Key Points
Question
Does use of low-concentration atropine eyedrops affect the incidence of myopia in children?
Findings
In this randomized clinical trial that included 474 children aged 4 to 9 years without myopia, nightly use of 0.05% atropine, 0.01% atropine, and placebo eyedrops resulted in a 2-year cumulative incidence of myopia of 28.4%, 45.9%, and 53.0%, respectively. The difference between 0.05% atropine and placebo was statistically significant.
Meaning
Although 0.05% atropine eyedrops resulted in a significantly lower incidence of myopia at 2 years compared with placebo, further research is needed to replicate the findings and to understand whether this represents a delay or prevention of myopia.
Abstract
Importance
Early onset of myopia is associated with high myopia later in life, and myopia is irreversible once developed.
Objective
To evaluate the efficacy of low-concentration atropine eyedrops at 0.05% and 0.01% concentration for delaying the onset of myopia.
Design, Setting, and Participants
This randomized, placebo-controlled, double-masked trial conducted at the Chinese University of Hong Kong Eye Centre enrolled 474 nonmyopic children aged 4 through 9 years with cycloplegic spherical equivalent between +1.00 D to 0.00 D and astigmatism less than −1.00 D. The first recruited participant started treatment on July 11, 2017, and the last participant was enrolled on June 4, 2020; the date of the final follow-up session was June 4, 2022.
Interventions
Participants were assigned at random to the 0.05% atropine (n = 160), 0.01% atropine (n = 159), and placebo (n = 155) groups and had eyedrops applied once nightly in both eyes over 2 years.
Main Outcomes and Measures
The primary outcomes were the 2-year cumulative incidence rate of myopia (cycloplegic spherical equivalent of at least −0.50 D in either eye) and the percentage of participants with fast myopic shift (spherical equivalent myopic shift of at least 1.00 D).
Results
Of the 474 randomized patients (mean age, 6.8 years; 50% female), 353 (74.5%) completed the trial. The 2-year cumulative incidence of myopia in the 0.05% atropine, 0.01% atropine, and placebo groups were 28.4% (33/116), 45.9% (56/122), and 53.0% (61/115), respectively, and the percentages of participants with fast myopic shift at 2 years were 25.0%, 45.1%, and 53.9%. Compared with the placebo group, the 0.05% atropine group had significantly lower 2-year cumulative myopia incidence (difference, 24.6% [95% CI, 12.0%-36.4%]) and percentage of patients with fast myopic shift (difference, 28.9% [95% CI, 16.5%-40.5%]). Compared with the 0.01% atropine group, the 0.05% atropine group had significantly lower 2-year cumulative myopia incidence (difference, 17.5% [95% CI, 5.2%-29.2%]) and percentage of patients with fast myopic shift (difference, 20.1% [95% CI, 8.0%-31.6%]). The 0.01% atropine and placebo groups were not significantly different in 2-year cumulative myopia incidence or percentage of patients with fast myopic shift. Photophobia was the most common adverse event and was reported by 12.9% of participants in the 0.05% atropine group, 18.9% in the 0.01% atropine group, and 12.2% in the placebo group in the second year.
Conclusions and Relevance
Among children aged 4 to 9 years without myopia, nightly use of 0.05% atropine eyedrops compared with placebo resulted in a significantly lower incidence of myopia and lower percentage of participants with fast myopic shift at 2 years. There was no significant difference between 0.01% atropine and placebo. Further research is needed to replicate the findings, to understand whether this represents a delay or prevention of myopia, and to assess longer-term safety.
Trial Registration
Chinese Clinical Trial Registry: ChiCTR-IPR-15006883
This randomized placebo-controlled trial examines the efficacy of low-concentration atropine eyedrops (0.05% and 0.01% concentration) for delaying the onset of myopia in children vs placebo.
Introduction
Myopia is a worldwide public health threat with increasing prevalence across many regions in past decades, especially in East Asia.1,2,3,4 Individuals with myopia have excessive globe elongation and higher risks of sight-threatening complications that lead to poor vision and even blindness.5 Myopia is irreversible once it has developed and the earlier its onset, the greater the likelihood of high myopia (spherical equivalent indicated more myopia than −6.00 D) occurring later in life.6,7,8 Prevention or delay of myopia onset may have the potential to improve long-term visual outcomes.
Lifestyle modifications, such as increasing the time spent on outdoor activities, can delay the onset of myopia.9,10,11 A randomized trial in China found that increasing outdoor class time by 40 minutes daily reduced the 3-year cumulative incidence of myopia from 39.5% to 30.4% in first-grade children.9 Although encouraging children to spend more time outdoors is a practical and generalizable approach, additional strategies are warranted, especially for children at high risk of developing myopia.
Low-concentration atropine eyedrops are effective in reducing myopia progression, have been widely adopted in Asia,12 and are being further evaluated in randomized trials in many countries (eAppendix tables 1-2 in Supplement 1). Low-concentration (0.01%-0.05%) atropine can reduce myopia progression, but studies of 0.01% atropine have produced inconsistent findings.13,14,15 Of note, whether atropine treatment is effective in delaying myopia onset remains unknown. A retrospective study suggested that low-concentration atropine should be helpful for delaying myopia onset.16 However, randomized trials are needed to provide robust evidence on efficacy and safety. The Low-Concentration Atropine for Myopia Prevention (LAMP2) trial was conducted to assess the efficacy of low-concentration atropine in delaying myopia onset among children.
Methods
This study was a randomized, placebo-controlled, double-masked trial. The study was conducted at the Chinese University of Hong Kong Eye Centre. The first recruited participant started treatment on July 11, 2017, and the last participant was enrolled on June 4, 2020. The date of the final follow-up session was June 4, 2022. The study conformed to the tenets of the Declaration of Helsinki and the trial protocol (Supplement 2) was approved by the ethics committee of the Hong Kong Eye Hospital, with all procedures conducted in accordance with the former. The study complied with Good Clinical Practice standard. Verbal assent was obtained from participating children and written informed consent was obtained from their parents or guardians. The statistical analysis plan is shown in Supplement 3.
Participants
This study recruited children without myopia who were aged 4 to 9 years with cycloplegic spherical equivalent between +1.00 and 0.00 D, astigmatism of less than −1.00 D (mean of both eyes), anisometropia of less than 2.00 D, and at least 1 parent whose spherical equivalent indicated more myopia than −3.00 D. It excluded children with ocular diseases (eg, cataracts, congenital retinal diseases, amblyopia, strabismus), those who had previously used atropine or pirenzepine for myopia management, those who were using orthokeratology lenses or other optical methods for myopia management, those with allergies to atropine, and those with systemic diseases (eg, endocrine, cardiac, respiratory diseases) and developmental anomalies.
Randomization
An independent statistician performed block randomization via computer-generated random numbers with block sizes of 3, 6, or 9 to balance randomization confidentially. The randomization sequence was predetermined to conceal treatment group. The sample was divided into 8 strata, which were defined by sex, mean spherical equivalent in both eyes, and age. The latter 2 factors divided the sample into 4 groups: children aged 4 to 6 years with spherical equivalent greater than +0.50 D and less than or equal to +1.00 D, children aged 4 to 6 years with spherical equivalent greater than +0.00 D and less than or equal to +0.50 D, children aged 7 to 9 years with spherical equivalent greater than +0.50 D and less than or equal to +1.00 D, and children aged 7 to 9 years with spherical equivalent greater than +0.00 D and less than or equal to +0.50 D. The sample size in the strata ranged from 50 to 90 participants.
Treatment Concealment and Masking
Eligible children were randomized to the treatment and placebo groups by an independent research nurse who was masked to both treatment groups and participants’ ocular data. The project coordinator, statistician, research nurse, study investigators, participants, and parents/guardians of participants were all masked to the trial medications administered. The investigators included a nurse for logistic coordination; an optometrist for visual acuity, spherical equivalent, accommodation, and near points measurements; a technician for axial length and other ocular imaging examinations; a research assistant for pupil size measurement and another research assistant for the questionnaire; and an ophthalmologist for the ocular assessment and consultation after dilation of the pupils. Trial medications were prepackaged identically in the same type of bottle and were precoded confidentially by the drug manufacturer. The randomization sequence was predetermined by a statistician to conceal randomization. All of the investigators were masked. Analyses were independently conducted by another statistician. Randomization groups were only revealed at 2 years after preliminary analyses were completed.
Interventions
Treatments included atropine sulfate at either 0.05% or 0.01% concentration (0.5-mL unit concentration, preservative-free), and the placebo was 0.9% sodium chloride (0.5-mL unit concentration, preservative-free). All enrolled participants were randomized in a 1:1:1 ratio to receive 0.05% atropine, 0.01% atropine, or placebo eyedrops once nightly in both eyes at their baseline visit (Figure 1). Participants were then followed up using the same schedule under identical examination protocols: at 2 weeks after baseline for monitoring and then at 4, 8, 12, 16, 20, and 24 months after baseline. All eyedrops were prepared for mono-dose administration by Aseptic Innovative Medicine. The shelf life for each batch of eyedrops was 2 years. The manufacturer provided certificates of analysis for each type of eyedrops with assurances for their concentration, stability, and sterility, and the Hong Kong Department of Health granted their certification for drug trials.
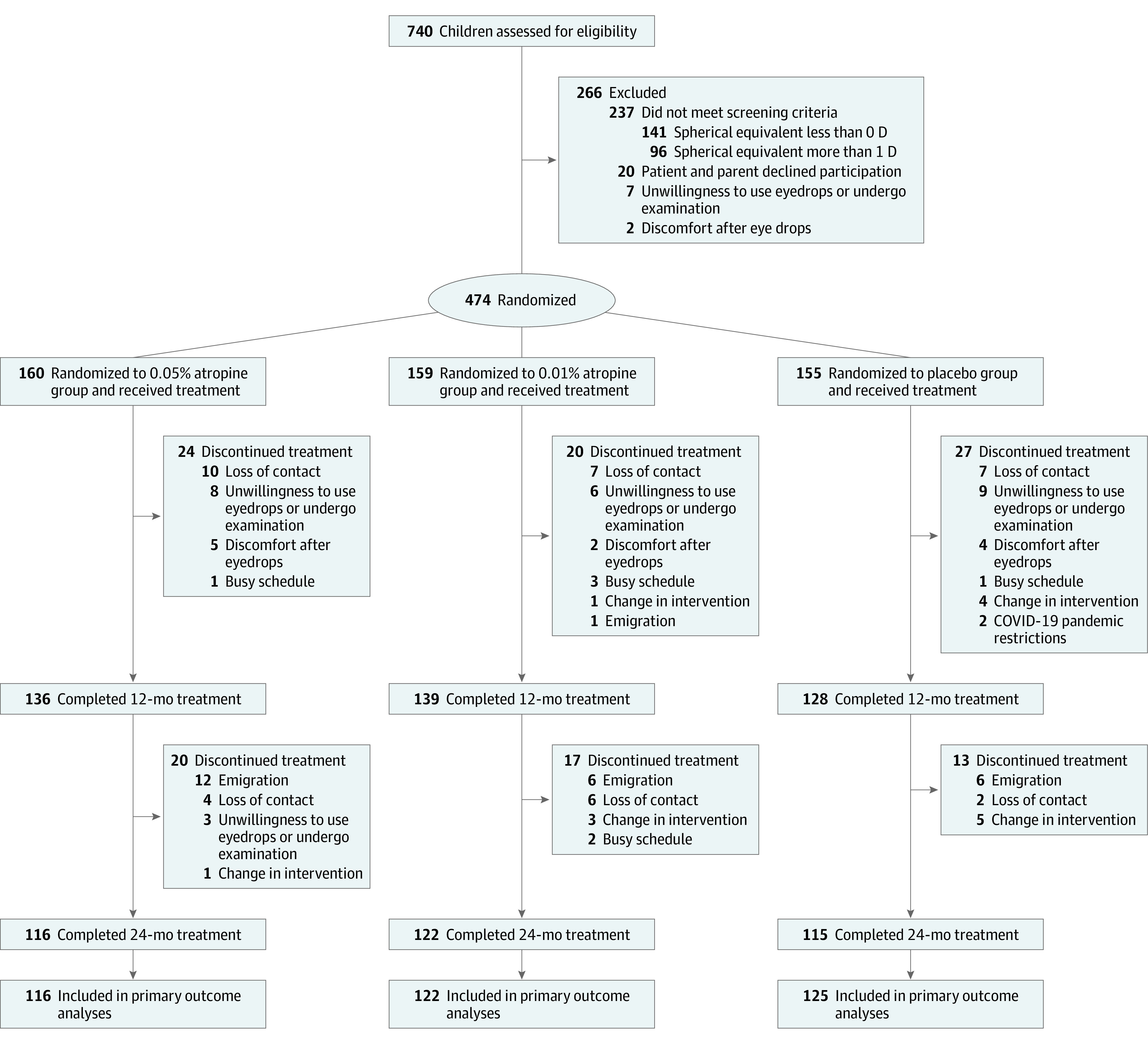
Figure 1. Patient Flow in the LAMP2 Trial of Atropine for Delaying Onset of Myopia.
Outcomes
The primary outcomes were the cumulative incidence of myopia (defined as cycloplegic spherical equivalent of at least −0.50 D in either eye17) and the percentage of participants with fast myopic shift (defined as spherical equivalent myopic shift of at least 1.00 D) over 2 years. Secondary outcomes included changes in spherical equivalent and axial length over 2 years. Exploratory outcomes included time to myopia onset and changes in accommodation, pupil diameters, and visual acuity. Adverse events included the need of photochromic glasses or progressive glasses, photophobia, allergic conjunctivitis, and hospitalization.
Measurements
Distance best-corrected visual acuity was measured using the logMAR chart (Nidek) with a box luminance in adequate room lights. Near visual acuity was assessed using best-corrected distance spectacle correction with a reduced logMAR reading chart placed 40 cm away. The near point of accommodation and accommodation amplitude were measured according to the Royal Air Force near point rule (Haag-Streit UK). Mesopic and photopic pupil sizes were measured using an OPD-Scan III unit (Nidek). Cycloplegic autorefraction was performed using a Nidek ARK-510A autorefractor unit following the cycloplegia regimen composed of 1% cyclopentolate (Cyclogyl, Alcon-Convreur) and 1% tropicamide (Santen).13 Spherical equivalent was calculated as spherical power plus half of the cylinder power. Ocular axial length was measured on a Zeiss IOL Master unit (Carl Zeiss Meditec Inc) using noncontact partial coherence interferometry.
Participating children were offered photochromic glasses (which darkened on exposure to UV light or sunlight) for daily wear if they experienced glare or if their parents/guardians were worried about excessive exposure to light and progressive glasses (reading aids) if they experienced difficulties with near vision. If participants developed myopia during follow-up, they were prescribed best-corrected spectacles but remained in the same respective groups for the 2-year study period. Moreover, parental spherical equivalent using noncycloplegic autorefraction and axial length data were collected from both parents of each child.
Questionnaires and Compliance Assessments
At baseline and 12- and 24-month follow-up visits, all participants were assessed using the Chinese version of the 25-item National Eye Institute Visual Function Questionnaire18 and validated questionnaires about time spent outdoors and near work activities.2 At each visit, participants and their parents/guardians were given an opportunity to report any medical illnesses or adverse effects in an open-ended format. Parents and guardians were also specifically asked about symptoms related to allergies, blurred near vision, glare, or visual loss, in addition to whether participants had been ill or hospitalized since the previous visit. Any adverse events, regardless of whether they appeared relevant to atropine use, were documented.
Each participant’s family kept a diary regarding their trial medication use. Adherence levels were determined based on mean weekly atropine use as reported by participants. Participants with a 75% adherence rate (ie, a mean of 5.25 days/week) over the 24-month period were classified as having good adherence.
Sample Size Calculation
To calculate the required number of study participants, the myopia onset rates over 2 years for 0.05% atropine, 0.01% atropine, and placebo groups were estimated based on the pilot data to be 6.6%, 14.6%, and 20.0%, respectively. A final sample size of 375 participants (125 per group) was selected to achieve 90% power at a .05 significance level. The initial sample size of 474 participants (158 per group) was selected after factoring in a projected attrition rate of 20%.
Statistical Analyses
Participants’ baseline demographic information, clinical characteristics (ocular parameters), adverse events, and Visual Function Questionnaire results were summarized using descriptive statistics. Continuous variables were reported in terms of means and SDs and categorical variables were reported in frequencies and percentages. Ocular parameters were analyzed using mean values from both eyes.
For the analyses of primary outcomes, which were participant-based, logistic regression, including trend analysis, was used to test the effectiveness of atropine on primary outcomes. In the sensitivity analysis, covariate-adjusted analyses with the adjustment of baseline age, sex, baseline spherical equivalent, outdoor time, near work time, and parental myopia were also performed. Differences in the cumulative myopia incidence rates among the 3 treatment groups were calculated using exact unconditional methods based on the Farrington-Manning score statistic.9,19
For analyses of the secondary outcomes, which were eye-based, generalized estimating equations were used to adjust the correlation between both eyes of an individual using the differences in values between the baseline visit and the designated follow-up visit of secondary outcomes as the dependent variables. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Sensitivity analyses were conducted to control for potential confounding factors; analyses for secondary outcomes were repeated with adjustments for baseline age, sex, baseline spherical equivalent/axial length, outdoor time, near work time, and parental myopia. The analyses for right/left eye were also performed to test the consistency of the results using linear regression model.
For analyses of exploratory outcomes, which were eye-based, generalized estimating equations with the adjustment of age and sex were used to compare the differences in values between the baseline visit and the designated follow-up visit of secondary outcomes as the dependent variables. To compare the time to myopia onset among participants of the 3 treatment groups, the Kaplan-Meier method was used to estimate the survival curves and log-rank test. A number-at-risk table was also completed.
For dropout analyses, univariate logistic regression was used to test for associations between baseline variables and dropping out. Further interaction tests between these significant factors and the treatment groups were conducted to explore the effect of treatment groups on dropouts.
All statistical tests were performed by 2-sided test. Two-sided P values less than .05 were considered statistically significant. All statistical analyses were performed using R statistical software, version 4.0.0 (R Foundation for Statistical Computing), and SPSS, version 24.0 (IBM).
Results
Participant Disposition and Baseline Characteristics
Of the 740 individuals assessed for eligibility, 474 were enrolled (Figure 1). Among the 266 ineligible individuals, 237 had refractive errors outside the eligible range, 20 declined to participate in the study, 7 were not willing to administer eyedrops or undergo examinations, and 2 reported discomfort when receiving eyedrops for cycloplegia and were not enrolled for randomization. Of the 474 randomized participants, 160 were randomized to the 0.05% atropine group, 159 were randomized to the 0.01% atropine group, and 155 were randomized to the placebo group. Table 1 presents the baseline characteristics of the 3 groups. Details of time spent outdoors and near work (including homework, reading, and use of cell phones, computers, video games, and television) are presented in eTable 1 in Supplement 1.
Table 1. Baseline Demographic and Clinical Characteristics in a Study of the Effect of Low-Concentration Atropine Eyedrops vs Placebo on Myopia Incidence in Children.
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| 0.05% Atropine (n = 160) | 0.01% Atropine (n = 159) | Placebo (n = 155) | |
| Age, y | 6.86 (1.42) | 6.88 (1.35) | 6.75 (1.27) |
| Sex, No. (%) | |||
| Male | 81 (50.6) | 78 (49.1) | 78 (50.3) |
| Female | 79 (49.4) | 81 (50.9) | 77 (49.7) |
| Body mass index | 15.66 (2.60) | 15.58 (2.10) | 15.47 (2.00) |
| Spherical equivalent, Da | 0.50 (0.33) | 0.51 (0.33) | 0.53 (0.31) |
| Axial length, mmb | 22.82 (0.72) | 22.89 (0.70) | 22.80 (0.64) |
| Central corneal thickness, μmc | 556.07 (32.13) | 555.78 (31.58) | 553.14 (31.71) |
| Intraocular pressure, mm Hgd | 15.89 (2.01) | 15.85 (2.00) | 15.97 (1.91) |
| Pupil size, mme | |||
| Photopic | 3.55 (0.57) | 3.57 (0.61) | 3.69 (0.72) |
| Mesopic | 6.36 (0.68) | 6.34 (0.74) | 6.53 (0.74) |
| Accommodation amplitude, Df | 13.34 (2.69) | 13.60 (2.67) | 13.33 (2.74) |
| Visual acuity, logMARg | |||
| Distance | 0.03 (0.09) | 0.02 (0.09) | 0.03 (0.07) |
| Near | 0.04 (0.10) | 0.03 (0.10) | 0.02 (0.09) |
| Outdoor activity, h/dh | 1.48 (0.51) | 1.50 (0.48) | 1.46 (0.48) |
| Near work, D h/di | 10.55 (3.55) | 10.20 (3.64) | 10.54 (3.69) |
| Parents with myopia, No. (%) | |||
| 1 | 66 (37.4) | 62 (39.0) | 58 (41.2) |
| 2 | 94 (62.6) | 97 (61.0) | 97 (58.8) |
| Baseline visit before January 1, 2020, No. (%) | 94 (58.8) | 102 (64.2) | 92 (59.4) |
Spherical equivalent gives an estimate of the refractive error of the eye. It is calculated as spherical power plus half of the cylinder power.
Axial length is the length of the eyeball and a main determining factor for refractive status, with the increased axial length being the major cause of myopia. The mean (SD) axial length in children in Hong Kong is 23.08 (0.92) mm.20
Central corneal thickness (CCT) is the thickness of the central cornea. The mean (SD) CCT of emmetropic eyes in Chinese children is 557.98 (33.77) μm.21
Intraocular pressure is the fluid pressure of the eye. It is a measurement of the force exerted by the aqueous humor on the internal surface area of the anterior eye; reference range, 10-21 mm Hg.
Photopic pupil size is measured under medium to high light conditions and mesopic pupil size is measured under low light conditions. In children aged 4 to 12 years in Hong Kong, the mean (SD) photopic pupil size was 3.75 (0.82) mm and mesopic pupil size was 6.66 (0.69) mm.13
Accommodation amplitude is a measure of the ability of the eye to change the refractive power/curvature of the lens to focus from far to near distances. The mean (SD) accommodation amplitude in Hong Kong among children aged 4 to 12 years was 12.10 (2.48) D.13
Visual acuity is a measure of the vision at a given distance. The normal visual acuity by logMAR values is ≤0.1 (range, 0.1 to −0.2; lower values indicate better visual acuity). Distance and near visual acuity describe the vision at a distance of 6 m and 40 cm.
Outdoor activity includes outdoor exercise plus outdoor leisure activity. An increase in outdoor activities can help delay the onset and progression of myopia in children.
Diopter hours were calculated using the following formula: 3×(homework + reading + playing cell phone) + 2×(using computer + playing video game) + 1×(watching television). This summation represents an attempt to weigh the child's visual activities according to the amount of accommodation in diopters required to perform them. More diopter hours per day may be associated with higher chance of developing myopia.22
In total, 121 participants (25.5%) dropped out during the 2-year follow-up period (Figure 1). The main reasons for dropping out included loss of contact (36/121 [29.8%]), unwillingness to administer eyedrops or complete ocular examinations (26/121 [21.5%]), and emigration (25/121 [20.7%]). A total of 14 participants (11.6%) discontinued the study to switch to other treatments after their myopia progressed. There were no significant differences among the 3 groups between participants who completed follow-up and those who dropped out in terms of demographics and baseline parameters (eTable 2 in Supplement 1). Overall dropout rates were similar among the 3 groups over 2 years (eTable 3 in Supplement 1) and were not associated with treatment group (eTable 4 in Supplement 1). The 1-year outcomes of those who completed the 2-year follow-up and those who dropped out after the first year are presented in eTable 5 in Supplement 1.
Primary Outcomes: Myopia Incidence and Percentage With Fast Myopic Shift
The 2-year cumulative incidence rates of myopia were 28.4% (33/116 participants) for the 0.05% atropine group, 45.9% (56/122) for the 0.01% atropine group, and 53.0% (61/115) for the placebo group (P for trend <.001; Table 2). The percentages of participants with fast myopic shift at 2 years were 25.0% (29/116) for the 0.05% atropine group, 45.1% (55/122) for the 0.01% atropine group, and 53.9% (62/115) for the placebo group (P for trend <.001; Table 2).
Table 2. Primary Outcomes of Myopia Incidence and Participants With Fast Myopic Shift Over 2 Years.
| Follow-up | No./total No. (%) | P value | Difference (95% CI), % | P valuec | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.05% Atropine | 0.01% Atropine | Placebo | For trenda | For trend (adjusted)b | 0.05% Atropine vs 0.01% atropine | 0.05% Atropine vs placebo | 0.01% Atropine vs placebo | 0.05% Atropine vs 0.01% atropine | 0.05% Atropine vs placebo | 0.01% Atropine vs placebo | |
| Cumulative myopia incidence (defined as spherical equivalent ≤−0.50 D) | |||||||||||
| 4 mo | 1/138 (0.7) | 4/127 (3.1) | 8/130 (6.2) | .02 | .01 | 2.4 (−1.1 to 7.2) | 5.4 (1.4 to 11.0) | 3.0 (−2.5 to 8.9) | .15 | .01 | .25 |
| 8 mo | 8/132 (6.1) | 20/131 (15.3) | 21/120 (17.5) | .007 | .001 | 9.2 (1.8 to 17.1) | 11.4 (3.7 to 19.9) | 2.2 (−7.0 to 11.7) | .02 | .004 | .63 |
| 12 mo | 13/136 (9.6) | 35/139 (25.2) | 39/128 (30.5) | <.001 | <.001 | 15.6 (6.9 to 24.5) | 20.9 (11.6 to 30.4) | 5.3 (−5.4 to 16.0) | <.001 | <.001 | .33 |
| 16 mo | 23/124 (18.5) | 43/132 (32.6) | 44/124 (35.5) | .004 | .001 | 14.0 (3.4 to 24.4) | 16.9 (6.0 to 27.7) | 2.9 (−8.7 to 14.5) | .01 | .003 | .62 |
| 20 mod | 26/95 (27.4) | 36/92 (39.1) | 38/88 (43.2) | .03 | .01 | 11.8 (−1.7 to 24.9) | 15.8 (2.0 to 29.2) | 4.1 (−10.3 to 18.2) | .09 | .03 | .58 |
| 24 mo | 33/116 (28.4) | 56/122 (45.9) | 61/115 (53.0) | <.001 | <.001 | 17.5 (5.2 to 29.2) | 24.6 (12.0 to 36.4) | 7.1 (−5.6 to 19.6) | .005 | <.001 | .27 |
| Participants with fast myopic shift (defined as spherical equivalent myopic shift ≥0.50 D over the first 12 mo and ≥1.00 D over 24 mo) | |||||||||||
| 12 mo | 39/136 (28.7) | 68/139 (48.9) | 81/128 (63.3) | <.001 | <.001 | 20.2 (8.8 to 31.2) | 34.6 (22.9 to 45.3) | 14.4 (2.4 to 25.9) | <.001 | <.001 | .02 |
| 24 mo | 29/116 (25.0) | 55/122 (45.1) | 62/115 (53.9) | <.001 | <.001 | 20.1 (8.0 to 31.6) | 28.9 (16.5 to 40.5) | 8.8 (−3.9 to 21.3) | .001 | <.001 | .17 |
P values for trend were calculated via logistic regression without adjustment.
Adjusted P values for trend were calculated via logistic regression with adjustment of baseline age, sex, baseline spherical equivalent/axial length, outdoor time, near work time, and parental myopia.
P values were calculated by exact unconditional methods based on the Farrington-Manning score statistic.
A proportion of participants did not attend the 20-month visit because of infection control restrictions during the COVID-19 pandemic.
Compared with placebo, 0.05% atropine significantly reduced both cumulative myopia incidence at 2 years (difference, 24.6% [95% CI, 12.0%-36.4%]; P < .001) and the percentage of participants with fast myopic shift at 2 years (difference, 28.9% [95% CI, 16.5%-40.5%]; P < .001). Compared with 0.01% atropine, 0.05% atropine significantly reduced both cumulative myopia incidence at 2 years (difference, 17.5% [95% CI, 5.2%-29.2%]; P = .005) and the percentage of participants with fast myopic shift at 2 years (difference, 20.1% [95% CI, 8.0%-31.6%]; P = .001). There was no significant difference between the 0.01% atropine and placebo groups at 2 years in myopia incidence (difference, 7.1% [95% CI, −5.6% to 19.6%]; P = .27) or percentage of participants with fast myopic shift (difference, 8.8% [95% CI, −3.9% to 21.3%]; P = .17). Results of sensitivity analyses showed similar trends (eTables 6-11 in Supplement 1).
Secondary Outcomes: Changes in Spherical Equivalent and Axial Length
At 2 years, the mean myopic shifts in spherical equivalent were −0.46 (95% CI, −0.58 to −0.33) D in the 0.05% atropine group, −0.84 (95% CI, −0.98 to −0.70) D in the 0.01% atropine group, and −1.01 (95% CI, −1.15 to −0.87) D in the placebo group (P < .001; Table 3, Figure 2, and eFigure 1 in Supplement 1). The mean axial length elongations were 0.48 (95% CI, 0.42-0.53) mm in the 0.05% atropine group, 0.63 (95% CI, 0.57-0.70) mm in the 0.01% atropine group, and 0.70 (95% CI, 0.64-0.76) mm in the placebo group (P < .001; Table 3, Figure 2, and eFigure 1 in Supplement 1). Spherical equivalent and axial length at follow-up visits over 2 years are reported in eTable 12 in Supplement 1.
Table 3. Secondary Outcomes of Changes in Spherical Equivalent and Axial Length Over 2 Years.
| Follow-up | 0.05% Atropine | 0.01% Atropine | Placebo | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Mean (95% CI)b | No. of patients | Mean (95% CI)b | No. of patients | Mean (95% CI)b | For trendc | Overallc | 0.05% Atropine vs 0.01% atropine | 0.05% Atropine vs placebo | 0.01% Atropine vs placebo | |
| Changes in spherical equivalent, D | |||||||||||
| 2 wk | 143 | 0.17 (0.13 to 0.21) | 138 | 0.09 (0.05 to 0.12) | 136 | 0.01 (−0.02 to 0.05) | <.001 | <.001 | .01 | <.001 | .007 |
| 4 mo | 138 | 0.16 (0.11 to 0.21) | 127 | −0.01 (−0.06 to 0.04) | 130 | −0.15 (−0.21 to −0.10) | <.001 | <.001 | <.001 | <.001 | .002 |
| 8 mo | 132 | 0.01 (−0.05 to 0.08) | 131 | −0.24 (−0.31 to −0.17) | 120 | −0.37 (−0.45 to −0.28) | <.001 | <.001 | <.001 | <.001 | .04 |
| 12 mo | 136 | −0.11 (−0.18 to −0.04) | 139 | −0.38 (−0.46 to −0.30) | 128 | −0.55 (−0.64 to −0.45) | <.001 | <.001 | <.001 | <.001 | .03 |
| 16 mo | 124 | −0.25 (−0.35 to −0.15) | 132 | −0.56 (−0.66 to −0.46) | 124 | −0.69 (−0.80 to −0.59) | <.001 | <.001 | <.001 | <.001 | .11 |
| 20 moa | 95 | −0.38 (−0.51 to −0.24) | 92 | −0.71 (−0.85 to −0.56) | 88 | −0.83 (−0.98 to −0.69) | <.001 | <.001 | .003 | <.001 | .39 |
| 24 mo | 116 | −0.46 (−0.58 to −0.33) | 122 | −0.84 (−0.98 to −0.70) | 115 | −1.01 (−1.15 to −0.87) | <.001 | <.001 | <.001 | <.001 | .19 |
| Changes in axial length, mm | |||||||||||
| 2 wk | 140 | 0.01 (0.00 to 0.02) | 136 | 0.02 (0.01 to 0.02) | 135 | 0.04 (0.03 to 0.05) | .001 | .003 | .35 | .001 | .005 |
| 4 mo | 137 | 0.08 (0.06 to 0.10) | 125 | 0.11 (0.10 to 0.13) | 129 | 0.16 (0.13 to 0.18) | <.001 | <.001 | .06 | <.001 | .01 |
| 8 mo | 131 | 0.16 (0.14 to 0.19) | 129 | 0.24 (0.21 to 0.27) | 120 | 0.30 (0.27 to 0.34) | <.001 | <.001 | <.001 | <.001 | .04 |
| 12 mo | 135 | 0.25 (0.22 to 0.28) | 137 | 0.34 (0.31 to 0.37) | 127 | 0.40 (0.36 to 0.45) | <.001 | <.001 | <.001 | <.001 | .09 |
| 16 mo | 123 | 0.33 (0.29 to 0.38) | 130 | 0.46 (0.41 to 0.51) | 123 | 0.51 (0.46 to 0.56) | <.001 | <.001 | <.001 | <.001 | .18 |
| 20 moa | 94 | 0.42 (0.36 to 0.48) | 92 | 0.56 (0.50 to 0.63) | 87 | 0.60 (0.54 to 0.66) | <.001 | <.001 | .001 | <.001 | .74 |
| 24 mo | 114 | 0.48 (0.42 to 0.53) | 120 | 0.63 (0.57 to 0.70) | 115 | 0.70 (0.64 to 0.76) | <.001 | <.001 | <.001 | <.001 | .30 |
A proportion of participants did not attend the 20-month visit because of the infection control restriction during the COVID-19 pandemic.
Mean and 95% CI values were calculated with data from both eyes.
P values were generated by the generalized estimating equation model with the adjustment of baseline age, sex, baseline spherical equivalent/axial length, outdoor time, near work time, and parental myopia.
Figure 2. Spherical Equivalent Myopic Shifts and Axial Length Elongation Over 2 Years.
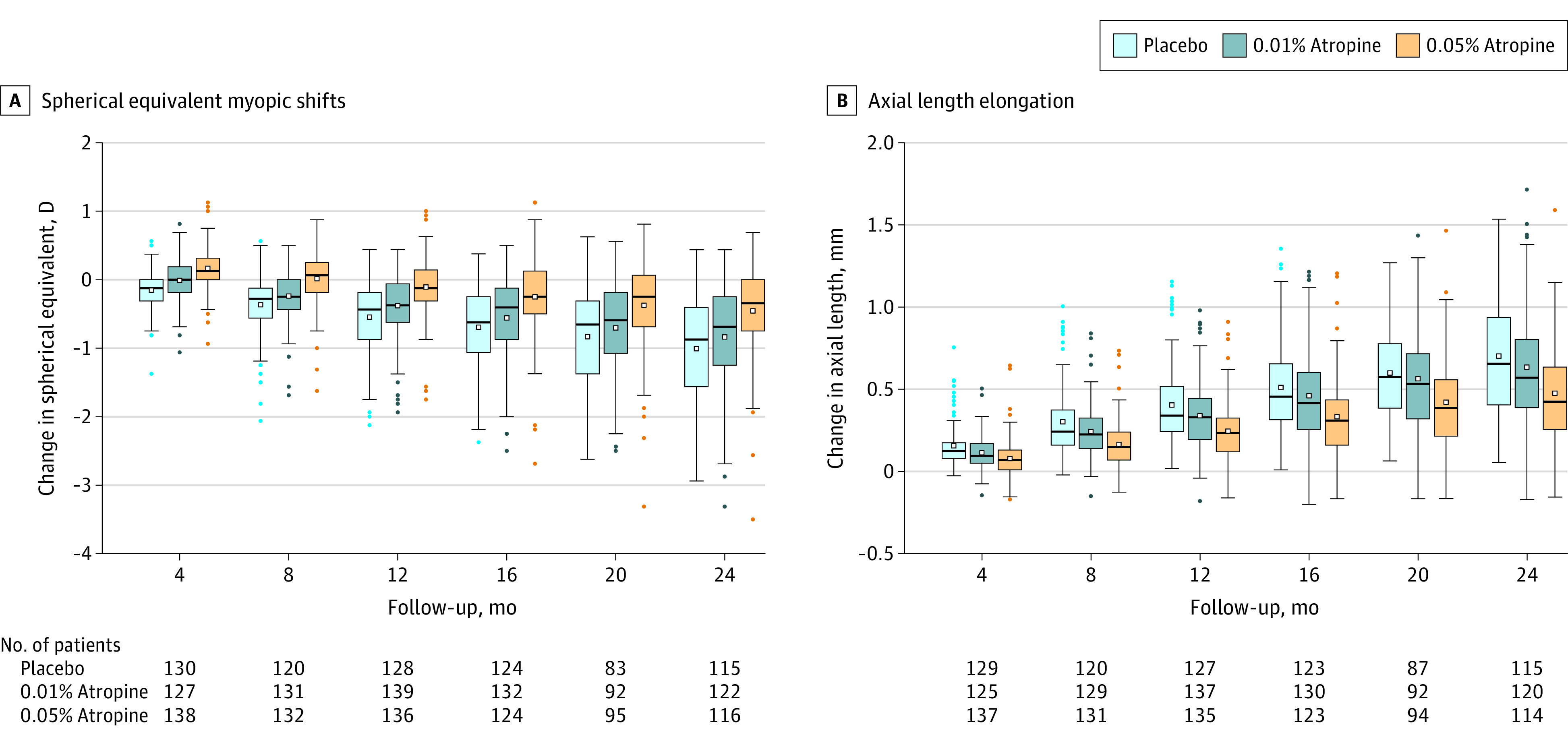
The horizontal line within the box represents the median value and the dot within the box represents the mean value. The top end of the box represents the 75th quartile and the bottom of the box represents the 25th quartile. The whiskers end at the upper and lower adjacent values, the location of the furthest point that is within 1.5 IQRs of the first and third quartiles. Dots represent outside values.
Multiple comparisons revealed that 0.05% atropine decreased spherical equivalent myopic shift and axial length elongation significantly more than 0.01% atropine (P < .001 for spherical equivalent shift and for axial length elongation) and placebo (P < .001 for spherical equivalent shift and for axial length elongation) at 2 years (Table 3). The difference between the 0.01% atropine and placebo groups did not reach statistical significance at 2 years (P = .19 for spherical equivalent shift and P = .30 for axial length elongation). Further sensitivity analyses showed similar trends (eTable 13 in Supplement 1).
Exploratory Outcomes: Time to Myopia Onset and Changes in Accommodation, Pupil Diameter, and Visual Acuity
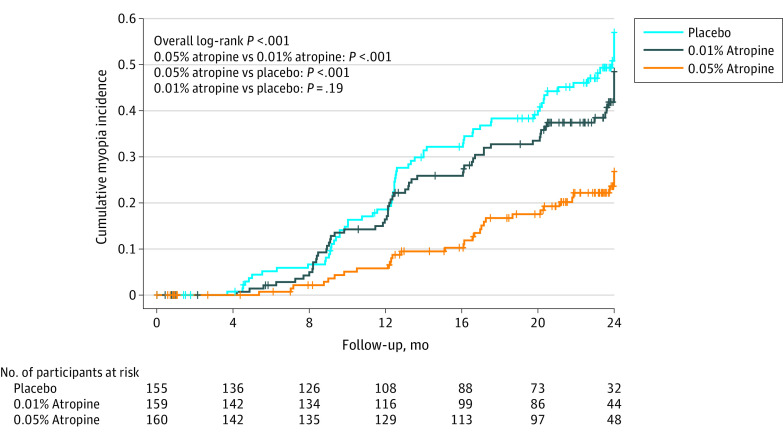
The 2-year cumulative incidence of myopia differed significantly among the 3 groups (P < .001; Figure 3). The cumulative myopia incidence in the 0.05% atropine group was significantly lower than that of the 0.01% atropine group (P < .001) and placebo group (P < .001) at 2 years, which suggested a delay of myopia onset in the 0.05% atropine group compared with both the 0.01% atropine group and placebo group. There was no significant difference between the 0.01% atropine and placebo groups at 2 years in cumulative myopia incidence (P = .19), suggesting similar myopia onset time between the 2 groups.
Figure 3. Time to Myopia Onset by Treatment Group.
The median (IQR) observation time was 23.77 (18.57-24.6) months for the 0.05% atropine group, 23.80 (20.63-24.57) months for the 0.01% atropine group, and 23.13 (19.83-24.47) months for the placebo group.
The changes in accommodation, pupil size, and visual acuity are reported in eTable 14 in Supplement 1 and the mean values of these parameters at all follow-up visits are reported in eTable 15 in Supplement 1.
Tolerability and Adverse Events
Adverse events are reported in eTable 16 in Supplement 1. Photophobia was the most common adverse event and was reported by 15 participants (12.9%) in the 0.05% atropine group, 23 (18.9%) in the 0.01% atropine group, and 14 (12.2%) in the placebo group in the second year. None of the 16 severe adverse events requiring hospitalization were related to atropine treatment. No significant differences were observed among groups in vision-related quality of life (P > .05; eTable 17 in Supplement 1).
Discussion
Among children aged 4 to 9 years without myopia, randomization to nightly use of 0.05% atropine eyedrops compared with placebo resulted in a significantly lower incidence of myopia and lower percentage of participants with fast myopic shift at 2 years; there was no significant difference between 0.01% atropine and placebo.
Low-concentration atropine is one of the few interventions that can be initiated in high-risk children without myopia to delay the potential onset of myopia.16 Of note, the results in this study could not be interpreted as prevention or decreasing future risk of myopia, because the study duration was only 2 years. Furthermore, results of this study (Figures 2 and 3) suggested a delay of myopia onset and progression, rather than prevention. Nevertheless, this study provides evidence for atropine as an additional strategy for delaying myopia onset beyond increasing time spent outdoors. Although increasing outdoor time offers an effective approach for general populations of children, the addition of low-concentration atropine could be considered for children at high risk of developing myopia.
In this study, 0.01% atropine did not achieve a statistically significant difference compared with placebo. This result suggests that the concentration of 0.01% atropine might not be strong enough to achieve sufficient treatment effects. Such results were consistent with the LAMP1 study, which reported no effect of 0.01% atropine on ocular axial elongation.13,15,23 Of note, the current study observed good tolerability for both 0.05% atropine and 0.01% atropine without severe adverse events, which is consistent with previous reports.13,14,15,23,24,25,26,27,28,29,30,31,32,33,34
Limitations
This study has several limitations. First, there was a potential for unmasking participants due to atropine-induced mydriasis and cycloplegia. In addition, assessment of treatment masking success was not conducted in the study. Nevertheless, rates of reported photophobia, near-vision disturbances, and vision-related quality of life were similar across all groups. Furthermore, the investigators responsible for assessing all of the outcome measures were always masked and both cycloplegic refraction and biometry examinations were performed only after the child had completed the bilateral cycloplegic regimen. Second, the study population was homogeneous in that the participants were all Chinese, so the generalizability of the results to other populations may be limited, especially for individuals with different iris pigmentation. Third, because this was a single-center study, external validation that included multiple centers was warranted. Fourth, the participant dropout rate was higher than anticipated, which could potentially hamper the statistical power and lead to bias. However, analyses suggested that dropout was not related to treatment group, and sensitivity analyses suggested that dropouts did not affect the main results. Although type II error was not an issue for the primary analyses, type I error was still possible. However, the results of using different outcomes measures consistently suggested that 0.05% atropine was superior to 0.01% atropine and placebo in delaying myopia onset. Fifth, the sample size was determined based on the primary outcomes and might lack statistical power for the exploratory and secondary outcomes, so caution is warranted in interpreting the results of these analyses, particularly adverse event analyses. Sixth, the study was conducted in part during the COVID-19 pandemic, which was found to be associated with increasing myopia risk in children and could have diminished the effectiveness of myopia treatment.35 However, the proportion of children who were enrolled before the COVID-19 pandemic was similar across the 3 groups. Seventh, information on treatment adherence was collected from parents by self-report, which could induce recall bias. Eighth, the duration of the study did not allow for measuring of the final myopic state of the participants, with the long-term effects of low-concentration atropine on myopia prevention requiring additional years of follow-up. The participants in this study are currently in their third year of follow-up (Supplement 2), with the total intended follow-up duration of 6 years to evaluate a longer-term effect.
Conclusions
Among children aged 4 to 9 years without myopia, randomization to nightly use of 0.05% atropine eyedrops compared with placebo resulted in a significantly lower incidence of myopia and lower percentage of participants with fast myopic shift at 2 years; there was no significant difference between 0.01% atropine and placebo. Further research is needed to replicate the findings, to understand whether this represents a delay or prevention of myopia, and to assess longer-term safety.
eAppendix Table 1. Summary of Randomized Controlled Trials (RCTs) in Atropine for Myopia Control
eAppendix Table 2. Summary of Ongoing Randomized Controlled Trials (RCTs) in Atropine for Myopia Control
eAppendix References
eTable 1. Outdoor Time and Near Work over Two Years
eTable 2. Baseline Demographics and Clinical Characteristics in 0.05% Atropine, 0.01% Atropine and Placebo Groups for Those Who Completed 2 Years versus Those Who Did Not Complete 2 Years of Treatment
eTable 3. Dropout Rate and Compliance over Two Years
eTable 4. Association between Dropout and Baseline Characteristics
eTable 5. Comparison of Baseline and First Year Outcomes between Those Discontinued after the First Year and Those Completed Two Years
eTable 6. Myopia Incidence over Two Years Using Different Cut-off Points to Define Myopia
eTable 7. Myopia Incidence over Two Years (Including Those Who Had Become Myopic before Discontinuing Treatment)
eTable 8. Myopia Incidence over Two Years (Including the Missing Data for Those Who Had Missed the Follow-up Visit before 24-month but Had Completed Two Years)
eTable 9. Myopia Incidence over Two Years (Including the Missing Data for Those Who Missed the Follow-up or Discontinued Treatment)
eTable 10. Proportion with Fast Myopic Shift over Two Years Using Different Cut-off Points to Define Fast Myopic Shift
eTable 11. Proportion with Fast Myopic Shift over Two Years (Including the Missing Data for Those Who Missed the Follow-up or Discontinued Treatment)
eTable 12. Spherical Equivalent and Axial Length at Follow-Up Visits over Two years
eTable 13. Changes in Spherical Equivalent and Axial Length over Two Years Using the Right or Left Eyes
eTable 14. Changes in Accommodation, Pupil Diameter, and Visual Acuity over Two Years
eTable 15. Accommodation, Pupil Diameter, and Visual Acuity at Follow-Up Visits over Two years
eTable 16. Adverse Events over Two Years
eTable 17. Visual Function Questionnaire Scores over Two Years
Trial protocol
Statistical analysis plan
Data sharing statement
References
- 1.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739-1748. doi: 10.1016/S0140-6736(12)60272-4 [DOI] [PubMed] [Google Scholar]
- 2.Yam JC, Tang SM, Kam KW, et al. High prevalence of myopia in children and their parents in Hong Kong Chinese population: the Hong Kong Children Eye Study. Acta Ophthalmol. 2020. doi: 10.1111/aos.14350 [DOI] [PubMed] [Google Scholar]
- 3.Dolgin E. The myopia boom. Nature. 2015;519(7543):276-278. doi: 10.1038/519276a [DOI] [PubMed] [Google Scholar]
- 4.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. doi: 10.1167/iovs.61.4.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua SY, Sabanayagam C, Cheung YB, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016;36(4):388-394. doi: 10.1111/opo.12305 [DOI] [PubMed] [Google Scholar]
- 7.Pärssinen O, Kauppinen M. Risk factors for high myopia: a 22-year follow-up study from childhood to adulthood. Acta Ophthalmol. 2019;97(5):510-518. doi: 10.1111/aos.13964 [DOI] [PubMed] [Google Scholar]
- 8.Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology. 2018;125(6):863-877. doi: 10.1016/j.ophtha.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 9.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in china: a randomized clinical trial. JAMA. 2015;314(11):1142-1148. doi: 10.1001/jama.2015.10803 [DOI] [PubMed] [Google Scholar]
- 10.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125(8):1239-1250. doi: 10.1016/j.ophtha.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 11.He X, Sankaridurg P, Wang J, et al. Time outdoors in reducing myopia: a school-based cluster randomized trial with objective monitoring of outdoor time and light intensity. Ophthalmology. 2022;129(11):1245-1254. doi: 10.1016/j.ophtha.2022.06.024 [DOI] [PubMed] [Google Scholar]
- 12.Leshno A, Farzavandi SK, Gomez-de-Liaño R, Sprunger DT, Wygnanski-Jaffe T, Mezer E. Practice patterns to decrease myopia progression differ among paediatric ophthalmologists around the world. Br J Ophthalmol. 2020;104(4):535-540. doi: 10.1136/bjophthalmol-2019-314752 [DOI] [PubMed] [Google Scholar]
- 13.Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113-124. doi: 10.1016/j.ophtha.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 14.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. doi: 10.1016/j.ophtha.2011.07.031 [DOI] [PubMed] [Google Scholar]
- 15.Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the Low-Concentration Atropine for Myopia Progression (LAMP) study: phase 2 report. Ophthalmology. 2020;127(7):910-919. doi: 10.1016/j.ophtha.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 16.Fang PC, Chung MY, Yu HJ, Wu PC. Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocul Pharmacol Ther. 2010;26(4):341-345. doi: 10.1089/jop.2009.0135 [DOI] [PubMed] [Google Scholar]
- 17.Negrel AD, Maul E, Pokharel GP, Zhao J, Ellwein LB. Refractive error study in children: sampling and measurement methods for a multi-country survey. Am J Ophthalmol. 2000;129(4):421-426. doi: 10.1016/S0002-9394(99)00455-9 [DOI] [PubMed] [Google Scholar]
- 18.Chan CW, Wong D, Lam CL, McGhee S, Lai WW. Development of a Chinese version of the National Eye Institute Visual Function Questionnaire (CHI-VFQ-25) as a tool to study patients with eye diseases in Hong Kong. Br J Ophthalmol. 2009;93(11):1431-1436. doi: 10.1136/bjo.2009.158428 [DOI] [PubMed] [Google Scholar]
- 19.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9(12):1447-1454. doi: 10.1002/sim.4780091208 [DOI] [PubMed] [Google Scholar]
- 20.Yam JC, Tang SM, Kam KW, et al. High prevalence of myopia in children and their parents in Hong Kong Chinese Population: the Hong Kong Children Eye Study. Acta Ophthalmol. 2020;98(5):e639-e648. doi: 10.1111/aos.14350 [DOI] [PubMed] [Google Scholar]
- 21.Wei W, Fan Z, Wang L, Li Z, Jiao W, Li Y. Correlation analysis between central corneal thickness and intraocular pressure in juveniles in Northern China: the Jinan city eye study. PLoS One. 2014;9(8):e104842. doi: 10.1371/journal.pone.0104842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zadnik K, Satariano WA, Mutti DO, Sholtz RI, Adams AJ. The effect of parental history of myopia on children’s eye size. JAMA. 1994;271(17):1323-1327. doi: 10.1001/jama.1994.03510410035029 [DOI] [PubMed] [Google Scholar]
- 23.Yam JC, Zhang XJ, Zhang Y, et al. Three-year clinical trial of Low-Concentration Atropine for Myopia Progression (LAMP) Study: continued versus washout: phase 3 report. Ophthalmology. 2022;129(3):308-321. doi: 10.1016/j.ophtha.2021.10.002 [DOI] [PubMed] [Google Scholar]
- 24.Chia A, Lu QS, Tan D. Five-year clinical trial on Atropine for the Treatment of Myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391-399. doi: 10.1016/j.ophtha.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 25.Li FF, Zhang Y, Zhang X, et al. Age effect on treatment responses to 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine for myopia progression study. Ophthalmology. 2021;128(8):1180-1187. doi: 10.1016/j.ophtha.2020.12.036 [DOI] [PubMed] [Google Scholar]
- 26.Hieda O, Hiraoka T, Fujikado T, et al. ; ATOM-J. Study Group . Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. 2021;65(3):315-325. doi: 10.1007/s10384-021-00822-y [DOI] [PubMed] [Google Scholar]
- 27.Azuara-Blanco A, Logan N, Strang N, et al. Low-dose (0.01%) atropine eye-drops to reduce progression of myopia in children: a multicentre placebo-controlled randomised trial in the UK (CHAMP-UK)-study protocol. Br J Ophthalmol. 2020;104(7):950-955. doi: 10.1136/bjophthalmol-2019-314819 [DOI] [PubMed] [Google Scholar]
- 28.Sacchi M, Serafino M, Villani E, et al. Efficacy of atropine 0.01% for the treatment of childhood myopia in European patients. Acta Ophthalmol. 2019;97(8):e1136-e1140. doi: 10.1111/aos.14166 [DOI] [PubMed] [Google Scholar]
- 29.Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology. 2022;129(3):322-333. doi: 10.1016/j.ophtha.2021.10.016 [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Fang PC, Yang IH, et al. Prevention of myopia progression with 0.05% atropine solution. J Ocul Pharmacol Ther. 2006;22(1):41-46. doi: 10.1089/jop.2006.22.41 [DOI] [PubMed] [Google Scholar]
- 31.Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178-1184. doi: 10.1001/jamaophthalmol.2020.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu A, Stapleton F, Wei L, et al. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol. 2020;104(11):1535-1541. doi: 10.1136/bjophthalmol-2019-315440 [DOI] [PubMed] [Google Scholar]
- 33.Saxena R, Dhiman R, Gupta V, et al. Atropine for the treatment of childhood myopia in India: multicentric randomized trial. Ophthalmology. 2021;128(9):1367-1369. doi: 10.1016/j.ophtha.2021.01.026 [DOI] [PubMed] [Google Scholar]
- 34.Lee SS, Lingham G, Blaszkowska M, et al. Low-concentration atropine eyedrops for myopia control in a multi-racial cohort of Australian children: a randomised clinical trial. Clin Exp Ophthalmol. 2022;50(9):1001-1012. doi: 10.1111/ceo.14148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi KY, Chun RKM, Tang WC, To CH, Lam CS, Chan HH. Evaluation of an optical defocus treatment for myopia progression among schoolchildren during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2143781. doi: 10.1001/jamanetworkopen.2021.43781 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix Table 1. Summary of Randomized Controlled Trials (RCTs) in Atropine for Myopia Control
eAppendix Table 2. Summary of Ongoing Randomized Controlled Trials (RCTs) in Atropine for Myopia Control
eAppendix References
eTable 1. Outdoor Time and Near Work over Two Years
eTable 2. Baseline Demographics and Clinical Characteristics in 0.05% Atropine, 0.01% Atropine and Placebo Groups for Those Who Completed 2 Years versus Those Who Did Not Complete 2 Years of Treatment
eTable 3. Dropout Rate and Compliance over Two Years
eTable 4. Association between Dropout and Baseline Characteristics
eTable 5. Comparison of Baseline and First Year Outcomes between Those Discontinued after the First Year and Those Completed Two Years
eTable 6. Myopia Incidence over Two Years Using Different Cut-off Points to Define Myopia
eTable 7. Myopia Incidence over Two Years (Including Those Who Had Become Myopic before Discontinuing Treatment)
eTable 8. Myopia Incidence over Two Years (Including the Missing Data for Those Who Had Missed the Follow-up Visit before 24-month but Had Completed Two Years)
eTable 9. Myopia Incidence over Two Years (Including the Missing Data for Those Who Missed the Follow-up or Discontinued Treatment)
eTable 10. Proportion with Fast Myopic Shift over Two Years Using Different Cut-off Points to Define Fast Myopic Shift
eTable 11. Proportion with Fast Myopic Shift over Two Years (Including the Missing Data for Those Who Missed the Follow-up or Discontinued Treatment)
eTable 12. Spherical Equivalent and Axial Length at Follow-Up Visits over Two years
eTable 13. Changes in Spherical Equivalent and Axial Length over Two Years Using the Right or Left Eyes
eTable 14. Changes in Accommodation, Pupil Diameter, and Visual Acuity over Two Years
eTable 15. Accommodation, Pupil Diameter, and Visual Acuity at Follow-Up Visits over Two years
eTable 16. Adverse Events over Two Years
eTable 17. Visual Function Questionnaire Scores over Two Years
Trial protocol
Statistical analysis plan
Data sharing statement