Abstract
Different breeds of domestic and junglefowl differ in foraging strategies indicating that domestication resulted in modified energy saving behavioral strategies. In the present study we investigated foraging strategies and foraging-related behavior in 4 lines of laying hens differing in phylogenetic origin and laying performance to analyze a possible relationship between foraging and the level of egg production. High performing brown and white pure bred lines were compared with their low performing brown and white counterparts. To control possible effects on behavior other than genetic effects, all hens were reared and kept in an identical environment. A total of 72 hens from each line were kept in 6 compartments with 12 hens per compartment, respectively. Observations were done for 3 times during one laying period. Foraging strategy was tested by a contrafreeloading (CFL) paradigm. CFL describes a behavior in which animals prefer food that requires effort to obtain, although at the same time food is freely available. The hens were offered a commercial standard diet in one trough and a mixture of wood shavings and commercial standard diet in another trough. The behavior of hens was video recorded and the activity level of individual hens in the litter area was recorded by an antenna-transponder system. The high performing layers showed less CFL and foraging-related behavior compared with their low performing counterparts in both the white and brown layers. Despite differences in CFL, all hens showed a preference for the commercial standard diet compared to the mixture of wood-shavings. Our results show an association between foraging strategy and level of egg production. This suggests that a high level of egg production is accompanied by behaviors enabling the hens to satisfy their higher energy demand more efficiently. Saving energy by reduced activity probably allows them to reallocate energy into reproduction, that is, laying performance.
Key words: laying performance, chicken, activity, foraging, genetic line
INTRODUCTION
Animals evolved under natural selection allocate their available resources optimally between important functions such as growth, reproduction, maintenance and immunity in order to maximize their fitness. Domestication results in an increased reallocation of resources toward traits desired by humans such as growth or reproduction. Such a reallocation of resources significantly increases if artificial selection is targeting on production efficiency. Even if feeding of livestock meets their nutritional demand regarding their production traits, resources available for other traits might become limited or physiological limits, for example, to ingest nutrients (Beilharz et al., 1993; Beilharz and Nitter, 1998; Schütz and Jensen, 2001; Rauw, 2009). Thus, intensive selection for productivity traits may have led to undesired side effects, such as behavioral and physiological changes, as predicted by the resource allocation theory (Beilharz et al., 1993; reviewed by Rauw et al., 1998).
In the fowl, domestication led to an increase in laying rate and, thus, reallocation of available resources toward reproduction (Morris and Taylor, 1967). The ancestor of domestic fowl, the junglefowl, lays around 12 eggs per yr (Morejohn, 1967) but modern domestic hens selected for egg production lay more than 300 eggs per yr (Lieboldt et al., 2015a). Thus, modern domestic hens have to allocate much more energy to the eggs compared to their ancestors. Whether this led to behavioral changes in hens had been tested in a variety of studies (Väisänen et al., 2005a; Jensen, 2014). Compared to junglefowl, White Leghorn hens kept larger distance to stimulus birds and had shorter nearest neighbor distances in a novel pen (Väisänen and Jensen, 2003). The authors concluded that domesticated White Leghorn layers may have greater problems in adapting to a new environment. Schütz and Jensen (2001) compared junglefowl, bantam, a domestic breed that has not undergone selection for production traits, and a White Leghorn laying hybrid. Domestic hens showed less behaviors of high energetic cost, such as foraging and social interactions. The authors concluded that the reduction of such behaviors allows the laying hens to save energy that can be reallocated to production traits.
Foraging is a behavior directly linked to production efficiency. Thus, it is likely that an increase in production efficiency will lead to changes in foraging strategies. A test paradigm that often has been used to test foraging strategies in fowl is contrafreeloading (CFL). CFL describes a behavior of animals in which the animals are willing to perform foraging behavior although food is also freely available (Jensen, 1963; Inglis et al., 1997; Lindqvist et al., 2002). This implies a separate motivation for foraging, which is independent of satiation. CFL had been tested in hens by different experimental procedures: Duncan and Hughes (1972) used operant conditioning techniques where the birds were given the opportunity to get access to food by pecking on a disk while food was freely available. Harlander-Matauschek and Häusler (2009) offered food, wood-shavings and feathers on flat dishes, in open holes or holes covered with transparent plastic foils, which required increasing efforts to access the different objects. Schütz and Jensen (2001) offered freely accessible food and food mixed with wood-shavings in a preference test. All of these studies showed that chickens work for access to food even if freely accessible food is offered simultaneously. Schütz and Jensen (2001) conclude that their results are generally consistent with the idea that selection for high production has caused a reallocation of resources with modified behavioral strategies as a consequence.
By comparing CFL in different strains, Schütz and Jensen (2001) and Väisänen et al. (2005b) showed that modern laying hens (White Leghorn) showed less CFL compared to junglefowl and bantam (Schütz and Jensen, 2001; Väisänen et al., 2005b). Thus, these studies demonstrated that domestication has led to an increased laying performance and, simultaneously, is associated with changes in foraging strategies that can be explained by resource allocation theory. This theory proposes that evolutionary adaptation has resulted in optimal energy allocation between maintenance and reproductive processes in order to maximize evolutionary fitness in wild animals (Beilharz et al., 1993). The studies mentioned above, however, used different strains that differed in performance traits and at the same time in phylogenetic origin, with the main focus on effects of domestication (Schütz and Jensen, 2001; Väisänen and Jensen, 2003; Väisänen et al., 2005b). Regarding possible associations between selection for production efficiency and behavioral traits these studies are of a limited value because it has been shown that chickens of different phylogenetic origins also significantly differ in behavior (Potts, 2012; Jensen and Wright, 2022).
During the last 6 decades, selection for high egg production led to a dramatic increase in production efficiency of modern laying hens (Speedy, 2002). This resulted in an increased energy demand (Morris and Taylor, 1967; Höhne et al., 2017). For the same breeds used in our study Lieboldt et al. (2015a) described: in the 23rd to 35th wk of age hens with high laying intensity (90.2–94.8%) and high egg mass (49.3–50.3 g/hen/d) showed higher food intake (104–115 g/hen/d) compared to low performing hens (laying intensity: 51.3–60.7%, egg mass: 22.4–28.2 g/hen/d, food intake: 75–92 g/hen/d). Food energy is primarily converted in egg production during laying period (Morris and Taylor, 1967). Artificial selection of egg production resulted in an increase in food intake and more efficient food conversion (low performance: 3.35–2.50 kg/kg vs. high performance: 2.16–1.93 kg/kg) (Willems et al., 2013; Lieboldt et al., 2015a). Nevertheless, it still can create a competitive situation between performance and fitness-related traits regarding energy allocation because limits in the ingestion of nutrients are reached (Schütz and Jensen, 2001). Thus, in animals with a high performance resources are drawn off from fitness-related traits to cover their increasing energy needs (Van der Waaij, 2004) and this imbalance in resource allocation may lead to physiological problems and behavioral changes (Rauw et al., 1998). Other behavioral differences related to differences in production efficiency have been observed in learning performance and social traits. In a feeding-reward context, high performing compared to low performing hens showed a higher learning efficiency and a reduced social motivation (Dudde et al., 2018a,b).
A reallocation of resources to production traits might be associated with reduced resources available for example to maintain a high state of health or may be associated with behavioral changes that may cause or increase the risks of unfavored behaviors such as feather pecking. Thus, such changes also might be relevant regarding production issues.
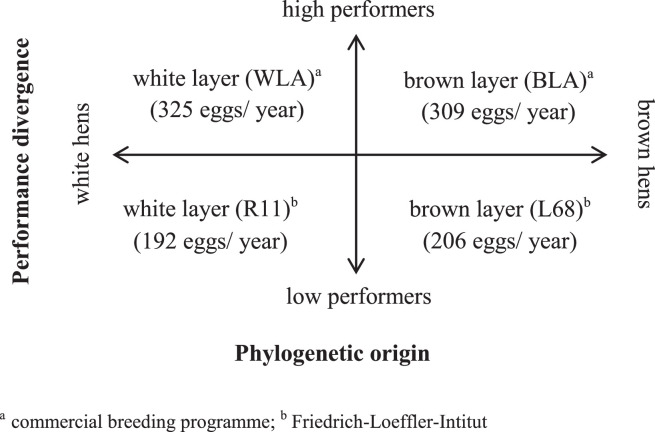
In the present study we used a model to experimentally disentangle effects of phylogeny and selection to obtain a better understanding of associations between foraging behavior and performance. Pairs of closely related pure breeding lines within strains of 2 phylogenetic origins (white and brown egg layer strains) which differ in laying performance (Figure 1) were used to study the effects of laying performance on CFL and foraging-related behavior. To rule out other effects on behavior in addition to effects of selection of egg production, all hens were reared in a controlled environment. This 4-line model has been established at the Friedrich-Loeffler-Institut (FLI) for studying possible effects of both level of productivity and phylogenetic origin (Lieboldt et al., 2015a,b, 2016; Polasky et al., 2016; Höhne et al., 2017; Dudde et al., 2018a,b, 2020; Eusemann et al., 2018, 2020).
Figure 1.
The “4-line-model” used in the experiment consists of pure bred laying hens differing in their level of egg production and phylogenetic origin.
Based on previous knowledge on the effect of laying performance on the behavior in hens, we supposed changes in foraging strategies related to different levels of egg production. These differences should be apparent in hens of both phylogenetic origins. Because Red junglefowls differed in their foraging behavior from domestic fowl, we hypothesized, that high performing lines will show less effort to obtain food, that is, they will prefer freely available food in CFL and will show less foraging behavior.
MATERIALS AND METHODS
All experiments were performed in accordance with the German Animal Protection Law. All birds were housed and managed according to general farming procedures for laying hens.
Birds and Housing Conditions
We used a pair of brown layers with a high performing line (BLA) and a low performing line (L68), and a pair of white layers with also a high performing line (WLA) and a low performing line (R11). Both high performing lines were pure bred layer lines from a commercial breeding program. The low performing lines are kept as nonselected breeds for genetic conservation at the FLI. The 2 white layer lines (WLA and R11) are of White Leghorn origin and phylogenetically closely related, but distant from the brown lines, the Rhode Island Red higher performing line (BLA) and its low performing counterpart L68 (Lyimo et al., 2014) (Figure 1, for details on the layer lines see Lieboldt et al., 2015a).
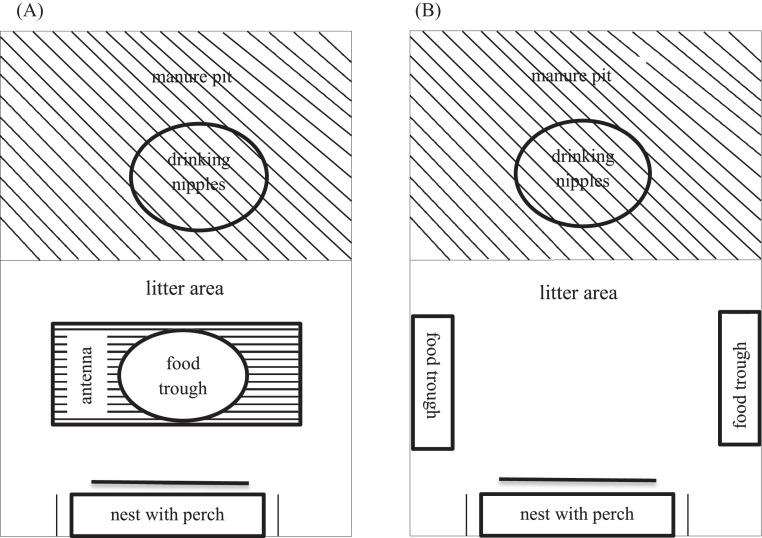
Chickens of each line were hatched on the same day at the research station of the FLI and were separately raised in floor housing until 16th wk of age. Rearing compartments (6 m × 4 m) were littered with wood-shavings and straw and were equipped with perches. Food and water were offered ad libitum. A standard light program was applied during rearing period. On the first 2 d of life, light was provided for 24 h. It was reduced to 15 h on d 3 and to 9 h in wk 7 until 16. At wk 16 of age, hens from each line (brown-high: n = 72, brown-low: n = 72, white-high: n = 72, white-low: n = 72) were randomly allocated to 6 pens (12 hens per pen; 1 × 2 m) resulting in 24 pens. Half of each pen was littered with wood-shavings, the other half was a manure pit covered with a perforated plastic floor. Each pen was equipped with a nest, a perch, a food trough in the litter area and nipple drinkers located on the manure pit (Figure 2). From 16th wk of age the light period was increased in steps of 30 min/wk from 9 h to 14 h and stayed constant for the rest of laying phase.
Figure 2.
(A) Schematic drawing of the experimental compartments during measurements with the antenna-transponder system (see the text for further explanations); and (B) during the foraging strategy test (see the text for further explanations).
Periods of Investigation
All recordings were done during 3 observation periods, each lasting 3 wk. In the first period (P1), from the 17th to 19th wk of age, the hens did not start egg laying. In the second period (P2), from the 35th to the 37th wk of age, the hens reached the maximum of egg production. In the last period (P3), from the 51st to the 53rd wk of age, the egg production already decreased.
In the first week of each observation periods, the activity of hens in the feeding area was recorded by an antenna-transponder system, in the second or the third week the foraging strategy test (CLF) was done and recorded by video observation (Table 1).
Table 1.
Timing of the observation.
| Periods of investigation | Week of age | Observation |
|---|---|---|
| P1 | 17th | Antenna-transponder system |
| 18th | Foraging strategy test | |
| 19th | Foraging strategy test | |
| P2 | 35th | Antenna-transponder system |
| 36th | Foraging strategy test | |
| 37th | Foraging strategy test | |
| P3 | 51st | Antenna-transponder system |
| 52nd | Foraging strategy test | |
| 53rd | Foraging strategy test |
Data Collection and Calculation
The activity of hens in the feeding area was recorded for 1 wk by an antenna-transponder system. Each hen was equipped with a RFID-transponder that was fixated on the right leg (Antenna and Chip GlastagHITAGS3, 15 mm × 13 mm × 3 mm; Gantner Pigeon Systems GmbH, Schruns, Austria). In each compartment one antenna (length: 90 cm, width: 30 cm, height: 3 cm) was placed below the food through in the feeding area (Figure 2A). The presence of the hens in the antenna area was registered in a maximal distance of 5 to 8 cm from the antenna.
The recordings of the antenna-transponder system were analyzed from 6 am to 3 pm for each day of observation for the following parameters:
Activity Around Feeder. Frequency of changes across the antenna area (feeder area). A change was defined as 2 registrations by the antenna with a time interval of more than 2.5 s between registrations.
Time at Feeding Area. Time (minutes) a hen was registered by the antenna at the food trough per hour. In case of time intervals less than 2.5 s this was counted as the same stay.
After the recordings of the antenna-transponder, the foraging strategy test (CFL) was done according to Schütz and Jensen (2001). The test was carried out in the second or the third week of each of the 3 observation periods. During this time the round trough in which the commercial standard diet was fed was removed (Figure 2A). And 2 new troughs were installed in which free and the mixed food of the CLF was offered in parallel (Figure 2B). The free food was the commercial standard diet as offered outside the test periods, the mixed food was the commercial standard diet mixed with wood shavings in a ratio of 1:3. The 2 test troughs were placed on 2 different sides in the litter area (Figure 2B) and the side at which the 2 types of food were offered was changed each day during the week of CLF. The litter area and the 2 troughs were recorded using infrared video cameras (Model VTC-E220IRP, color camera for corner mount with IR-LEDs; SANTEC BW AG, Ahrensburg, Germany) connected to a commercial PC. From the week of video recordings, we used the second and third day for analysis, respectively. Every morning both types of food were reweighed. At least 30 min after food reweight 6 h during the light period were analyzed using instantaneous sampling with 30 min intervals regarding the following behaviors:
Free food: number of hens at trough with free food.
Mixed food: number of hens at trough with mixed food.
Foraging: number of hens scratching or pecking in the litter area.
Litter area: number of hens in the litter area.
The number of hens observed at either of the 2 troughs were expressed as the percentage of hens at mixed food in relation to hens at free food ((mixed food/free food) × 100).
In addition to behavioral recordings, the food consumption was calculated for each type of food after each observation day by reweighing. The consumption of both types of food was expressed as the ratio mixed/free food consumption ((mixed/free food consumption) × 100).
Statistical Analysis
For each time sample from video recordings the percentage of hens performing the respective behavior was calculated and from these data the mean values per observation period were calculated on group level.
All data were analyzed with a general linear model (Glimmix procedure in SAS 9.4, 2002–2012; SAS Institute Inc., Cary, NC) with PO (brown/white), LP (high/low), and LP (PO) as fixed effects. Group nested within period was included as repeated factor.
Differences were regarded as statistically significant at P ≤ 0.05. Differences between means were tested using Tukey-Kramer test adjusted for multiple comparisons.
RESULTS
Food Consumption
The total food consumption (g/hen and day) was higher in the high performing lines compared to the low performing lines (P1: BLA = 71 g, WLA = 70 g, L68 = 54 g, R11 = 53 g; P2: BLA = 119 g, WLA = 117 g, L68 = 102 g, R11 = 90 g; P3: BLA = 116 g, WLA = 117 g, L68 = 94 g, R11 = 100 g).
Activity in the Feeding Area
The time at feeding area and the activity around feeder were influenced by performance (phylogenetic origin) (P < 0.05, Table 2). The white high performing hens spent the least time at feeder area and were least active at feeder, followed by the white-low and brown-low hens. White-low hens showed the highest values for time at feeding area and activity around feeder.
Table 2.
Measurements of contrafreeloading (ratio mixed/free food, ratio mixed/free food consumption), foraging behavior (foraging, litter area), and behavior of hens in the feeding area (time at feeding area, activity around feeder) (LS-means, n = 288) differing in phylogenetic origin (PO) and laying performance (LP).
| Effect | Ratio mixed/free food (%)1 | Ratio mixed/free food consumption (%)2 | Foraging (%)3 | Litter area (%)4 | Time at feeding area (min/h)5 | Activity around feeder (n)6 | |
|---|---|---|---|---|---|---|---|
| PO | Brown | 11.3 | 19.3 | 10.1b | 63.5a | 7.7 | 41.0 |
| White | 16.0 | 14.7 | 14.7a | 56.5b | 7.4 | 41.7 | |
| SEMd | 2.3 | 2.2 | 0.7 | 2.2 | 0.2 | 1.1 | |
| P value | 0.155 | 0.151 | <0.001 | 0.026 | 0.240 | 0.665 | |
| LP | High | 8.7b | 12.0b | 9.1b | 51.9b | 6.7b | 34.3b |
| Low | 18.5a | 21.9a | 15.6a | 68.1a | 8.4a | 48.4a | |
| SEMd | 2.3 | 2.2 | 0.7 | 2.2 | 0.2 | 1.1 | |
| P value | 0.004 | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | |
| LP (PO) | Brown-high | 6.7 | 12.8 | 7.9b | 55.7 | 7.4b | 38.3b |
| Brown-low | 15.8 | 25.8 | 12.2a | 71.3 | 8.1ab | 43.8b | |
| White-high | 10.7 | 11.3 | 10.4b | 48.1 | 6.0c | 30.3c | |
| White-low | 21.3 | 18.0 | 19.1a | 64.8 | 8.8a | 53.1a | |
| SEMd | 4.6 | 4.5 | 1.5 | 4.4 | 0.4 | 2.2 | |
| P value | 0.814 | 0.319 | 0.044 | 0.858 | 0.003 | <0.001 | |
The number of hens observed at either of the 2 troughs were expressed as the percentage of hens at mixed food in relation to hens at free food.
The consumption of mixed and free food was expressed as the ratio mixed/free food consumption.
The number of hens scratching or pecking in the litter area.
The number of hens in the litter area.
Time (minutes) a hen was registered by the antenna at the food trough per hour. In case of time intervals less than 2.5 s this was counted as the same stay.
Frequency of changes across the antenna area (feeder area). A change was defined as 2 registrations by the antenna with a time interval of more than 2.5 s between registrations.
Different small letters indicate significant differences (P ≤ 0.05).
Standard error of the mean.
Contrafreeloading
The ratio mixed/free food and the ratio mixed/free food consumption showed that all hens prefer the free food compared to the mixed food (Table 2). Both parameters were significantly affected by performance (P < 0.05, Table 2) but not by phylogenetic origin or performance (phylogenetic origin) (P > 0.05, Table 2). Hens with higher egg production showed a lower ratio of hens at the mixed/free food and also of the ratio in consumption of mixed/free food compared to the lower performing hens.
Foraging Behavior
Foraging behavior was influenced by phylogenetic origin, performance, and performance (phylogenetic origin) (P < 0.05, Table 2). In both, the white and brown strains, the high performing hens showed less foraging compared to the low performing hens.
The percentage of hens observed in the litter area was affected by performance (P < 0.001) and phylogenetic origin (P < 0.05, Table 2). Low performing hens were more frequently observed in the litter area compared to high performing hens and brown hens more often compared to white hens. Performance (phylogenetic origin) did not affect the stay in litter area (P > 0.05).
DISCUSSION
This study aimed to disentangle possible effects of laying performance and phylogenetic origin on contrafreeloading and other food-related behaviors in laying hens. Within the phylogenic groups (brown and white layers), hens of lines with higher egg production showed less foraging behavior. In addition, high performing hens showed less contrafreeloading, were observed less often and were less active in the litter area. This confirms our hypothesis that genetic selection for high performance is associated with a reduction of foraging-related behaviors.
Despite these differences in foraging-related behaviors, all hens of our 4 lines showed comparable low levels of contrafreeloading independent of laying performance. All hens were less interested in hidden food, for example, food mixed with wood shavings, than in a standard free available food. This finding partly is in contrast to the results of Schütz and Jensen (2001). They tested the preference of Red junglefowl, Swedish Bantam, and White Leghorn hybrids for freely accessible food and food mixed with wood-shavings in a choice feeding experiment. Similar to the Red junglefowl, the Swedish Bantam fed about twice as much food from the mixed diet than the White Leghorn hybrid, which fed more from the freely available food. The Swedish Bantam is a domestic breed which has been selected for body size and feather color but not for high egg production whereas the White Leghorn hybrid is a common commercial breed for egg production. Schütz and Jensen (2001) concluded that reduction of CFL is influenced by selection for performance rather than by domestication. Our results confirm this conclusion. However, the lines we used in our study differed in 2 aspects compared to the lines used by Schütz and Jensen (2001): The low performing hens still lay more eggs compared to the Swedish Bantam and the Red junglefowl. In addition, the phylogenetic distances between Red junglefowl, Swedish Bantam, and White Leghorn hybrid likely are larger than between the low and high performing hens within the white and brown layers used in our study. Comparable to our results, in a study by Harlander-Matauschek and Häusler (2009) hens of 2 lines of White Leghorn divergently selected for feather pecking also preferred the easily accessible food instead of working for food in a hole-in-the-wall test.
This low CFL in our and the study by Harlander-Matauschek and Häusler (2009) is in contrast to the findings of Duncan and Hughes (1972) and of Harlander-Matauschek et al. (2006). In these studies, hens of high laying performance showed high levels of CFL as assessed by an operant conditioning test and hens showed a high level of operant responses even toward empty troughs. These divergent results may be related to the different methods used. In an operant conditioning test, the animals learn a certain task to get a reward. High productive laying hens have been shown to learn more efficiently in a feeding-reward context compared to less productive hens, possibly because they are higher motivated to receive feed (Dudde et al., 2018a). The different results from the studies mentioned above may have also resulted from keeping the birds in cages. Under such barren environmental conditions, the operating system may have represented a reward on its own. In contrast, hens in the present and the studies by Harlander-Matauschek and Häusler (2009) and Schütz and Jensen (2001) were kept in barns, that is, pens with plain earth as ground or littered with wood-shavings. The possibility to forage in these littered housings may have led to reduced foraging motivation compared to cages (Blokhuis, 1989), possibly resulting in a reduced CLF.
High performing hens were less observed in the litter area in which the food was offered and, consequently, they stayed longer on the plastic grid covering the manure pit, the perch or in the nest. These areas neither allows foraging (no litter present) nor feeding (no trough present) and hens are most likely to use this area to rest. In addition, we recorded less foraging in high performing hens. These results indicate that, in line with our hypothesis, selection for high productivity is associated with a reduction of foraging most likely in favor of resting (on the elevated plastic grid, the perch or in the nest). This finding corresponds to the results of Braastad and Katle (1989) who found a negative correlation between food pecking and laying performance in White Leghorns. The current study showed less foraging was not related to less food intake. In contrast, the high performers showed less foraging but a higher food consumption. Thus, the increased laying performance seems to be linked with a more efficient feeding strategy. It cannot be excluded that the differences between the hens of the 4 strains in our study were affected by the time of video observation of the foraging strategy test. Videos were analyzed for 6 h at least 30 min after food reweight in the morning. Thus, differences between hens might have resulted from a different distribution of foraging and feeding activities across daytime. However, also Schütz and Jensen (2001) found less foraging but higher food consumption in high performing hens compared to Red junglefowls with lower egg production.
The measurements of the antenna-transponder system used in the current study leads to a similar conclusion as the foraging strategy test. A reduced effort for foraging in high performing hens is also reflected in their behavior related to the feeder. In the white layers, high performing hens spent less time at feeder area and were less active at feeder compared to low performing hens. The difference between high and low performing hens was less distinct in the brown layers. The lower time spent for feeding by the high performing lines in parallel with a higher food intake compared to the low performing lines supports our conclusion based on video observations that increased laying performance seems to be linked with a more efficient feeding strategy.
A better learning strategy in a feeding-reward context in high performers compared to low performers and reduced social motivation were also observed related to performance (Dudde et al., 2018a). It is quite possible that these behavioral modifications are also linked. More efficient learning in the high performing hens may be a strategy to optimize foraging resulting in decrease CFL and more efficient feeding strategy.
Foraging also is discussed in relation to feather pecking. According to the foraging theory, feather pecking is a redirected food pecking and foraging activity (Blokhuis, 1989). This hypothesis has been challenged in various experiments. While the foraging theory implies a negative correlation between foraging and feather pecking, de Haas et al. (2010) reported more foraging in a high feather pecking line. There was no relationship between foraging and feather pecking in other studies (Newberry et al., 2007; Bessei et al., 2018). Feather pecking is both an animal welfare and an economic problem. Thus, it seems worthwhile to further elucidate possible associations between feather pecking and foraging in relation to laying performance.
We could show, that, beyond domestication, breeding for performance also fundamentally changes behavioral traits. Further studies on possible side effects of selection for production efficiency seem promising to better understand and possibly prevent behavioral problems in laying hen husbandry.
CONCLUSIONS
The results of the present study confirm the hypothesis, that lines with high egg production show a reduced foraging-related behavior. Since this effect occurred in 2 phylogenetic strains which have been selected for many generations it can be assumed that the effect on foraging-related behavior is based on the common effect of selection on laying performance. This can be explained by a shift of energy into reproduction (i.e., laying performance) in case available energy and its metabolism are limited resulting in a lower investment of energy into redundant behaviors such as foraging.
ACKNOWLEDGMENTS
We would like to thank Silke Werner, Oliver Sanders, and Silvia Wittig for technical assistance. We are grateful to Sabine Dippel for her help in the statistical analysis of the data.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
REFERENCES
- Beilharz R., Nitter G. The missing E: the role of the environment in evolution and animal breeding. J. Anim. Breed. Gen. 1998;115:439–453. [Google Scholar]
- Beilharz R.G., Luxford B.G., Wilkinson J.L. Quantitative genetics and evolution: is our understanding of genetics sufficient to explain evolution? J. Anim. Breed. Gen. 1993;110:161–170. doi: 10.1111/j.1439-0388.1993.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Bessei W., Lutz V., Kjaer J.B., Grashorn M., Bennewitz J. Relationships between foraging and open-field activity in young chicks and feather pecking in adult birds: results of analyses using quantitative genetics and structural equation models. Eur. Poult. Sci. 2018;82 pp. 242. [Google Scholar]
- Blokhuis H.J. The effect of a sudden change in floor type on pecking behaviour in chicks. Appl. Anim. Behav. Sci. 1989;22:65–73. [Google Scholar]
- Braastad B.O., Katle J. Behavioural differences between laying hen populations selected for high and low efficiency of food utilisation. Br. Poult. Sci. 1989;30:533–544. doi: 10.1080/00071668908417177. [DOI] [PubMed] [Google Scholar]
- De Haas E.N., Nielsen B.L., Buitenhuis A.J., Rodenburg T.B. Selection on feather pecking affects response to novelty and foraging behaviour in laying hens. Appl. Anim. Behav. Sci. 2010;124:90–96. [Google Scholar]
- Dudde A., Krause E.T., Matthews L.R., Schrader L. More than eggs – relationship between productivity and learning in laying hens. Front. Psychol. 2018;9:2000. doi: 10.3389/fpsyg.2018.02000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudde A., Schrader L., Weigend S., Matthews L.R., Krause T. More eggs but less social and more fearful? Differences in behavioral traits in relation to the phylogenetic background and productivity level in laying hens. Appl. Anim. Behav. Sci. 2018;209:65–70. [Google Scholar]
- Dudde A., Weigend S., Krause E.T., Jansen S., Habig Ch., Schrader L. Chickens in motion – effect of egg production level and pen size on the motor abilities and bone stability of laying hens (Gallus gallus forma domestica) Appl. Anim. Behav. Sci. 2020;227 [Google Scholar]
- Duncan I.J.H., Hughes B.O. Free and operant feeding in domestic fowls. Anim. Behav. 1972;20:775–777. doi: 10.1016/s0003-3472(72)80150-7. [DOI] [PubMed] [Google Scholar]
- Eusemann B.K., Baulain U., Schrader L., Thöne-Reineke Ch., Petow S. Radiographic examination of keel bone damage in living laying hens of different strains kept in two housing systems. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0194974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusemann B.K., Patt A., Schrader L., Weigend S., Thöne-Reineke Ch., Petow S. The role of egg production in the etiology of keel bone damage in laying hens. Front. Vet. Sci. 2020;7:81. doi: 10.3389/fvets.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlander-Matauschek A., Baes C., Bessei W. The demand of laying hens for feathers and wood shavings. Appl. Anim. Behav. Sci. 2006;101:102–110. [Google Scholar]
- Harlander-Matauschek A., Häusler K. Understanding feather eating behaviour in laying hens. Appl. Anim. Behav. Sci. 2009;117:35–41. [Google Scholar]
- Höhne A., Schrader L., Weigend S., Petow S. Ghrelin plasma concentration does not covary with energy demand in adult laying hens. Domest. Anim. Endocinol. 2017;61:77–83. doi: 10.1016/j.domaniend.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Inglis I.R., Forkman B., Lazarus J. Free food or earned food? A review and fuzzy model of contrafreeloading. Anim. Behav. 1997;53:1171–1191. doi: 10.1006/anbe.1996.0320. [DOI] [PubMed] [Google Scholar]
- Jensen G.D. Preference for bar pressing over “freeloading” as a function of number rewarded presses. J. Exp. Psychol. 1963;65:451–454. doi: 10.1037/h0049174. [DOI] [PubMed] [Google Scholar]
- Jensen P. Behavior genetics and the domestication of animals. Annu. Rev. Anim. Biosci. 2014;2:85–104. doi: 10.1146/annurev-animal-022513-114135. [DOI] [PubMed] [Google Scholar]
- Jensen P. and Wright D., Chapter 2 - Behavioral genetics and animal domestication, Editor(s): Grandin T., General Behavior of Domestic Animals, 3rd ed., Academic Press, Cambridge, MA, 2022, 49-93.
- Lieboldt M.-A., Frahm J., Halle I., Görs S., Schrader L., Weigend S., Preisinger R., Metges C., Breves G., Dänicke S. Metabolic and clinical response to Escherichia coli lipopolysaccharide in layer pullets of different genetic backgrounds supplied with graded dietary L-arginine. Poult. Sci. 2016;95:595–611. doi: 10.3382/ps/pev359. [DOI] [PubMed] [Google Scholar]
- Lieboldt M.-A., Halle I., Frahm J., Schrader L., Baulain U., Henning M., Preisinger R., Dänicke S., Weigend S. Phylogenic versus selection effects on growth development, egg laying and egg quality in purebred laying hens. Eur. Poult. Sci. 2015;79 [Google Scholar]
- Lieboldt M.-A., Halle I., Frahm J., Schrader L., Weigend S., Preisinger R., Dänicke S. Effects of long-term graded L-arginine supply on growth development, egg laying and egg quality in four genetically diverse purebred layer lines. J. Poult. Sci. 2015;53:8–21. doi: 10.2141/jpsa.0150067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist C.E.S., Schütz K.E., Jensen P. Red jungle fowls have more contrafreeloading than white leghorn layers: effect of food deprivation and consequences for information gain. Behaviour. 2002;139:1195–1209. [Google Scholar]
- Lyimo C.M., Weigend A., Msoffe P.L., Eding H., Simianer H., Weigend S. Global diversity and genetic contributions of chicken populations from African, Asian and European regions. Anim. Gen. 2014;45:836–848. doi: 10.1111/age.12230. [DOI] [PubMed] [Google Scholar]
- Morejohn G.V. Breakdown of isolation mechanisms in two species of captive junglefowl (Gallus gallus and Gallus sonneratii) Evolution. 1967;22:576–582. doi: 10.1111/j.1558-5646.1968.tb03993.x. [DOI] [PubMed] [Google Scholar]
- Morris B.A., Taylor T.G. The daily food consumption of laying hens in relation to egg formation. Br. Poult. Sci. 1967;8:251–257. doi: 10.1080/00071666708415676. [DOI] [PubMed] [Google Scholar]
- Newberry R.C., Keeling L.J., Estevez I., Bilcik B. Behaviour when young as a predictor of severe feather pecking in adult laying hens: the redirected foraging hypothesis revisited. Appl. Anim. Behav. Sci. 2007;107:262–274. [Google Scholar]
- Polasky Ch., Weigend S., Schrader L., Berndt A. Non-specific activation of CD8α-characterised γd T cells in PBL cultures of different chicken lines. Vet. Immunol. Immunopathol. 2016;179:1–7. doi: 10.1016/j.vetimm.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Potts A. Reaktion Books LTD; London, UK: 2012. Chicken. [Google Scholar]
- Rauw W.M. CABI; Cambridge, MA: 2009. Resource Allocation Theory Applied to Farm Animal Production. [Google Scholar]
- Rauw W.M., Kanis E., Noordhuizen-Stassen E.N., Grommers F.J. Undesirable side effects of selection for high production efficiency in farm animals: a review. Livestock Prod. Sci. 1998;56:15–33. [Google Scholar]
- Schütz K.E., Jensen P. Effects of resource allocation on behavioural strategies: a comparison of red junglefowl (Gallus gallus) and two domesticated breeds of poultry. Ethology. 2001;107:753–765. [Google Scholar]
- Speedy A.W. FAO, FAO Expert Consultation and Workshop; Bangkok, Thailand: 2002. pp. 9–29. (Overview of world feed protein needs and supply, Protein Sources for the Animal Feed Industry). [Google Scholar]
- Väisänen J., Häkansson J., Jensen P. Social interactions in Red Junglefowl (Gallus gallus) and White Leghorn layers in stable groups and after re-grouping. Br. Poult. Sci. 2005;46:156–168. doi: 10.1080/00071660500062638. [DOI] [PubMed] [Google Scholar]
- Väisänen J., Jensen P. Social versus exploration and foraging motivation in young red junglefowl (Gallus gallus) and White Leghorn layers. Appl. Anim. Behav. Sci. 2003;84:139–158. [Google Scholar]
- Väisänen J., Lindqvist C., Jensen P. Co-segregation of behavior and production related traits in an F3 intercross between junglefowl and White Leghorn laying hens. Livstock Prod. Sci. 2005;94:149–158. [Google Scholar]
- Van der Waaij E.H. A resource allocation model describing consequences of artificial selection under metabolic stress. J. Anim. Sci. 2004;82:973–981. doi: 10.2527/2004.824973x. [DOI] [PubMed] [Google Scholar]
- Willems O.W., Miller S.P., Wood B.J. Aspects of selection for feed efficiency in meat producing poultry. Worlds Poult. Sci. J. 2013;69:77–88. [Google Scholar]