Key Points
Question
What are the operating characteristics of the CAPTURE ([chronic obstructive pulmonary disease] COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk) screening tool (composed of 5 questions and selective use of peak expiratory flow rate) to identify undiagnosed, clinically significant COPD among US primary care patients?
Findings
In this cross-sectional study of 4325 patients from US primary care settings, the CAPTURE tool demonstrated a sensitivity of 48.2% and a specificity of 88.6% for detecting clinically significant COPD, which was defined by spirometry results showing airflow obstruction of moderate severity or accompanied by a history of acute respiratory illness.
Meaning
In US primary care, the CAPTURE screening tool did not perform well identifying undiagnosed, clinically significant COPD.
Abstract
Importance
Chronic obstructive pulmonary disease (COPD) is underdiagnosed in primary care.
Objective
To evaluate the operating characteristics of the CAPTURE (COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk) screening tool for identifying US primary care patients with undiagnosed, clinically significant COPD.
Design, Setting, and Participants
In this cross-sectional study, 4679 primary care patients aged 45 years to 80 years without a prior COPD diagnosis were enrolled by 7 primary care practice–based research networks across the US between October 12, 2018, and April 1, 2022. The CAPTURE questionnaire responses, peak expiratory flow rate, COPD Assessment Test scores, history of acute respiratory illnesses, demographics, and spirometry results were collected.
Exposure
Undiagnosed COPD.
Main Outcomes and Measures
The primary outcome was the CAPTURE tool’s sensitivity and specificity for identifying patients with undiagnosed, clinically significant COPD. The secondary outcomes included the analyses of varying thresholds for defining a positive screening result for clinically significant COPD. A positive screening result was defined as (1) a CAPTURE questionnaire score of 5 or 6 or (2) a questionnaire score of 2, 3, or 4 together with a peak expiratory flow rate of less than 250 L/min for females or less than 350 L/min for males. Clinically significant COPD was defined as spirometry-defined COPD (postbronchodilator ratio of forced expiratory volume in the first second of expiration [FEV1] to forced vital capacity [FEV1:FVC] <0.70 or prebronchodilator FEV1:FVC <0.65 if postbronchodilator spirometry was not completed) combined with either an FEV1 less than 60% of the predicted value or a self-reported history of an acute respiratory illness within the past 12 months.
Results
Of the 4325 patients who had adequate data for analysis (63.0% were women; the mean age was 61.6 years [SD, 9.1 years]), 44.6% had ever smoked cigarettes, 18.3% reported a prior asthma diagnosis or use of inhaled respiratory medications, 13.2% currently smoked cigarettes, and 10.0% reported at least 1 cardiovascular comorbidity. Among the 110 patients (2.5% of 4325) with undiagnosed, clinically significant COPD, 53 had a positive screening result with a sensitivity of 48.2% (95% CI, 38.6%-57.9%) and a specificity of 88.6% (95% CI, 87.6%-89.6%). The area under the receiver operating curve for varying positive screening thresholds was 0.81 (95% CI, 0.77-0.85).
Conclusions and Relevance
Within this US primary care population, the CAPTURE screening tool had a low sensitivity but a high specificity for identifying clinically significant COPD defined by presence of airflow obstruction that is of moderate severity or accompanied by a history of acute respiratory illness. Further research is needed to optimize performance of the screening tool and to understand whether its use affects clinical outcomes.
This cross-sectional study evaluates the operating characteristics of the CAPTURE screening tool by identifying US primary care patients with undiagnosed, clinically significant COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is an underdiagnosed leading cause of death and morbidity, particularly in primary care settings.1 Undiagnosed patients experience impaired health status and greater risk of acute respiratory events, health care use, and all-cause mortality.2,3 Although there is insufficient evidence to support COPD screening in asymptomatic individuals, identifying patients with respiratory symptoms has been suggested.4
The COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) screening tool uses 5 symptom and exposure questions plus, for a subset of patients, peak expiratory flow rates with sex-specific thresholds to detect patients with undiagnosed COPD who are candidates for immediate therapy, labeled as having clinically significant COPD.5
Based on published recommendations, clinically significant COPD was defined as those who have a postbronchodilator airflow obstruction plus a forced expiratory volume in the first second of expiration (FEV1) less than 60% of the predicted value or a history of acute respiratory illnesses within the past 12 months.6,7 Since development of the screening tool and initiation of the CAPTURE study, the criteria for starting maintenance therapy (the basis for the clinically significant label) evolved to include respiratory symptoms and exacerbation risk independent of the severity of airway obstruction.8
The CAPTURE screening tool exhibited good operating characteristics during initial validation.5 Questions from the CAPTURE screening tool have been tested in global, cross-sectional analyses of select populations,9,10,11 but with limited use of the peak expiratory flow rate component. The current multicenter, cross-sectional study was designed to validate the predictive accuracy of the full tool in identifying patients with previously undiagnosed, clinically significant COPD in a diverse US primary care population.
Methods
Study Design
The full study methods, including those for this cross-sectional data collection, have been described.12 The study protocol and the statistical analysis plan appear in Supplement 1. Patients were enrolled between October 12, 2018, and April 1, 2022, and completed a single study visit at primary care practices within 7 experienced primary care practice–based research networks (eFigure 1 in Supplement 2) with practices that vary in size, rural vs urban location, and patient characteristics (race and ethnicity and socioeconomic status) (eTable 1 in Supplement 2). One hundred practices enrolled patients based on interest and available space to complete the study visit. The study was approved by the Weill Cornell and University of Michigan institutional review boards and by the 7 primary care practice–based research network institutional review boards. All patients provided written informed consent.
Participants
Eligible patients were identified and recruited by either approaching clinic patients on the day of a visit, or by contacting patients with future appointments via US mail, email, or telephone. The method used by each primary care practice–based research network was based on prior experience and requirements of the local clinic, health system, and institutional review board.
Enrolled patients were men and women aged 45 years to 80 years who were able to complete a visit in English or Spanish, were willing to comply with all study procedures, were available for 1-year follow-up, did not have a self-reported COPD diagnosis, and did not report being treated for an acute respiratory illness with antibiotics or systemic steroids within the prior 30 days.12 To ensure safety during spirometry, patients were excluded if they had a self-reported eye, chest, or abdominal procedure or an acute myocardial infarction or stroke within the prior 30 days.
Study Procedures
Enrolled patients provided demographics, medical history, COPD Assessment Test scores (a patient-reported health status measure),13 modified Medical Research Council dyspnea score,14 history of respiratory illnesses within the prior 12 months,15 smoking status and smoking history,16 and current inhaled medication use. Sex, race, and ethnicity were self-selected by patients from a list of fixed categories for the purpose of examining performance of the CAPTURE screening tool by subgroups. Height was self-reported and weight was measured at the study visit.
The questionnaire aspect of the CAPTURE screening tool queried patients on the following concepts: (1) exposure to dirty or polluted air, smoke, secondhand smoke, or dust; (2) breathing changes with seasons, weather, or air quality; (3) breathing difficulty with activity; (4) tiring easily compared with others the same age; and (5) missing work, school, or other activities due to colds, bronchitis, or pneumonia (eFigure 2 in Supplement 2). The questionnaire aspect of the CAPTURE screening tool was self-completed by all patients. Questionnaire scores range from 0 to 6 and a higher score represents greater respiratory exposure, symptoms, or acute respiratory illnesses.
Peak expiratory flow rate was recorded using the asmaPlan (Vitalograph Ltd) mechanical peak expiratory flow rate meter with SafeTway disposable mouthpieces (Vitalograph Ltd). Three peak expiratory flow rate maneuvers were performed, and the highest rate was used to determine the screening results. A positive screening result was defined as (1) a CAPTURE questionnaire score of 5 or 6 or (2) a questionnaire score of 2, 3, or 4 with a peak expiratory flow rate of less than 350 L/min for males or less than 250 L/min for females.5 All other findings were considered negative screening results.
All enrolled patients underwent spirometry following published standards17 using the Easy On-PC spirometer (ndd Medical Technologies Inc) performed by primary care practice–based research network research coordinators trained and certified in spirometry performance. The spirometry results were centrally reviewed for quality and adjudication. The initial spirometry values were considered prebronchodilator unless the participant reported inhaling a bronchodilator within the previous 2 hours (these spirometry values were considered postbronchodilator). For patients with a prebronchodilator ratio of FEV1 to forced vital capacity (FEV1:FVC) less than 0.70 or an FEV1 less than 80% of the predicted value, a postbronchodilator measurement was performed 15 to 20 minutes after inhalation of 2 puffs of albuterol HFA (180 μg) using an AeroChamber Plus Flow-Vu Spacer (AbbVie).
Forced vital capacity and FEV1 were directly measured and the percentages of the predicted values for FEV1 and FVC were calculated using National Health and Nutrition Examination Survey prediction equations18 programmed into the spirometer. For people of multiple races or with unknown race, prediction equations for people of White race were used.
Throughout this report, unless specified otherwise, the FEV1 and FVC values are from postbronchodilator spirometry for patients who completed postbronchodilator spirometry, and from prebronchodilator spirometry for all other patients. Clinically significant COPD was defined as spirometry-defined COPD (postbronchodilator ratio of FEV1:FVC <0.70 or prebronchodilator FEV1:FVC <0.65 if postbronchodilator spirometry was not completed) combined with either an FEV1 less than 60% of the predicted value or a self-reported history of an acute respiratory illness within the past 12 months.
During the COVID-19 pandemic, sites were given an option for parts of the study visit (informed consent and questionnaires other than the CAPTURE screening tool) to be completed remotely.
Outcomes
The primary outcome was the CAPTURE tool’s sensitivity and specificity for identifying patients with undiagnosed, clinically significant COPD.
The prespecified secondary outcomes presented here include (1) the sensitivity and specificity for detecting clinically significant COPD in the subgroups defined by sex, race and ethnicity, rural or urban location, and education level; (2) positive predictive value (PPV) and negative predictive value (NPV) for detecting clinically significant COPD; (3) area under the receiver operating characteristic curve (AUROC) for varying thresholds of the CAPTURE questionnaire score and peak expiratory flow rate for defining a positive screening result for clinically significant COPD; and (4) all of the primary and secondary outcomes evaluated for the detection of spirometry-defined COPD (including COPD that was not clinically significant).
The secondary outcomes not reported here include the primary and secondary outcomes evaluated for the detection of COPD that was not clinically significant, and AUROC for combinations of patient and site characteristics that optimally identify those with clinically significant COPD, spirometry-defined COPD, and COPD that was not clinically significant.
Post hoc exploratory outcomes included (1) sensitivity and specificity for detecting previously undiagnosed patients with clinically significant COPD in subgroups defined by age, body mass index, smoking status, asthma history or inhaled respiratory medication use, cardiac comorbidity, enrollment in a practice with a residency training program, and enrollment in a practice with in-house spirometry; (2) unadjusted and covariate-adjusted associations of patient and site characteristics with screening tool sensitivity and specificity for detecting clinically significant COPD; (3) sensitivity and specificity for detecting clinically significant COPD before and during the COVID-19 pandemic; (4) comparison of patients with clinically significant COPD who had positive vs negative results with the CAPTURE screening tool; (5) comparison of patients without clinically significant COPD who had positive vs negative results with the CAPTURE screening tool; (6) the number needed to screen to identify 1 patient with clinically significant COPD or 1 patient with spirometry-defined COPD; and (7) describing patients with other respiratory conditions related to symptoms or exacerbation risk.
Statistical Analysis
Patients were excluded from the analysis if the data needed for positive or negative screening classification were missing, or if the spirometry did not pass the quality review (Figure 1). In addition, patients were excluded who had only prebronchodilator spirometry results with an FEV1:FVC between 0.65 and 0.70 in which case airflow obstruction was considered indeterminate, and when there was a discrepancy greater than 5% between the predicted FEV1 value calculated by the demographics input on the spirometer and the predicted FEV1 value recalculated centrally using case report form demographics (Figure 1).
Figure 1. Cohort Development and Participant Classification in the CAPTURE Trial.
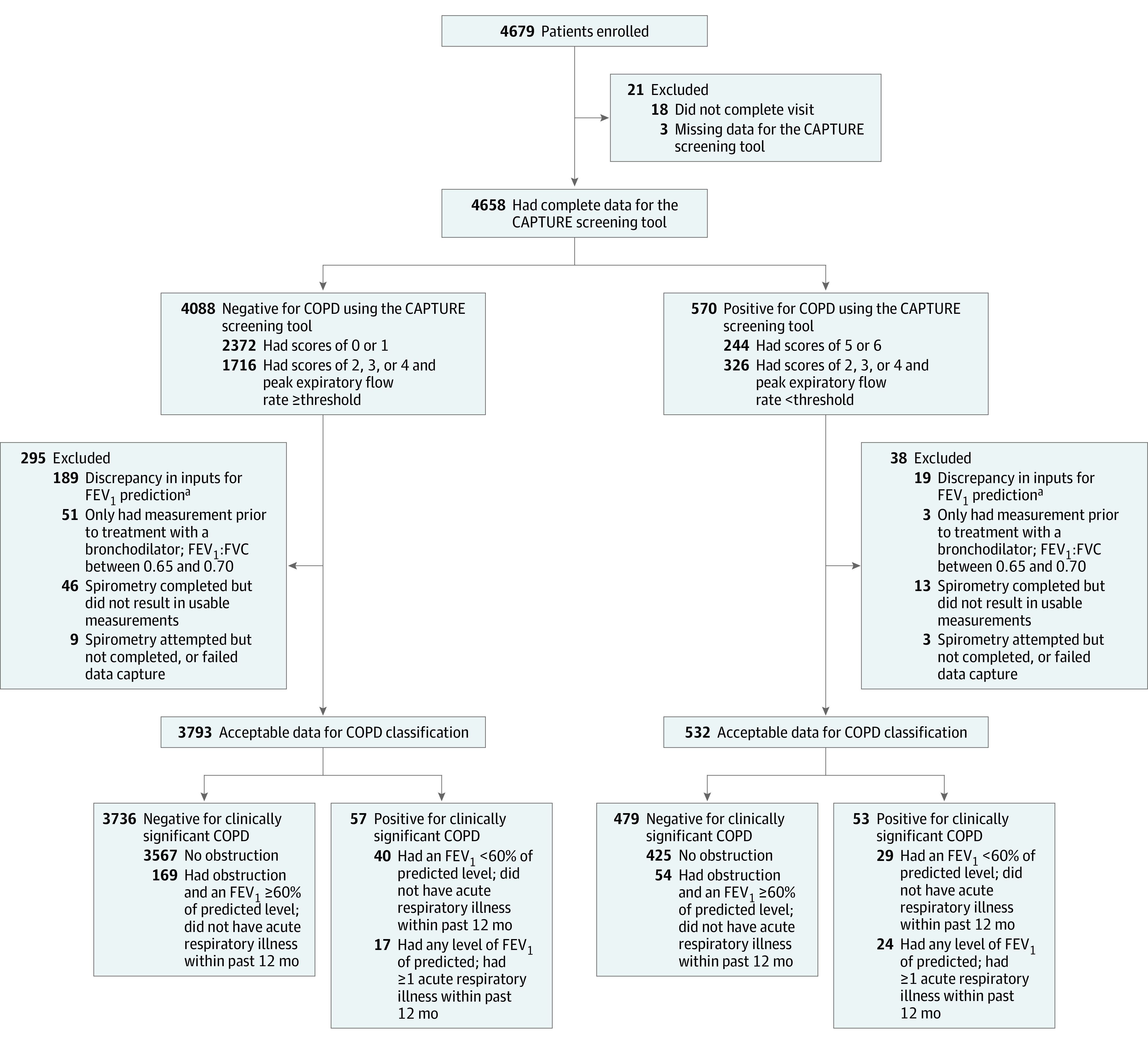
The number of patients approached for enrollment was not available because these data were not collected at all study sites. Not all patients approached were eligible because screening included queries related to self-reported prior chronic obstructive pulmonary disease (COPD) diagnosis, recent myocardial infarction, recent respiratory infection treated with antibiotics, and cataract surgery. CAPTURE indicates COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk; FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity.
aAt analysis time, it was found that FEV1 prediction inputs (sex, race, age, and height) recorded during spirometry sometimes disagreed with the values recorded separately in the study forms, and the correct values could not be verified. Patients with a resulting discrepancy of greater than 5% in the FEV1 predicted value were excluded from the analysis.
Sensitivity, specificity, PPV, and NPV estimates assumed independent Bernoulli outcomes for each patient. All estimates and hypothesis tests assumed independence between patients; there was no adjustment for correlation within study sites. The 95% CIs were calculated using the exact binomial (Clopper-Pearson) method.19 A bootstrap 95% CI was calculated for the AUROC.
All P values were 2-sided and P < .05 was considered statistically significant. P values were not adjusted for multiple testing. Because of the potential for type I error due to multiple testing, findings for the analyses of the secondary outcomes should be interpreted as exploratory. The analyses were performed using R version 4.1 (R Foundation for Statistical Computing)20 and the pROC package.21
Results
Participants
Between October 12, 2018, and April 1, 2022, 4679 patients were enrolled, of whom 354 (7.6%) had inadequate data and were excluded from the analysis of the CAPTURE screening tool’s operating characteristics (Figure 1 and eTable 2 in Supplement 2). Of the 4325 patients with adequate data for analysis, 63.0% were women, the mean age was 61.6 years (SD, 9.1 years), 49.9% had a body mass index of 30 or greater (calculated as weight in kilograms divided by height in meters squared), 44.6% had ever smoked cigarettes, 18.3% reported a prior asthma diagnosis or use of inhaled respiratory medications, 13.2% currently smoked cigarettes, and 10.0% reported at least 1 cardiovascular comorbidity.
Of the 4325 patients, 532 (12.3%) had a positive screening result for clinically significant COPD (Figure 1 and eFigure 3A in Supplement 2). Of these 532 patients with a positive screening result for clinically significant COPD, 233 (43.8%) had the result by CAPTURE questionnaire alone (score of 5 or 6) and 299 (56.2%) had a questionnaire score of 2, 3, or 4 combined with a low peak expiratory flow rate. Of all 4325 screened patients, 43.7% had CAPTURE questionnaire scores of 2, 3, or 4 requiring a peak expiratory flow rate measurement to determine final screening status. Patients with a positive screening result for clinically significant COPD were significantly more likely (P < .001 for all comparisons) than patients with a negative screening result to have asthma or use daily inhaled respiratory medications (43.6% vs 14.8%, respectively), currently smoke (25.1% vs 11.5%), have COPD Assessment Test scores of 10 or greater (77.3% vs 30.6%), and report 1 or more respiratory illnesses within the prior 12 months (24.7% vs 7.2%) (Table 1 and Table 2).
Table 1. Demographics, Medical History, and Medication Use of Enrolled Patients by Screening Result and Chronic Obstructive Pulmonary Disease (COPD) Status.
| Characteristic | Overall (N = 4325)a | No clinically significant COPD (n = 4215)a,b | Clinically significant COPD (n = 110)b | |||
|---|---|---|---|---|---|---|
| Negative screening result (n = 3793) | Positive screening result (n = 532) | Negative screening result (n = 3736) | Positive screening result (n = 479) | Negative screening result (n = 57)a | Positive screening result (n = 53)c | |
| Demographics | ||||||
| Age, mean (SD), y | 61.6 (9.1) | 61.4 (9.3) | 61.6 (9.1) | 61.1 (9.2) | 65.3 (8.6) | 64.1 (9.8) |
| Sex, No. (%)d | ||||||
| Female | 2373 (62.6) | 352 (66.2) | 2350 (62.9) | 322 (67.2) | 23 (40.4) | 30 (56.6) |
| Male | 1420 (37.4) | 180 (33.8) | 1386 (37.1) | 157 (32.8) | 34 (59.6) | 23 (43.4) |
| Race, No. (%) | ||||||
| Black or African American | 972 (25.6) | 192 (36.1) | 955 (25.6) | 176 (36.7) | 17 (29.8) | 16 (30.2) |
| White | 2415 (63.7) | 274 (51.5) | 2381 (63.8) | 241 (50.3) | 34 (59.6) | 33 (62.3) |
| Do not know or prefer not to answer | 255 (6.7) | 32 (6.0) | 253 (6.8) | 30 (6.3) | 2 (3.5) | 2 (3.8) |
| Othere | 148 (3.9) | 34 (6.4) | 144 (3.9) | 32 (6.7) | 4 (7.0) | 2 (3.8) |
| Ethnicity, No. (%) | ||||||
| Hispanic or Latino | 504 (13.3) | 49 (9.2) | 501 (13.4) | 46 (9.6) | 3 (5.3) | 3 (5.7) |
| Non-Hispanic or Non-Latino | 3196 (84.3) | 458 (86.3) | 3142 (84.2) | 409 (85.6) | 54 (94.7) | 49 (92.5) |
| Do not know or prefer not to answer | 90 (2.4) | 24 (4.5) | 90 (2.4) | 23 (4.8) | 0 | 1 (1.9) |
| Body mass index ≥30, No. (%)f | 1856 (48.9) | 303 (57.0) | 1832 (49.0) | 275 (57.4) | 24 (42.1) | 28 (52.8) |
| Education, No. (%) | ||||||
| Less than high school | 327 (8.6) | 86 (16.2) | 320 (8.6) | 78 (16.3) | 7 (12.3) | 8 (15.1) |
| High school or GED | 837 (22.1) | 157 (29.5) | 827 (22.2) | 138 (28.8) | 10 (17.5) | 19 (35.8) |
| Vocational school or some college | 914 (24.2) | 148 (27.8) | 900 (24.2) | 136 (28.4) | 14 (24.6) | 12 (22.6) |
| College degree | 1039 (27.5) | 101 (19.0) | 1024 (27.5) | 94 (19.6) | 15 (26.3) | 7 (13.2) |
| Professional or graduate degree | 664 (17.6) | 40 (7.5) | 653 (17.5) | 33 (6.9) | 11 (19.3) | 7 (13.2) |
| Employment, No. (%) | ||||||
| Working part-time or full-time | 1753 (46.3) | 182 (34.3) | 1736 (46.5) | 165 (34.5) | 17 (29.8) | 17 (32.7) |
| Not working for reason other than disability | 1644 (43.4) | 214 (40.4) | 1609 (43.1) | 187 (39.1) | 35 (61.4) | 27 (51.9) |
| Not working due to disability | 390 (10.3) | 134 (25.3) | 385 (10.3) | 126 (26.4) | 5 (8.8) | 8 (15.4) |
| Health insurance, No. (%) | ||||||
| Private only | 1444 (38.1) | 120 (22.6) | 1431 (38.3) | 112 (23.4) | 13 (22.8) | 8 (15.1) |
| Public only | 1320 (34.8) | 267 (50.2) | 1294 (34.6) | 241 (50.3) | 26 (45.6) | 26 (49.1) |
| Both public and private | 870 (22.9) | 111 (20.9) | 853 (22.8) | 95 (19.8) | 17 (29.8) | 16 (30.2) |
| None | 110 (2.9) | 22 (4.1) | 109 (2.9) | 19 (4.0) | 1 (1.8) | 3 (5.7) |
| Other or do not know | 49 (1.3) | 12 (2.3) | 49 (1.3) | 12 (2.5) | 0 | 0 |
| Medical history and medication use | ||||||
| Asthma history and respiratory medication use, No. (%) | ||||||
| No asthma history | 3350 (88.4) | 370 (69.8) | 3312 (88.8) | 340 (71.1) | 38 (66.7) | 30 (57.7) |
| Not using respiratory medications | 3228 (85.2) | 299 (56.4) | 3197 (85.7) | 278 (58.2) | 31 (54.4) | 21 (40.4) |
| Using ≥1 respiratory medications | 122 (3.2) | 71 (13.4) | 115 (3.1) | 62 (13.0) | 7 (12.3) | 9 (17.3) |
| Asthma history | 438 (11.6) | 161 (30.3) | 419 (11.2) | 138 (28.9) | 19 (33.3) | 22 (42.3) |
| Not using any respiratory medications | 174 (4.6) | 41 (7.7) | 171 (4.6) | 37 (7.7) | 3 (5.3) | 4 (7.7) |
| Using short-acting bronchodilator | 126 (3.3) | 47 (8.9) | 120 (3.2) | 41 (8.6) | 6 (10.5) | 6 (11.5) |
| Using daily inhaled respiratory medication | 138 (3.6) | 72 (13.6) | 128 (3.4) | 60 (12.6) | 10 (17.5) | 12 (23.1) |
| Cardiac comorbidity, No. (%)g | 332 (8.8) | 98 (18.5) | 317 (8.5) | 86 (18.0) | 15 (26.3) | 12 (23.1) |
| Cigarette smoking, No. (%) | ||||||
| Never | 2178 (57.5) | 213 (40.2) | 2158 (57.8) | 194 (40.6) | 20 (35.1) | 19 (36.5) |
| Former | 1175 (31.0) | 184 (34.7) | 1150 (30.8) | 162 (33.9) | 25 (43.9) | 22 (42.3) |
| Current | 435 (11.5) | 133 (25.1) | 423 (11.3) | 122 (25.5) | 12 (21.1) | 11 (21.2) |
| Short-acting bronchodilator, No. (%) | 323 (8.5) | 170 (32.0) | 304 (8.1) | 144 (30.1) | 19 (33.3) | 26 (49.1) |
| Respiratory maintenance, No. (%) | ||||||
| None | 3613 (95.3) | 434 (81.7) | 3570 (95.6) | 396 (82.8) | 43 (75.4) | 38 (71.7) |
| Long-acting β-agonist and inhaled corticosteroid | 111 (2.9) | 55 (10.4) | 100 (2.7) | 48 (10.0) | 11 (19.3) | 7 (13.2) |
| Inhaled corticosteroid alone | 57 (1.5) | 29 (5.5) | 55 (1.5) | 26 (5.4) | 2 (3.5) | 3 (5.7) |
| Other combination of inhaled corticosteroid, long-acting β-agonist, and long-acting muscarinic agonist | 10 (0.3) | 13 (2.4) | 9 (0.2) | 8 (1.7) | 1 (1.8) | 5 (9.4) |
Abbreviation: GED, General Educational Development test (a high school equivalency credential).
Data were missing for less than 0.5% of patients in each column.
COPD was defined by a postbronchodilator ratio of forced expiratory volume in the first second of expiration to forced vital capacity (FEV1:FVC) less than 0.70 or a prebronchodilator FEV1:FVC less than 0.65 in patients who did not complete postbronchodilator spirometry, and was considered clinically significant if the patient also had either 1 or more acute respiratory illnesses within the past 12 months or an FEV1 less than 60% of the predicted value.
Data were missing for 1 patient in this column.
Selected by patients from a list of fixed categories (male or female).
American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or multiple race categories.
Calculated as weight in kilograms divided by height in meters squared.
Angina (heart or chest pain), coronary artery disease, myocardial infarction, coronary artery bypass graft surgery, angioplasty or cardiac stents, or congestive heart failure.
Table 2. Symptom and Physiological Characterization of Enrolled Patients by Screening Result and Chronic Obstructive Pulmonary Disease (COPD) Status.
| Overall (N = 4325)a | No clinically significant COPD (n = 4215)a,b | Clinically significant COPD (n = 110)a,b | ||||
|---|---|---|---|---|---|---|
| Negative screening result (n = 3793) | Positive screening result (n = 532) | Negative screening result (n = 3736) | Positive screening result (n = 479) | Negative screening result (n = 57) | Positive screening result (n = 53) | |
| COPD Assessment Testc | ||||||
| Score, mean (SD) | 7.6 (6.0) | 16.2 (8.1) | 7.6 (6.0) | 16.2 (8.2) | 10.3 (5.9) | 16.0 (7.9) |
| Score ≥10, No. (%) | 1159 (30.6) | 411 (77.3) | 1131 (30.3) | 368 (76.8) | 28 (49.1) | 43 (81.1) |
| Walks slower than others or stops to catch breath (modified Medical Research Council dyspnea score ≥2), No. (%)d | 417 (11.0) | 234 (44.1) | 405 (10.9) | 217 (45.4) | 12 (21.1) | 17 (32.1) |
| Acute respiratory illness within past 12 mo, No. (%)e | 274 (7.2) | 131 (24.7) | 257 (6.9) | 107 (22.4) | 17 (29.8) | 24 (45.3) |
| FEV1:FVC, mean (SD), %f | 78.7 (6.2) | 75.3 (9.1) | 79.0 (5.7) | 77.1 (7.0) | 60.0 (7.9) | 59.0 (9.8) |
| FVC, mean (SD), % of predicted valuef | 89.8 (14.8) | 80.7 (16.4) | 90.0 (14.6) | 81.6 (16.6) | 73.5 (16.3) | 73.3 (12.7) |
| FEV1, mean (SD), % of predicted valuef | 91.5 (15.6) | 78.3 (17.4) | 92.0 (15.0) | 80.7 (16.1) | 57.7 (14.6) | 56.3 (12.6) |
| Disease classification, No. (%) | ||||||
| No COPD | 3567 (94.0) | 425 (79.9) | 3567 (95.5) | 425 (88.7) | ||
| COPD that was not clinically significant | 169 (4.5) | 54 (10.2) | 169 (4.5) | 54 (11.3) | ||
| Clinically significant COPD | 57 (1.5) | 53 (10.0) | 57 (100) | 53 (100) | ||
| GOLD classification, No. (%)g | ||||||
| No COPD | 3567 (94.0) | 425 (79.9) | 3567 (95.5) | 425 (88.7) | ||
| Group A (COPD Assessment Test score <10 and no or a mild exacerbation history) | 146 (3.8) | 28 (5.3) | 120 (3.2) | 18 (3.8) | 26 (45.6) | 10 (18.9) |
| Group B (COPD Assessment Test score ≥10 and no or a mild exacerbation history) | 72 (1.9) | 66 (12.4) | 49 (1.3) | 36 (7.5) | 23 (40.4) | 30 (56.6) |
| Group C (COPD Assessment Test score <10 and a moderate or severe exacerbation history) | 3 (0.1) | 0 | 3 (5.3) | 0 | ||
| Group D (COPD Assessment Test score ≥10 and a moderate or severe exacerbation history) | 5 (0.1) | 13 (2.4) | 5 (8.8) | 13 (24.5) | ||
| Post hoc classification based on spirometry results and respiratory symptom assessment, No. (%) | ||||||
| A normal spirometry resulth and COPD Assessment Test score <10 | 2147 (56.6) | 60 (11.3) | 2147 (57.5) | 60 (12.5) | ||
| A normal spirometry result, COPD Assessment Test score ≥10, and never smoked | 418 (11.0) | 76 (14.3) | 418 (11.2) | 76 (15.9) | ||
| A normal spirometry result, COPD Assessment Test score ≥10, and formerly or currently smoked | 409 (10.8) | 109 (20.5) | 409 (11.0) | 109 (22.8) | ||
| Preserved ratio impaired spirometryi | 591 (15.6) | 180 (33.8) | 591 (15.8) | 180 (37.6) | ||
| Any spirometry-defined COPD | 226 (6.0) | 107 (20.1) | 169 (4.5) | 54 (11.3) | 57 (100) | 53 (100) |
Data were missing for less than 0.5% of patients in each column.
COPD was defined by a postbronchodilator ratio of forced expiratory volume in the first second of expiration to forced vital capacity (FEV1:FVC) less than 0.70 or a prebronchodilator FEV1:FVC less than 0.65 in patients who did not complete postbronchodilator spirometry, and was considered clinically significant if the patient also had either 1 or more acute respiratory illnesses within the past 12 months or an FEV1 less than 60% of the predicted value.
Scores range from 0 to 40; higher scores indicate worse symptoms. A score of 10 or greater represents significant respiratory symptom burden.8
Scores range from 0 to 4; higher scores indicate worse breathlessness.
Patients were asked, “Over the last 12 months, have you had episodes of chest troubles (cough, phlegm, or shortness of breath) requiring treatment with antibiotics and/or steroids?”
Measured postbronchodilator for patients who completed postbronchodilator spirometry. For all others, it was measured prebronchodilator.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) groupings are based on published standards.8
No COPD and FEV1 was 80% or greater of the predicted value.
No COPD and FEV1 was less than 80% of the predicted value.
Of the 4325 patients, 333 (7.7%) had spirometry-defined COPD (110 [2.5%] had clinically significant COPD and 223 [5.2%] had COPD that was not clinically significant; eFigure 3B in Supplement 2). Of the 333 patients with spirometry-defined COPD, 174 (52.3%) were in Global Initiative for Chronic Obstructive Lung Disease8 (GOLD) group A (had less severe symptoms defined by a COPD Assessment Test score <10 and no or a mild exacerbation history within the prior year), 138 (41.4%) were in group B (had more severe symptoms defined by a COPD Assessment Test score ≥10 and no or a mild exacerbation history within the prior year), 3 (0.9%) were in group C (had less severe symptoms defined by a COPD Assessment Test score <10 and a moderate or severe exacerbation history within the prior year), and 18 (5.4%) were in group D (had more severe symptoms defined by a COPD Assessment Test score ≥10 and a moderate or severe exacerbation history within the prior year).
Of the 110 patients with clinically significant COPD, 41 (37.3%) had at least 1 acute respiratory illness within the past 12 months and 69 (62.7%) had no acute respiratory illness within the past 12 months and satisfied the definition of clinical significance based only on airflow obstruction with an FEV1 less than 60% of the predicted value. Nearly two-thirds (n = 71, 64.5%) of the 110 with clinically significant COPD had a COPD Assessment Test score of 10 or greater; 36 (32.7%) were categorized as being in GOLD group A; 53 (48.2%) in GOLD group B; 3 in GOLD group C (2.7%); and 18 (16.4%) in GOLD group D.
Primary Outcome
Of the 110 patients (2.5% of 4325) with undiagnosed, clinically significant COPD, 53 had a positive screening result with a sensitivity of 48.2% (95% CI, 38.6%-57.9%) and a specificity of 88.6% (95% CI, 87.6%-89.6%).
Secondary Outcomes
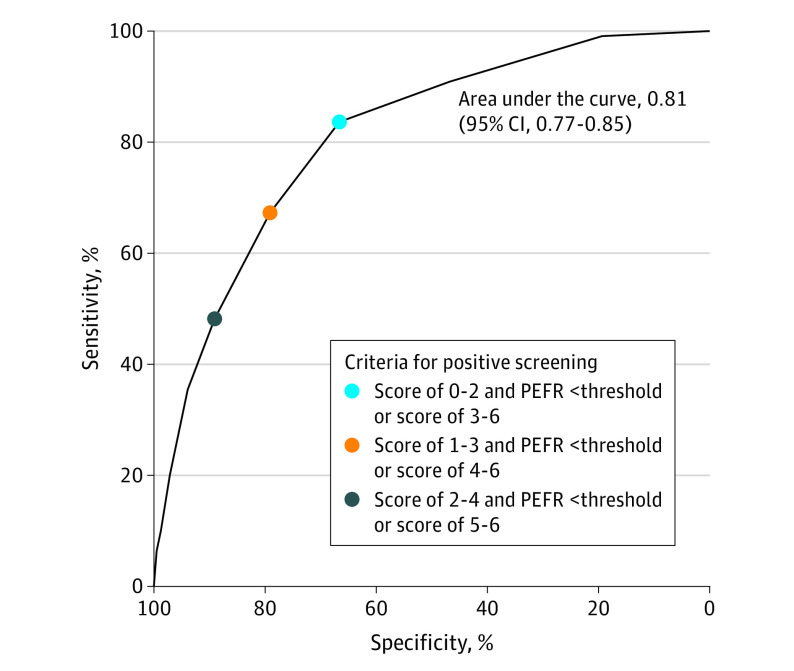
The PPV for clinically significant COPD was 10.0% (95% CI, 7.6%-12.8%) and the NPV was 98.5% (95% CI, 98.1%-98.9%). The results for sensitivity, specificity, PPV, and NPV among the prespecified subgroups appear in eFigures 4-5 in Supplement 2. The sensitivity and specificity for identifying clinically significant COPD are illustrated in Figure 2 using alternative thresholds for the CAPTURE questionnaire score to define, in combination with peak expiratory flow rate for intermediate scores, a positive screening result (AUROC, 0.81; 95% CI, 0.77-0.85). For example, improving sensitivity to 67.3% (while lowering specificity to 79.1%) could be accomplished by defining a positive screening result as a CAPTURE questionnaire score of 4, 5, or 6 or a questionnaire score of 1, 2, or 3 with a peak expiratory flow rate below the sex-specific threshold (eTable 3 in Supplement 2). The AUROCs for the CAPTURE questionnaire score and peak expiratory flow rate are shown separately in eFigure 6 in Supplement 2.
Figure 2. Receiver Operating Characteristic Curve for Detecting Clinically Significant Chronic Obstructive Pulmonary Disease (COPD) Under Alternative Definitions of a Positive Screening Result.
The curve was obtained by shifting the COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) questionnaire score thresholds that determine who is positive or negative for clinically significant COPD based on the questionnaire alone, and who requires peak expiratory flow rate (PEFR) to determine positivity. The PEFR thresholds of 350 L/min for males and 250 L/min for females were used throughout. Three of the points on the curve are labeled to illustrate how the criteria for CAPTURE positivity shift along the curve. Sensitivity indicates the probability of meeting defined screen-positive criteria in those with clinically significant COPD. Specificity indicates the probability of not meeting defined screen-positive criteria in those without clinically significant COPD.
The sensitivity of the CAPTURE screening tool for spirometry-defined COPD was 32.1% (95% CI, 27.1%-37.4%) and the specificity was 89.4% (95% CI, 88.4%-90.3%). The values for the prespecified subgroups are illustrated in eFigure 7 in Supplement 2. The sensitivity and specificity for identifying spirometry-defined COPD across a range of questionnaire score thresholds combined with peak expiratory flow rate to define a positive screening result had an AUROC of 0.69 (95% CI, 0.66-0.72; eFigure 8A in Supplement 2). The sensitivity and specificity of the CAPTURE questionnaire alone and by peak expiratory flow rate alone are illustrated in eFigure 8 in Supplement 2. The PPV of the CAPTURE screening tool for detecting any spirometry-defined COPD was 20.1% (95% CI, 16.8%-23.8%) and the NPV was 94.0% (95% CI, 93.2%-94.8%).
Post Hoc Outcomes
The sensitivity and specificity for detecting clinically significant COPD in additional subgroups appear in eFigure 9 in Supplement 2. The subgroup differences in sensitivity and specificity were not explained by age, sex, or smoking status (eFigure 10 and eTable 4 in Supplement 2).
Comparing operating characteristics for those who were enrolled prior to onset of the COVID-19 pandemic in the US (March 2020) and after resumption of enrollment in the CAPTURE study (August 2020), the sensitivity dropped significantly (P = .02) from 54.1% (95% CI, 43.0%-65.0%; 46 of 85 patients) to 28.0% (95% CI, 12.1%-49.4%; 7 of 25 patients), whereas the specificity significantly increased (P < .001) from 86.3% (95% CI, 85.0%-87.6%; 2351 of 2724 patients) to 92.9% (95% CI, 91.5%-94.1%; 1385 of 1491 patients).
Among patients without a prior asthma diagnosis or use of daily inhaled respiratory medicines, the sensitivity was 40.4% (95% CI, 27.0%-54.9%; 21 of 52 patients), the specificity was 92.0% (95% CI, 91.0%-92.9%; 3197 of 3475 patients), the PPV was 7.0% (95% CI. 4.4%-10.5%; 21 of 299 patients), and the NPV was 99.0% (95% CI, 98.6%-99.3; 3197 of 3228 patients). The difference in sensitivity was not statistically significant (P = .15) between patients with an asthma history and use of daily inhaled respiratory medications and those without such a history; the difference in specificity was significant (P < .001). Under a range of varying questionnaire thresholds combined with peak expiratory flow rate to define a positive screening result in the group without asthma or use of daily inhaled respiratory medications, the AUROC was 0.82 (95% CI, 0.77-0.88).
Among the patients with clinically significant COPD, those with a positive screening result compared with those with a negative screening result had a higher proportion with COPD Assessment Test scores of 10 or greater (81.1% vs 49.1%, respectively; P < .001), and a higher proportion in the GOLD B, C, and D groups (81.1% vs 54.4%; P = .002) (Table 2). The mean FEV1 percentage of the predicted value was not significantly different (P = .70) in the patients with a positive screening result for clinically significant COPD (mean, 56.3 [SD, 12.6]) compared with in the patients with a negative screening result (mean, 57.7 [SD, 14.6]). Of the patients with a positive screening result, 10.0% had clinically significant COPD compared with 1.5% of patients with a negative screening result (Figure 3 and eFigure 3C-D in Supplement 2). Another 10.2% of patients with a positive screening result had spirometry-defined COPD but not clinically significant COPD (3.4% in GOLD group A and 6.8% in GOLD group B) compared with 4.5% of patients with a negative screening result (3.2% in GOLD group A and 1.3% in GOLD group B).
Figure 3. Spirometry- and Symptom-Based Classification of Patients With a Positive or Negative Screening Result for Detecting Clinically Significant Chronic Obstructive Pulmonary Disease (COPD).
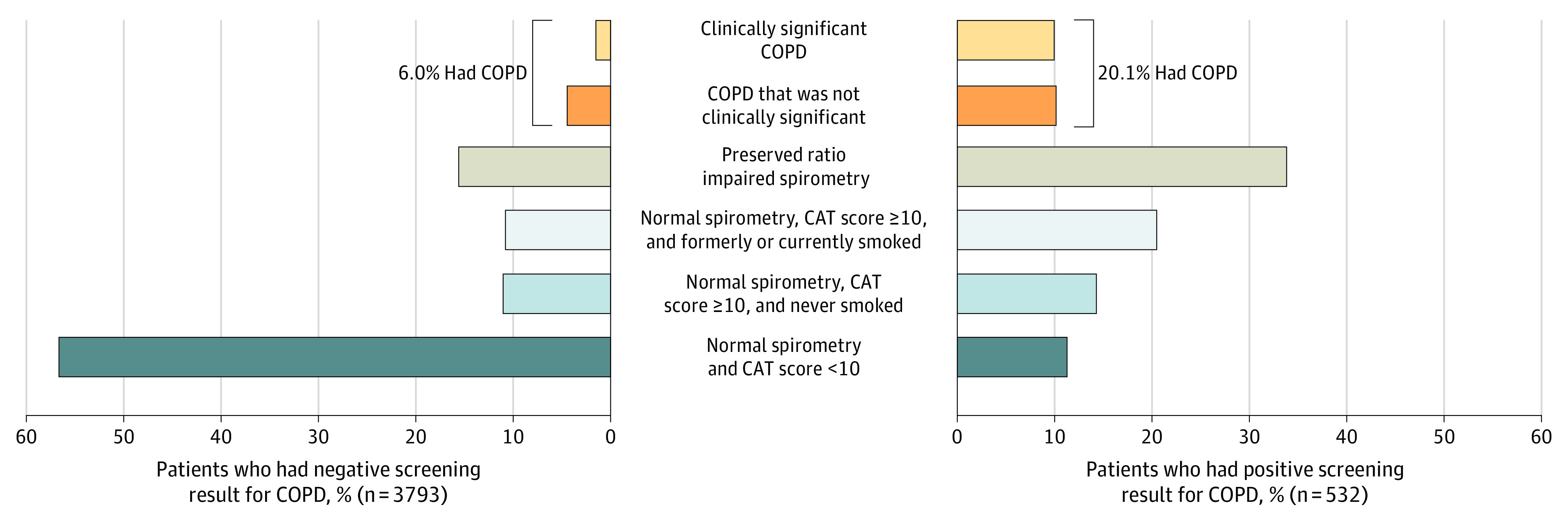
COPD was defined a postbronchodilator ratio of forced expiratory volume in the first second of expiration to forced vital capacity (FEV1:FVC) less than 0.70 or a prebronchodilator FEV1:FVC less than 0.65 in patients who did not complete postbronchodilator spirometry, and was considered clinically significant if the patient also had either 1 or more acute respiratory illnesses within the past 12 months or an FEV1 less than 60% of the predicted value. Clinically significant COPD was measured using spirometry with an FEV1 less than 60% of the predicted value or a history of acute respiratory illness within the past 12 months. A preserved ratio impaired spirometry result indicates no spirometry-defined COPD and an FEV1 less than 80% of the predicted value. A normal spirometry result indicates no spirometry-defined COPD or a preserved ratio impaired spirometry result. CAT indicates COPD Assessment Test.
Patients with a positive screening result but without clinically significant COPD were significantly (P < .001 for all comparisons) more likely to self-report race as Black or African American (36.7% vs 25.6% of patients with a negative screening result), have attained lower education status (45.1% vs 30.8%), be unemployed due to disability (26.4% vs 10.3%), receive public insurance (70.1% vs 57.5%), report cardiac comorbidity (18.0% vs 8.5%), report a history of smoking (59.4% vs 42.2%), use inhaled respiratory medications (30.1% used short-acting bronchodilator vs 8.1% and 17.2% used daily maintenance medication vs 4.4%), had a COPD Assessment Test score of 10 or greater (76.8% vs 30.3%), report a modified Medical Research Council dyspnea score of 2 or greater (45.4% vs 10.9%), report acute respiratory illnesses within the past 12 months (22.4% vs 6.9%), and exhibit a lower FEV1 percentage of the predicted value (mean, 80.7 [SD, 16.1] vs 92.0 [SD, 15.0]; Table 1 and Table 2).
In this primary care population, 4325 patients were screened to identify 53 patients with clinically significant COPD (number needed to screen, 81.6) and to identify 107 patients with spirometry-defined COPD (number needed to screen, 40.4).
Among the 4215 patients without clinically significant COPD, 771 (18.3%) had a preserved ratio impaired spirometry result, which was defined as FEV1:FVC of 0.70 or greater with an FEV1 less than 80% of the predicted value22 (Figure 3 and eFigure 3C-D in Supplement 2). Of the 4215 patients, an additional 518 (12.3%) were symptomatic for COPD (COPD Assessment Test score ≥10) with status as currently or formerly smoking cigarettes but with a normal spirometry result.23 Among patients with a positive screening result, 88.7% had either COPD, a preserved ratio impaired spirometry result, or were symptomatic but with a normal spirometry result compared with 43.4% of patients with a negative screening result.
Discussion
The CAPTURE screening tool, which combined questions about respiratory exposure, symptoms, and acute respiratory illnesses with selective use of peak expiratory flow rate and predefined thresholds for a positive screening result within a broad US primary care population, had a low sensitivity but a high specificity for identifying clinically significant COPD (defined as airflow obstruction plus either an FEV1 <60% of the predicted value or had ≥1 respiratory illnesses within the past 12 months). Sensitivity was influenced by the high proportion of individuals with airflow obstruction, but limited respiratory symptoms; specificity was influenced by high prevalence of respiratory symptoms among those with a normal spirometry result. Further research is needed to potentially optimize performance of the screening tool and to understand whether its use affects clinical outcomes.
Overall, this study demonstrates the challenge of identifying undiagnosed patients with COPD in primary care. The definition of clinically significant COPD used to develop the CAPTURE screening tool was based on published therapeutic recommendations that focused on patients with at least moderate airflow obstruction or the presence of acute respiratory illnesses within the past 12 months.6,7 The CAPTURE screening tool exhibited low sensitivity to identify these patients. Since the design and initiation of the CAPTURE study, the criteria for initiating maintenance therapy (the basis for the definition of clinically significant COPD) evolved to emphasize respiratory symptoms and exacerbation risk. The GOLD therapeutic strategy8 recommends maintenance pharmacotherapy for patients meeting criteria in 3 of the GOLD multidimensional categories (groups B-D) based on symptoms (COPD Assessment Test score or modified Medical Research Council dyspnea score) and prior exacerbation history, but not severity of airflow obstruction. Patients with a positive screening result and previously undiagnosed COPD exhibited more respiratory symptoms and a greater likelihood of acute respiratory illnesses within the prior year, and there was a greater proportion who had spirometry-defined COPD characterized by GOLD groups B, C, and D.
Improving the sensitivity of the CAPTURE screening tool requires multiple considerations. Balancing sensitivity and specificity is important to maintaining PPV and NPV while considering implementation burden. Improving sensitivity using the current questions would require more assessments of peak expiratory flow rate and more referrals for spirometry evaluation, which may introduce challenges in implementation of the screening tool in primary care. Another approach would be to consider using an alternate definition of clinically significant COPD that would be congruent with the current GOLD recommendations8 for treatment initiation. Evaluation is ongoing to optimize the approach and composition of the CAPTURE screening tool’s questions.
The sensitivity of the CAPTURE screening tool for identifying clinically significant COPD was higher (though without statistical significance), and the specificity was lower among those with self-reported prior asthma diagnoses and use of daily inhaled respiratory medications. Other research has reported mislabeling of COPD as asthma in primary care practices.24 A portion of patients self-labeled as having asthma or using inhaled medications did have airflow obstruction, suggesting they could be candidates for reevaluation of their respiratory diagnosis. The sensitivity of the CAPTURE screening tool was higher and the specificity was lower in patients enrolled before the COVID-19 pandemic compared with those enrolled later. In addition, there were differences in race and educational and socioeconomic status in patients with a positive screening result vs those with a negative screening result that highlight potential continuing inequities in respiratory symptom burden as well its recognition in primary care.25,26,27,28
Patients with a positive screening result included individuals with a variety of alternate respiratory diagnoses. Patients with a preserved ratio impaired spirometry result, and those who are symptomatic but have a normal spirometry result, have been shown to be at risk for health care use and mortality.23,29,30,31 Another smaller percentage of patients exhibited airflow obstruction, but did not meet the predefined criteria for clinically significant COPD. Given the level of respiratory symptoms and respiratory illnesses or exacerbation-like events in these patients with a positive screening result, additional diagnostic evaluation may be warranted to identify patients with undiagnosed COPD for whom available or future therapeutic strategies are appropriate.
There have been many attempts at devising innovative screening or case finding tools to identify undiagnosed COPD.32,33 Many of these attempts have been limited by their narrow scope in high-risk populations.34 The CAPTURE screening tool was specifically developed for use in general primary care populations. The operating characteristics of the screening tool in the current study may be compared with those found for identifying GOLD groups8 B, C, and D in 3 low- and middle-income countries where those with prior COPD were not excluded from screening.10 The sensitivity and specificity in the current study were numerically not different from those found in Peru and Uganda, whereas the sensitivity was lower in the current study and the specificity was higher than in Nepal.
Sensitivity and specificity were lower in the current study than in the original CAPTURE validation study,5 which likely reflects a difference in the patient populations between the studies. The current study enrolled a broader, primary care population and excluded patients with known COPD diagnoses, whereas the original CAPTURE study enrolled patients already diagnosed with COPD; such patients were likely more symptomatic than patients with undiagnosed COPD.
Limitations
This study has limitations. First, the screening tool results reflect operating characteristics in a regionally diverse US-based primary care cohort which, although broad, may not reflect the whole US population.
Second, prior diagnoses of COPD and asthma, regular use of inhaled medications, and respiratory illnesses within the prior 12 months were all self-reported by the patients (self-reported exacerbations have previously been shown to be accurate35).
Third, the definition for clinically significant COPD used in the design of the CAPTURE screening tool reflects older criteria that focused on the severity of airflow obstruction and prior respiratory illnesses. More recent therapeutic strategies are based on symptoms and respiratory illness.8
Conclusions
Within this US primary care population, the CAPTURE screening tool had a low sensitivity but a high specificity for identifying clinically significant COPD defined by presence of airflow obstruction that is of moderate severity or accompanied by a history of acute respiratory illness. Further research is needed to optimize performance of the screening tool and to understand whether its use affects clinical outcomes.
Trial protocol and statistical analysis plan
eAbbreviations
eFigure 1. Geographic distribution of participating PBRNs
eFigure 2. The CAPTURE screening tool
eFigure 3. Screening results and classification of patients based on spirometry and symptom assessment
eFigure 4. Sensitivity and specificity of the screening tool for clinically significant COPD in pre-specified subgroups
eFigure 5. Positive and negative predictive value of the screening tool for clinically significant COPD in pre-specified subgroups
eFigure 6. ROC curves for varying thresholds of the CAPTURE questionnaire score and PEFR separately to detect clinically significant COPD
eFigure 7. Sensitivity and specificity of the screening tool for spirometry-defined COPD in pre-specified subgroups
eFigure 8. ROC curves for varying thresholds defining a positive screen to detect spirometry-defined COPD
eFigure 9. Sensitivity and specificity of the screening tool for clinically significant COPD in additional subgroups
eFigure 10. Unadjusted and covariate-adjusted associations of patient and site characteristics with screening tool sensitivity and specificity for clinically significant COPD
eTable 1. Characteristics of participating practices
eTable 2. Characteristics of excluded and included patients
eTable 3. Operating characteristics of the screening tool for detecting clinically significant COPD across a range of thresholds defining a positive screen
eTable 4. Unadjusted and covariate-adjusted associations of patient and site characteristics with screening tool sensitivity and specificity for clinically significant COPD
eReference
Nonauthor collaborators
Data sharing statement
References
- 1.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(5)(suppl):121S-126S. doi: 10.1378/chest.121.5_suppl.121S [DOI] [PubMed] [Google Scholar]
- 2.Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed obstructive lung disease in the United States: associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12(12):1788-1795. doi: 10.1513/AnnalsATS.201506-388OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863-868. doi: 10.1136/thx.2009.115725 [DOI] [PubMed] [Google Scholar]
- 4.Mangione CM, Barry MJ, Nicholson WK, et al. ; US Preventive Services Task Force . Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2022;327(18):1806-1811. doi: 10.1001/jama.2022.5692 [DOI] [PubMed] [Google Scholar]
- 5.Martinez FJ, Mannino D, Leidy NK, et al. ; High-Risk-COPD Screening Study Group . A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(6):748-756. doi: 10.1164/rccm.201603-0622OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qaseem A, Wilt TJ, Weinberger SE, et al. ; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society . Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191. doi: 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]
- 7.Rennard S, Thomashow B, Crapo J, et al. Introducing the COPD Foundation Guide for Diagnosis and Management of COPD, recommendations of the COPD Foundation. COPD. 2013;10(3):378-389. doi: 10.3109/15412555.2013.801309 [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease (GOLD) science committee . The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. Accessed April 25, 2022. https://goldcopd.org
- 9.Pan Z, Dickens AP, Chi C, et al. Accuracy and cost-effectiveness of different screening strategies for identifying undiagnosed COPD among primary care patients (≥40 years) in China: a cross-sectional screening test accuracy study: findings from the Breathe Well group. BMJ Open. 2021;11(9):e051811. doi: 10.1136/bmjopen-2021-051811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddharthan T, Pollard SL, Quaderi SA, et al. ; GECo Study Investigators . Discriminative accuracy of chronic obstructive pulmonary disease screening instruments in 3 low- and middle-income country settings. JAMA. 2022;327(2):151-160. doi: 10.1001/jama.2021.23065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamaki K, Sakihara E, Miyata H, et al. Utility of self-administered questionnaires for identifying individuals at risk of COPD in Japan: the OCEAN (Okinawa COPD casE finding AssessmeNt) study. Int J Chron Obstruct Pulmon Dis. 2021;16:1771-1782. doi: 10.2147/COPD.S302259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yawn BP, Han M, Make BM, et al. Protocol summary of the COPD Assessment in Primary Care To Identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) validation in primary care study. Chronic Obstr Pulm Dis. 2021;8(1):60-75. doi: 10.15326/jcopdf.2020.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CM, Clifton M, Fiarbairn AS, et al. Standardized questionaires on respiratory symptoms. BMJ. 1960;2:1665. doi: 10.1136/bmj.2.5213.166513688719 [DOI] [Google Scholar]
- 15.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32-43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services . Methodology for the PATH Study data tables and figures: tobacco use measures. Accessed April 28, 2022. https://www.icpsr.umich.edu/files/NAHDAP/pathstudy/Methodology-PATH-TablesFigures-Waves1-5.pdf
- 17.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 19.Clopper CJ, Pearson ES. The use of confidence or difucial limits illustrated in the case of the binomial. Biometrika. 1934;26:404-413. doi: 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 20.R Project . A language and environment for statitical computing. Accessed January 19, 2023. https://www.R-project.org
- 21.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regan EA, Lynch DA, Curran-Everett D, et al. ; Genetic Epidemiology of COPD (COPDGene) Investigators . Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539-1549. doi: 10.1001/jamainternmed.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodruff PG, Barr RG, Bleecker E, et al. ; SPIROMICS Research Group . Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811-1821. doi: 10.1056/NEJMoa1505971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maselli DJ, Hardin M, Christenson SA, et al. Clinical approach to the therapy of asthma-COPD overlap. Chest. 2019;155(1):168-177. doi: 10.1016/j.chest.2018.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan JK, Ancy KM, Oromendia C, et al. ; SPIROMICS Investigators . Characterizing COPD symptom variability in the stable state utilizing the evaluating respiratory symptoms in COPD instrument. Chronic Obstr Pulm Dis. 2022;9(2):195-208. doi: 10.15326/jcopdf.2021.0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ejike CO, Dransfield MT, Hansel NN, et al. Chronic obstructive pulmonary disease in America’s Black population. Am J Respir Crit Care Med. 2019;200(4):423-430. doi: 10.1164/rccm.201810-1909PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo H, Brigham EP, Allbright K, et al. Racial segregation and respiratory outcomes among urban Black residents with and at risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;204(5):536-545. doi: 10.1164/rccm.202009-3721OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ejike CO, Woo H, Galiatsatos P, et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021;203(8):987-997. doi: 10.1164/rccm.202002-0253OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan ES, Fortis S, Regan EA, et al. ; COPDGene Investigators . Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am J Respir Crit Care Med. 2018;198(11):1397-1405. doi: 10.1164/rccm.201804-0663OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan ES, Balte P, Schwartz JE, et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA. 2021;326(22):2287-2298. doi: 10.1001/jama.2021.20939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washio Y, Sakata S, Fukuyama S, et al. Risks of mortality and airflow limitation in Japanese individuals with preserved ratio impaired spirometry. Am J Respir Crit Care Med. 2022;206(5):563-572. doi: 10.1164/rccm.202110-2302OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han MK, Steenrod AW, Bacci ED, et al. Identifying patients with undiagnosed COPD in primary care settings: insight from screening tools and epidemiologic studies. Chronic Obstr Pulm Dis. 2015;2(2):103-121. doi: 10.15326/jcopdf.2.2.2014.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haroon S, Jordan RE, Fitzmaurice DA, Adab P. Case finding for COPD in primary care: a qualitative study of the views of health professionals. Int J Chron Obstruct Pulmon Dis. 2015;10:1711-1718. doi: 10.2147/COPD.S84247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su KC, Ko HK, Chou KT, et al. An accurate prediction model to identify undiagnosed at-risk patients with COPD: a cross-sectional case-finding study. NPJ Prim Care Respir Med. 2019;29(1):22. doi: 10.1038/s41533-019-0135-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quint JK, Donaldson GC, Hurst JR, Goldring JJ, Seemungal TR, Wedzicha JA. Predictive accuracy of patient-reported exacerbation frequency in COPD. Eur Respir J. 2011;37(3):501-507. doi: 10.1183/09031936.00035909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eAbbreviations
eFigure 1. Geographic distribution of participating PBRNs
eFigure 2. The CAPTURE screening tool
eFigure 3. Screening results and classification of patients based on spirometry and symptom assessment
eFigure 4. Sensitivity and specificity of the screening tool for clinically significant COPD in pre-specified subgroups
eFigure 5. Positive and negative predictive value of the screening tool for clinically significant COPD in pre-specified subgroups
eFigure 6. ROC curves for varying thresholds of the CAPTURE questionnaire score and PEFR separately to detect clinically significant COPD
eFigure 7. Sensitivity and specificity of the screening tool for spirometry-defined COPD in pre-specified subgroups
eFigure 8. ROC curves for varying thresholds defining a positive screen to detect spirometry-defined COPD
eFigure 9. Sensitivity and specificity of the screening tool for clinically significant COPD in additional subgroups
eFigure 10. Unadjusted and covariate-adjusted associations of patient and site characteristics with screening tool sensitivity and specificity for clinically significant COPD
eTable 1. Characteristics of participating practices
eTable 2. Characteristics of excluded and included patients
eTable 3. Operating characteristics of the screening tool for detecting clinically significant COPD across a range of thresholds defining a positive screen
eTable 4. Unadjusted and covariate-adjusted associations of patient and site characteristics with screening tool sensitivity and specificity for clinically significant COPD
eReference
Nonauthor collaborators
Data sharing statement