium, France, Switzerland, Austria, Italy (Torino department), Spain; Baltic/Central/East: Estonia, Lithuania, Poland, Czech Republic, Hungary, and Slovenia]. Most data stem from a longitudinal mortality follow-up at the individual level after a census. Deaths and person-years were classified according to 5-year age groups and follow up periods. For a few countries (Czech Republic, Hungary, Poland, Spain), separate, unlinked data sources were used for deaths and population-at-risk by age, sex and education. Most countries provide data about the entire national population, except England/Wales and France, for which large representative samples were used, and Italy and Spain, for which selected regions were included (Turin province and Barcelona, respectively). We used mortality data for total cancer and specific cancer sites (although in some countries, data were not always available for all cancer sites) by education level, biological sex and age for individuals aged 40–79 years across multiple points in time over the period 1990–2015,26 Supplementary Table S1. Overall availability of cancer-specific data is provided in Supplementary Table S3. Age-standardised mortality rates (ASMR) per 100,000 person-years were calculated using the European Standard Population year 2013,27 and presented by educational-level, country, sex and cancer-type. Educational-level was used as an indicator of socioeconomic position and measured at the individual level, with “lower”, “intermediate”, and “higher” education corresponding to the 1997 International Standard Classification of Education (ISCED, categories 0–2, 3–4, 5–6).28 Persons with missing information on educational attainment were excluded (this percentage varied between 0 and 11%, depending on the country).
Both absolute and relative educational inequalities in cancer mortality were assessed in each country to represent different aspects of inequalities, using rate differences (RD) and rate ratios (RR) in the ASMRs between the lower-, or intermediate-, and higher-education groups (the latter being the reference). When the main focus was on the comparison between specific-cancer types (i.e., Fig. 3 and Supplementary Figure S4), rather than on geographical heterogeneity, and it was necessary to summarize RRs across countries, Poisson regression models were fitted, adjusting for age, with random-effects at the country-level acting on the intercept and with robust estimation of the standard error to account for overdispersion. Data are presented for the most recent period, i.e., between 1998 and 2015, depending on the country, or for the time series available, as specified. Cancer- and country-specific estimated annual percentage changes (EAPC) over the period 1990–2015 were calculated by fitting a linear regression model, where the logarithm of the age-standardised mortality rates was regressed on time. This model assumes linear trends over the whole period of observation, even when countries have a varying time-span of available data. The assumptions underlying our linear regression model, e.g., Normality and homogeneity of the variance of residuals, and linearity of the temporal predictor have been assessed using residual plots and QQ plots. As a sensitive analysis, we have carried out a join point analysis, which however did not perform better than our main analysis that assume linearity of the temporal trends. To summarize EAPC in cancer mortality across countries, a random-effects model was used, with country-level random effects and with estimates weighted according to the population-size of the country. The share of total cancer mortality linked with less-than-higher educational levels, i.e., lower- and intermediate-, was estimated by assuming a scenario based on the concept of plausible minimal risk where these groups have the same mortality rates as the higher-educated group, within each age-category. Confidence intervals (CIs) for the above describes statistics were computed using bootstrapping and reported as indicated. Throughout the rest of the text, the term “inequalities” is used to signify “educational inequalities in cancer mortality”. Analyses were carried out in R and the code can be made available upon request.
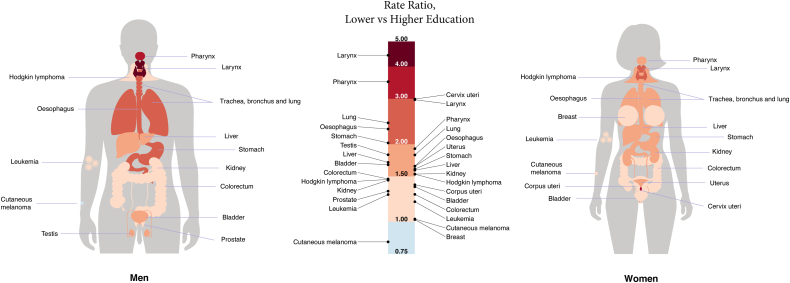
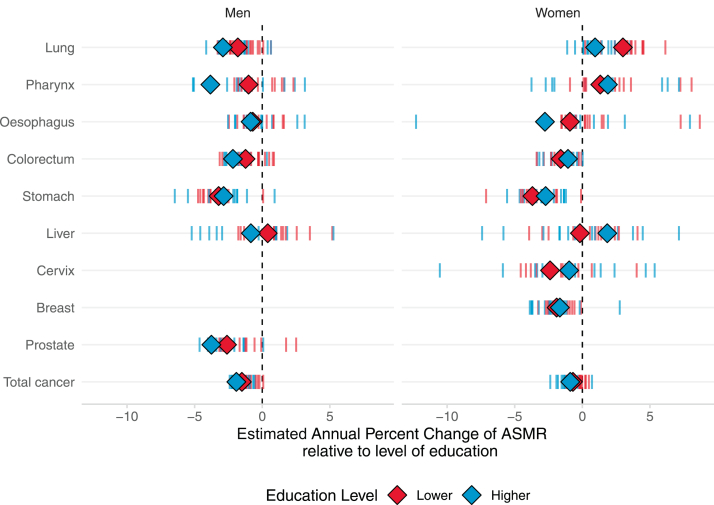
Fig. 3.
Relative educational inequalities in cancer mortality between lower and higher educated by anatomical site and sex, for the last period of observation. Footnote: this infographic corresponds to the forest plot shown in Supplementary Figure S4. Pooled rate ratio estimates and corresponding 95% CIs are obtained by fitting a Poisson regression model adjusted for age, with random-effects at the country-level acting on the intercept and with robust estimation of the standard error to take into account for overdispersion of the data.
Role of the funding source
The funding sources had no role in the collection, study design, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article.
Results
National profiles of education
There was substantial variability in education levels across countries, with the highest proportion of lower-education found in Spain (66% in men, 72% in women) and the lowest in Switzerland (13%) among men and Estonia (15%) among women, Supplementary Figure S1.
Inequalities between- and within-countries
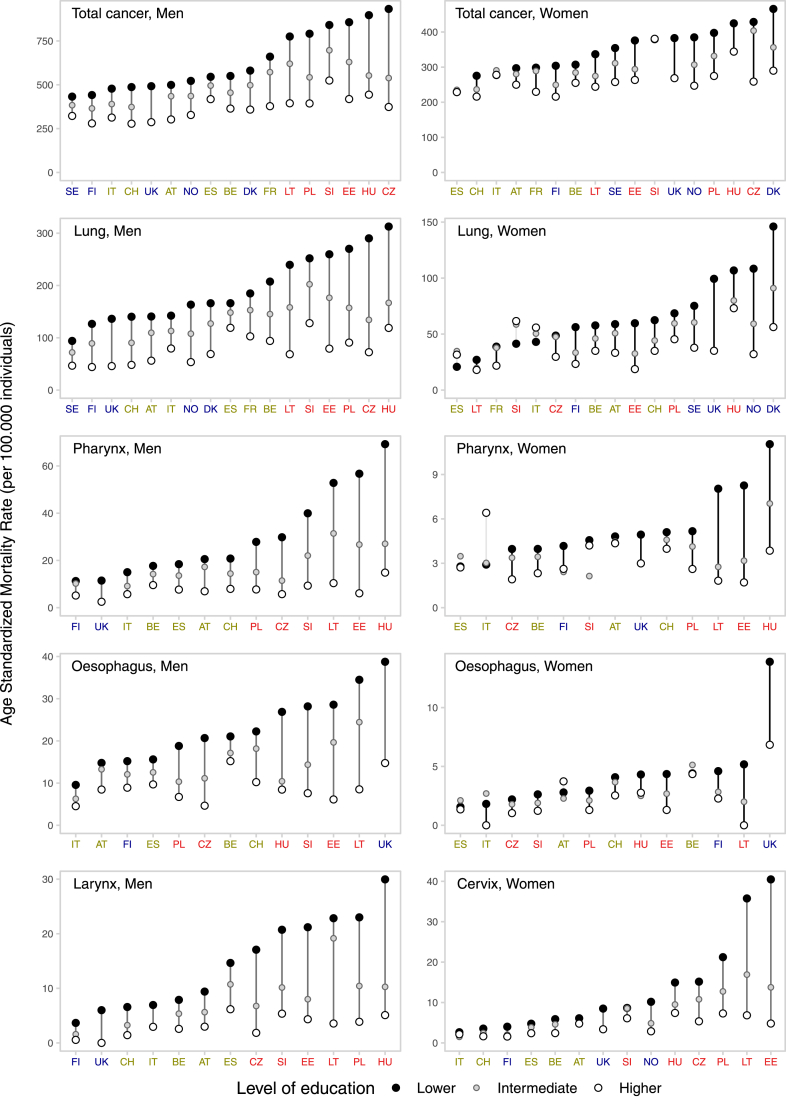
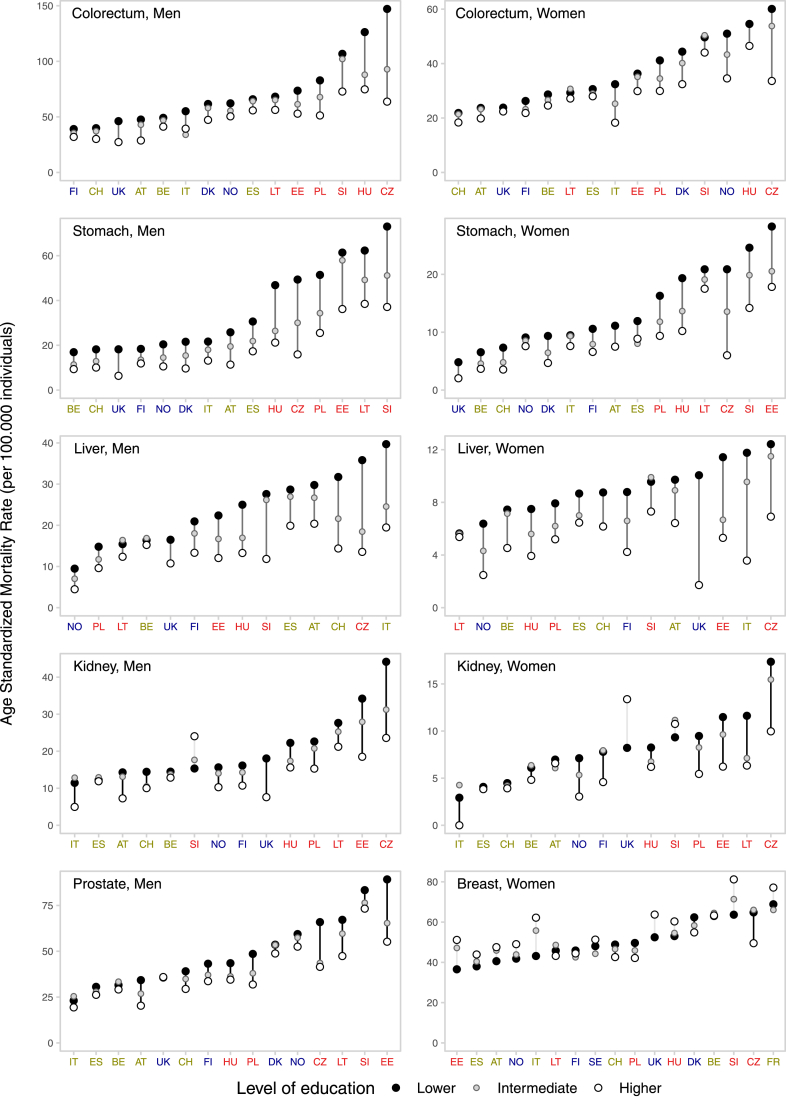
Fig. 1 and Supplementary Figures S2 and S3 present inequalities between and within countries for all cancers combined and for type-specific cancers, by sex for the last period of observation. Inequalities were observed for most cancer forms, in all countries and for both sexes, with lower-education groups exhibiting consistently elevated ASMRs than higher-education groups, and in-between values for intermediate-education groups, i.e., therefore following a clear socioeconomic gradient. However, the magnitude of the inequalities varied greatly across countries, with two major patterns of between-country variation observed. Firstly, for most cancer types the ASMRs variability among higher-education groups was relatively narrow, and much of the variability in between-country inequalities was confined to differences among lower-education groups. This pattern was observed for total cancer, for cancer of the lung, cervix, pharynx, larynx, and oesophagus (for the latter three, the pattern was less pronounced among women). Secondly, for cancer of the colorectum, stomach, and liver in both sexes, and prostate, bladder, and kidney among men, higher ASMRs among lower-education groups in a country generally corresponded also to higher ASMRs among higher-education groups, with rate differences progressively widening for countries at the higher risk of cancer mortality. For the remaining cancer types, no clear between-country pattern was observed.
Fig. 1.
Educational inequalities between and within countries in total cancer mortality, and for type-specific cancers, in Europe, by sex, for the last period of observation. Footnote: in each panel, countries are ordered (from left-to-right) according to an increasing level of ASMR for lower-educated. The period of observation varies between 1998 and 2015, depending on the country. North; West/South; Baltic/Central/Eastern.
Geographic profile of inequalities in total cancer mortality
Absolute and relative inequalities in total cancer mortality were generally greater in the Baltic/Central/Eastern European countries and lesser in the South, although among women the largest inequalities were observed in the North, Fig. 1 and Supplementary Figure S3A. Among men, rate differences in total cancer mortality between lower- and higher-educated groups varied substantially, with values of RD per 100,000 ranging from 110 (95% CIs: 98–123) in Sweden to 559 (546–573) in the Czech Republic. These differences were largely accounted for by differences in the ASMRs among lower-educated men, which ranged from 432 (425–440) per 100,000 in Sweden to 933 (925–940) per 100,000 in the Czech Republic. The range of ASMRs among higher-educated men in fact varied much less, i.e., from 278 (269–286) per 100,000 in Switzerland to 524 (494–555) per 100,000 in Slovenia. Among women, large absolute differences in total cancer mortality were observed in the Baltic/Central/Eastern area—RD per 100,000: 170 (153–185) in the Czech Republic; 123 (115–130) in Poland; 112 (77–146) in Estonia—as well as in Northern Europe, with Denmark reaching the highest levels [RD = 176/100,000 (162–189)] and with high values also observed in Norway [RD = 138/100,000 (116–157)] and England/Wales [RD = 114/100,000 (63–161)]. Conversely, among women from Italy, Spain and Slovenia, there was no evidence of substantial inequalities. Similar patterns were generally found when considering relative inequalities (in men, RR lower- versus higher-education >1.6 in all Baltic/Central/Eastern European countries, reaching 2.5 in the Czech Republic; in women, RR ≥ 1.4 in all five Northern European countries, and in Poland, Czech Republic, Lithuania and Estonia).
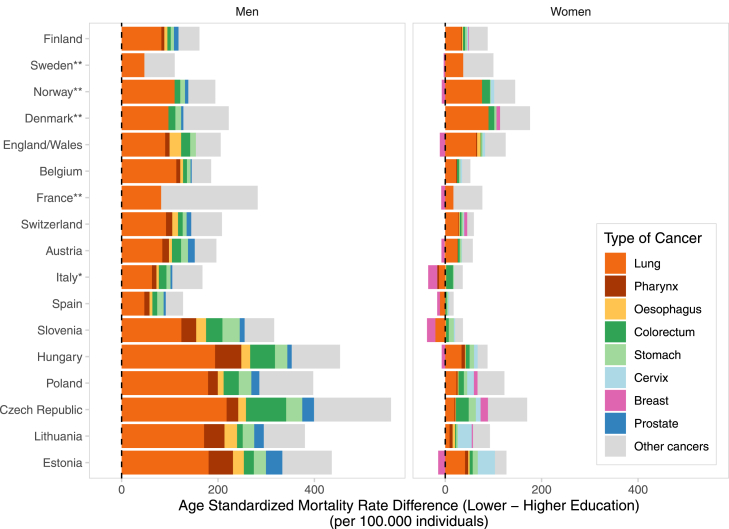
Contribution of specific cancers to inequalities in total cancer mortality
Lung cancer was, by far, the most important contributor to absolute inequalities in total cancer mortality everywhere in men and in most European countries in women, although the specific contribution varied by country and sex (men: from 29.1% in France to 61.1% in Belgium; women: from 9.7% in Lithuania to 56.3% in England/Wales), Fig. 2. Exceptions were women from the Czech Republic and Lithuania, where lung cancer ranked 2nd (in the Czech Republic: 11.1%, after colorectum, 15.6%; in Lithuania: 9.7%, after cervix, 31.2%). The other major contributors were colorectum (men: 3.1–14.9%; women: 1.2–15.6%), pharynx (men: 3.8–12.0%; women: 0.9–6.7%), stomach (men: 3.9–11.3%; women: 1.1–11.3%), and cervical cancer (2.7–31.7%). Among women from Slovenia, Spain and Italy, the balance between positive and negative inequalities across all specific-cancer types amounted to an approximately null value of total cancer inequalities.
Fig. 2.
Contribution of frequent type-specific cancers to absolute educational inequalities in cancer mortality, by sex, for the last period of observation 1998–2015. Lower- vs higher-educated. Footnotes: ∗ The coverage for Italy was limited to the department of Torino. ∗∗ For Norway, Denmark, Sweden and France, it was possible to estimate the contribution to total cancer mortality only for certain specific-cancer types, due to data unavailability. In women from Slovenia, Spain and Italy, the balance between positive and negative inequalities across all specific-cancer types amounted to an approximately null value of total cancer inequalities.
Relative inequalities in cancer-specific mortality
Relative inequalities of lower- versus higher-educated groups for specific cancer sites in 18 European countries combined are shown in Fig. 3 and Supplementary Figure S4, whereas the between-country variability of the corresponding results is displayed in Supplementary Figure S3AC. Although RR for lower- vs higher-educated exceeded 1 for almost all examined cancer types, particularly high values were observed for smoking-related, e.g., lung [RR = 2.4 (95% CIs: 2.1–2.8), men; RR = 1.8 (1.5–2.1), women], larynx [RR = 4.4 (3.0–6.6), men; RR = 3.0 (2.0–4.5), women], pharynx [RR = 3.5 (2.7–4.5), men; RR = 1.9 (1.4–2.6), women], oesophagus [RR = 2.3 (1.8–3.0), men; RR = 1.6 (1.2–2.2), women] and infection-related cancers, e.g., cervix [RR = 3.0 (2.5–3.6)], stomach [RR = 2.0 (1.8–2.2), men; RR = 1.6 (1.4–1.8), women] and liver [RR = 1.7 (1.5–2.0), men; RR = 1.6 (1.4–1.8), women]. Breast cancer and melanoma did not show a clear social gradient. Nevertheless, there was substantial between-country variability in the estimates, with notably large RRs among men for pharyngeal cancer in Estonia [RR = 9.3 (5.7–19.2)] and laryngeal cancer in the Czech Republic [RR = 9.3 (6.4–14.6)], and among women for cervical cancer, larynx and pharynx in Estonia [RRs = 8.4 (4.8–15.5), 5.9 (0.0–14.6), and 4.8 (1.8–18.7), respectively] and for liver cancer in England/Wales [RR = 5.9 (1.9–15.7)].
Inequalities over time
Estimated changes in mortality rates over time during the period 1990 and 2015 for total cancer and type-specific cancers, by education level and sex, are shown in Fig. 4 and by country in Supplementary Figure S5. Overall, declines in cancer mortality for total cancer were observed in both sexes, but yearly decreasing rates were greater among higher-educated [EAPC = −1.9% (95% CIs: −2.2% to −1.6%), men; −0.9% (−1.2% to −0.6%), women] than lower-educated [EAPC = −1.5% (−1.7% to −1.3%), men; −0.7% (−0.9% to −0.5%), women].
Fig. 4.
Estimatedannual percentage change during the period 1990–2015 for total cancer and for the most frequent type-specific cancer, by country and sex. Footnote: Lines represent country-specific values. Diamonds represent the weighted average of the estimated annual percentage change across all study-countries, by education level.
Gender inequalities
Although absolute and relative inequalities were almost everywhere higher in men compared to women, this situation is rapidly becoming more unfavourable to women. Among men, declines were generally observed for most frequent cancer sites, including lung, pharynx, liver, colorectum, and prostate, with exceptions for some specific cancer/country combinations. Conversely, among women, increases in lung cancer mortality were evident for all educational levels in most countries, but were faster among lower-education groups, especially in the Nordic countries, the Baltic/Central/East area, France, and Spain. Also, diverging trends, with increases among lower-educated and decreases or relatively stable rates among higher-educated groups were observed among women from certain countries for cancer of the liver, pharynx, oesophagus and cervix, and among men for pharynx, colorectum, liver, and prostate, Supplementary Figure S5.
Proportion of cancer deaths associated with lower educational levels
Table 1 shows the proportion of total cancer mortality associated with a lower- or intermediate-education level by cancer type, sex and region in Europe, during 2004–2015. In men, 32.2% (31.6%–32.7%) of total cancer deaths are associated with a lower or intermediate education level, but this proportion reaches up to 45.5% (44.8%–46.2%) in countries from the Baltic/Central/East region. In women, 15.9% (14.9%–16.9%) of total cancer deaths are associated with a lower or intermediate educational level, with proportions reaching 23.8% (22.3%–25.3%) in the Baltic/Central/East area. The highest proportions of cancer deaths associated with lower or intermediate education were found for smoking- and infections-related cancers, particularly in Eastern/Baltic countries in both sexes and in Northern Europe among women.
Table 1.
Percentage of total cancer mortality associated with less-than-higher educational level in European regions, by sex, for the last period of observation.
| North % (95% CI) | West/South % (95% CI) | Baltic/Central/East % (95% CI) | All % (95% CI) | |
|---|---|---|---|---|
| A) Men | ||||
| Total cancer | 23.8 (22.6, 24.9) | 22.0 (21.0, 22.8) | 45.5 (44.6, 46.0) | 32.2 (31.5, 32.6) |
| Lung | 46.5 (44.6, 48.6) | 30.7 (29.1, 32.3) | 61.1 (60.0, 62.2) | 45.9 (44.9, 46.7) |
| Pharynx | 45.4 (33.2, 57.4) | 49.7 (45.9, 53.5) | 71.9 (69.5, 74.3) | 61.8 (59.6, 63.9) |
| Larynx | 75.4 (57.4, 94.1) | 53.6 (48.4, 58.8) | 79.4 (76.6, 82.1) | 67.2 (64.3, 70.0) |
| Oesophagus | 31.0 (20.1, 41.9) | 30.9 (26.2, 35.5) | 63.6 (60.0, 67.3) | 44.8 (41.8, 47.7) |
| Colorectum | 14.1 (9.8, 18.4) | 14.2 (11.2, 17.1) | 37.1 (34.9, 39.2) | 24.9 (23.1, 26.5) |
| Stomach | 31.9 (25.2, 38.6) | 37.3 (33.2, 41.2) | 45.9 (43.0, 48.8) | 41.3 (39.0, 43.6) |
| Liver | 28.5 (19.4, 37.9) | 25.3 (21.2, 29.5) | 41.7 (37.3, 46.1) | 30.8 (27.9, 33.9) |
| Prostate | 9.2 (4.4, 13.9) | 12.3 (8.1, 16.5) | 25.1 (21.8, 28.2) | 17.3 (15.0, 19.7) |
| Bladder | 32.5 (17.5, 47.2) | 20.6 (15.8, 25.4) | 42.7 (39.0, 46.5) | 30.1 (27.0, 33.3) |
| Kidney | 26.4 (16.7, 36.3) | 7.1 (0.6, 13.4) | 32.8 (28.6, 36.9) | 22.2 (18.7, 25.7) |
| Leukemia | 11.2 (−0.9, 23.5) | 9.7 (3.5, 15.9) | 22.3 (17.1, 27.6) | 15.6 (11.7, 19.6) |
| B) Women | ||||
| Total cancer | 20.3 (18.9, 21.6) | 4.9 (3.0, 6.7) | 23.8 (22.2, 25.1) | 15.9 (14.8, 16.8) |
| Lung | 43.6 (41.1, 46.2) | −3.6 (−8.6, 1.4) | 25.4 (21.9, 29.0) | 20.3 (18.0, 22.7) |
| Pharynx | 12.1 (−17.2, 41.4) | 8.8 (−7.5, 25.3) | 44.7 (34.2, 55.4) | 27.5 (18.4, 36.5) |
| Larynx | 71.8 (11.4, 132.1) | 22.1 (−10.6, 55.3) | 44.8 (22.0, 67.8) | 37.5 (19.1, 55.8) |
| Oesophagus | 31.1 (8.6, 54.1) | 9.4 (−6.9, 26.0) | 45.3 (30.8, 60.0) | 26.1 (15.5, 37.1) |
| Colorectum | 16.7 (11.3, 21.9) | 8.9 (3.3, 14.5) | 24.6 (20.1, 29.0) | 17.4 (14.2, 20.5) |
| Stomach | 24.3 (13.8, 34.8) | 24.1 (15.2, 33.1) | 42.1 (36.5, 47.7) | 33.5 (28.8, 38.4) |
| Liver | 41.5 (27.9, 55.3) | 25.4 (15.3, 35.6) | 32.9 (23.5, 42.6) | 29.3 (22.5, 36.1) |
| Cervix | 43.2 (26.0, 60.3) | 42.4 (32.7, 52.4) | 53.7 (48.5, 59.0) | 50.4 (45.9, 55.0) |
| Kidney | 32.8 (18.5, 46.9) | 9.0 (−5.2, 23.2) | 35.0 (25.7, 44.3) | 26.0 (18.5, 33.4) |
| Uterus | 3.9 (−40.4, 47.7) | 10.6 (−6.5, 27.6) | 45.2 (29.6, 60.7) | 26.3 (14.9, 37.7) |
| Corpus uteri | 14.1 (−1.1, 29.2) | 22.1 (10.7, 33.3) | 24.2 (14.9, 33.4) | 22.7 (15.8, 29.5) |
Footnote: We have assumed a counterfactual scenario, based on the concept of plausible minimal risk, where all educational groups (lower- and intermediate-education) have the same level of risk of cancer death as the most educated group, within each age group.
Discussion
The results of this study allow a number of conclusions to be drawn that are of immediate public health relevance to several generations of European citizens. Firstly, everywhere lower-educated individuals systematically suffer from higher mortality rates for nearly all cancer types, relative to their more highly-educated counterparts, with a social gradient of increasing risk of death with diminishing education level.
Secondly, the extent of the inequalities nevertheless varies markedly geographically and over time. The large international heterogeneity of the inequalities is predominantly attributable to between-country differences in cancer mortality rates among lower-educated groups, given that corresponding rates among the higher educated are more homogeneous and relatively low. This is an important addition to the current knowledge on the topic, from which the following implications arise. At the individual-level, the most advantaged in society are relatively protected against cancer mortality independently of where in Europe they live, whereas for the less educated majority, the country of residence matters significantly with respect to cancer mortality. At the country-level, reducing cancer mortality among lower socioeconomic individuals is crucial to lower national average rates of cancer mortality.
Thirdly, within each country, inequalities were almost always higher in men compared to women, for total and specific-cancer sites. However, the rapidly increasing lung cancer rates (and other smoking- and alcohol-related cancers, such as pharynx, oesophageal and liver cancer) among lower-educated women are a reason for concern, driving an exacerbation of socioeconomic inequalities in cancer among women.22,29 Despite their well-established social safety net with egalitarian health and social policies, the Nordic countries are not spared from socioeconomic inequalities; conversely, they show among the highest levels and most unfavourable trends in inequalities in lung cancer mortality among women in Europe.
The observed inequalities in cancer mortality are linked to events that occurred over the previous decades: from individual and collective behaviours, customs and social interactions linked to the exposure to cancer risk factors and to cancer incidence, to the availability and access to early diagnosis and screening programme and to effective treatments. These factors are in large part socially determined, depending on the way the society is structured and healthcare services are organized and delivered.18 The pathway to inequalities in mortality varies also across cancer types, reflecting a different balance of how inequalities impact across the different phases of the cancer continuum. A study from France has contributed to clarify this issue by estimating the respective contribution of socioeconomic inequalities in incidence and in lethality to socioeconomic inequalities in cancer mortality.23 For lung cancer, and other smoking-related cancers, inequalities in the distribution of the most important risk factor (i.e., tobacco smoking) may represent a large contribution to inequalities in mortality,30 given also that the prognosis of the disease is relatively poor. The different stages of the tobacco-smoking epidemic across socioeconomic groups and along gender lines thus underlie the observed patterns of inequalities for these cancers.31 In our analysis, lung cancer was the main driver of total cancer inequalities in the majority of the 18 European study-countries. Despite the potential impact of effective tobacco control measures,32 the disease is still one of the most frequently diagnosed cancer types worldwide.2 A similar reasoning is applicable to alcohol-drinking, which is associated with cancer of the liver, colorectum and breast, and strongly interacts with tobacco-smoking for cancers of the upper respiratory and digestive tract.33
For other cancer types, socioeconomic inequalities in access to healthcare and to highly cost-effective screening and early diagnosis and treatment interventions likely play a substantial role in exacerbating inequalities in cancer mortality. Cervical cancer, for instance, is characterised by a strong socioeconomic gradient between- and within-countries, which primarily reflects inequalities in the availability, access and uptake of effective screening programmes, which can detect and remove precancerous lesions and thus reduce incidence34 (the impact of HPV vaccination is not yet visible in the present data). Cervical cancer mortality is rising in some Baltic/Central/Eastern countries,35 but our study shows that the geographical imbalance is almost exclusively due to between-country differences among lower-educated women. Conversely, the risk of dying from cervical cancer for higher-educated women is quite strikingly similar across European countries, likely because they can benefit from effective cancer screening and treatment programmes.36 A strong social gradient exists also for colorectal cancer, particularly pronounced in both sexes in the Baltic/Central/Eastern area and, among women, in Norway and Denmark. The incidence of this common cancer in some European countries is still rising among younger cohorts,37 and factors such as smoking, alcohol consumption, diet, obesity and differential access to screening, early detection, and timely and effective treatment, may explain the observed inequalities.
Even for cancers for which general declines in mortality were observed, trends were consistently less favourable among lower-educated, compared to their highly-educated counterparts, with rates often stable or even increasing, as the former are less likely to benefit from progress in prevention and treatment.38 Mortality rates of stomach cancer declined across all education groups and sexes, likely due to a general improvement in the living conditions and a consequent decreasing prevalence of Helicobacter pylori,39,40 but levels remain high in Baltic/Central/Eastern. The picture for liver cancer—for which the main causes are hepatitis B and C viruses41,42 and excessive alcohol drinking43—is less favourable, as increases are seen, especially among lower-educated groups, in some countries, including in North Europe.
Higher-educated women usually have higher breast cancer incidence rates, compared to lower-educated, likely due to a delayed age at childbearing, lower parity, and less breastfeeding, which are important risk factors for breast cancer. In the present study, there was not a clear social gradient for breast cancer mortality, in agreement with previous reports from high/medium-income settings.3 Trends of breast cancer mortality have been declining in the past decades in Europe for all educational-groups and are now converging to similar levels. Despite the lower incidence, lower-socioeconomic groups thus alarmingly suffer from similarly high mortality rates as those observed among their more affluent fellow citizens, perhaps due to a limited access and use of screening and timely and effective treatment.44 Only for melanoma, mortality rates were systematically higher among the higher-education group, particularly for men, likely due to higher levels of leisure activity involving high exposure levels to ultraviolet radiation therefore causing higher incidence of the disease.45
Among the limitations of this study is the fact that similar education levels may represent different levels of socioeconomic position in different countries, as participants were born in different periods of time and subjected to different educational systems. Lower-education levels may not adequately represent a situation of socioeconomic disadvantage in certain populations where small inequalities were found. However, the fact that cancer rates were quite homogeneous (and relatively low) across countries among highly-educated group for most cancer types, with systematic social gradient, suggests that education level may capture sufficiently well the relative socioeconomic advantage in cancer mortality across the studied populations.46,47 Ideally, multiple indicators of socioeconomic position (either summarized in a composite index, either analysed separately in parallel, at the individual and aggregate level) should be used to explore diverse aspects of socioeconomic inequalities in cancer, but such data are alas not as yet available on a large and comparative scale. The use of a standardised variable for education as a measure of socioeconomic condition here demonstrates to stratify populations relatively well according to the risk of dying from cancer, and has a number of recognized advantages, particularly with respect to ecological indicators but also compared to other individual indicators (like income or occupation), i.e., it is easier to collect on a routine basis; it is a good proxy of health literacy, it is relevant for both men and women and for all countries; it suffers less from the risk of reverse causation; and it usually remains fixed throughout life, after formal education is attained.25,26 Another possible limitation may be the inclusion only of individuals aged 40–79 years, although this age-group nevertheless represents around 70% of all cancer deaths in Europe.2 Also, the analyses on the most recent time-period varied by over a decade depending on the country, which may have led to a more unfavourable comparison for countries with older data, like the Czech Republic, given the general decline of the rates.
Among the other strengths, this study includes individual data for the entire population (within the study age range) for most of the study-countries (and large representative samples for the reminder countries). This is especially important given that most of the research on the topic has been conducted at a single country-level and often based on cohort studies, which are affected by the “healthy cohort bias”, and may lack sufficient representation for disadvantaged individuals. In addition, the possibility to dispose and analyse data for the whole population for many countries allowed to detect and quantify precisely significant associations between education level and cancer mortality for many cancer types (see rate ratios in Supplementary Figure S4), this demonstrating the adequacy of the sample size of this study.
The large geographical and temporal variability of socioeconomic inequalities in cancer reported here implies that these inequalities are not immutable but rather can be potentially modified and reduced, at least to the lowest levels observed in certain countries for higher socioeconomic groups. From a global perspective, cancer is gradually replacing cardiovascular disease as the main cause of premature mortality,48 and there is a pressing need to refocus efforts in tackling inequalities in present national cancer policies. We have measured the extent of socioeconomic inequalities in cancer mortality, finding that a substantial proportion of cancer deaths are associated with a lower socioeconomic position (>30% in men and >15% in women, but up to 46% and 24%, respectively in Baltic/Central/East-Europe). Although this observation cannot be interpreted directly in causal terms, it shows that reducing the excess cancer mortality among the lower socioeconomic groups would lead to a broad reduction in the burden and suffering from the disease for many individuals, and would also narrow between-country inequalities in cancer mortality.21 Actions on the social determinants of health, including intersectoral interventions, may contribute to reduce socioeconomic inequalities in cancer over a medium-range time horizon, if adequately adapted to country-specific situations. We recommend an equitable implementation of effective cancer policies across the cancer continuum, i.e., that all interventions and cancer control programmes, from prevention to treatment—particularly where effective prevention measures exist, e.g., tobacco policies for smoking-related cancers and vaccination/screening for cervical cancer—should be explicitly designed to reduce socioeconomic inequalities in cancer.49 The present study also calls for action to strengthen routine surveillance systems to measure and monitor socioeconomic inequalities in cancer, via population-based cancer registries or studies linking information on cancer with multiple indicators of socioeconomic position if possible or, at least, education level. This is of even more urgency at present, given the Covid-19 pandemic has further exacerbated existing social and health inequalities in Europe and worldwide.50
Contributors
SV and WN conceptualised and designed the study. DG, HC, and WN performed the statistical analyses. SV wrote the initial manuscript. SV, WN, DG, FB, HC, JM, PV, MM, VL, OG, EDV, PM, HBH, PD, MB, ML, and BA contributed to data interpretation. All authors contributed to the article and approved the final version.
Data sharing statement
The datasets analyzed during the current study are not publicly available due to restrictions imposed by several national data providers, but may be made available from the corresponding author on reasonable request.
Disclosures
The authors report no conflicts of interest.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Declaration of interest
We declare no competing interests.
Acknowledgements
The permission of the Office for National Statistics (ONS) to use the Longitudinal Study is gratefully acknowledged, as is the help provided by staff of the Centre for Longitudinal Study Information & User Support (CeLSIUS). CeLSIUS is supported by the ESRC Census of Population Programme (award reference ES/K000365/1). The authors alone are responsible for the interpretation of the data. This work contains statistical data from ONS, which is Crown Copyright. The use of the ONS statistical data in this work does not imply the endorsement of the ONS in relation to the interpretation or analysis of the statistical data. This work uses research datasets, which might not exactly reproduce National Statistics aggregates. Aggregated data on deaths and person years by age group, sex and level of education for Hungary between 1999 and 2012 were obtained and harmonized by Erasmus MC as part of the DEMETRIQ project and LIFEPATH project. The mortality data for Switzerland were obtained from the Swiss National Cohort, which is based on mortality and census data provided by the Federal Statistical Office and supported by the Swiss National Science Foundation. Mortality data for Slovenia were provided by the Statistical Office of the Republic of Slovenia and supported by the Slovenian Research Agency (research core funding No. P3-0429, Slovenian Research Programme for Comprehensive Cancer Control SLORApro). We acknowledge Eva-Maria Asamer, Carme Borell, Guesepe Costa, Ramune Kalediene, Olle Lundberg, Gwenn Menvielle, Jitka Rychtaříková, Heine Strand, Chris White, Bodgan Wojtyniak for providing data. We would like to thank Morena Sarzo from IARC for the production of the infographic shown in Fig. 3.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2022.100551.
Appendix A. Supplementary data
References
- 1.Bray F., Colombet M., Mery L., et al. International Agency for Research on Cancer; Lyon: 2020. Cancer incidence in five continents, Vol. XI. IARC Scientific Publication No. 166; p. 2021. [Google Scholar]
- 2.Ferlay J.E.M., Lam F., Colombet M., et al. International Agency for Research on Cancer; Lyon, France: 2020. Global cancer observatory: cancer today. [Google Scholar]
- 3.Vaccarella S., Lortet-Tieulent J., Saracci R., Conway D., Straif K., Wild C. Reducing social inequalities in cancer: evidence and priorities for research (IARC Scientific Publication No. 168). International Agency for Research on Cancer, Lyon. 2019. http://publicationsiarcfr/580 Available from: Chapter 6. [PubMed]
- 4.Rachet B., Ellis L., Maringe C., et al. Socioeconomic inequalities in cancer survival in England after the NHS cancer plan. Br J Cancer. 2010;103(4):446–453. doi: 10.1038/sj.bjc.6605752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stringhini S., Carmeli C., Jokela M., et al. Socioeconomic status and the 25×25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet. 2017;389(10075):1229–1237. doi: 10.1016/S0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallo V., Mackenbach J.P., Ezzati M., et al. Social inequalities and mortality in Europe--results from a large multi-national cohort. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0039013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart C.L., Hole D.J., Gillis C.R., Smith G.D., Watt G.C., Hawthorne V.M. Social class differences in lung cancer mortality: risk factor explanations using two Scottish cohort studies. Int J Epidemiol. 2001;30(2):268–274. doi: 10.1093/ije/30.2.268. [DOI] [PubMed] [Google Scholar]
- 8.Menvielle G., Kunst A.E., Stirbu I., et al. Educational differences in cancer mortality among women and men: a gender pattern that differs across Europe. Br J Cancer. 2008;98(5):1012–1019. doi: 10.1038/sj.bjc.6604274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway D.I., Brenner D.R., McMahon A.D., et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case-control studies from 27 countries. Int J Cancer. 2015;136(5):1125–1139. doi: 10.1002/ijc.29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries E., Arroyave I., Pardo C. Time trends in educational inequalities in cancer mortality in Colombia, 1998–2012. BMJ Open. 2016;6(4) doi: 10.1136/bmjopen-2015-008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanthomme K., Vandenheede H., Hagedoorn P., Gadeyne S. Evolution of educational inequalities in site-specific cancer mortality among Belgian men between the 1990s and 2000s using a “fundamental cause” perspective. BMC Cancer. 2017;17(1):470. doi: 10.1186/s12885-017-3461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Launoy G., Coleman M.P., Zadnik V. Springer; 2021. Social environment and cancer in Europe. [Google Scholar]
- 13.Mihor A., Tomsic S., Zagar T., Lokar K., Zadnik V. Socioeconomic inequalities in cancer incidence in Europe: a comprehensive review of population-based epidemiological studies. Radiol Oncol. 2020;54(1):1–13. doi: 10.2478/raon-2020-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Exarchakou A., Kipourou D.K., Belot A., Rachet B. Socio-economic inequalities in cancer survival: how do they translate into number of life-years lost? Br J Cancer. 2022;126(10):1490–1498. doi: 10.1038/s41416-022-01720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vineis P., Avendano-Pabon M., Barros H., et al. Special report: the biology of inequalities in health: the lifepath consortium. Front Public Health. 2020;8:118. doi: 10.3389/fpubh.2020.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaccarella S., Weiderpass E., Vineis P. Present and future of health inequalities: rationale for investing in the biological capital. EClinicalMedicine. 2020;19 doi: 10.1016/j.eclinm.2020.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmot M. Bloomsbury; London, UK: 2015. The health gap. [Google Scholar]
- 18.Marmot M., Allen J., Goldblatt P. Institute of Health Equity, University College London; London: 2010. Fair society healthy lives (the Marmot review). 242. [Google Scholar]
- 19.https://www.who.int/health-topics/health-equity WHOHEAo
- 20.https://sdgs.un.org/2030agenda UNTOWTAfSDAo
- 21.https://ec.europa.eu/health/sites/health/files/non_communicable_diseases/docs/eu_cancer-plan_en.pdf
- 22.Ginsburg O., Horton R. A Lancet Commission on women and cancer. Lancet (London, England) 2020;396(10243):11–13. doi: 10.1016/S0140-6736(20)31479-3. [DOI] [PubMed] [Google Scholar]
- 23.Bryere J., Tron L., Menvielle G., Launoy G. The respective parts of incidence and lethality in socioeconomic differences in cancer mortality. An analysis of the French network cancer registries (FRANCIM) data. Int J Equity Health. 2019;18(1):189. doi: 10.1186/s12939-019-1087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derette K., Rollet Q., Launay L., Launoy G., Bryere J. Evolution of socioeconomic inequalities in cancer incidence between 2006 and 2016 in France: a population-based study. Eur J Cancer Prev. 2022;31(5):473–481. doi: 10.1097/CEJ.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 25.Mackenbach J., Menvielle G., Jasilionis D., de Gelder R. 2015. Measuring educational inequalities in mortality statistics. [Google Scholar]
- 26.Mackenbach J.P., Rubio Valverde J., Bopp M., et al. Progress against inequalities in mortality: register-based study of 15 European countries between 1990 and 2015. Eur J Epidemiol. 2019;34(12):1131–1142. doi: 10.1007/s10654-019-00580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad O.B., Boschi-Pinto C., Lopez A.D., Murray C.J., Lozano R., Inoue M. World Health Organization; Geneva: 2001. Age standardization of rates: a new WHO standard. 9(10) [Google Scholar]
- 28.Statistics UIf International standard classification of education: ISCED 2011. Comp Soc Res. 2012;30 [Google Scholar]
- 29.Ginsburg O., Bray F., Coleman M.P., et al. The global burden of women's cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casetta B., Videla A.J., Bardach A., et al. Association between cigarette smoking prevalence and income level: a systematic review and meta-analysis. Nicotine Tob Res. 2017;19(12):1401–1407. doi: 10.1093/ntr/ntw266. [DOI] [PubMed] [Google Scholar]
- 31.Long D., Mackenbach J., Martikainen P., et al. Smoking and inequalities in mortality in 11 European countries: a birth cohort analysis. Popul Health Metr. 2021;19(1):3. doi: 10.1186/s12963-021-00247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization WH . 2003. WHO framework convention on tobacco control. [Google Scholar]
- 33.Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:3–1383. [PMC free article] [PubMed] [Google Scholar]
- 34.Vaccarella S., Lortet-Tieulent J., Plummer M., Franceschi S., Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49(15):3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Vaccarella S., Franceschi S., Zaridze D., et al. Preventable fractions of cervical cancer via effective screening in six Baltic, central, and eastern European countries 2017-40: a population-based study. Lancet Oncol. 2016;17(10):1445–1452. doi: 10.1016/S1470-2045(16)30275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Prez V., Jolidon V., Willems B., Cullati S., Burton-Jeangros C., Bracke P. Cervical cancer screening programs and their context-dependent effect on inequalities in screening uptake: a dynamic interplay between public health policy and welfare state redistribution. Int J Equity Health. 2021;20(1):211. doi: 10.1186/s12939-021-01548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araghi M., Soerjomataram I., Bardot A., et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;4(7):511–518. doi: 10.1016/S2468-1253(19)30147-5. [DOI] [PubMed] [Google Scholar]
- 38.Link B.G., Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;Spec No:80–94. [PubMed] [Google Scholar]
- 39.Howson C.P., Hiyama T., Wynder E.L. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 40.de Martel C., Forman D., Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42(2):219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Organization WH . World Health Organization; 2017. Global hepatitis report 2017. [Google Scholar]
- 42.Nagel G., Linseisen J., Boshuizen H.C., et al. Socioeconomic position and the risk of gastric and oesophageal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Epidemiol. 2007;36(1):66–76. doi: 10.1093/ije/dyl275. [DOI] [PubMed] [Google Scholar]
- 43.Grittner U., Kuntsche S., Gmel G., Bloomfield K. Alcohol consumption and social inequality at the individual and country levels—results from an international study. Eur J Public Health. 2013;23(2):332–339. doi: 10.1093/eurpub/cks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duggan C., Trapani D., Ilbawi A.M., et al. National health system characteristics, breast cancer stage at diagnosis, and breast cancer mortality: a population-based analysis. Lancet Oncol. 2021;22(11):1632–1642. doi: 10.1016/S1470-2045(21)00462-9. [DOI] [PubMed] [Google Scholar]
- 45.Gibson J.A.G., Dobbs T.D., Griffiths R., et al. The association of smoking and socioeconomic status on cutaneous melanoma: a population-based, data-linkage, case-control study. Br J Dermatol. 2020;182(5):1136–1147. doi: 10.1111/bjd.18526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulhánová I., Bacigalupe A., Eikemo T.A., et al. Why does Spain have smaller inequalities in mortality? An exploration of potential explanations. Eur J Public Health. 2014;24(3):370–377. doi: 10.1093/eurpub/cku006. [DOI] [PubMed] [Google Scholar]
- 47.Mackenbach J.P. Nordic paradox, Southern miracle, Eastern disaster: persistence of inequalities in mortality in Europe. Eur J Public Health. 2017;27(suppl_4):14–17. doi: 10.1093/eurpub/ckx160. [DOI] [PubMed] [Google Scholar]
- 48.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 49.Vaccarella S., Lortet-Tieulent J., Saracci R., et al. Reducing social inequalities in cancer: setting priorities for research. CA Cancer J Clin. 2018;68(5):324–326. doi: 10.3322/caac.21463. [DOI] [PubMed] [Google Scholar]
- 50.Barnard S., Fryers P., Fitzpatrick J., et al. Inequalities in excess premature mortality in England during the COVID-19 pandemic: a cross-sectional analysis of cumulative excess mortality by area deprivation and ethnicity. BMJ Open. 2021;11(12) doi: 10.1136/bmjopen-2021-052646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.