Summary
Background
Herpes simplex virus type 2 (HSV-2) infection is a globally prevalent, life-long, sexually transmitted infection. This study characterized HSV-2 seroprevalence in Europe for various at-risk populations and proportions of HSV-2 detection in genital ulcer disease (GUD) and in genital herpes. Data on neonatal herpes and HSV-2's contribution to HIV transmission were also reviewed.
Methods
Cochrane and PRISMA guidelines were followed to systematically review, synthesize, and report HSV-2 related findings. The search was conducted in PubMed and Embase databases up to February 20, 2022. Any publication reporting data on the outcome measures was included. Meta-analyses and meta-regressions were conducted.
Findings
211 relevant reports were identified, including 12 overall incidence measures, 294 overall (813 stratified by factors such as age and sex) seroprevalence measures, 13 overall (15 stratified by sex) proportions of HSV-2 detection in clinically diagnosed GUD, and 70 overall (183 stratified by factors such as age and sex) proportions of HSV-2 detection in laboratory-confirmed genital herpes. Pooled mean seroprevalence was 12.4% (95% CI: 11.5–13.3%) among general populations, 27.8% (95% CI: 17.5–39.4%) among men who have sex with men, 46.0% (95% CI: 40.1–51.8%) among people living with HIV and people in HIV discordant couples, and 63.2% (95% CI: 55.5–70.6%) among female sex workers. Most measures showed heterogeneity in HSV-2 seroprevalence. The pooled mean seroprevalence among general populations increased with age and was 0.65-fold (95% CI: 0.58–0.74) lower in men than women. Seroprevalence decreased by 1% per calendar year. Pooled mean proportions of HSV-2 detection in GUD and in genital herpes were 22.0% (95% CI: 15.3–29.6%) and 66.0% (95% CI: 62.9–69.1%), respectively. HSV-2 detection in genital herpes cases was 1.21-fold (95% CI: 1.10–1.32) higher in men compared to women and decreased by 1% per calendar year. Incidence of neonatal herpes indicated an increasing trend.
Interpretation
Although seroprevalence is declining, a significant proportion of Europe's population is infected with HSV-2. HSV-2 accounts for approximately one-fifth of GUD cases and two-thirds of genital herpes cases. Findings support the need to invest in HSV-2 vaccine development, and sexual and reproductive health services.
Funding
Qatar National Research Fund [NPRP 9-040-3-008] and pilot funding from the Biomedical Research Program at Weill Cornell Medicine in Qatar supported this study.
Keywords: Epidemiology, Herpes simplex virus, HSV-2, Genital herpes, Genital ulcer, Sexually transmitted infection, Prevalence, Europe
Research in context.
Evidence before this study
Herpes simplex virus type 2 (HSV-2) infection is a globally prevalent sexually transmitted infection that causes mild to severe disease. Despite over 30 years of active research, the global epidemiology of this infection remains inadequately characterized. A study by Yousuf et al1 investigated epidemiology of HSV-1 in Europe, a closely related infection to HSV-2. The study found that although HSV-1 seroprevalence is declining, it is becoming the leading cause of genital herpes in Europe.1 Global estimates for HSV-2 and HSV-1 infections have been published by James et al2 using mathematical modelling. These indicated that, in Europe in 2016, 10.7% of females and 5.3% of males 15–49 years of age were infected with HSV-2, and 60.6% of females and 40.1% of males 0–49 years of age were infected with HSV-1.2 Prior to starting this study, a PubMed search was conducted using a broad search strategy (using the keywords “Herpes Simplex”[MeSH] AND “Review”[Publication Type]) with no language or time limitations. No systematic review and meta-analytics investigating epidemiology of HSV-2 infection in Europe were identified.
Added value of this study
By implementing rigorous methodologies, this study provided an analytical assessment of the epidemiology of HSV-2 infection in Europe. After searching international electronic databases, a large body of data was identified, facilitating a range of analyses. Approximately 12% of the adult population of Europe is chronically infected with HSV-2, but seroprevalence is declining by 1% per calendar year. Sexual risk behaviour, age, sex, and subregion within Europe explained a large proportion of the variation in seroprevalence in this continent. HSV-2 was the aetiological cause of 22% of clinically diagnosed genital ulcer disease cases and 66% of laboratory-confirmed genital herpes. The contribution of HSV-2 infection (as opposed to HSV-1 infection) to genital herpes cases was declining by 1% per calendar year.
Implications of all the available evidence
Though slowly declining, HSV-2 disease burden remains sizable in Europe, against a background of non-specific, and no targeted, public health interventions to prevent and control transmission of this infection. In the context of serious HSV-2 disease sequelae affecting reproductive, sexual, and psychosocial health, these findings highlight the public health value of accelerating HSV-2 vaccine development as a fundamental solution to tackle this infection in Europe and elsewhere. HSV-2 research and surveillance should be expanded by conducting national population-based seroprevalence surveys and monitoring aetiology of genital ulcer disease and genital herpes.
Introduction
Herpes simplex virus type 2 (HSV-2) infection is a life-long, incurable, sexually (or vertically) transmitted infection (STI) that is prevalent worldwide.2 Although asymptomatic in most people, HSV-2 infection can cause a range of symptoms and adverse health outcomes such as recurrent genital lesions and serious neonatal infections.3, 4, 5 HSV-2 infection is characterized by frequent subclinical shedding and reactivations6 that are linked to increased sexual transmission and acquisition of HIV,7,8 perhaps causing epidemiological synergy between these two infections.7,9,10
Inadequate understanding of HSV-2 epidemiology and its consequences for sexual and reproductive health and the HIV epidemic, call for further research and for preventive and control measures.3,4,11 The World Health Organization (WHO) issued Global Health Sector Strategies on STIs12,13 to eliminate STIs as a main public health concern by 2030, through integration of preventive and control measures. WHO and global partners are prioritizing efforts to develop an HSV vaccine.2,14,15
Global estimates published by James et al2 using mathematical modelling indicated that, in Europe in 2016, 10.7% of females and 5.3% of males 15–49 years of age were infected with HSV-2, and 60.6% of females and 40.1% of males 0–49 years of age were infected with HSV-1. However, despite over three decades of active HSV-2 epidemiology research, no specific systematic review and meta-analytics investigating epidemiology of HSV-2 infection in Europe were published.
Accordingly, we conducted a systematic review and meta-analytics to characterize HSV-2 epidemiology in Europe. Pooled mean HSV-2 antibody prevalence (seroprevalence) was estimated for various at-risk populations, including general populations (such as antenatal clinic attendees, blood donors, and pregnant women), intermediate-risk populations (such as prisoners, people who inject drugs, and truck drivers), higher-risk populations (such as female sex workers (FSWs), men who have sex with men (MSM), male sex workers, and transgender people), STI clinic attendees and symptomatic populations, people living with HIV and people in HIV discordant couples, infertility clinic attendees and women with ectopic pregnancies, and other populations (such as cervical cancer patients and their spouses) (Box S1). Pooled mean proportions of HSV-2 virus detection in clinically diagnosed genital ulcer disease (GUD) cases and in laboratory-confirmed genital herpes cases were also estimated. Data on neonatal herpes and HSV-2's contribution to HIV transmission were also reviewed.
Both overall measures and stratified measures were extracted from relevant studies included in this review. Since our aim was to understand the natural heterogeneity that exists in HSV-2 epidemiology, such as the variation in seroprevalence by age, measures were extracted and stratified by key epidemiological factors known to affect the natural epidemiology of this infection.16, 17, 18, 19 Meta-regression analyses were conducted on these stratified measures to estimate effects of these epidemiological factors on both HSV-2 seroprevalence and proportion of HSV-2 detection in genital herpes. The meta-regression analyses were also conducted to investigate temporal trends and sources of between-study heterogeneity. This analytical approach combining meta-regressions with meta-analyses allows the generation of fundamental inferences about the epidemiology of this infection based on understanding the sources of variations that exist in available measures.
Methods
The methodology used in this study was based on that developed in a series of published systematic reviews and meta-analyses characterizing the epidemiology of HSV-1 and HSV-2 infections in other regions and countries.1,16, 17, 18, 19, 20, 21, 22, 23, 24 Therefore, no study protocol was registered in PROSPERO for this specific study. Study methods are described in Box 1 and summarized below.
Box 1. Description of the methodology of this study.
| Methodology | Description |
|---|---|
| Data source and search strategy |
|
| Study selection and inclusion and exclusion criteria |
|
| Data extraction and data synthesis |
|
| Quality assessments | The Cochrane-informed approach for risk of bias assessment included:
|
| Meta-analyses |
|
| Meta-regressions |
|
Abbreviations: ELISA = Enzyme-linked immunosorbent type-specific assay, GUD = Genital ulcer disease, HSV-1 = Herpes simplex virus type 1, HSV-2 = Herpes simplex virus type 2, STI = Sexually transmitted infection.
Data sources and search strategy
This systematic review was informed by the Cochrane Collaboration Handbook25 and its findings are reported as per the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Table S1).26,27 The search was first conducted in PubMed and Embase databases on January 12, 2021, with no language or time limitations, and then updated on February 20, 2022. The definition of Europe included 53 countries classified into subregions as informed by the WHO and United Nations Geoscheme definitions for Europe (Box 1).28,29 Search strategies are indicated in Box 1 and explicitly included in Table S2.
Study selection and inclusion and exclusion criteria
Screening steps and inclusion and exclusion criteria are described in Box 1. Using the reference manager Endnote (Thomson Reuters, USA), citations were imported from PubMed and Embase databases and duplicate citations were excluded. Title and abstract screening was performed to identify relevant and potentially relevant publications. Full texts of relevant and potentially relevant publications were retrieved. Bibliography screening of relevant publications and reviews was conducted to identify additional potentially relevant publications.
Inclusion criteria involved any publication with a minimum sample size of 10, reporting primary data on HSV-2 antibody incidence and/or seroprevalence, proportion of HSV-2 detection in clinically diagnosed GUD, and proportion of HSV-2 detection in laboratory-confirmed genital herpes. Exclusion criteria included case reports, case series, reviews, editorials, commentaries, and qualitative studies. Measures reporting seroprevalence in infants aged <6 months were excluded as their antibodies can be maternal in origin.
Data extraction and data synthesis
Screening of retrieved citations and articles was conducted by A.A., A.M.M.O., M.N.K., and M.H. All citations and articles retrieved were first independently screened by M.N.K., then, following the updated search, citations and articles retrieved were divided among A.A., A.M.M.O., and M.H. and once more independently screened. Data extraction was performed by A.M.M.O., A.A., and M.N.K. Double extraction was performed by M.H. Discrepancies were discussed in consultation with L.J.A.-R. to reach consensus. The extracted variables are indicated in Box 1 and listed in Box S2.
Overall outcome measures and their stratified measures were extracted, provided sample size in each stratum is ≥10. In occasions in which the subsample sizes for specific strata were not available, this subsample size was set at the total sample size of the study divided by the number of strata. Data from included studies were collected between 1968 and 2019 and were published between 1977 and 2021. The year of data collection was missing for a small proportion of studies, but it was imputed by adjusting the year of publication using the median difference with the year of data collection in studies with reported year of data collection.
Stratification hierarchy for incidence and seroprevalence measures in descending order of priority was population type, sex, and age group (<20 years old, 20–29 years old, 30–39 years old, 40–49 years old, 50–59 years old, ≥60 years old, and mixed ages). Stratification hierarchy for GUD and genital herpes included genital herpes episode status (first episode versus recurrent episodes) and study site (hospital versus STI clinic). All relevant data are presented in the manuscript and its Supplementary Material file. Detailed database of extracted variables can be obtained by contacting the authors.
Quality assessments
Due to known limitations of HSV-2 assays,30, 31, 32 a quality assessment of the assay in each relevant study was conducted with the assistance of Professor Rhoda Ashley–Morrow of the University of Washington – a leading expert in HSV-2 serological assays who has investigated and evaluated the validity and reliability of different assays for three decades. Professor Ashley–Morrow contributed as a compensated consultant. Details of each assay in each study were shared with Professor Ashley–Morrow. Validity and reliability of each assay was based on her expert judgment. Only studies with valid and reliable assays were included in the systematic review.
Each study was then assessed for precision and risk of bias (ROB) as informed by the Cochrane approach.25 Precision of a study was classified as low versus high based on the sample size (<200 versus ≥200). ROB of a study was classified as low versus high based on the sampling method (probability-based versus non-probability-based) and response rate (≥80% versus <80% or unclear) (Box 1). Information on precision and ROB were utilized to provide summary statistics of the precision and quality of studies. These variables were also included in the meta-regressions to investigate their effect on observed seroprevalence.
Meta-analyses and meta-regressions
All meta-analyses were conducted in R version 4.41.333 using the “meta” package,34 as described in Box 1. To account for both sampling variation and heterogeneity in effect size, meta-analyses were conducted using the DerSimonian-Laird random-effects model,35 with the Freeman-Tukey double arcsine transformation to stabilize the variance,36 after factoring applicability of this transformation.37
Heterogeneity was assessed based on Cochran's Q statistic, I2 heterogeneity measure, and prediction interval. Extracted measures were described using summary statistics, including medians, ranges, and pooled mean estimates and their 95% confidence intervals (CI) for HSV-2 seroprevalence and for proportions of HSV-2 detection in GUD and in genital herpes. Forest plots of all meta-analyses were generated by population type classification and by subregion for the general populations.
Univariable and multivariable regressions of log-transformed seroprevalence measures were conducted in Stata/SE version 1638 using the “metareg” package39 (Box 1). All stratified seroprevalence measures were utilized in all population types. Variables included in the univariable meta-regressions for HSV-2 seroprevalence were population type, age group, sex, European subregion/country, country's income, assay type (Western blot, ELISA, and monoclonal antibody), sample size, sampling method, response rate, year of data collection, year of publication, year of data collection category (<1995, 1995–2005, >2005), and year of publication category (<2000, 2000–2010, >2010). Variables included in the univariable meta-regressions for proportion of HSV-2 detection in genital herpes were age group, sex, genital herpes episode status, European subregion/country, sample size, year of data collection category (<1995, 1995–2005, >2005), and year of publication category (<2000, 2000–2010, >2010). Variables in the univariable analyses with a p-value <0.1 were included in the multivariable meta-regression models.
Four multivariable meta-regression models were implemented for each of HSV-2 seroprevalence and proportion of HSV-2 detection in genital herpes. The first and second models included the year of data collection as categorical and continuous linear variables, respectively. The third and fourth models included the year of publication as categorical and continuous linear variables, respectively. Variables in the multivariable analyses with a p-value ≤0.05 were considered significant predictors. These four models were implemented to account for the collinearity between year of data collection and year of publication, and to investigate whether the trends may have been appropriately described using an average linear trend. Although the year of data collection is more relevant than the year of publication, we added analyses using the year of publication because it has no missing entries.
Subgroup analyses were also conducted for HSV-2 seroprevalence including only studies among general populations, as well as for proportion of HSV-2 detection in genital herpes, including only studies with reported genital herpes episode status.
Neonatal herpes and role of HSV-2 in HIV transmission
A broad search of the literature was conducted to identify and extract epidemiological data on neonatal herpes in Europe (Table S3). PubMed and Embase were searched using the keywords “Neonatal herpes[MeSH] OR Neonatal herpes[Text])” and “(exp neonatal herpes) OR (neonatal herpes.mp.)”, respectively, with no language or time limitations. This search was conducted on August 16, 2022. Moreover, based on search terms used in a recent systematic review8 and as informed by two decades of investigating the overlapping epidemiology of HSV-2 and HIV,7, 8, 9, 10,20,40, 41, 42 a narrative review was conducted of estimates for the contribution of HSV-2 to HIV transmission in Europe.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results
Search results and scope of evidence
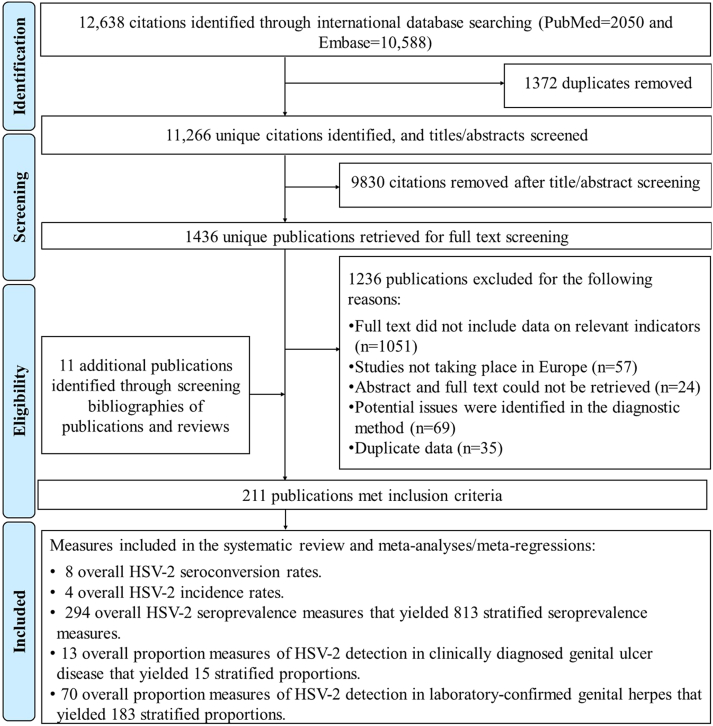
Fig. 1 describes the study selection process following PRISMA guidelines. Overall, 12,638 citations were identified (PubMed = 2050 and Embase = 10,588). After de-duplication and title and abstract screening, 1436 unique citations were identified as relevant or potentially relevant for further screening. Full text screening of these citations identified 200 relevant publications. Eleven additional relevant publications were identified through bibliography screening, including one thesis.
Fig. 1.
Flow chart of article selection for this systematic review of HSV-2 epidemiology in Europe, per PRISMA guidelines.27
In total, data were extracted from 211 publications that met the inclusion criteria. Extracted outcome measures included: 8 overall HSV-2 seroconversion rates, 4 overall HSV-2 incidence rates, 294 overall and 813 stratified HSV-2 seroprevalence measures, 13 overall and 15 stratified proportion measures of HSV-2 detection in GUD, and 70 overall and 183 stratified proportion measures of HSV-2 detection in genital herpes.
HSV-2 incidence overview
Table S4 summarizes extracted seroconversion rates (n (number of measures) = 8) and incidence rates (n = 4). These measures were extracted from cohort studies with follow-up durations ranging between 42 days and 6 years. The studies were conducted in pregnant women, healthy adolescents, soldiers, STI clinic attendees, MSM, and MSM living with HIV. Across all populations, seroconversion rate ranged between 0.3 and 17.0% and incidence rate ranged between 0.1 and 1.3 per 100 person-years.
HSV-2 seroprevalence overview
Tables S5 and S6 list overall extracted seroprevalence measures (n = 294). The earliest study was published in 1984, but the majority of studies (n = 162, 55.1%) were published between 2000 and 2010. Most studies (n = 251, 85.4%) used convenience sampling.
Descriptive statistics of all stratified seroprevalence measures by population type classification are presented in Table 1. HSV-2 seroprevalence ranged between 0.0 and 95.0% with a median of 11.3% among general populations (n = 626), between 2.0 and 32.8% with a median of 11.9% among intermediate-risk populations (n = 12), between 33.3 and 89.3% with a median of 59.6% among FSWs (n = 16), between 5.9 and 56.0% with a median of 34.5% among MSM (n = 12), between 2.0 and 84.9% with a median of 25.0% among STI clinic attendees and symptomatic populations (n = 74), between 9.0 and 94.0% with a median of 43.9% among people living with HIV and people in HIV discordant couples (n = 35), and between 2.0 and 24.0% with a median of 12.0% among infertility clinic attendees and women with ectopic pregnancies (n = 7). Descriptive statistics of stratified seroprevalence measures among only general populations are presented in Table 2.
Table 1.
Pooled mean estimates for HSV-2 seroprevalence among different at-risk populations in Europe by sex.
| Population type | Outcome measures |
Sample size |
HSV-2 seroprevalence (%) |
Pooled mean HSV-2 seroprevalence |
Heterogeneity measures |
|||
|---|---|---|---|---|---|---|---|---|
| Total n |
Total N |
Range | Median | Mean (%) (95% CI) |
Qa (p-value) | I2b (%) (95% CI) |
Prediction intervalc (%) | |
| General populations | 626 | 154,957 | 0.0–95.0 | 11.3 | 12.4 (11.5–13.3) | 13127.0 (p < 0.001) | 95.2 (95.0–95.5) | 0.0–38.8 |
| Women | 359 | 88,907 | 0.0–85.7 | 12.5 | 14.0 (12.8–15.3) | 7690.7 (p < 0.001) | 95.3 (95.0–95.6) | 0.2–41.8 |
| Men | 186 | 38,478 | 0.0–40.8 | 8.5 | 8.2 (7.2–9.2) | 2272.7 (p < 0.001) | 91.9 (91.0–92.6) | 0.1–24.9 |
| Mixed | 81 | 27,572 | 0.0–95.0 | 14.7 | 15.8 (12.8–19.2) | 1964.4 (p < 0.001) | 95.9 (95.4–96.4) | 0.0–50.4 |
| Intermediate-risk populationsd | 12 | 3119 | 2.0–32.8 | 11.9 | 12.3 (7.3–18.4) | 241.4 (p < 0.001) | 95.4 (93.5–96.8) | 0.0–40.7 |
| Womene | 1 | 67 | - | - | 9.0 (3.4–18.5) | - | - | - |
| Men | 6 | 1558 | 8.1–28.1 | 17.1 | 17.0 (11.5–23.3) | 41.8 (p < 0.001) | 88.0 (76.4–93.9) | 2.1–41.4 |
| Mixed | 5 | 1494 | 2.0–32.9 | 5.1 | 8.2 (1.6–19.1) | 93.7 (p < 0.001) | 95.7 (92.5–97.6) | 0.0–63.5 |
| Higher-risk populations | 28 | 9058 | 5.9–89.3 | 50.7 | 47.2 (37.6–57.0) | 2276.4 (p < 0.001) | 98.8 (98.6–99.0) | 4.4–93.0 |
| FSWs | 16 | 2047 | 33.3–89.3 | 59.6 | 63.2 (55.5–70.6) | 149.4 (p < 0.001) | 90.0 (85.3–93.1) | 30.7–90.3 |
| MSM | 12 | 7011 | 5.9–56.0 | 34.5 | 27.8 (17.5–39.4) | 1177.8 (p < 0.001) | 99.1 (98.8–99.2) | 0.2–75.8 |
| STI clinic attendees and symptomatic populationsf | 74 | 19,487 | 2.0–84.9 | 25.0 | 26.9 (23.1–30.8) | 1725.8 (p < 0.001) | 95.8 (95.2–96.3) | 2.8–62.8 |
| Women | 29 | 6136 | 4.8–60.0 | 23.0 | 25.5 (20.8–30.5) | 422.5 (p < 0.001) | 93.4 (91.5–94.8) | 5.0–54.5 |
| Men | 21 | 6144 | 2.0–35.0 | 18.5 | 17.4 (13.6–21.6) | 340.4 (p < 0.001) | 94.1 (92.2–95.6) | 3.2–39.3 |
| Mixed | 24 | 7207 | 8.0–84.9 | 31.0 | 38.3 (29.9–47.0) | 667.8 (p < 0.001) | 96.6 (95.7–97.2) | 4.2–81.2 |
| People living with HIV and people in HIV discordant couples | 35 | 3660 | 9.0–94.0 | 43.9 | 46.0 (40.1–51.8) | 386.3 (p < 0.001) | 91.2 (88.8–93.1) | 15.0–78.8 |
| Women | 12 | 784 | 17.4–94.0 | 56.5 | 55.3 (41.4–68.8) | 138.3 (p < 0.001) | 92.0 (88.0–94.7) | 7.8–97.1 |
| Men | 9 | 1370 | 31.0–62.0 | 50.0 | 46.4 (39.5–53.3) | 64.9 (p < 0.001) | 87.7 (78.7–92.9) | 24.1–69.5 |
| Mixed | 14 | 1506 | 9.0–59.6 | 39.6 | 38.5 (31.7–45.5) | 126.0 (p < 0.001) | 89.7 (84.5–93.1) | 14.3–66.1 |
| Infertility clinic attendees and women with ectopic pregnancies | 7 | 766 | 2.0–24.0 | 12.0 | 11.7 (5.8–19.2) | 49.8 (p < 0.001) | 87.9 (77.5–93.5) | 0.0–43.0 |
| Women | 7 | 766 | 2.0–24.0 | 12.0 | 11.7 (5.8–19.2) | 49.8 (p < 0.001) | 87.9 (77.5–93.5) | 0.0–43.0 |
| Other populations | 31 | 27,929 | 6.7–44.4 | 17.5 | 21.2 (17.4–25.2) | 508.0 (p < 0.001) | 94.1 (92.6–95.3) | 4.4–45.7 |
| Women | 13 | 2052 | 11.4–42.1 | 16.7 | 20.1 (15.4–25.3) | 93.3 (p < 0.001) | 87.1 (79.8–91.8) | 5.2–41.2 |
| Men | 4 | 535 | 6.7–44.4 | 24.6 | 23.1 (7.8–43.4) | 78.8 (p < 0.001) | 96.2 (92.9–98.0) | 0.0–100 |
| Mixed | 14 | 25,342 | 9.3–43.9 | 21.8 | 21.8 (16.1–28.0) | 296.5 (p < 0.001) | 95.6 (94.0–96.8) | 3.4–49.5 |
Abbreviations: CI = Confidence interval, FSWs = Female sex workers, HIV = Human immunodeficiency virus, HSV-2 = Herpes simplex virus type 2, MSM = Men who have sex with men, STI = Sexually transmitted infection.
Q: The Cochran's Q statistic is a measure assessing the existence of heterogeneity in seroprevalence.
I2: A measure that assesses the magnitude of between-study variation that is due to actual differences in seroprevalence across studies, rather than sampling variation.
Prediction interval: A measure that estimates the distribution (95% interval) of true seroprevalence around the estimated mean.
Intermediate-risk populations include populations who presumably have frequent sexual contacts with populations engaging in high sexual risk behaviour and have therefore a higher risk of exposure to HSV-2 than the general population. These comprise prisoners, people who inject drugs, and truck drivers, among others.
No meta-analysis was conducted due to the small number of studies (n < 3).
Symptomatic populations include patients with STI clinical manifestations.
Table 2.
Pooled mean estimates for HSV-2 seroprevalence among general populations in Europe.
| Population classification | Outcome measures |
Sample size |
HSV-2 seroprevalence (%) |
Pooled mean HSV-2 seroprevalence |
Heterogeneity measures |
|||
|---|---|---|---|---|---|---|---|---|
| Total n | Total N | Range | Median | Mean (%) (95% CI) |
Qa (p-value) | I2b (%) (95% CI) |
Prediction intervalc (%) | |
| European countries | ||||||||
| Belgium | 18 | 3477 | 7.0–27.7 | 12.5 | 13.3 (10.8–15.9) | 74.7 (p < 0.001) | 77.2 (64.4–85.5) | 4.7–25.2 |
| Bulgaria | 12 | 2508 | 2.0–40.0 | 20.0 | 19.1 (12.7–26.4) | 182.1 (p < 0.001) | 94.0 (91.2–95.9) | 0.9–51.3 |
| Croatia | 17 | 2651 | 0.0–32.0 | 8.0 | 9.7 (6.1–14.0) | 153.2 (p < 0.001) | 89.6 (84.9–92.8) | 0.0–32.2 |
| Czech Republic | 12 | 2570 | 2.0–8.5 | 4.0 | 4.8 (3.5–6.4) | 30.9 (p = 0.001) | 64.4 (33.9–80.8) | 1.2–10.5 |
| Estonia | 20 | 3049 | 1.8–34.0 | 17.9 | 16.5 (12.1–21.4) | 222.2 (p < 0.001) | 91.4 (88.2–93.8) | 1.4–42.4 |
| Finland | 32 | 8089 | 0.5–51.7 | 16.5 | 16.0 (12.3–20.0) | 556.0 (p < 0.001) | 94.4 (93.0–95.5) | 1.0–42.6 |
| France | 12 | 9389 | 12.5–28.3 | 17.1 | 17.3 (15.4–19.2) | 35.0 (p < 0.001) | 68.6 (42.8–82.7) | 11.2–24.4 |
| Germany | 65 | 23,609 | 0.0–25.0 | 11.5 | 11.5 (10.1–13.0) | 673.1 (p < 0.001) | 90.5 (88.6–92.1) | 2.7–25.2 |
| Hungary | 17 | 3012 | 0.0–12.2 | 2.7 | 3.6 (2.0–5.5) | 103.5 (p < 0.001) | 84.5 (76.6–89.8) | 0.0–14.2 |
| Israel | 29 | 4126 | 0.0–33.1 | 7.7 | 9.5 (7.1–12.2) | 218.2 (p < 0.001) | 87.2 (82.7–90.5) | 0.6–26.1 |
| Italy | 60 | 4706 | 0.0–95.0 | 13.0 | 17.3 (12.1–23.1) | 2333.4 (p < 0.001) | 97.5 (97.1–97.8) | 0.0–67.7 |
| Netherlands | 59 | 10,596 | 1.0–77.0 | 11.2 | 14.0 (10.9–17.4) | 652.5 (p < 0.001) | 91.1 (89.3–92.6) | 0.0–43.5 |
| Norway | 22 | 2775 | 0.0–37.0 | 13.4 | 12.4 (8.5–16.9) | 165.7 (p < 0.001) | 87.3 (82.1–91.0) | 0.1–36.7 |
| Poland | 18 | 2871 | 4.0–12.9 | 8.0 | 8.3 (7.0–9.7) | 28.9 (p = 0.035) | 41.2 (0.0–66.4) | 4.5–13.0 |
| Romania | 12 | 1070 | 0.0–38.3 | 14.6 | 13.1 (7.4–20.1) | 73.7 (p < 0.001) | 85.1 (75.6–90.9) | 0.0–44.7 |
| Russian Federation | 18 | 4829 | 1.4–41.3 | 16.7 | 14.8 (9.6–20.8) | 461.5 (p < 0.001) | 96.3 (95.2–97.2) | 0.0–46.8 |
| Spain | 24 | 5519 | 0.0–40.8 | 4.0 | 6.3 (3.2–10.3) | 532.5 (p < 0.001) | 95.7 (94.5–96.6) | 0.0–34.9 |
| Sweden | 53 | 12,103 | 0.9–40.0 | 18.0 | 18.2 (15.4–21.3) | 967.2 (p < 0.001) | 94.6 (93.6–95.5) | 2.6–43.1 |
| Switzerland | 13 | 4150 | 10.6–31.8 | 18.1 | 19.1 (16.4–21.9) | 43.0 (p < 0.001) | 72.1 (51.3–84.1) | 10.2–29.9 |
| Turkey | 32 | 4674 | 2.1–62.9 | 8.2 | 13.5 (8.2–19.8) | 1130.1 (p < 0.001) | 97.3 (96.7–97.7) | 0.0–58.0 |
| UK | 46 | 25,381 | 0.0–33.9 | 6.2 | 6.7 (5.3–8.2) | 614.8 (p < 0.001) | 92.7 (91.1–94.0) | 0.4–19.0 |
| Otherd | 27 | 13,128 | 3.4–26.0 | 11.3 | 10.8 (8.6–13.1) | 344.1 (p < 0.001) | 92.4 (90.1–94.2) | 2.1–24.8 |
| European subregion/country | ||||||||
| Eastern Europe | 90 | 16,897 | 0.0–41.3 | 8.5 | 9.6 (7.8–11.5) | 1546.0 (p < 0.001) | 94.2 (93.4–95.0) | 0.0–32.2 |
| Southern Europe | 121 | 22,635 | 0.0–95.0 | 9.4 | 12.2 (9.6–15.1) | 3559.1 (p < 0.001) | 96.6 (96.3–96.9) | 0.0–50.8 |
| Western Europe | 174 | 51,372 | 0.0–77.0 | 12.5 | 13.2 (12.0–14.4) | 1764.9 (p < 0.001) | 90.2 (89.0–91.2) | 2.2–30.8 |
| Northern Europe | 176 | 52,064 | 0.0–51.7 | 14.0 | 13.5 (12.0–15.1) | 4421.1 (p < 0.001) | 96.0 (95.7–96.4) | 0.5–37.9 |
| Israel, Turkey, and mixed countries | 65 | 11,989 | 0.0–62.9 | 8.1 | 11.3 (8.5–14.4) | 1433.3 (p < 0.001) | 95.5 (94.8–96.1) | 0.0–42.5 |
| Age group | ||||||||
| <20 years | 45 | 7346 | 0.0–78.6 | 4.5 | 5.9 (3.5–8.8) | 842.6 (p < 0.001) | 94.8 (93.7–95.6) | 0.0–32.6 |
| 20–29 years | 109 | 23,057 | 1.4–67.8 | 9.0 | 10.7 (9.2–12.4) | 1407.1 (p < 0.001) | 92.3 (91.2–93.3) | 0.4–30.7 |
| 30–39 years | 92 | 13,291 | 0.0–68.4 | 14.2 | 15.0 (12.6–17.5) | 1410.2 (p < 0.001) | 93.5 (92.6–94.4) | 0.4–42.2 |
| 40–49 years | 34 | 5939 | 2.6–85.7 | 15.0 | 15.8 (11.3–20.8) | 815.8 (p < 0.001) | 96.0 (95.1–96.7) | 0.0–50.7 |
| 50–59 years | 19 | 3814 | 4.6–29.6 | 14.2 | 13.3 (10.5–16.3) | 117.1 (p < 0.001) | 84.6 (77.3–89.6) | 3.3–28.2 |
| ≥60 years | 21 | 3192 | 0.0–60.9 | 12.3 | 15.5 (9.7–22.3) | 255.8 (p < 0.001) | 92.2 (89.4–94.2) | 0.0–53.0 |
| Mixed | 306 | 98,318 | 0.0–95.0 | 11.9 | 12.7 (11.4–14.0) | 7650.8 (p < 0.001) | 96.0 (95.8–96.3) | 0.0–39.3 |
| Year of data collection category | ||||||||
| <1995 | 166 | 46,557 | 0.0–85.7 | 11.2 | 12.6 (10.7–14.6) | 5487.4 (p < 0.001) | 97.0 (96.7–97.2) | 0.0–44.8 |
| 1995–2005 | 373 | 98,318 | 0.0–95.0 | 11.9 | 12.9 (11.8–14.0) | 6746.2 (p < 0.001) | 94.5 (94.1–94.8) | 0.2–37.7 |
| >2005 | 87 | 16,750 | 0.0–41.3 | 8.8 | 9.8 (8.2–11.6) | 884.8 (p < 0.001) | 90.3 (88.6–91.7) | 0.2–29.2 |
| Year of publication category | ||||||||
| <2000 | 91 | 24,236 | 0.0–85.7 | 14.0 | 15.5 (12.3–18.9) | 4109.4 (p < 0.001) | 97.8 (97.6–98.0) | 0.0–54.8 |
| 2000–2010 | 383 | 94,471 | 0.0–95.0 | 11.0 | 12.0 (11.0–13.1) | 6442.2 (p < 0.001) | 94.1 (93.7–94.4) | 0.1–36.4 |
| >2010 | 152 | 36,250 | 0.0–43.6 | 10.9 | 11.3 (9.9–12.9) | 2328.2 (p < 0.001) | 93.5 (92.8–94.2) | 0.3–32.0 |
| All studies | 626 | 154,957 | 0.0–95.0 | 11.3 | 12.4 (11.5–13.3) | 13,127.0 (p < 0.001) | 95.2 (95.0–95.5) | 0.0–38.8 |
Abbreviations: CI = Confidence interval, HSV-2 = Herpes simplex virus type 2, UK = United Kingdom of Great Britain and Northern Ireland.
Q: The Cochran's Q statistic is a measure assessing the existence of heterogeneity in seroprevalence.
I2: A measure that assesses the magnitude of between-study variation that is due to actual differences in seroprevalence across studies, rather than sampling variation.
Prediction interval: A measure that estimates the distribution (95% interval) of true seroprevalence around the estimated mean.
Other countries: Denmark, Greece, Serbia, Slovakia, Slovenia, and multi-country studies.
Quality assessments
The quality assessments of diagnostic methods excluded 69 publications due to potential validity issues in diagnostic assays (Fig. 1). Results of the quality assessments of included seroprevalence measures are summarized in Table S7. Out of 294 studies, 210 (71.4%) were of high precision, whereas 43 (14.6%) were of low ROB in the sampling method domain, and 18 (6.1%) were of low ROB in the response rate domain. Eighty-four (28.6%) studies were of low precision, whereas 251 studies (85.4%) were of high ROB in the sampling method domain, and 24 (8.2%) were of high ROB in the response rate domain. Six (2.0%) studies were of low ROB in both quality domains, while 15 (5.1%) studies were of high ROB in both quality domains. For 252 (85.7%) studies, the ROB assessment for the response rate domain was “unclear.” Notably, in the meta-regressions for HSV-2 seroprevalence (note below), only the ROB in the sampling method domain was statistically significantly associated with HSV-2 seroprevalence whereas precision and ROB response rate domain were not. The effect of sampling method was also relatively small.
Estimates of pooled mean HSV-2 seroprevalence
Table 1 summarizes the pooled mean HSV-2 seroprevalence by sex across at-risk populations. Among general populations, the pooled mean was 14.0% (95% CI: 12.8–15.3%) in women and 8.2% (95% CI: 7.2–9.2%) in men. The pooled mean was highest at 63.2% (95% CI: 55.5–70.6%) among FSWs, 46.0% (95% CI: 40.1–51.8%) among people living with HIV and people in HIV discordant couples, and 27.8% (95% CI: 17.5–39.4%) among MSM. Among STI clinic attendees and symptomatic populations, the pooled mean was 25.5% (95% CI: 20.8–30.5%) in women and 17.4% (95% CI: 13.6–21.6%) in men.
Table 2 summarizes the pooled mean HSV-2 seroprevalence among different subpopulations within the general populations. Across age groups, the pooled mean increased from 5.9% (95% CI: 3.5–8.8%) among <20 years old individuals to 15.5% (95% CI: 9.7–22.3%) among ≥60 years old individuals. By country, the pooled mean was lowest in Hungary at 3.6% (95% CI: 2.0–5.5%) and highest in Bulgaria and Switzerland at 19.1% (95% CI: 12.7–26.4%) and 19.1% (95% CI: 16.4–21.9%), respectively.
Heterogeneity was evident in all meta-analyses (p-value < 0.01) with wide prediction intervals (Tables 1 and 2). The prediction interval estimates the distribution (95% interval) of true seroprevalence around the estimated mean. The 95% CI quantifies the uncertainty in the mean seroprevalence. Since I2 measures the magnitude of between-study variation that is due to true differences in seroprevalence across studies rather than sampling variation, an I2 >50% indicates that heterogeneity was due to true differences in seroprevalence rather than sampling variation. Forest plots confirmed substantial heterogeneity in stratified seroprevalence measures (Fig. S1).
Predictors of HSV-2 seroprevalence and sources of between-study heterogeneity
Tables 3 and S8 show results of univariable and multivariable meta-regression analyses for HSV-2 seroprevalence. All four multivariable regression models generated similar results and explained about 40% of seroprevalence variation.
Table 3.
Univariable and multivariable meta-regression analyses for HSV-2 seroprevalence in Europe, using the year of data collection as the temporal variable instead of the year of publication.
| Outcome measures | Sample size | Univariable analysis |
Multivariable analysisa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1b: Time as a categorical variable model |
Model 2c: Time as a linear variable model |
|||||||||||
| Total n | Total N | RR (95% CI) | p-value | LR test p-valued | Adjusted R2 (%) | ARR (95% CI) | p-value | ARR (95% CI) | p-value | |||
| Population characteristics | Population type | General populations | 626 | 154,957 | 1.00 | - | <0.001 | 27.72 | 1.00 | - | 1.00 | - |
| Intermediate-risk populationse | 12 | 3119 | 0.93 (0.61–1.44) | 0.751 | 1.06 (0.71–1.59) | 0.768 | 1.06 (0.71–1.60) | 0.776 | ||||
| FSWs | 16 | 2047 | 5.13 (3.58–7.33) | <0.001 | 4.20 (3.01–5.86) | <0.001 | 4.55 (3.25–6.36) | <0.001 | ||||
| MSM | 12 | 7011 | 1.99 (1.32–3.00) | <0.001 | 2.66 (1.80–3.93) | <0.001 | 2.52 (1.70–3.73) | <0.001 | ||||
| STI clinic attendees and symptomatic populationsf | 74 | 19,487 | 2.07 (1.73–2.47) | 0.001 | 1.92 (1.61–2.29) | <0.001 | 1.90 (1.59–2.27) | <0.001 | ||||
| People living with HIV and people in HIV discordant couples | 35 | 3660 | 3.69 (2.88–4.72) | <0.001 | 3.41 (2.68–4.33) | <0.001 | 3.37 (2.65–4.30) | <0.001 | ||||
| Infertility clinic attendees and women with ectopic pregnancies | 7 | 766 | 0.96 (0.53–1.72) | 0.884 | 0.93 (0.53–1.61) | 0.791 | 0.99 (0.57–1.73) | 0.972 | ||||
| Other populations | 31 | 27,929 | 1.70 (1.30–2.22) | <0.001 | 1.71 (1.32–2.22) | <0.001 | 1.49 (1.16–1.92) | 0.002 | ||||
| Age group | <20 years | 48 | 7799 | 1.00 | - | <0.001 | 8.41 | 1.00 | - | 1.00 | - | |
| 20–29 years | 117 | 28,019 | 1.77 (1.29–2.41) | <0.001 | 1.80 (1.38–2.34) | <0.001 | 1.78 (1.37–2.32) | <0.001 | ||||
| 30–39 years | 98 | 19,576 | 2.55 (1.85–3.51) | <0.001 | 2.70 (2.06–3.54) | <0.001 | 2.67 (2.03-3.51) | <0.001 | ||||
| 40–49 years | 35 | 13,743 | 2.35 (1.59–3.46) | <0.001 | 2.79 (2.01–3.89) | <0.001 | 2.76 (1.98–3.84) | <0.001 | ||||
| 50–59 years | 19 | 3814 | 2.14 (1.35–3.39) | 0.001 | 2.58 (1.75–3.81) | <0.001 | 2.66 (1.80–3.93) | <0.001 | ||||
| ≥60 years | 22 | 9220 | 2.57 (1.63–4.03) | <0.001 | 2.60 (1.76–3.83) | <0.001 | 2.62 (1.77–3.87) | <0.001 | ||||
| Mixed | 474 | 136,805 | 2.73 (2.06–3.61) | <0.001 | 2.01 (1.57–2.57) | <0.001 | 2.05 (1.60–2.62) | <0.001 | ||||
| Sex | Women | 437 | 100,759 | 1.00 | - | <0.001 | 6.10 | 1.00 | - | 1.00 | - | |
| Men | 238 | 55,096 | 0.69 (0.60–0.79) | <0.001 | 0.65 (0.57–0.73) | <0.001 | 0.65 (0.58–0.74) | <0.001 | ||||
| Mixed | 138 | 63,121 | 1.26 (1.07–1.49) | 0.005 | 0.98 (0.84–1.14) | 0.777 | 0.98 (0.84–1.14) | 0.798 | ||||
| European subregion | Eastern Europe | 101 | 17,863 | 1.00 | - | <0.001 | 3.19 | 1.00 | - | 1.00 | - | |
| Southern Europe | 164 | 31,963 | 1.52 (1.22–1.89) | <0.001 | 1.22 (1.01-1.48) | 0.039 | 1.18 (0.98–1.43) | 0.086 | ||||
| Western Europe | 225 | 85,317 | 1.68 (1.37–2.07) | <0.001 | 1.37 (1.14–1.63) | 0.001 | 1.33 (1.12–1.59) | 0.002 | ||||
| Northern Europe | 233 | 67,636 | 1.51 (1.23–1.85) | <0.001 | 1.23 (1.03–1.48) | 0.023 | 1.16 (0.96–1.39) | 0.121 | ||||
| Mixed regions | 90 | 16,197 | 1.26 (0.98–1.62) | 0.076 | 0.91 (0.74–1.13) | 0.400 | 0.90 (0.73–1.12) | 0.344 | ||||
| Country's income | UMIC | 87 | 15,429 | 1.00 | - | 0.326 | 0.03 | - | - | - | - | |
| HIC | 721 | 202,919 | 1.03 (0.84–1.26) | 0.769 | - | - | - | - | ||||
| Mixed | 5 | 628 | 1.80 (0.84–3.89) | 0.132 | - | - | - | - | ||||
| Study methodology characteristics | Assay type | Western Blot | 80 | 23,545 | 1.00 | - | 0.541 | 0.00 | - | - | - | - |
| ELISA | 725 | 190,632 | 0.99 (0.80–1.24) | 0.940 | - | - | - | - | ||||
| Monoclonal antibody | 8 | 4799 | 1.39 (0.74–2.60) | 0.306 | - | - | - | - | ||||
| Sample sizeg | <200 | 106 | 6947 | 1.00 | - | 0.013 | 1.24 | 1.00 | - | 1.00 | - | |
| ≥200 | 707 | 212,029 | 0.78 (0.65–0.95) | 0.013 | 1.00 (0.85–1.18) | 0.979 | 0.98 (0.83–1.16) | 0.854 | ||||
| Sampling method | Probability-based | 210 | 62,803 | 1.00 | - | <0.001 | 3.04 | 1.00 | - | 1.00 | - | |
| Non-probability-based | 603 | 156,173 | 1.31 (1.15–1.51) | <0.001 | 1.25 (1.09–1.44) | 0.001 | 1.20 (1.05–1.38) | 0.008 | ||||
| Response rate | ≥80% | 41 | 13,331 | 1.00 | - | 0.009 | 1.09 | 1.00 | - | 1.00 | - | |
| <80% | 102 | 28,738 | 1.06 (0.78–1.44) | 0.716 | 1.01 (0.78–1.3) | 0.944 | 1.02 (0.79–1.32) | 0.869 | ||||
| Unclear | 670 | 176,907 | 0.82 (0.62–1.07) | 0.135 | 0.80 (0.64–1.00) | 0.053 | 0.82 (0.65–1.02) | 0.078 | ||||
| Temporal variables | Year of data collection category | <1995 | 226 | 61,250 | 1.00 | - | 0.268 | 0.09 | 1.00 | - | - | - |
| 1995–2005 | 465 | 111,095 | 1.07 (0.93–1.23) | 0.362 | 1.12 (1.00–1.27) | 0.056 | - | - | ||||
| >2005 | 122 | 46,631 | 0.93 (0.76–1.13) | 0.454 | 0.82 (0.69–0.97) | 0.021 | - | - | ||||
| Year of data collection | 813 | 218,976 | 0.99 (0.99–1.00) | 0.181 | 0.181 | 0.13 | - | - | 0.99 (0.98–1.00) | 0.013 | ||
Abbreviations: ARR = Adjusted risk ratio, CI = Confidence interval, ELISA = Enzyme-linked immunosorbent type-specific assay, FSWs = Female sex workers, HIC = High-income countries, HIV = Human immunodeficiency virus, HSV-2 = Herpes simplex virus type 2, LR = Likelihood ratio, MSM = Men who have sex with men, RR = Risk ratio, STI = Sexually transmitted infection, UMIC = Upper-middle-income countries.
Models 1 and 2 used only year of data collection as the temporal variable due to collinearity between year of publication and year of data collection. The same analyses using year of publication are presented in Table S8.
Variance explained by multivariable model 1 (adjusted R2) = 41.28%.
Variance explained by multivariable model 2 (adjusted R2) = 40.51%.
Factors in the univariable analyses with a p-value <0.1 were included in the multivariable analysis.
Intermediate-risk populations include populations who presumably have frequent sexual contacts with populations engaging in high sexual risk behaviour and have therefore a higher risk of exposure to HSV-2 than the general population. These comprise prisoners, people who inject drugs, and truck drivers, among others.
Symptomatic populations include patients with STI clinical manifestations.
Sample size denotes the sample size of each study population found in the original publication.
The model including year of data collection as a continuous linear variable explained 40.5% of seroprevalence variation (Table 3). Population type variable alone explained most variation (27.7%), followed by age group variable (8.4%). Compared to general populations, HSV-2 seroprevalence was 4.55-fold (95% CI: 3.25–6.36) higher among FSWs, 3.37-fold (95% CI: 2.65–4.30) higher among people living with HIV and people in HIV discordant couples, 2.52-fold (95% CI: 1.70–3.73) higher among MSM, and 1.90-fold (95% CI: 1.59–2.27) higher among STI clinic attendees and symptomatic populations.
Seroprevalence increased rapidly for young persons, but plateaued by 30–39 years of age. Seroprevalence in men was 0.65-fold (95% CI: 0.58–0.74) lower compared to women. Compared to Eastern Europe, HSV-2 seroprevalence was highest in Western Europe (1.33-fold (95% CI: 1.12–1.59) higher). HSV-2 seroprevalence decreased by 0.99-fold (95% CI: 0.98–1.00) per calendar year, indicating that seroprevalence has been declining at a rate of 1% per calendar year.
Among study methodology characteristics, assay type, sample size, and response rate were not associated with HSV-2 seroprevalence; however, sampling method was. Compared to studies using probability-based sampling, seroprevalence was 1.20-fold (95% CI: 1.05–1.38) higher in studies using non-probability-based sampling.
Subgroup meta-regression analyses were conducted including only studies among general populations (Table S9). The analyses confirmed similar findings to those of the main analyses including all population types.
Overview and meta-analyses of HSV-2 detection in genital ulcer disease and in genital herpes
Tables 4 and S10 summarize extracted proportions of HSV-2 detection in clinically diagnosed GUD and in laboratory-confirmed genital herpes. The proportion of HSV-2 detection in GUD (n = 15) ranged from 2.0 to 48.1% with a median of 20.9% and a pooled mean of 22.0% (95% CI: 15.3–29.6%). The proportion of HSV-2 detection in genital herpes (n = 183) ranged from 12.0 to 100.0% with a median of 66.7% and a pooled mean of 66.0% (95% CI: 62.9–69.1%). The meta-analyses showed heterogeneity in proportions and wide prediction intervals (Table 4). Forest plots confirmed substantial heterogeneity in the stratified proportions of HSV-2 detection in GUD and in genital herpes (Fig. S2).
Table 4.
Pooled proportions of HSV-2 virus detection in clinically diagnosed GUD and in laboratory-confirmed genital herpes in Europe.
| Population type | Outcome measures |
Sample size |
Proportion of HSV-2 detection (%) |
Pooled proportion of HSV-2 detection (%) |
Heterogeneity measures |
|||
|---|---|---|---|---|---|---|---|---|
| Total n | Total N |
Range | Median | Mean (95% CI) | Qa (p-value) | I2b (%) (95% CI) |
Prediction Intervalc (%) | |
| Patients with clinically diagnosed GUD | ||||||||
| Sex | ||||||||
| Womend | 2 | 201 | - | - | 23.9 (17.4–31.1) | - | - | - |
| Mend | 2 | 153 | - | - | 17.6 (11.8–24.1) | - | - | - |
| Mixed | 11 | 3819 | 2.0–48.1 | 22.3 | 22.2 (13.3–32.6) | 282.9 (p < 0.001) | 96.5 (95.0–97.5) | 0.0–66.0 |
| All patients with GUD | 15 | 4173 | 2.0–48.1 | 20.9 | 22.0 (15.3–29.6) | 300.1 (p < 0.001) | 95.3 (93.6–96.6) | 1.2–57.2 |
| Patients with laboratory-confirmed genital herpes | ||||||||
| Sex | ||||||||
| Women | 51 | 7368 | 12.0–92.0 | 54.5 | 55.4 (50.5–60.2) | 692.0 (p < 0.001) | 92.8 (91.3–94.0) | 24.3–84.4 |
| Men | 48 | 5190 | 25.0–96.0 | 70.7 | 69.8 (65.2–74.2) | 580.7 (p < 0.001) | 91.9 (90.1–93.4) | 38.6–93.5 |
| Mixed | 84 | 10,765 | 16.7–100 | 71.4 | 69.7 (64.6–74.6) | 2227.9 (p < 0.001) | 96.3 (95.8–96.7) | 23.0–99.7 |
| Age | ||||||||
| <25 years | 7 | 1638 | 32.8–70.0 | 42.5 | 42.4 (35.3–49.7) | 25.3 (p < 0.001) | 76.3 (50.2–88.7) | 22.0–64.2 |
| ≥25 years | 15 | 1865 | 44.3–90.3 | 60.0 | 62.1 (56.0–68.0) | 57.5 (p < 0.001) | 75.7 (59.8–85.3) | 38.7–83.0 |
| Mixed | 161 | 19,820 | 12.0–100 | 68.2 | 67.2 (63.8–70.6) | 3317.0 (p < 0.001) | 95.2 (94.7–95.6) | 25.3–97.5 |
| Genital herpes episode status | ||||||||
| First episode | 57 | 9135 | 12.0–92.8 | 52.0 | 52.3 (47.7–56.8) | 643.6 (p < 0.001) | 91.3 (89.5–92.8) | 21.7–82.0 |
| Recurrent episode | 13 | 1907 | 22.7–96.0 | 85.0 | 83.2 (73.2–91.2) | 134.9 (p < 0.001) | 91.1 (86.6–94.1) | 37.9–100 |
| Unspecified status | 113 | 12,281 | 24.8–100 | 71.0 | 70.6 (66.9–74.1) | 1863.1 (p < 0.001) | 94.0 (93.2–94.7) | 32.7–97.1 |
| European subregion/country | ||||||||
| Southern Europee | 20 | 1881 | 24.8–100 | 77.6 | 75.3 (64.4–84.9) | 636.3 (p < 0.001) | 97.0 (96.2–97.6) | 23.4–100 |
| Western Europe | 11 | 1016 | 45.6–94.2 | 77.3 | 74.2 (63.3–83.9) | 96.9 (p < 0.001) | 89.7 (83.6–93.5) | 32.5–99.6 |
| Northern Europe | 142 | 20,141 | 12.0–96.0 | 67.1 | 66.1 (62.8–69.4) | 2776.4 (p < 0.001) | 94.9 (94.4–95.4) | 27.7–95.4 |
| Israel | 10 | 285 | 25.0–52.4 | 35.0 | 33.5 (27.9–39.2) | 6.5 (p = 0.689) | 0.0 (0.0–62.4) | 27.0–40.2 |
| Sample size | ||||||||
| <200 | 49 | 2032 | 12.0–94.3 | 58.7 | 57.1 (50.1–64.0) | 512.6 (p < 0.001) | 90.6 (88.5–92.4) | 13.6–95.0 |
| ≥200 | 134 | 21,291 | 22.7–100 | 68.3 | 68.8 (65.5–72.1) | 3423.9 (p < 0.001) | 96.1 (95.7–96.5) | 30.2–96.6 |
| Year of publication category | ||||||||
| <2000 | 50 | 4179 | 25.0–100 | 71.0 | 71.6 (65.9–76.9) | 754.2 (p < 0.001) | 93.5 (92.2–94.6) | 30.4–98.6 |
| 2000–2010 | 87 | 13,125 | 12.0–100 | 64.5 | 64.8 (59.6–69.8) | 1818.2 (p < 0.001) | 95.3 (94.6–95.8) | 19.2–98.3 |
| >2010 | 46 | 6019 | 16.7–92.8 | 63.8 | 61.7 (57.1–66.2) | 805.8 (p < 0.001) | 94.4 (93.3–95.4) | 32.2–87.3 |
| Year of data collection category | ||||||||
| <1995 | 69 | 8285 | 25.0–100 | 68.2 | 68.2 (63.0–73.2) | 1728.6 (p < 0.001) | 96.1 (95.5–96.6) | 25.2–98.2 |
| 1995–2005 | 68 | 9692 | 12.0–100 | 72.7 | 67.6 (61.8–73.2) | 1355.0 (p < 0.001) | 95.1 (94.3–95.7) | 21.5–99.2 |
| >2005 | 46 | 5346 | 16.7–92.8 | 62.2 | 59.7 (55.1–64.2) | 456.1 (p < 0.001) | 90.1 (87.7–92.1) | 31.0–85.3 |
| All patients with genital herpes | 183 | 23,323 | 12.0–100.0 | 66.7 | 66.0 (62.9–69.1) | 3944.1 (p < 0.001) | 95.4 (95.0–95.8) | 25.2–96.5 |
Abbreviations: CI = Confidence interval, GUD = Genital ulcer disease, HSV-2 = Herpes simplex virus type 2.
Q: The Cochran's Q statistic is a measure assessing the existence of heterogeneity in proportion of HSV-2 virus detection.
I2: A measure assessing the magnitude of between-study variation that is due to true differences in proportion of HSV-2 virus detection across studies, rather than sampling variation.
Prediction interval: A measure quantifying the distribution 95% interval of true proportions of HSV-2 virus detection around the estimated pooled mean.
No meta-analysis was done due to the small number of studies (n < 3).
Southern Europe includes one measure from Eastern Europe.
Predictors of HSV-2 detection in genital herpes and sources of between-study heterogeneity
Tables 5 and S11 show results of univariable and multivariable meta-regression analyses for the proportion of HSV-2 detection in genital herpes. All four multivariable regression models generated similar results and explained over 50% of the variation in this proportion.
Table 5.
Univariable and multivariable meta-regression analyses for proportion of HSV-2 virus detection in laboratory-confirmed genital herpes in Europe, using the year of data collection as the temporal variable instead of the year of publication.
| Outcome measures | Samples | Univariable analysis |
Multivariable analysisa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1b: Time as a categorical variable model |
Model 2c: Time as a linear variable model |
||||||||||
| Total n | Total N | RR (95%CI) | p-value | LR test p-valued | Adjusted R2 (%) | ARR (95%CI) | p-value | ARR (95%CI) | p-value | ||
| Age group | <25 years | 7 | 1638 | 1.00 | - | 0.005 | 10.09 | 1.00 | - | 1.00 | - |
| ≥25 years | 15 | 1865 | 1.41 (1.05–1.88) | 0.021 | 1.53 (1.23–1.89) | <0.001 | 1.54 (1.25–1.89) | <0.001 | |||
| Mixed | 161 | 19,820 | 1.50 (1.18–1.93) | 0.001 | 1.15 (0.94–1.40) | 0.180 | 1.18 (0.98–1.42) | 0.087 | |||
| Sex | Women | 51 | 7368 | 1.00 | - | 0.001 | 10.39 | 1.00 | - | 1.00 | - |
| Men | 48 | 5190 | 1.23 (1.08–1.39) | 0.001 | 1.21 (1.10–1.33) | <0.001 | 1.21 (1.10–1.32) | <0.001 | |||
| Mixed | 84 | 10,765 | 1.21 (1.08–1.35) | 0.001 | 1.12 (1.01–1.23) | 0.028 | 1.07 (0.97–1.18) | 0.180 | |||
| Genital herpes episode status | First episode | 57 | 9135 | 1.00 | - | <0.001 | 27.34 | 1.00 | - | 1.00 | - |
| Recurrent episode | 13 | 1907 | 1.54 (1.30–1.82) | <0.001 | 1.51 (1.30–1.75) | <0.001 | 1.52 (1.32–1.75) | <0.001 | |||
| Unspecified status | 113 | 12,281 | 1.31 (1.19–1.44) | <0.001 | 1.39 (1.26–1.53) | <0.001 | 1.33 (1.21–1.46) | <0.001 | |||
| European subregion/country | Southern Europee | 20 | 1881 | 1.00 | - | <0.001 | 13.30 | 1.00 | - | 1.00 | - |
| Western Europe | 11 | 1016 | 1.01 (0.81–1.26) | 0.909 | 1.13 (0.95–1.35) | 0.164 | 1.08 (0.91–1.28) | 0.373 | |||
| Northern Europe | 142 | 20,141 | 0.91 (0.79–1.05) | 0.185 | 0.94 (0.83–1.06) | 0.311 | 0.88 (0.78–0.99) | 0.030 | |||
| Israel | 10 | 285 | 0.49 (0.37–0.64) | <0.001 | 0.44 (0.35–0.56) | <0.001 | 0.44 (0.35–0.56) | <0.001 | |||
| Sample sizef | <200 | 49 | 2032 | 1.00 | - | 0.014 | 0.53 | 1.00 | - | 1.00 | - |
| ≥200 | 134 | 21,291 | 1.15 (1.03–1.29) | 0.014 | 1.09 (1.00–1.19) | 0.054 | 1.14 (1.04–1.24) | 0.004 | |||
| Year of data collection category | <1995 | 69 | 8285 | 1.00 | - | 0.104 | 5.03 | 1.00 | - | - | - |
| 1995–2005 | 68 | 9692 | 1.01 (0.90–1.13) | 0.863 | 0.97 (0.88–1.06) | 0.507 | - | - | |||
| >2005 | 46 | 5346 | 0.89 (0.79–1.01) | 0.067 | 0.86 (0.77–0.96) | 0.010 | - | - | |||
| Year of data collection | 183 | 23,323 | 0.99 (0.99–1.00) | 0.001 | 0.001 | 11.12 | - | - | 0.99 (0.99–1.00) | 0.001 | |
Abbreviations: ARR = Adjusted risk ratio, CI = Confidence interval, HSV-2 = Herpes simplex virus type 2, LR = Likelihood ratio, RR = Risk ratio.
Models 1 and 2 used only year of data collection as the temporal variable due to collinearity between year of publication and year of data collection. The same analyses using year of publication are presented in Table S11.
Variance explained by the final multivariable model 1 (adjusted R2) = 53.89%.
Variance explained by the final multivariable model 2 (adjusted R2) = 58.15%.
Factors in the univariable analyses with a p-value <0.1 were included in the multivariable analysis.
Southern Europe includes one measure from Eastern Europe.
Sample size denotes the sample size of the study population found in the original publication.
The model including year of data collection as a continuous linear variable explained 58.2% of the genital herpes proportion variation (Table 5). Genital herpes episode status (first versus recurrent episodes) explained most variation (27.3%), followed by European subregion, sex, and age, each at just over 10% of the variation.
Compared to those with first episode genital herpes, the proportion of HSV-2 detection was 1.52-fold (95% CI: 1.32–1.75) higher in those with recurrent genital herpes (Table 5). Compared to <25 years old individuals, the proportion of HSV-2 detection in genital herpes was 1.54-fold (95% CI: 1.25–1.89) higher in ≥25 years old individuals. Men had a 1.21-fold (95% CI: 1.10–1.32) higher proportion of HSV-2 detection compared to women. Israel had the lowest proportion of HSV-2 detection. The proportion of HSV-2 detection decreased by 0.99-fold (95% CI: 0.99–1.00) per calendar year, indicating that seroprevalence has been declining at a rate of 1% per calendar year.
Subgroup meta-regression analyses were conducted for each of studies of first episode genital herpes and studies of recurrent genital herpes (not shown). Both analyses suggested declining HSV-2 contribution to both, but the effects did not reach statistical significance, perhaps due to the smaller number of studies included in the analyses.
Neonatal herpes
Neonatal herpes is a rare condition but results in significant morbidity and mortality.43 Incidence of neonatal herpes infection worldwide during 2010–2015 was estimated using mathematical modelling at 10.3 cases per 100,000 live births.44 During the same duration, incidence in Europe was estimated at 8.9 cases per 100,000 live births, just below the global incidence rate.44 By type of infection, incidence in Europe was estimated at 5.2 cases per 100,000 live births for HSV-1 and at 3.8 cases per 100,000 live births for HSV-2.44
Tens of studies have documented occurrence of neonatal herpes for over 4 decades in Europe in countries such as Denmark,45 France,43 Germany,46,47 Ireland,48 Israel,49, 50, 51 the Netherlands,52, 53, 54, 55, 56, 57, 58 Poland,59,60 Russia,61 Spain,62 Sweden,63, 64, 65 Switzerland,66 Turkey,67 and the United Kingdom (UK).48,68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 However, surveillance for this condition remains overall limited with no data being reported for the majority of countries in Europe. Reporting quality also varies widely from reports indicating sporadic cases to well-conducted epidemiologic studies based on surveillance systems and reporting estimates for incidence at a national level, such as in the Netherlands52, 53, 54, 55, 56, 57 and the UK.48,69,71, 72, 73, 74, 75
In the Netherlands, incidence of neonatal herpes during 1981–1985, 1987–1991, 1992–1998, 1999–2005, 2006–2011, and 2012–2015 was reported at 2.9,52 2.0,53 2.4,54 3.2,55 4.7,56 and 4.857 cases, respectively, per 100,000 live births. The studies suggested a trend of slowly increasing neonatal herpes over time. Of 179 cases reported during 1981–2015, 117 (65.4%) were due to HSV-1, 34 (19.0%) were due to HSV-2, and the remaining 28 (15.6%) had unknown HSV type.52, 53, 54, 55, 56, 57
In the UK, incidence of neonatal herpes during 1986–1991, 2004–2015, 2006–2012, and 2012, was reported at 1.7,75 11.9,70 17.5,68 and 9.669 cases, respectively, per 100,000 live births. In the UK and Ireland during 2019–2021, incidence was estimated at 6.9 cases per 100,000 live births.48 Overall, studies suggested an increasing trend. Of 59 reported cases in the UK during 2019–2021, 29 (49.2%) were due to HSV-1, 25 (42.4%) were due to HSV-2, and the remaining 5 (8.5%) had unknown HSV type.48
In Denmark, incidence of neonatal herpes was reported during 1977–1984 and 1984–1991 at 2.445 and 4.645 cases, respectively, per 100,000 live births. In Germany, it was reported during 2017–2018 at 2.4 cases per 100,000 live births.46 In Israel, it was reported during 2001–2007 at 8.4 cases per 100,000 live births.51 In Poland, it was reported during 2014–2019 at 69.0 cases per 100,000 live births.59 Poland also reported an outbreak of neonatal herpes in a hospital setting that affected 11 new-borns.60 In Sweden, it was reported during 1989–2000 at 7.1 cases per 100,000 live births.63 In Switzerland, it was reported during 2002–2008 at 1.6 cases per 100,000 live births.66 Examination of the trend in all available incidence measures suggested an increasing trend of neonatal herpes in Europe (Fig. S3).
Role of HSV-2 in HIV transmission
Direct, observational studies of the contribution of HSV-2 to HIV infections in Europe are lacking. A systematic review done in 20178 of the effect of HSV-2 infection on HIV acquisition (i.e., susceptibility) identified only one such study, a nested case–control study in MSM in the Netherlands.79 This study reported data from which the unadjusted odds ratio (OR) for HIV incidence in those with HSV-2 infection compared to those without could be calculated. The unadjusted OR was calculated to be 4.3 (95% CI: 1.9–9.9) for prevalent (existing) HSV-2 infection, and 2.2 (95% CI: 0.4–12.2) for incident (recently acquired) HSV-2 infection. However, for the OR for incident HSV-2 on HIV, it was not known whether HSV-2 infection preceded HIV or not.
By pooling all adjusted risk ratio (ARR) study estimates, which met certain quality criteria (including: HSV-2 infection known to have preceded HIV infection), from any setting worldwide, the 2017 systematic review found an ARR of 2.7 (95% CI: 2.2–3.4; n = 22) for prevalent HSV-2 on HIV acquisition in general populations, and an ARR of 4.7 (95% CI: 2.2–10.1; n = 6) for incident HSV-2 on HIV acquisition in general populations. The RR for HIV transmission (i.e., infectiousness) due to HSV-2 (any setting) has been estimated to be 1.3 (range: 1.0–1.9).80 Studies (done in settings outside of Europe) suggest that during ulcerative episodes the risks of HIV acquisition and transmission are even higher.81,82
A recent epidemiological analysis of the population attributable fraction (PAF) of sexually-acquired incident HIV attributable to HSV-2 among 15–49 years old in the general populations was recently completed for all six WHO regions,9 including Europe. Here, the classic epidemiological formula for the PAF was used to apply the pooled ARRs of HSV-2 on HIV acquisition from the 2017 systematic review8 to HSV-2 infection data for Europe for 2016. An overall PAF of 11.6% (95% uncertainty interval (UI): 7.0–19.4%) was calculated for Europe, of which 10.6% (95% UI: 5.9–18.3%) was estimated to be due to prevalent HSV-2 and 1.0% (95% UI: 0.4–2.2%) due to incident HSV-2. These estimates were similar to those for other WHO regions, with the exception of the WHO African and Americas regions, which had higher estimated PAFs due to higher HSV-2 infection rates. There is the potential for residual confounding in the pooled ARR estimates, which may have inflated PAF estimates, however only the contribution of HSV-2 to increased HIV susceptibility was considered (and not also transmissibility), potentially under-estimating the PAF.
A subsequent model-based analysis of the PAF, which included the WHO European region,80 found similar PAF estimates to those obtained from the classic formula (11.2% [95% UI: 7.9–13.8%] for Europe 2009–2018), and even higher (26.8% [95% UI: 16.2–35.8%] for Europe for 2009–2018) PAF estimates when additionally considering the contribution of HSV-2 to HIV transmissibility.
Discussion
HSV-2 seroprevalence in the adult general population of Europe averaged at about 12% over the last three decades, similar to that found in Asia,17 but lower than that in Africa at 37%,18 in Latin America and the Caribbean (LAC) at 21%,19 and in the United States of America (USA), at about 15%.83, 84, 85, 86 HSV-2 seroprevalence has been declining in Europe at a rate of 1% per calendar year over the last three decades. Similar declines were observed, but at a higher rate of 2% per calendar year in Africa,18 Asia,17 LAC,19 and the USA.83, 84, 85, 86 Since HSV-2 seroprevalence is a proxy for population sexual risk behaviour,20,41,42,87, 88, 89 these declines may be explained by less risky sex following recognition of the HIV epidemic,90, 91, 92, 93 improved STI awareness,94 increasing access to HIV/STI services,12,95 and/or changes in the structure of sexual networks following changes in socio-economic conditions.17 Use of antiviral suppressive therapy is unlikely to be substantial in Europe and such therapy, if used, is not likely to be used in the long-term. Therefore, use of such therapy may not have appreciably affected HSV-2 transmission to explain the declining trends.
These results demonstrated generic patterns in HSV-2 epidemiology that are also observed in other world regions.17, 18, 19,83, 84, 85, 86 There was strong hierarchy in seroprevalence based on sexual risk behaviour classification, with the highest seroprevalence observed in FSWs and MSM and the lowest in the general populations, similar to what is observed for other STIs.96,97 Men had 35% lower seroprevalence than women, attesting to a higher bio-anatomical susceptibility to this infection among women.98,99 Seroprevalence increased rapidly with age after sexual debut, but plateaued by the mid-30s. There was also evidence for some variability by subregion and country, with Western Europe having the highest seroprevalence.
HSV-2 was the aetiological cause of 22% of clinically diagnosed GUD and 66% of laboratory-confirmed genital herpes. While these rates are considerable and similar to those observed in North America,100, 101, 102 they are lower than those found in Africa,18 Asia,17 and LAC.19 The contribution of HSV-2 infection (as opposed to HSV-1 infection) to genital herpes is also declining at a rate of 1% per calendar year. This decline may reflect not only declining HSV-2 seroprevalence, but also increasing transmission of HSV-1 through oral sex.103 Indeed, findings of this study are consistent with findings of a study of similar design for HSV-1 infection that estimated HSV-1's contribution to genital herpes at 34%, increasing by 1% per calendar year.1
HSV-2 had a higher detection rate in recurrent genital herpes than in first-episode genital herpes, consistent with more persistent reactivations in the genital tract for HSV-2 than for HSV-1 infection.104 Consequently, and since our estimates are for the proportion of HSV-1 versus HSV-2 among people who present to care and receive a diagnosis of symptomatic genital herpes, not among all people with genital HSV-2 or HSV-1 infection, this finding will underestimate the proportion of genital herpes due to HSV-1. HSV-2 was found more common in genital herpes among older individuals and men than among younger individuals and women. This finding may reflect decreasing childhood HSV-1 infection and increasing transmission of HSV-1 through oral sex among younger persons, and especially young women.101,103
Findings suggested an increasing trend of incidence of neonatal herpes in Europe, opposite to the decreasing trend of HSV-2 seroprevalence, perhaps reflecting the increasing trend of HSV-1-caused genital herpes in this region,1 and increasing maternal age associated with higher HSV-2 seroprevalence.44 Incident HSV-1 appears more readily transmissible to the neonate than HSV-2.65 HSV-2 infection appears to facilitate a considerable number of HIV transmissions in Europe, particularly in higher-risk populations where HIV is concentrated, but not as much as its contribution to increasing HIV transmission in Africa.7,9
This study has limitations. No data were available for 27 of the 53 European countries. However, data were available for all large countries. The 26 European countries for which data were available constituted 80.2% of the total population of the European region. Despite the availability of a large number of seroprevalence measures in Europe, larger than those available in other regions,16,17,19 there are still significant gaps in available data. Most seroprevalence measures were in general populations, but there were not sufficient measures in key populations such as FSWs and MSM. Among the general populations, 23.2% (n = 42) of studies were on pregnant women and 7.7% (n = 14) were on blood donors, but these populations may not be representative of the wider general population.105
The majority of studies were conducted between 1995 and 2005, with a declining trend in availability of studies in recent years. A significant proportion of studies was conducted on relatively small samples; 28.6% of studies had a sample size of <200. More studies were conducted in Western and Northern Europe than in Eastern and Southern Europe. Most studies did not use probability-based sampling. Use of diagnostic assays varied over time.
While studies differed by assay type, sample size, sampling method, and response rate, none of these study characteristics, apart from sampling method, had an effect on observed seroprevalence. The effect of sampling method was also relatively small–studies using non-probability sampling reported on average 1.20-fold higher seroprevalence. There was heterogeneity in effect sizes of included studies, but much of this heterogeneity was subsequently explained by the meta-regressions. Publication bias was not assessed due to methodological issues in assessing it for studies pooling proportions.106
Most studies of HSV-2 virus detection in genital herpes did not differentiate between first episode and recurrent episodes. This distinction is important considering the different contributions of HSV-2 versus HSV-1 infections to first episode versus recurrent episodes.1,17,24 Most studies were conducted before or in 2005 with decreasing number of studies in recent years. Most studies were conducted in Northern Europe with only small number of studies conducted elsewhere in Europe.
Much of the studies of neonatal herpes were conducted in the Netherlands and the UK, with only small number of studies from other countries. More systematic research needs to be conducted to better understand epidemiology of neonatal herpes in Europe. Neonatal herpes is a serious condition with significant mortality and long-term sequela, which necessitates the recontinued surveillance and monitoring of new cases to inform prevention efforts of herpes infections, including HSV-2. Surveillance for neonatal herpes remains limited in Europe with no data being reported for the majority of countries. Only a few countries have estimates for incidence of neonatal herpes based on large national studies.
A strength of this study is the availability of a large volume of HSV-2 data, larger than that found in other regions,16,17,19 which facilitated an array of analyses. Building on these compiled data, a future direction of this work is to conduct mathematical modelling studies to quantitatively characterize the transitioning epidemiology of HSV-2 in Europe and to estimate its epidemiologic indicators such as incidence, past, present, and future, similar to that done recently for the USA.83
Conclusions
HSV-2 seroprevalence is declining in Europe, but at a relatively slow rate of 1% per calendar year, lower than that in other world regions. A significant proportion of Europe's population is chronically infected with this virus, which causes over 20% of GUD cases and nearly 70% of genital herpes cases in this part of the world. HSV-2 infection appears to facilitate the transmission of a considerable number of HIV infections. Meanwhile, there is an increasing trend of neonatal herpes in Europe, but mostly driven by HSV-1 infection. These findings demonstrate the need to accelerate HSV-2 vaccine development and universal access to sexual and reproductive health services. HSV-2 research and surveillance need also to be expanded by conducting national population-based seroprevalence surveys and monitoring incidence of neonatal herpes and aetiology of GUD and genital herpes cases.
Contributors
A.A., A.M.M.O., M.N.K., and M.H. conducted the systematic search, data extraction, data analysis, and interpretation of the results. A.A. and A.M.M.O. wrote the first draft of the paper with L.J.A.-R. L.J.A.-R. conceived the study and led the data extraction and analysis and interpretation of the results. K.J.L. contributed to the neonatal herpes review and drafted the contribution of HSV-2 to HIV transmission. All authors contributed to drafting and revising the manuscript.
Data sharing statement
All relevant data are presented in the manuscript and its Supplementary Material file.
Declaration of interests
K.J.L. has received funding from GSK and the World Health Organization for work outside of the submitted work. Otherwise all authors declare no competing interests.
Acknowledgements
The authors gratefully acknowledge Professor Emeritus Rhoda Ashley Morrow of the University of Washington, for her support in assessing the quality of study diagnostic methods. The authors are also grateful to Ms. Adona Canlas for administrative support. The authors are grateful for pilot funding by the Biomedical Research Program and infrastructure support provided by the Biostatistics, Epidemiology, and the Biomathematics Research Core at Weill Cornell Medicine-Qatar. K.J.L. is a member of the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at the University of Bristol. This publication was also made possible by NPRP grant number 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. The findings achieved herein are solely the responsibility of the authors.
Funding: This work was supported by the Qatar National Research Fund [NPRP 9-040-3-008] and by pilot funding from the Biomedical Research Program at Weill Cornell Medicine in Qatar.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2022.100558.
Appendix A. Supplementary data
References
- 1.Yousuf W., Ibrahim H., Harfouche M., Abu Hijleh F., Abu-Raddad L. Herpes simplex virus type 1 in Europe: systematic review, meta-analyses and meta-regressions. BMJ Glob Health. 2020;5(7) doi: 10.1136/bmjgh-2020-002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James C., Harfouche M., Welton N.J., et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315–329. doi: 10.2471/BLT.19.237149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas J.M., Jr., Berman S.M. Screening for HSV-2 infection in STD clinics and beyond: a few answers but more questions. Sex Transm Dis. 2009;36(11):729–731. doi: 10.1097/OLQ.0b013e3181c04dea. [DOI] [PubMed] [Google Scholar]
- 4.Johnston C., Corey L. Current concepts for genital herpes simplex virus infection: diagnostics and pathogenesis of genital tract shedding. Clin Microbiol Rev. 2016;29(1):149. doi: 10.1128/CMR.00043-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gnann J.W., Jr., Whitley R.J. Genital herpes. N Engl J Med. 2016;375(7):666–674. doi: 10.1056/NEJMcp1603178. [DOI] [PubMed] [Google Scholar]
- 6.Wald A., Zeh J., Selke S., et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Raddad L.J., Magaret A.S., Celum C., et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One. 2008;3(5) doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Looker K.J., Elmes J.A.R., Gottlieb S.L., et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17(12):1303–1316. doi: 10.1016/S1473-3099(17)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Looker K.J., Welton N.J., Sabin K.M., et al. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data. Lancet Infect Dis. 2020;20(2):240–249. doi: 10.1016/S1473-3099(19)30470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omori R., Nagelkerke N., Abu-Raddad L.J. HIV and herpes simplex virus type 2 epidemiological synergy: misguided observational evidence? A modelling study. Sex Transm Infect. 2018;94(5):372–376. doi: 10.1136/sextrans-2017-053336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb S.L., Giersing B.K., Hickling J., et al. Meeting report: initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics, March 2017. Vaccine. 2019;37(50):7408–7418. doi: 10.1016/j.vaccine.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Global health sector strategy on sexually transmitted infections 2016-2021. https://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/
- 13.World Health Organization Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. https://www.who.int/publications/i/item/9789240053779
- 14.Gottlieb S.L., Deal C.D., Giersing B., et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine. 2016;34(26):2939–2947. doi: 10.1016/j.vaccine.2016.03.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb S.L., Giersing B., Boily M.C., et al. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: key findings from the World Health Organization Consultation on HSV Vaccine Impact Modelling. Vaccine. 2019;37(50):7336–7345. doi: 10.1016/j.vaccine.2017.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AlMukdad S., Farooqui U.S., Harfouche M., Aldos L., Abu-Raddad L.J. Epidemiology of herpes simplex virus type 2 in Canada, Australia, and New Zealand: systematic review, meta-analyses, and meta-regressions. Sex Transm Dis. 2022;49(6):403–413. doi: 10.1097/OLQ.0000000000001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AlMukdad S., Harfouche M., Wettstein A., Abu-Raddad L.J. Epidemiology of herpes simplex virus type 2 in Asia: a systematic review, meta-analysis, and meta-regression. Lancet Reg Health West Pac. 2021;12 doi: 10.1016/j.lanwpc.2021.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harfouche M., Abu-Hijleh F.M., James C., Looker K.J., Abu-Raddad L.J. Epidemiology of herpes simplex virus type 2 in sub-Saharan Africa: systematic review, meta-analyses, and meta-regressions. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harfouche M., Maalmi H., Abu-Raddad L.J. Epidemiology of herpes simplex virus type 2 in Latin America and the Caribbean: systematic review, meta-analyses and metaregressions. Sex Transm Infect. 2021;97(7):490–500. doi: 10.1136/sextrans-2021-054972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Raddad L.J., Schiffer J.T., Ashley R., et al. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics. 2010;2(4):173–182. doi: 10.1016/j.epidem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaabane S., Harfouche M., Chemaitelly H., Schwarzer G., Abu-Raddad L.J. Herpes simplex virus type 1 epidemiology in the Middle East and North Africa: systematic review, meta-analyses, and meta-regressions. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-018-37833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harfouche M., Chemaitelly H., Abu-Raddad L.J. Herpes simplex virus type 1 epidemiology in Africa: systematic review, meta-analyses, and meta-regressions. J Infect. 2019;79(4):289–299. doi: 10.1016/j.jinf.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Khadr L., Harfouche M., Omori R., Schwarzer G., Chemaitelly H., Abu-Raddad L.J. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis. 2019;68(5):757–772. doi: 10.1093/cid/ciy562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukik L., Alyafei M., Harfouche M., Abu-Raddad L.J. Herpes simplex virus type 1 epidemiology in Latin America and the Caribbean: systematic review and meta-analytics. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0215487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J., Green S. John Wiley & Sons; 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization WHO regional offices. https://www.who.int/countries Available at:
- 29.World Atlas Europe countries and regions. https://www.worldatlas.com/articles/the-four-european-regions-as-defined-by-the-united-nations-geoscheme-for-europe.html
- 30.Ashley R.L. Performance and use of HSV type-specific serology test kits. Herpes. 2002;9(2):38–45. [PubMed] [Google Scholar]
- 31.Ashley-Morrow R., Nollkamper J., Robinson N.J., Bishop N., Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect. 2004;10(6):530–536. doi: 10.1111/j.1469-0691.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 32.Arshad Z., Alturkistani A., Brindley D., Lam C., Foley K., Meinert E. Tools for the diagnosis of herpes simplex virus 1/2: systematic review of studies published between 2012 and 2018. JMIR Public Health Surveill. 2019;5(2) doi: 10.2196/14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RStudio Team RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
- 34.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 35.Borenstein M.H.,L.V., Higgins J.P.T., Rothstein H.R. John Wiley & Sons, Ltd; Chichester, UK: 2009. Introduction to meta-analysis. [Google Scholar]
- 36.Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607–611. [Google Scholar]
- 37.Schwarzer G., Chemaitelly H., Abu-Raddad L.J., Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476–483. doi: 10.1002/jrsm.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.StataCorp . StataCorp LLC; College Station, TX: 2019. Stata statistical software: release 16. [Google Scholar]
- 39.Harbord R.M., Higgins J.P.T. Meta-regression in Stata. STATA J. 2008;8(4):493–519. [Google Scholar]
- 40.Chemaitelly H., Weiss H.A., Abu-Raddad L.J. HSV-2 as a biomarker of HIV epidemic potential in female sex workers: meta-analysis, global epidemiology and implications. Sci Rep. 2020;10(1):19293. doi: 10.1038/s41598-020-76380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouyoumjian S.P., Heijnen M., Chaabna K., et al. Global population-level association between herpes simplex virus 2 prevalence and HIV prevalence. AIDS. 2018;32(10):1343–1352. doi: 10.1097/QAD.0000000000001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omori R., Abu-Raddad L.J. Sexual network drivers of HIV and herpes simplex virus type 2 transmission. AIDS. 2017;31(12):1721–1732. doi: 10.1097/QAD.0000000000001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renesme L. [Neonatal herpes: epidemiology, clinical manifestations and management. Guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF)] Gynecol Obstet Fertil Senol. 2017;45(12):691–704. doi: 10.1016/j.gofs.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Looker K.J., Magaret A.S., May M.T., et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health. 2017;5(3):e300–e309. doi: 10.1016/S2214-109X(16)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fonnest G., de la Fuente Fonnest I., Weber T. Neonatal herpes in Denmark 1977-1991. Acta Obstet Gynecol Scand. 1997;76(4):355–358. doi: 10.1111/j.1600-0412.1997.tb07992.x. [DOI] [PubMed] [Google Scholar]
- 46.Kidszun A., Bruns A., Schreiner D., et al. Characteristics of neonatal herpes simplex virus infections in Germany: results of a 2-year prospective nationwide surveillance study. Arch Dis Child Fetal Neonatal Ed. 2022;107(2):188–192. doi: 10.1136/archdischild-2021-321940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobczak H., Gebauer W., Schutz E. Herpes simplex-infection in the newborn. Klin Pädiatr. 1985;197(2):141–145. doi: 10.1055/s-2008-1033948. [DOI] [PubMed] [Google Scholar]
- 48.Fidler K., Heath P., Dudley J., Yan G., Lynn R. Neonatal HSV disease in infants under 90 days of age in UK and Ireland: BPSU study interim analysis. Arch Dis Child. 2021;106(SUPPL 1):A390–A391. [Google Scholar]
- 49.Leventon-Kriss S., Rannon L., Smetana Z., Yoffe R. Genital herpes simplex infections in Israel: 1973 throughout 1980. Isr J Med Sci. 1982;18(9):941–946. [PubMed] [Google Scholar]
- 50.Leventon-Kriss S., Rannon L., Smetana Z., Yoffe R. Increase in laboratory-confirmed cases of herpes genitalis and neonatal herpes infections in Israel. Isr J Med Sci. 1983;19(10):946–949. [PubMed] [Google Scholar]
- 51.Koren A., Tasher D., Stein M., Yossepowitch O., Somekh E. Neonatal herpes simplex virus infections in Israel. Pediatr Infect Dis J. 2013;32(2):120–123. doi: 10.1097/INF.0b013e3182717f0b. [DOI] [PubMed] [Google Scholar]
- 52.van der Meijden W., Dumas A. Consensus preventie van herpes neonatorum. Ned Tijdschr Geneeskd. 1988;131:2030–2034. [PubMed] [Google Scholar]
- 53.van Everdingen J.J., Peeters M.F., ten Have P. Neonatal herpes policy in The Netherlands. Five years after a consensus conference. J Perinat Med. 1993;21(5):371–375. doi: 10.1515/jpme.1993.21.5.371. [DOI] [PubMed] [Google Scholar]
- 54.Gaytant M.A., Steegers E.A., van Cromvoirt P.L., Semmekrot B.A., Galama J.M. [Incidence of herpes neonatorum in Netherlands] Ned Tijdschr Geneeskd. 2000;144(38):1832–1836. [PubMed] [Google Scholar]
- 55.Poeran J., Wildschut H., Gaytant M., Galama J., Steegers E., van der Meijden W. The incidence of neonatal herpes in The Netherlands. J Clin Virol. 2008;42(4):321–325. doi: 10.1016/j.jcv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Hemelaar S.J., Poeran J., Steegers E.A., van der Meijden W.I. Neonatal herpes infections in The Netherlands in the period 2006-2011. J Matern Fetal Neonatal Med. 2015;28(8):905–909. doi: 10.3109/14767058.2014.937691. [DOI] [PubMed] [Google Scholar]
- 57.van Oeffelen L., Biekram M., Poeran J., et al. Update on neonatal herpes simplex epidemiology in the Netherlands: a health problem of increasing concern? Pediatr Infect Dis J. 2018;37(8):806–813. doi: 10.1097/INF.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 58.Keuning M.W., van der Kuip M., van Hattem J.M., Pajkrt D. Inconsistent management of neonatal herpes simplex virus infections. Hosp Pediatr. 2019;9(10):808–812. doi: 10.1542/hpeds.2019-0001. [DOI] [PubMed] [Google Scholar]
- 59.Rząd M., Nitsch-Osuch A., Tyszko P.Z., et al. Congenital herpes simplex virus infection among hospitalized infants in Poland. Ann Agric Environ Med. 2021;28(4):612–616. doi: 10.26444/aaem/142999. [DOI] [PubMed] [Google Scholar]
- 60.Słowińska I., Wardecka J., Sułocka Z. [An epidemic focus of herpes simplex infection in a neonatal ward] Wiad Lek. 1982;35(1):25–30. [PubMed] [Google Scholar]
- 61.Ivanova R.A., Vasilyev V.V., Rogozina N.V., Grineva A.A., Ushakova G.M. Congenital herpes simplex: modern approach to prevention, diagnosis, and treatment. Child Infect. 2021;20(4):47–52. [Google Scholar]
- 62.Echevarría C., Echevarría J.M., Anda P., et al. [Congenital and perinatal infections caused by viral agents, Toxoplasma gondii and Treponema pallidum. Study of 2000 cases and analysis of 488 positive cases] Med Clin (Barc) 1987;88(4):129–134. [PubMed] [Google Scholar]
- 63.Engman M.L., Adolfsson I., Lewensohn-Fuchs I., Forsgren M., Mosskin M., Malm G. Neuropsychologic outcomes in children with neonatal herpes encephalitis. Pediatr Neurol. 2008;38(6):398–405. doi: 10.1016/j.pediatrneurol.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Forsgren M. Genital herpes simplex virus infection and incidence of neonatal disease in Sweden. Scand J Infect Dis Suppl. 1990;69:37–41. [PubMed] [Google Scholar]
- 65.Brown E.L., Gardella C., Malm G., et al. Effect of maternal herpes simplex virus (HSV) serostatus and HSV type on risk of neonatal herpes. Acta Obstet Gynecol Scand. 2007;86(5):523–529. doi: 10.1080/00016340601151949. [DOI] [PubMed] [Google Scholar]
- 66.Pascual A., Moessinger A., Gerber S., Meylan P. Neonatal herpes simplex virus infections in Switzerland: results of a 6-year national prospective surveillance study. Clin Microbiol Infect. 2011;17(12):1907–1910. doi: 10.1111/j.1469-0691.2011.03641.x. [DOI] [PubMed] [Google Scholar]
- 67.Sert U.Y., Ozgu-Erdinc A.S., Saygan S., Engin-Ustun Y. Herpes simplex infection during pregnancy, results of a tertiary referral center in Turkey. Z Geburtshilfe Neonatol. 2020;224(1):22–25. doi: 10.1055/a-0842-6941. [DOI] [PubMed] [Google Scholar]
- 68.Batra D., Davies P., Manktelow B.N., Smith C. The incidence and presentation of neonatal herpes in a single UK Tertiary Centre, 2006-2013. Arch Dis Child. 2014;99(10):916–921. doi: 10.1136/archdischild-2013-305335. [DOI] [PubMed] [Google Scholar]
- 69.Clarke E., Shone E., Wright C., Patel R. Neonatal herpes simplex virus infection-is the UK special anymore? Sex Transm Dis. 2014;41(SUPPL. 1):S60. [Google Scholar]
- 70.Sachane K., Ironton R., Pelosi E. Neonatal HSV-experience over a decade in a tertiary neonatal unit in UK. Arch Dis Child. 2017;102(Supplement 1):A189. [Google Scholar]
- 71.Kadambari S., Pollard A.J., Goldacre M.J., Goldacre R. Congenital viral infections in England over five decades: a population-based observational study. Lancet Infect Dis. 2020;20(2):220–229. doi: 10.1016/S1473-3099(19)30416-5. [DOI] [PubMed] [Google Scholar]
- 72.Lynn R., Hall S.M. The British Paediatric Surveillance Unit: activities and developments in 1990 and 1991. Commun Dis Rep CDR Rev. 1992;2(13):R145–R148. [PubMed] [Google Scholar]
- 73.Report from the PHLS Communicable Disease Surveillance Centre. Br Med J (Clin Res Ed) 1987;294(6568):361–362. doi: 10.1136/bmj.294.6568.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudd P., Peckham C. Infection of the fetus and the newborn: prevention, treatment and related handicap. Baillieres Clin Obstet Gynaecol. 1988;2(1):55–71. doi: 10.1016/s0950-3552(88)80063-4. [DOI] [PubMed] [Google Scholar]
- 75.Tookey P., Peckham C.S. Neonatal herpes simplex virus infection in the British Isles. Paediatr Perinat Epidemiol. 1996;10(4):432–442. doi: 10.1111/j.1365-3016.1996.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 76.Royal College of Paediatrics and Child Health British Paediatric Surveillance Unit-annual reports. https://www.rcpch.ac.uk/work-we-do/bpsu/annual-reports
- 77.Badawy M.K., Hurrell S., Baldwin C., Hassan H. Prolonged fever and hyperferritinaemia: a puzzling diagnosis of neonatal herpes simplex virus infection during COVID-19 pandemic. BMJ Case Rep. 2021;14(3) doi: 10.1136/bcr-2020-241405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McClean P., Davison S., Roche J., et al. Liver transplantation in neonatal liver failure due to herpes simplex virus. Pediatr Transplant. 2011;15:87. [Google Scholar]
- 79.Keet I.P., Lee F.K., van Griensven G.J., Lange J.M., Nahmias A., Coutinho R.A. Herpes simplex virus type 2 and other genital ulcerative infections as a risk factor for HIV-1 acquisition. Genitourin Med. 1990;66(5):330–333. doi: 10.1136/sti.66.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silhol R., Coupland H., Baggaley R.F., et al. What is the burden of heterosexually acquired HIV due to HSV-2? Global and regional model-based estimates of the proportion and number of HIV infections attributable to HSV-2 infection. J Acquir Immune Defic Syndr. 2021;88(1):19–30. doi: 10.1097/QAI.0000000000002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boily M.C., Baggaley R.F., Wang L., et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gray R.H., Wawer M.J., Brookmeyer R., et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 83.Ayoub H.H., Amara I., Awad S.F., Omori R., Chemaitelly H., Abu-Raddad L.J. Analytic characterization of the herpes simplex virus type 2 epidemic in the United States, 1950-2050. Open Forum Infect Dis. 2021;8(7):ofab218. doi: 10.1093/ofid/ofab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chemaitelly H., Nagelkerke N., Omori R., Abu-Raddad L.J. Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0214151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McQuillan G., Kruszon-Moran D., Flagg E.W., Paulose-Ram R. National Center for Health Statistics; Hyattsville, MD: 2018. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14-49: United States, 2015-2016, NCHS Data Brief, no 304. [PubMed] [Google Scholar]
- 86.Xu F., Sternberg M.R., Kottiri B.J., et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. J Am Med Assoc. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 87.van de Laar M.J., Termorshuizen F., Slomka M.J., et al. Prevalence and correlates of herpes simplex virus type 2 infection: evaluation of behavioural risk factors. Int J Epidemiol. 1998;27(1):127–134. doi: 10.1093/ije/27.1.127. [DOI] [PubMed] [Google Scholar]
- 88.Cowan F.M., Johnson A.M., Ashley R., Corey L., Mindel A. Antibody to herpes simplex virus type 2 as serological marker of sexual lifestyle in populations. Br Med J. 1994;309(6965):1325–1329. doi: 10.1136/bmj.309.6965.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Obasi A., Mosha F., Quigley M., et al. Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. J Infect Dis. 1999;179(1):16–24. doi: 10.1086/314555. [DOI] [PubMed] [Google Scholar]
- 90.Hallett T.B., Gregson S., Mugurungi O., Gonese E., Garnett G.P. Assessing evidence for behaviour change affecting the course of HIV epidemics: a new mathematical modelling approach and application to data from Zimbabwe. Epidemics. 2009;1(2):108–117. doi: 10.1016/j.epidem.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 91.Hallett T.B., Aberle-Grasse J., Bello G., et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex Transm Infect. 2006;82 Suppl 1:i1–i8. doi: 10.1136/sti.2005.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kilian A.H., Gregson S., Ndyanabangi B., et al. Reductions in risk behaviour provide the most consistent explanation for declining HIV-1 prevalence in Uganda. AIDS. 1999;13(3):391–398. doi: 10.1097/00002030-199902250-00012. [DOI] [PubMed] [Google Scholar]
- 93.Awad S.F., Abu-Raddad L.J. Could there have been substantial declines in sexual risk behavior across sub-Saharan Africa in the mid-1990s? Epidemics. 2014;8:9–17. doi: 10.1016/j.epidem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Loeber O., Reuter S., Apter D., van der Doef S., Lazdane G., Pinter B. Aspects of sexuality education in Europe–definitions, differences and developments. Eur J Contracept Reprod Health Care. 2010;15(3):169–176. doi: 10.3109/13625181003797280. [DOI] [PubMed] [Google Scholar]
- 95.Wijesooriya N.S., Rochat R.W., Kamb M.L., et al. Global burden of maternal and congenital syphilis in 2008 and 2012: a health systems modelling study. Lancet Glob Health. 2016;4(8):e525–e533. doi: 10.1016/S2214-109X(16)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Low N., Broutet N., Adu-Sarkodie Y., Barton P., Hossain M., Hawkes S. Global control of sexually transmitted infections. Lancet. 2006;368(9551):2001–2016. doi: 10.1016/S0140-6736(06)69482-8. [DOI] [PubMed] [Google Scholar]
- 97.Smolak A., Chemaitelly H., Hermez J.G., Low N., Abu-Raddad L.J. Epidemiology of chlamydia trachomatis in the Middle East and North Africa: a systematic review, meta-analysis, and meta-regression. Lancet Glob Health. 2019;7(9):e1197–e1225. doi: 10.1016/S2214-109X(19)30279-7. [DOI] [PubMed] [Google Scholar]
- 98.Wald A., Corey L. In: Human herpesviruses: biology, therapy, and immunoprophylaxis. Arvin A., Campadelli-Fiume G., Mocarski E., et al., editors. Cambridge University Press; Cambridge: 2007. Persistence in the population: epidemiology, transmission. [PubMed] [Google Scholar]
- 99.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11:24A–35A. [PubMed] [Google Scholar]
- 100.Dabestani N., Katz D.A., Dombrowski J., Magaret A., Wald A., Johnston C. Time trends in first-episode genital herpes simplex virus infections in an urban sexually transmitted disease clinic. Sex Transm Dis. 2019;46(12):795–800. doi: 10.1097/OLQ.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 101.Bernstein D.I., Bellamy A.R., Hook E.W., 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–351. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts C.M., Pfister J.R., Spear S.J. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30(10):797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 103.Ayoub H.H., Chemaitelly H., Abu-Raddad L.J. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med. 2019;17(1):57. doi: 10.1186/s12916-019-1285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gupta R., Warren T., Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 105.Golding J., Northstone K., Miller L.L., Davey Smith G., Pembrey M. Differences between blood donors and a population sample: implications for case-control studies. Int J Epidemiol. 2013;42(4):1145–1156. doi: 10.1093/ije/dyt095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.