Abstract
Background
The Food and Drug Administration (FDA)-approved submucosal injection of lifting agents such as ORISE® has become a widespread, routine and standard practice in endoscopic mucosal resection and endoscopic submucosal dissection of gastrointestinal lesions. Lifting agent granulomas result from transformation of injected material into a mass-forming amorphous hyaline-like material eliciting a strong foreign body giant cell reaction. This report of three cases shows how lifting agent granulomas can act as potential clinical and gross mimickers of invasive adenocarcinoma.
Cases Description
Three cases were identified in a six-month span based on the histological presence of a lifting agent granuloma in a colonic/colorectal resection specimen with associated clinical, imaging and gross concern for invasive malignancy. Each case resulted in an escalation of clinical and surgical management due to the suspicion of an unresectable neoplastic process that was at least partially involved by an exuberant granulomatous reaction due to the utilization of a lifting agent. Colonic transmural involvement and sub-serosal vascular infiltration by the granulomas are described.
Conclusions
Lifting agent granulomas have become a routine endoscopic technique to help achieve full resection of flat/sessile colorectal polyps and early-stage cancers. This report confirms that these granulomas exhibit colonic transmural involvement. Sub-serosal blood vessel involvement is reported for the first time. It is important to recognize the unique characteristics of these new synthetic lifting agents. Their propensity to develop a mass-forming granulomatous reaction has the potential to mimic invasive adenocarcinoma clinically, radiologically and pathologically. This can significantly impact patient care and management both clinically and surgically.
Keywords: Lifting, agent, granuloma, case report
Highlight box.
Key Findings
• Lifting agent granulomas may mimic invasive adenocarcinoma.
What is known and what is new?
• Submucosal injection of lifting agents aid in removal flat/sessile colorectal polyps and early cancers.
• Lifting agent granulomas represent a robust foreign-body giant cell reaction to development of amorphous hyaline-like material.
• The reported three cases highlight how lifting agent granulomas may present as a mass-forming lesions which may mimic invasive adenocarcinoma and potentially alter clinical and surgical management.
• Subserosal blood vessels involvement by lifting agent granulomas is reported for the first time.
What is the implication, and what should change now?
• As lifting agent use during endoscopy becomes more widespread, it is essential to recognize the unique characteristics of such agents and avoid alterations to surgical management resulting in potential unnecessary interventions.
• The need for clear communication and documentation of lifting agent use among members of the clinical care team is strongly advocated.
Introduction
Submucosal injection of lifting agents is often performed to aid in endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). EMR and ESD are endoscopic techniques used for removal of early-stage neoplasms (e.g., adenomas, intramucosal adenocarcinoma, superficially invasive adenocarcinoma) confined to the mucosa or submucosa within the gastrointestinal tract. Lifting agents are designed to be used for submucosal cushioning of polyps, adenomas, early-stage cancers or other gastrointestinal mucosal lesions prior to excision with a snare or other endoscopic device. This technique can aid in the removal of flat/sessile and difficult to remove lesions via EMR or ESD, reducing the need for surgical intervention. Endoscopic resection is less invasive, less expensive, and associated with decreased overall morbidity and mortality, shorter recovery times, and reduced complications when compared to surgical resection (1).
Additionally, submucosal injection allows for observation of the lesion as an important method to assess the potential for deeply invasive carcinoma. Difficulties encountered during attempted injection and snare resection alert the endoscopist to the possibility of deep submucosal invasion. Non-lifting areas are typically difficult to capture in the snare. Lesions may not lift due to submucosal invasion or because of submucosal fibrosis related to prior biopsy sampling or manipulation procedure (2). However, it has been reported that the “non-lifting sign” suffers from lower sensitivity (61.5%) and positive predictive value (80.0%) for invasive cancer in treatment-naive lesions, thereby rendering it inferior to direct endoscopic evaluation (3).
Traditionally, a normal saline solution, which may be tinted with a dye such as methylene blue, has been used as a lifting agent. However, saline is rapidly absorbed by the surrounding tissue thereby necessitating reinjection during an endoscopic procedure. Other solutions such as glycerol, hyaluronic acid, hydroxypropyl methylcellulose and hydroxyethyl starch have also been used (4). The recently Food and Drug Administration (FDA) approved synthetic submucosal lifting agents, Eleview® (2017) and ORISE® (2018), have gained popularity due to longer maintenance of submucosal fluid cushions and subsequent reduction in procedure times (5,6).
While other lifting agents have been associated with only minimal histological findings, ORISE® is known to be associated with the appearance of an amorphous mucin-like substance at day zero post-injection followed by the development of a robust foreign body-type giant cell reaction, coined “lifting agent granuloma”. These histological findings have been documented in the submucosa, muscular propria, and focally in the adventitia (7). We present a series of three cases in which the presence of lifting agent granulomas represented a clinical (via endoscopy and surgical resection) and/or gross mimic of invasive adenocarcinoma. We present the following cases in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-22-59/rc).
Case presentation
All cases in this review are previously unpublished and were identified by gastrointestinal pathologists and colorectal surgeons during a 6-month period at the University of Vermont Medical Center. They were chosen based on the histological presence of a lifting agent granuloma in a colorectal surgical resection specimen in addition to the clinical concern for invasive adenocarcinoma. The use of the lifting agent during prior colonoscopy procedures was confirmed.
The cases study protocol was approved by the Institutional Review Board (IRB) of The University of Vermont and The University of Vermont Medical Center and an exemption category 4iii (Waiver of HIPAA Authorization) was provided. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
A 58-year-old woman presented for colonoscopy due to personal history of adenomas. During colonoscopy a 30 mm sessile multilobed polyp was identified in the sigmoid colon. The polyp was lifted with ease using ORISE® and removed in a piece-meal fashion. Subsequent pathological evaluation demonstrated a well-differentiated adenocarcinoma with fragmentation precluding definitive assessment of submucosal invasion. About ten days later, the patient underwent a flexible sigmoidoscopy which revealed the polypectomy site with ulceration about 15 cm from the dentate line. No other masses or lesions were appreciated. Approximately one month later, the patient underwent a laparoscopic hand-assisted low anterior resection for sigmoid colon cancer. During the resection, there was difficulty firing the stapler at the distal margin and subsequently a submucosal mass was visible after opening the specimen at the level of the distal staple line. Therefore, an additional distal resection margin was obtained. The residual sessile polyp was identified at the original EMR site approximately 3 cm proximal to the distal staple line. The post-surgical course was uneventful, and a subsequent one-year follow-up colonoscopy revealed no evidence of malignancy.
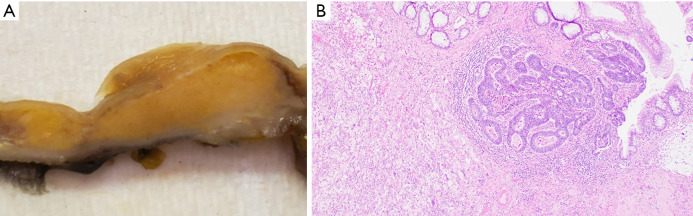
Upon gross pathological evaluation, a well-defined raised sub-mucosal solid yellow-tan lesion (1.2 cm × 1.1 cm × 0.8 cm) was noted extending to the original distal staple line (Figure 1A). Histological evaluation revealed a submucosal microscopic focus of residual adenocarcinoma measuring 1.0 mm in maximum dimension with an underlying mass-forming lifting agent granuloma measuring 1.2 cm in maximum dimension (Figure 1B).
Figure 1.
Lifting agent granuloma in a 58-year-old female (Case 1). (A) Gross pathology specimen, cross section of colonic wall with lesion. (B) Lifting agent granuloma underlying residual invasive adenocarcinoma (hematoxylin & eosin, 40×).
Case 2
A 61-year-old man underwent screening colonoscopy and was found to have a hepatic flexure polyp which was subsequently removed by EMR with the use of a synthetic lifting agent (ORISE®). Pathological evaluation demonstrated at least intramucosal adenocarcinoma; however, no tissue deeper than muscularis mucosae was present for evaluation. Approximately two weeks later the patient underwent another colonoscopy and was found to have a flat carpeting polyp in the area of prior EMR that could not be endoscopically retrieved. The polyp was described as not lifting well and there was concern for invasive carcinoma. No other masses or lesions were identified. He was subsequently referred for surgical intervention due to an endoscopically unresectable polyp. He underwent a laparoscopic hand-assisted right hemicolectomy. The post-operative course was uneventful, and the patient was scheduled to undergo follow-up colonoscopy in three years.
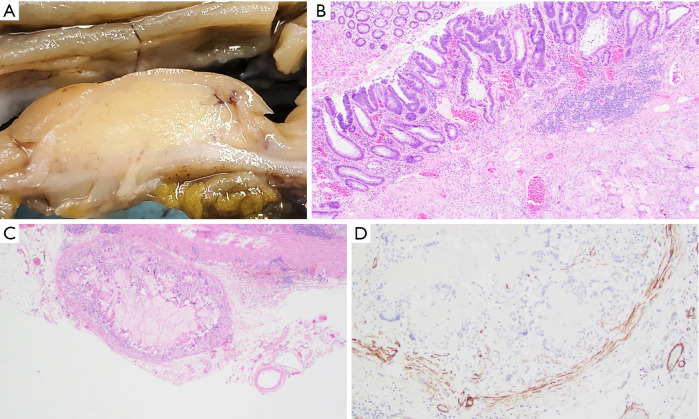
Gross pathological evaluation revealed an ill-defined mass (1.6 cm × 1.4 cm) that appears to invade the muscularis propria and abuts to the serosa (Figure 2A). Histological examination revealed a mass-forming lifting agent granuloma at the hepatic flexure with overlying residual adenoma (Figure 2B). Small discrete sub-serosal nodules were noted and represented involvement of blood vessels by the granulomatous process (Figure 2C). Positive immunohistochemical staining for smooth muscle actin (SMA) highlights the muscular wall of involved blood vessels (Figure 2D). No malignancy was identified.
Figure 2.
Lifting agent granuloma in a 61-year-old male (Case 2). (A) Gross pathology specimen, cross section of colonic wall with lesion. (B) Lifting agent granuloma underlying residual adenoma (hematoxylin & eosin, 40×). (C) Sub-serosal blood vessel infiltration by lifting agent granuloma (hematoxylin & eosin, 40×). (D) Positive smooth muscle actin immunohistochemical staining highlighting the muscular wall of sub-serosal blood vessel involved by lifting agent granuloma (immunohistochemistry, 100×).
Case 3
A 65-year-old man underwent screening colonoscopy during which a 25 mm polyp was identified in the ascending colon. A polypectomy was attempted, and eventually a piecemeal EMR was performed; however, the procedure was terminated prematurely due to patient discomfort. Approximately two months later another colonoscopy was performed during which the prior polypectomy site was visualized. The endoscopist used ORISE® in an attempt to lift the residual polyp, but this was unsuccessful due to underlying scarring. Ultimately, during this procedure the lesion was removed with a cold snare shaving technique and cold biopsy forceps. The lesion was also treated with a snare tip and Softcoag60 cautery after which no obvious residual polyp was present. Three months later another repeat colonoscopy was performed and demonstrated an unresectable polyp with central ulceration located at the prior polypectomy site. No other masses or lesions were identified. The patient was referred for surgical management due to a recurrent endoscopically unresectable polyp. He underwent a laparoscopic hand-assisted right hemicolectomy. The post-operative course was uneventful, and the patient was scheduled to undergo follow-up colonoscopy in three years.
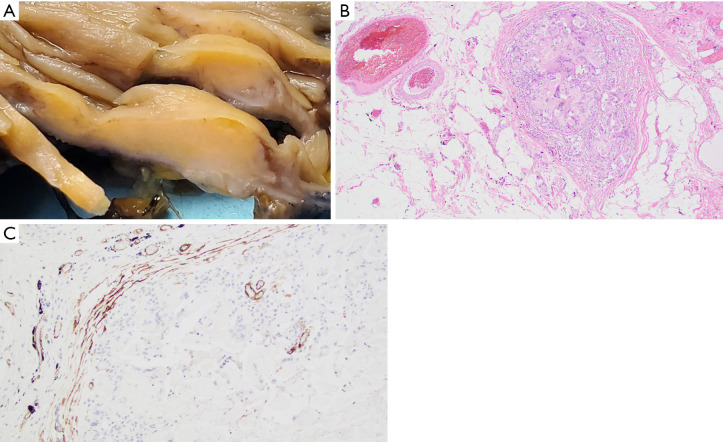
Subsequent pathological evaluation revealed an ill-defined tan mass (2.8 cm × 2.0 cm × 0.8 cm) within the ascending colon (Figure 3A). The mass appeared to grossly extend into the submucosa and through the colonic wall. Histological evaluation showed a mass-forming lifting agent granuloma underlying colonic mucosa. The lesion extended into the subserosa and involved a sub-serosal blood vessel (Figure 3B). Positive immunohistochemical staining for SMA highlights the muscular wall of the involved blood vessel (Figure 3C). There was no residual adenoma or malignancy identified.
Figure 3.
Lifting agent granuloma in a 65-year-old male (Case 3). (A) Gross pathology specimen, cross section of colonic wall with lesion. (B) Sub-serosal blood vessel infiltration by lifting agent granuloma (hematoxylin & eosin, 40×). (C) Positive smooth muscle actin immunohistochemical staining highlighting the muscular wall of sub-serosal blood vessel involved by lifting agent granuloma (immunohistochemistry, 100×).
Patient perspective
Case 1
I was more than glad to give my consent for this study. I consider myself fortunate to have the team I had to get me through this traumatic experience. Everyone was so kind and understanding and the care I received before, during and after was something I can never say thank you enough for. I hope this consent and study helps others.
Case 2
The patient did not provide his perspective.
Case 3
I was truly pleased with the confidence of the doctor and the staff from the time I entered the pre-op hallway. The confidence helped me to feel comfortable, knowing I was in good care. Physically, recovery was tough for me, and the post-op staff were so helpful, caring and compassionate.
Discussion
Submucosal injection of lifting agents (ORISE®, Eleview®) has gained popularity since their use was approved by the FDA. Several reports have described the endoscopic and histological features of the granulomatous reaction caused by ORISE® (7-11). Such a reaction is presumed to occur but has not been described yet in association with Eleview® (7). The transformation of the initial post-injection mucin-like appearance to an amorphous hyaline-like material eliciting a strong foreign body giant cell reaction is well-documented (7,8). Other entities that may cause a similar histological appearance include amyloidosis, pulse granulomata, and invasive adenocarcinoma (7,8,11). The ambiguity in endoscopic and gross appearance may arise from lateral and deep spread of the lifting agent within the colonic wall, thereby giving an endoscopic and/or radiologic appearance of a submucosal distortion in the region of EMR scars or more advanced invasive firm non-lifting lesion, potentially influencing and modifying subsequent clinical and surgical follow-up (12-14).
We herein describe three cases in which the use of lifting agents resulted in lifting agent granulomatous reactions which subsequently mimicked invasive adenocarcinoma either upon repeat endoscopy, during surgical resection, and/or at pathological gross evaluation. In the cases described, clinical management was impacted by the impression of invasive cancer which was ultimately felt to be a result of the granulomatous tissue associated with lifting agents. We also describe the presence of transmural and sub-serosal extension of the granulomatous reaction as well as intravascular involvement. Transmural infiltration has been recently described (13). One of the reported cases resulted in small bowel obstruction necessitating further surgical intervention. This study is limited by the small number of reported cases, for they represent patients that have been followed up at our institution. Details of the amount and technique of lifting agents injection are not available. Whether they influenced transmural and intravascular extension of the lifting agents cannot be ascertained.
In Case 1, additional distal margin was resected in a low anterior resection due to the presence of a lifting agent granuloma at the distal margin. The presence of this granulomatous reaction in the region of the known invasive adenocarcinoma acted as a mimic of invasive cancer and ultimately led to an extended resection. While in this case extending the margin did not compromise the ability to preserve sphincter function and bowel function, it is important to recognize that extension of a margin due to a mass-forming granulomatous reaction could result in decreased bowel function and as a result, quality of life.
In Case 2, a right hemicolectomy was performed due to an unresectable mass-forming lesion suspected to be residual/recurrent disease at the site of a prior biopsy with histopathological evidence of at least intramucosal adenocarcinoma.
In Case 3, a right hemicolectomy was performed due to an unresectable mass lesion with central ulceration in a site with previous histological evidence of a tubulovillous adenoma showing focal high-grade dysplasia, thereby raising the concern for underlying invasive adenocarcinoma.
Conclusions
The presented cases highlight the potential pitfall of lifting agents use mimicking an invasive carcinoma. A confirmation of previously described transmural infiltration is presented (13). To our knowledge, this is first time sub-serosal intravascular involvement is reported. As the use of lifting agents becomes more widespread, we propose the need for clear communication and documentation of their use during endoscopy. It is equally important to recognize the unique characteristics of these lifting agents and their potential to alter surgical management by mimicking invasive adenocarcinoma both clinically and pathologically.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol (Approval # STUDY00001920) and an exemption category 4iii (Waiver of HIPAA Authorization) was provided by the Institutional Review Board (IRB) of The University of Vermont and The University of Vermont Medical Center. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-22-59/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-22-59/coif). The authors have no conflicts of interest to declare.
References
- 1.Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol 2017;31:455-71. 10.1016/j.bpg.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Kim HG, Thosani N, Banerjee S, et al. Effect of prior biopsy sampling, tattoo placement, and snare sampling on endoscopic resection of large nonpedunculated colorectal lesions. Gastrointest Endosc 2015;81:204-13. 10.1016/j.gie.2014.08.038 [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi N, Saito Y, Sano Y, et al. Determining the treatment strategy for colorectal neoplastic lesions: endoscopic assessment or the non-lifting sign for diagnosing invasion depth? Endoscopy 2007;39:701-5. 10.1055/s-2007-966587 [DOI] [PubMed] [Google Scholar]
- 4.Castro R, Libânio D, Pita I, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol 2019;25:777-88. 10.3748/wjg.v25.i7.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotra M, Triadafilopoulos G, Friedland S. Utility and performance characteristics of a novel submucosal injection agent (Eleview(TM)) for endoscopic mucosal resection and endoscopic submucosal dissection. Transl Gastroenterol Hepatol 2018;3:32 . 10.21037/tgh.2018.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex DK, Broadley HM, Garcia JR, et al. SIC-8000 versus hetastarch as a submucosal injection fluid for EMR: a randomized controlled trial. Gastrointest Endosc 2019;90:807-12. 10.1016/j.gie.2019.06.040 [DOI] [PubMed] [Google Scholar]
- 7.Westbrook LM, Henn PA, Cornish TC. Lifting Agent Granuloma: Histologic Findings Following Use of ORISE Gel for Endoscopic Resections in the Gastrointestinal Tract. Am J Clin Pathol 2020;153:630-8. 10.1093/ajcp/aqz204 [DOI] [PubMed] [Google Scholar]
- 8.Castrodad-Rodríguez CA, Panarelli NC, Gersten AJ, et al. Features of endoscopic procedure site reaction associated with a recently approved submucosal lifting agent. Mod Pathol 2020;33:1581-8. 10.1038/s41379-020-0509-0 [DOI] [PubMed] [Google Scholar]
- 9.Sun BL. Submucosal lifting agent ORISE gel causes extensive foreign body granuloma post endoscopic resection. Int J Colorectal Dis 2021;36:419-22. 10.1007/s00384-020-03764-y [DOI] [PubMed] [Google Scholar]
- 10.Olivas AD, Setia N, Weber CR, et al. Histologic changes caused by injection of a novel submucosal lifting agent for endoscopic resection in GI lesions. Gastrointest Endosc 2021;93:470-6. 10.1016/j.gie.2020.06.056 [DOI] [PubMed] [Google Scholar]
- 11.Pezhouh MK, Burgart LJ, Chiu K, et al. Characterization of Novel Injectable Lifting Agents Used in Colonic Polyp Removal: An Emerging Amyloid Mimic. Am J Surg Pathol 2020;44:793-8. 10.1097/PAS.0000000000001435 [DOI] [PubMed] [Google Scholar]
- 12.Cypher L, Sun S, Forster E, et al. Submucosal lifting agent ORISE gel remnants histopathologically mimic mucin and malignancy: a case series. Am J Clin Pathol 2019;152:S73. [Google Scholar]
- 13.Colak Y, Hasan B, Tandon K, et al. Potential clinical complications of Orise™ gel use, a new submucosal lifting agent: experience from a tertiary care center and review of the literature. Ann Gastroenterol 2022;35:407-13. 10.20524/aog.2022.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahr RE, DeWitt JM, Zhang D, et al. Assessment of submucosal distortion and mass effect seen at follow-up after colorectal EMR with ORISE (with video). Gastrointest Endosc 2022;96:679-82. 10.1016/j.gie.2022.04.1344 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as