Abstract
Background
The oral soluble film (OSF) is a new drug delivery system. Whether montelukast sodium OSF has similar pharmacokinetic (PK) properties and bioequivalence to chewable tablet (CT) should be investigated.
Methods
This study, conducted at Haikou People’s Hospital, consisted of two trials: a randomized, open-label, single-dose, 3-sequence, 3-period crossover trial under fasting conditions and a randomized, open-label, single-dose, 2-sequence, 2-period crossover trial under fed conditions. Healthy volunteers were randomized 1:1:1 to receive single-dose oral montelukast sodium OSF without water, OSF, or CT with water in the fasting trial, and 1:1 to receive OSF or CT with water in the fed trial in each period, with a 7-day washout period. Randomization was performed according to random number tables generated using computer. Blood samples were collected over a 24-h period. Plasma drug concentrations were tested using high-performance liquid chromatography-tandem mass spectrometry. The primary PK parameters were maximum plasma drug concentration (Cmax), area under the plasma drug concentration-time curve (AUC) from t=0 to the last quantifiable concentration (AUC0–t), and AUC from t=0 to infinity (AUC0–∞). The other PK parameters included time to Cmax (Tmax), terminal elimination rate constant (λz), and half-life (t1/2). Safety was also assessed. Analysis of variance on log-transformed primary PK parameters was applied to analyze the bioequivalence between the OSF and CT. The bioequivalence margin was 80–125%.
Results
From November 2018 to January 2019, 30 subjects were included in each trial. The PK parameters between OSF and CT were numerically similar. All 90% confidence intervals (CIs) of the geometric mean ratio (GMR) for the primary PK parameters fell within 80–125%, confirming the bioequivalence of montelukast sodium OSF and CT under fasting and fed conditions. In the fasting trial, 6 (20%) adverse events (AEs) were reported, including 3 (10%) cases after OSF administration without water and 3 (10%) after OSF administration with water, with no serious AEs. No AEs were recorded in the fed trial.
Conclusions
Montelukast sodium OSF is bioequivalent to CT, with acceptable safety. The OSF is an alternative option of CT.
Trial Registration
ClinicalTrials.gov identifiers: NCT05528198 (the fasting trial) and NCT05531994 (the fed trial).
Keywords: Montelukast sodium, oral soluble film (OSF), bioequivalence
Highlight box.
Key findings
• In the present PKs and bioequivalence study, the PK parameters between montelukast sodium OSF and CT were numerically similar under fasting and fed conditions. It was also confirmed that montelukast sodium OSF is bioequivalent to CT, with acceptable safety.
What is known and what is new?
• Montelukast, a cysteinyl leukotriene type 1 receptor antagonist, is recommended for asthma treatment. OSF is a new drug delivery system that can be easily applied.
• This study reported the PK of montelukast sodium OSF for the first time and confirmed the bioequivalence between montelukast sodium OSF and CT.
What is the implication, and what should change now?
• Since montelukast sodium OSF and CT are bioequivalent, the OSF is an alternative option of CT.
Introduction
Asthma is a common disease characterized by reversible airflow obstruction and airway hyperresponsiveness (1-3). Montelukast, a cysteinyl leukotriene type 1 receptor antagonist, can alleviate leukotriene-mediated bronchoconstriction, increased vascular permeability, and mucus secretion, and is recommended for asthma treatment in both children and adults (4-6).
The oral soluble film (OSF) is a new drug delivery system that quickly releases the drug within a few seconds when placed in the mouth cavity or on the tongue (7,8). Moreover, it can be easily applied by different patients as well as those who cannot easily access water (8,9). Several studies have confirmed the bioequivalence between OSF and the reference formulations (e.g., sildenafil, clobazam, riluzole, and midazolam) (10-13).
To better meet the demand for clinical medications and patients, Qilu Pharmaceutical Co., Ltd. (Jinan, China) recently developed the montelukast sodium OSF. The bioequivalence of montelukast sodium OSF and the reference chewable tablet (CT) needs to be investigated. PK profiles of montelukast sodium differed between fasting and fed conditions (14). Thus, two trials were conducted to assess the bioequivalence under fasting and fed conditions in healthy Chinese volunteers. We present the following article in accordance with the CONSORT reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6485/rc).
Methods
Formulations and subject selection
The montelukast sodium OSF (5 mg/piece; lot No. 18L0011Q11, expiration date: 7 November 2020), manufactured and provided by Qilu Pharmaceutical Co., Ltd., and the reference montelukast sodium CT (5 mg/tablet; lot No. N032451; expiration date: 26 September 2019), marketed under the brand name Singulair® (Merck Sharp & Dohme Ltd., Kenilworth, NJ, USA), were used for the bioequivalence assessment.
Healthy adult males and females aged 18 years or older, weighing at least 50 kg for males and 45 kg for females, and with a body mass index between 18.6–28.5 kg/m2 (both inclusive), were eligible for inclusion. Volunteers were assessed by a comprehensive medical examination, including allergic history, medical history, smoking history, physical examination, vital signs (blood pressure, pulse rate, and temperature), laboratory tests [hematology, chemistry, coagulation function, urinalysis, human immunodeficiency virus, hepatitis B and C, syphilis diagnostic profile, and serum pregnancy test (females only), urine screening to test for drug abuse, alcohol breath testing], and 12-lead electrocardiography (ECG).
Volunteers with any history or evidence of the following were excluded: hypersensitivity to montelukast or any other components of the study drugs or their analogs, other leukotriene receptor antagonists, sulfonamides, or non-steroidal anti-inflammatory drugs; had clinically relevant diseases, drug or alcohol abuse, or excessive smoking. Furthermore, female volunteers who were pregnant, breastfeeding, or likely to become pregnant within 3 months were also excluded.
Study design and treatment
This study was performed at Haikou People’s Hospital in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice Guidelines of the International Conference on Harmonization. The study protocols were approved by the Ethics Committee of Haikou People’s Hospital [approval No. 2018-Lunshen-154 (the fasting trial) and 2018-Lunshen-153 (the fed trial)]. All volunteers provided written informed consent before study participation and were free to withdraw at any time.
The study consisted of two independent clinical trials: a randomized, open-label, single-dose, 3-sequence, 3-period crossover trial under fasting conditions, and a randomized, open-label, single-dose, 2-sequence, 2-period crossover trial under fed conditions. The washout period between each administration was 7 days in both trials. A total of 30 healthy volunteers were included in each study. Randomization was performed according to random number tables generated using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA).
The fasting trial
Subjects were randomly assigned (1:1:1) to three groups (ABC, BCA, and CAB). They were hospitalized on the day before drug administration and performed an overnight fast of at least 10 h during each period. Next, according to grouping, the subjects received a single dose of oral montelukast sodium OSF without (A) or with warm water 240 mL (B), or CT with warm water 240 mL (C). No food was permitted for 4 h post-dose. Standardized meals were provided at the appropriate time after drug administration. Water intake within 1 h before and after dosing and food or beverages containing xanthine, grapefruit, caffeine, and alcohol were also prohibited.
The fed trial
Subjects were randomly assigned (1:1) to either the TR group or the RT group. They were hospitalized on the day before drug administration and consumed a standardized, high-fat, and high-calorie (~950 kcal) breakfast (~30% carbohydrate, ~15% protein, and ~55% fat) 30 min before drug administration during each period. The meal was required to be consumed within 30 min. Then, the subjects received a single dose of oral montelukast sodium OSF (T) or CT (R) with warm water 240 mL according to grouping. The food and beverage restrictions were the same as those applied in the fasting trial.
Sample collection, processing, and pharmacokinetic (PK) assay
To determine the plasma concentrations of montelukast, blood samples were collected in ethylenediaminetetraacetic acid dipotassium (EDTA-K2) blood collection tubes at the following time points during each period: 0 (within 60 min pre-dose) and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, 8, 10, 12, and 24 h post-dose. The blood samples were immediately placed in an ice water mixture and centrifuged at 1,800 ×g and 4 ℃ for 10 min to separate the plasma within 1 h, and then temporarily stored at −20 ℃ and transferred to an ultra-low temperature freezer (−80 ℃) within 48 h for storage until analysis.
The plasma concentrations of montelukast were measured using high-performance liquid chromatography-tandem mass spectrometry, as previously validated by Shanghai Xihua Scientific Co., Ltd. (Shanghai, China). When a calculated concentration was higher than the upper limit of quantitation, the sample was diluted with control plasma and reanalyzed. The linearity range of the detection method for montelukast was 2.00–600 ng/mL and the assay had a lower limit of quantitation of 2.00 ng/mL.
Safety assessment
Safety assessments included physical examination, vital signs (blood pressure, pulse rate, and temperature), laboratory tests (hematology, chemistry, coagulation function, and urinalysis), 12-lead ECG, and the incidence of adverse events (AEs). Vital signs were monitored at admission, before administration (0 h), as well as at 3, 8, and 24 h after each drug administration. Physical examination, vital signs, laboratory tests [chemistry, hematology, urinalysis, and serum pregnancy test (females only)], and ECG were also performed at the end of the study or withdrawn ahead of time.
Statistical analysis
Assuming two one-sided tests with α=0.05, a power of 0.9, coefficient of variation (CV) intra-subject =20%, a mean ratio of the test and reference product of 0.95, and 90% CI within 80.00–125.00% for the bioequivalence, 24 subjects were required, according to the PASS software (version 11.0.7; NCSS, LLC, Kaysville, UT, USA). After considering the dropout rate of 20%, 30 subjects would be enrolled for each study.
The primary PK parameters were the maximum plasma drug concentration (Cmax), the area under the plasma drug concentration-time curve (AUC) from t=0 to the last quantifiable concentration (AUC0–t), and the AUC from t=0 to infinity (AUC0–∞). The other evaluated PK parameters included the time to Cmax (Tmax), the terminal elimination rate constant (λz), and the half-life (t1/2). The PK parameters of montelukast were calculated using Phoenix WinNonlin Software, v8.0 (Certara, Princeton, NJ, USA), according to the non-compartmental method. The linear trapezoidal rule was applied to calculate AUC0–t. AUC0–∞ was calculated as AUC0–t + Ct/λz (Ct: the last quantifiable concentration). λz was determined as the slope of the terminal log-linear portion of the concentration-time profile by regression analysis. The t1/2 was calculated as 0.693/λz. The PK parameters were expressed as mean ± standard deviation, except for Tmax, which was expressed as median (range). AEs were summarized as number (percentage).
The analysis of variance on log-transformed Cmax, AUC0–t, and AUC0–∞ was applied to analyze the bioequivalence between OSF and CT. The fixed factors included sequence, period, and drug. The random factor was the subject. For these PK parameters, a P<0.05 for two one-sided tests was considered statistically significant. OSF and CT were considered bioequivalent if the 90% confidence interval (CI) for the geometric mean ratio (GMR) of these three parameters fell within 80–125%. The coefficients of variation were also calculated.
Results
Subject population
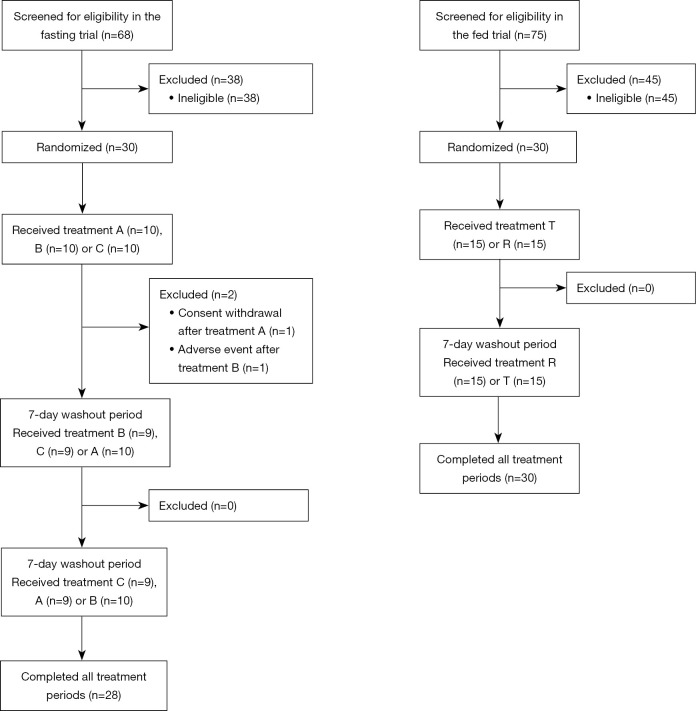
From December 2018 to January 2019, 68 subjects were screened in the fasting trial. Also, from November 2018 to December 2018, 75 subjects were screened in the fed trial. In total, 30 subjects were eligible in each trial. Two subjects withdrew from the fasting trial before the second period because of consent withdrawal and AE, respectively. All subjects were included in the analysis (Figure 1). Table 1 summarizes the demographic characteristics of the included subjects.
Figure 1.
Flow chart of the fasting and fed trials. Treatment A, B, C, T, and R were all a single dose of oral montelukast sodium. A, OSF without water; B, OSF with water; C, CT with water; T, OSF with water; R, CT with water. OSF, oral soluble film; CT, chewable tablet.
Table 1. Demographic characteristics of subjects in the fasting and fed trials.
| Variables | The fasting trial (n=30) | The fed trial (n=30) |
|---|---|---|
| Age (years) | 23.5±5.9 | 26.0±7.6 |
| Gender | ||
| Male | 21 [70] | 24 [80] |
| Female | 9 [30] | 6 [20] |
| Weight (kg) | 22.9±2.7 | 61.5±9.2 |
| Height (cm) | 167.1±9.1 | 166.5±7.8 |
| BMI (kg/m2) | 22.9±2.7 | 22.1±2.6 |
Data are presented as mean ± SD or number [%]. BMI = weight (kg)/[height (m)]2. BMI, body mass index; SD, standard deviation.
PK properties
In the fasting trial, the Cmax values were 299±75 ng/mL for OSF without water, 303±65 ng/mL for OSF with water, and 286±59 ng/mL for CT with water. The AUC0–t for OSF were 1,952.99±532.33 h·ng/mL (without water) and 1,886.62±496.79 h·ng/mL (with water). The AUC0–t for CT was 1,927.93±476.07 (with water). The AUC0–∞ were 2,023.64±571.97 h·ng/mL and 1,943.01±525.01 h·ng/mL for OSF without and with water, respectively, and 1,992.58±508.67 h·ng/mL for CT. The median Tmax values were 2.00 for OSF with or without water, respectively, and 2.50 for CT. The λz values were 0.14±0.02 h−1 (without water) and 0.15±0.02 h−1 (with water) for OSF, and 0.15±0.02 h−1 for CT with water. The OSF without and with water and CT with water had a t1/2 of 4.95±0.76, 4.82±0.56, and 4.80±0.49 h, respectively.
In the fed trial, the Cmax values were 155±33 ng/mL for OSF and 173±49 ng/mL for CT. The AUC0–t values were 1,768.55±428.64 vs. 1,815.82±407.94 h·ng/mL. The AUC0–∞ for OSF and CT were 1,805.55±394.98 and 1,884.41±437.14 h·ng/mL, respectively. Both products had a median Tmax of 5.00 h and mean λz of 0.15 ± 0.02 h−1. Also, the t1/2 was 4.78±0.79 vs. 4.71±0.60 h.
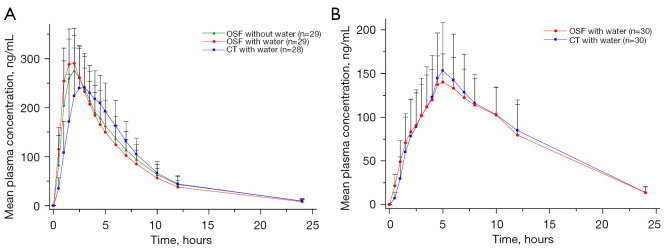
The PK parameters between montelukast sodium OSF and CT were numerically similar under fasting and fed conditions, and the data are summarized in Table 2. The mean plasma concentration-time profiles of montelukast are shown in Figure 2.
Table 2. PK parameters of montelukast after a single oral administration of OSF and CT under fasting and fed conditions.
| Parameters | Fasting condition | Fed condition | ||||
|---|---|---|---|---|---|---|
| OSF without water (n=29) | OSF with water (n=29) | CT with water (n=28) | OSF (n=30) | CT (n=30) | ||
| Cmax (ng/mL) | 299±75 | 303±65 | 286±59 | 155±33 | 173±49 | |
| AUC0–t (h·ng/mL) | 1,952.99±532.33 | 1,886.62±496.79 | 1,927.93±476.07 | 1,768.55±428.64 | 1,815.82±407.94 | |
| AUC0–∞ (h·ng/mL) | 2,023.64±571.97 | 1,943.01±525.01 | 1,992.58±508.67 | 1,805.55±394.98 | 1,884.41±437.14 | |
| Tmax (h) | 2.00 (1.00–3.00) | 2.00 (1.00–4.00) | 2.50 (2.00–6.00) | 5.00 (2.50–10.00) | 5.00 (2.50–12.00) | |
| λz (h−1) | 0.14±0.02 | 0.15±0.02 | 0.15±0.02 | 0.15±0.02 | 0.15±0.02 | |
| t1/2 (h) | 4.95±0.76 | 4.82±0.56 | 4.80±0.49 | 4.78±0.79 | 4.71±0.60 | |
Data are presented as the mean ± SD or median (minimum–maximum). PK, pharmacokinetic; OSF, oral soluble film; CT, chewable tablet; Cmax, maximum plasma drug concentration; AUC0–t, area under the plasma drug concentration-time curve from t=0 to the last quantifiable concentration; AUC0–∞, area under the plasma drug concentration-time curve from t=0 to infinity; Tmax, time to Cmax; λz, terminal elimination rate constant; t1/2, half-life; SD, standard deviation.
Figure 2.
Mean plasma concentration-time profiles of montelukast under fasting (A) and fed (B) conditions. Error bars represent SDs. OSF, oral soluble film; CT, chewable tablet; SD, standard deviation.
Bioequivalence assessment
In the fasting trial, bioequivalence evaluation of the primary PK parameters between OSF without water and CT with water showed that the GMR (90% CI) for Cmax, AUC0–t, and AUC0–∞ were 104.62% (99.47–110.04%), 101.33% (98.06–104.70%), and 101.51% (98.11–105.04%), respectively. As for OSF with water vs. CT with water, the results for Cmax, AUC0–t, and AUC0–∞ were 106.11% (100.88–111.60%), 97.84% (94.69–101.10%), and 97.52% (94.24–100.90%), respectively (Table 3).
Table 3. Bioequivalence assessment results of the OSF and CT under fasting and fed conditions.
| Parameters | GM (OSF) | GM (CT) | GMR (OSF/CT; %) | 90% CI (%) | CV (%) | Power (%) |
|---|---|---|---|---|---|---|
| OSF without water vs. CT with water under fasting conditions (n=29) | ||||||
| Cmax (ng/mL) | 290.02 | 277.21 | 104.62 | 99.47–110.04 | 11.34 | 100.00 |
| AUC0–t (h·ng/mL) | 1,878.30 | 1,853.70 | 101.33 | 98.06–104.70 | 7.33 | 100.00 |
| AUC0–∞ (h·ng/mL) | 1,941.37 | 1,912.40 | 101.51 | 98.11–105.70 | 7.64 | 100.00 |
| OSF with water vs. CT with water under fasting conditions (n=29) | ||||||
| Cmax (ng/mL) | 294.14 | 277.21 | 106.11 | 100.88–111.60 | 11.34 | 99.99 |
| AUC0–t (h·ng/mL) | 1,813.64 | 1,853.70 | 97.84 | 94.69–101.10 | 7.33 | 100.00 |
| AUC0–∞ (h·ng/mL) | 1,864.89 | 1,912.40 | 97.52 | 94.24–100.90 | 7.64 | 100.00 |
| OSF vs. CT under fed conditions (n=30) | ||||||
| Cmax (ng/mL) | 151.39 | 166.79 | 90.77 | 84.32–97.71 | 16.89 | 88.51 |
| AUC0–t (h·ng/mL) | 1,723.91 | 1,773.51 | 97.20 | 94.54–99.94 | 6.34 | 100.00 |
| AUC0–∞ (h·ng/mL) | 1,771.23 | 1,839.42 | 96.29 | 99.30–99.38 | 5.82 | 100.00 |
Analysis of variance on log-transformed Cmax, AUC0–t, and AUC0–∞ was used to analyze the bioequivalence between OSF and CT. The fixed factors included sequence, period, and drug. The random factor was the subject. OSF, oral soluble film; CT, chewable tablet; GM, geometric mean; GMR, geometric mean ratio; CI, confidence interval; CV, coefficient of variation; power, power of test; Cmax, maximum plasma drug concentration; AUC0–t, area under the plasma drug concentration-time curve from t=0 to the last quantifiable concentration; AUC0–∞, area under the plasma drug concentration-time curve from t=0 to infinity.
In the fed trial, the GMR (90% CI) for Cmax, AUC0–t, and AUC0–∞ were 90.77% (84.32–97.71%), 97.20% (94.54–99.94%), and 96.29% (93.30–99.38%), respectively (Table 3).
All 90% CIs of the GMR for the primary PK parameters fell within 80–125%. Thus, the bioequivalence of montelukast sodium OSF and CT under fasting and fed conditions was confirmed.
Safety assessment
In the fasting trial, all subjects received at least one dose of the study drug and were included in the safety analysis. A total of 6 (20%) AEs were reported, including 3 (10%) cases after OSF administration without water and 3 (10%) after OSF administration with water. Five (17%) cases had grade 1 AEs and did not receive any treatment. There was one (3%) case of a grade 2 AE (nasopharyngitis) after OSF administration with water, which was considered treatment-related. This subject recovered after treatment and withdrew from the trial because of the AE. Another AE of nasopharyngitis was also considered treatment-related. No serious AEs were reported during the study (Table 4). No AEs were recorded in the fed trial.
Table 4. AE profile.
| AE | Fasting condition (n=30) |
|---|---|
| Any grade | 6 [20] |
| Grade 1 | 5 [17] |
| Grade 2 | 1 [3] |
| Serious AEs | 0 |
| AEs possibly related to the drug treatment | 2 [7] |
| Nasopharyngitis | 2 [7] |
| AEs likely not related to the drug treatment | 4 [13] |
| Increased blood triglycerides | 2 [7] |
| Increased blood cholesterol | 1 [3] |
| Increased blood uric acid | 1 [3] |
Data are presented as number [%]. No AEs were reported under the fed conditions. AE, adverse event.
Discussion
This study evaluated the PK characteristics and the bioequivalence of montelukast sodium OSF and CT in healthy Chinese volunteers under fasting and fed conditions. The PK parameters of the two products were similar, and bioequivalence was confirmed. The results also indicated that both products were well tolerated.
Montelukast sodium OSF and CT had numerically similar PK parameters. Generally, these parameters were consistent with previous studies of montelukast sodium CT (15-17). Additionally, we found that Cmax decreased and Tmax increased under the fed conditions compared with the fasting conditions. In a previous study of montelukast sodium CT, Tmax also increased under fed conditions vs. fasting conditions (16). For AUC0–t, AUC0–∞, λz, and t1/2, the fasting or fed conditions had no impact on these parameters.
The National Medical Products Administration (NMPA) of China requires a bioequivalence acceptance range of 80% to 125% (18). In the present study, the 90% CI of the GMR for the primary endpoints Cmax, AUC0–t, and AUC0–∞ were all within 80% to 125%, indicating that montelukast sodium OSF and CT are bioequivalent under both fasting and fed conditions.
In this study, both formulations of montelukast were well tolerated by the healthy Chinese volunteers. In the fasting trial, the incidence of AEs was low (20%), and most AEs were in grade 1 and not treatment-related. No AEs were observed in the fed trial. These safety results were consistent with previous data on montelukast sodium CT (15-17). There were two treatment-related AEs; one was in grade 1 and did not require treatment, while the other was in grade 2 and recovered after treatment.
Conclusions
Montelukast sodium OSF and CT had similar PK parameters and good safety profiles under both fasting and fed conditions. Based on the regulatory criteria, montelukast sodium OSF is bioequivalent to CT.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Yunjie Yu (an employee of Qilu Pharmaceutical Co., Ltd.) and Yan Sun (a former employee of Qilu Pharmaceutical Co., Ltd.) for the provision of medical writing support.
Funding: This work was supported by Qilu Pharmaceutical Co., Ltd.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice Guidelines of the International Conference on Harmonization. The study protocols were approved by the Ethics Committee of Haikou People’s Hospital [approval No. 2018-Lunshen-154 (the fasting trial) and 2018-Lunshen-153 (the fed trial)]. All volunteers provided written informed consent before study participation and were free to withdraw at any time.
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6485/rc
Trial Protocol: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6485/tp
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6485/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6485/coif). GZ, LW, SH, HP, MT, and XH report that they received funding from Qilu Pharmaceutical Co., Ltd. XG, HH, YW, and ZL are employees of Qilu Pharmaceutical Co., Ltd. The authors have no other conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet 2018;391:783-800. 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 2.Aegerter H, Lambrecht BN. The Pathology of Asthma: What Is Obstructing Our View? Annu Rev Pathol 2022. [Epub ahead of print]. doi: . 10.1146/annurev-pathol-042220-015902 [DOI] [PubMed] [Google Scholar]
- 3.Castillo JR, Peters SP, Busse WW. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J Allergy Clin Immunol Pract 2017;5:918-27. 10.1016/j.jaip.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zubairi AB, Salahuddin N, Khawaja A, et al. A randomized, double-blind, placebo-controlled trial of oral montelukast in acute asthma exacerbation. BMC Pulm Med 2013;13:20. 10.1186/1471-2466-13-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ZM, Zhao DY, Xiang L, et al. Treatment of pediatric mild persistent asthma with low-dose budesonide inhalation suspension vs. montelukast in China. World J Pediatr 2021;17:619-25. 10.1007/s12519-021-00464-7 [DOI] [PubMed] [Google Scholar]
- 6.Montella S, Maglione M, De Stefano S, et al. Update on leukotriene receptor antagonists in preschool children wheezing disorders. Ital J Pediatr 2012;38:29. 10.1186/1824-7288-38-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahboob MBH, Riaz T, Jamshaid M, et al. Oral films: A comprehensive review. International Current Pharmaceutical Journal 2016;5:111-7. 10.3329/icpj.v5i12.30413 [DOI] [Google Scholar]
- 8.Khan QU, Siddique MI, Sarfraz M, et al. Oral Dispersible Films from Product Development to End-User Acceptability: A Review. Crit Rev Ther Drug Carrier Syst 2022;39:33-64. 10.1615/CritRevTherDrugCarrierSyst.2021036885 [DOI] [PubMed] [Google Scholar]
- 9.Sevinç Özakar R, Özakar E. Current Overview of Oral Thin Films. Turk J Pharm Sci 2021;18:111-21. 10.4274/tjps.galenos.2020.76390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dadey E. Bioequivalence of 2 Formulations of Sildenafil Oral Soluble Film 100 mg and Sildenafil Citrate (Viagra) 100 mg Oral Tablets in Healthy Male Volunteers. Am J Ther 2017;24:e373-80. 10.1097/MJT.0000000000000302 [DOI] [PubMed] [Google Scholar]
- 11.Heller AH, Wargacki S, Jung C, et al. Pharmacokinetics of clobazam oral soluble film. Epilepsia 2018;59:2153-61. 10.1111/epi.14581 [DOI] [PubMed] [Google Scholar]
- 12.Wymer J, Apple S, Harrison A, et al. Pharmacokinetics, Bioavailability, and Swallowing Safety With Riluzole Oral Film. Clin Pharmacol Drug Dev 2023;12:57-64. 10.1002/cpdd.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breithaupt MH, Krohmer E, Taylor L, et al. Oral bioavailability of microdoses and therapeutic doses of midazolam as a 2-dimensionally printed orodispersible film in healthy volunteers. Eur J Clin Pharmacol 2022;78:1965-72. 10.1007/s00228-022-03406-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merck. Singulair® (montelukast sodium). Prescribing Information and Patient Product Information for SINGULAIR. 2021. Available online: https://www.merck.com/research/singulair/ (Accessed January 7, 2023).
- 15.Cánovas M, Arcabell M, Martínez G, et al. Bioequivalence studies of film-coated tablet and chewable tablet generic formulations of montelukast in healthy volunteers. Arzneimittelforschung 2011;61:610-6. [DOI] [PubMed] [Google Scholar]
- 16.Pedroso P, Almeida S, Filipe A, et al. Bioequivalence studies for two different strengths of montelukast in healthy volunteers: 10 mg film-coated tablets and 5 mg chewable tablets. Drug Res (Stuttg) 2013;63:477-83. 10.1055/s-0033-1347235 [DOI] [PubMed] [Google Scholar]
- 17.Li W, Wang Y, Pei Y, et al. Pharmacokinetics and Bioequivalence Evaluation of Two Montelukast Sodium Chewable Tablets in Healthy Chinese Volunteers Under Fasted and Fed Conditions. Drug Des Devel Ther 2021;15:1091-9. 10.2147/DDDT.S298355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Medical Products Administration. Statistical Guideline for Bioequivalence Studies. Available online: https://www.nmpa.gov.cn/directory/web/nmpa/yaopin/ypggtg/ypqtgg/20181029173101911.html (Accessed October 12, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as