Abstract
Introduction
Influenza is a respiratory infection associated with a significant clinical burden globally. Adults aged ≥ 65 years are at increased risk of severe influenza-related symptoms and complications due to chronic comorbidity and immunosenescence. Annual influenza vaccination is recommended; however, current influenza vaccines confer suboptimal protection, in part due to antigen mismatch and poor durability. This systematic literature review characterizes the global clinical burden of seasonal influenza among adults aged ≥ 65 years.
Methods
An electronic database search was conducted and supplemented with a conference abstract search. Included studies described clinical outcomes in the ≥ 65 years population across several global regions and were published in English between January 1, 2012 and February 9, 2022.
Results
Ninety-nine publications were included (accounting for > 156,198,287 total participants globally). Clinical burden was evident across regions, with most studies conducted in the USA and Europe. Risk of influenza-associated hospitalization increased with age, particularly in those aged ≥ 65 years living in long-term care facilities, with underlying comorbidities, and infected with A(H3N2) strains. Seasons dominated by circulating A(H3N2) strains saw increased risk of influenza-associated hospitalization, intensive care unit admission, and mortality within the ≥ 65 years population. Seasonal differences in clinical burden were linked to differences in circulating strains.
Conclusions
Influenza exerts a considerable burden on adults aged ≥ 65 years and healthcare systems, with high incidence of hospitalization and mortality. Substantial influenza-associated clinical burden persists despite increasing vaccination coverage among adults aged ≥ 65 years across regions included in this review, which suggests limited effectiveness of currently available seasonal influenza vaccines. To reduce influenza-associated clinical burden, influenza vaccine effectiveness must be improved. Next generation vaccine production using mRNA technology has demonstrated high effectiveness against another respiratory virus—SARS-CoV-2—and may overcome the practical limitations associated with traditional influenza vaccine production.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02432-1.
Keywords: Burden of disease, Hospitalization, Mortality, mRNA vaccine, Older adults, Strain, Vaccination
Key Summary Points
| Why carry out the study? |
| Risk of influenza-associated clinical burden appeared to increase with age, particularly in those with existing comorbidities and infected with strain A(H3N2) |
| Influenza-related hospitalization and mortality in patients aged ≥65 years varied considerably by season, likely driven by dominant circulating influenza strain, population immunity rates, and the rigor of surveillance and reporting |
| What was learned from the study? |
| The presence of comorbidities was associated with increased rates of influenza-related hospitalization, ICU admissions, ER visits and mortality in patients aged ≥65 years |
| Innovative influenza vaccine design and production, e.g., implementation of mRNA vaccine technology, are required to overcome existing limitations and improve influenza vaccine effectiveness in adults aged ≥65 years |
Introduction
Influenza is a respiratory infection associated with a significant clinical burden globally [1]. Annually, there are an estimated one billion cases of influenza in the general population, of which three to five million cases are severe [2]. The clinical burden is exacerbated by limited regional access to annual influenza vaccines, including limited coverage within some racial and ethnic groups [3, 4]. Furthermore, inadequate influenza surveillance infrastructure, testing practices, and healthcare services can exacerbate influenza clinical burden in low- and middle-income countries (LMICs) [5].
Older adults aged ≥ 65 years are at an increased risk of severe influenza symptoms and the development of serious complications due to chronic comorbidity and age-associated decline in immune function, i.e., immunosenescence [6]. The US Centers for Disease Control and Prevention (CDC) estimated that 70–85% of influenza-related deaths and 50–70% of influenza-related hospitalizations during the 2010–2011 and 2019–2020 seasons were among those aged ≥ 65 years [7]. Similar estimates were reported from the World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC) [8, 9]. Older adults are a heterogenous population, in terms of general lifestyle (active to sedentary), their health status, living arrangements, healthcare support requirements, and access to healthcare resources. To improve clinical outcomes through targeted vaccination of those most at risk, a better understanding of how these risk factors influence severe influenza-related clinical outcomes in older adults is needed.
Influenza vaccines are fundamental to disease prevention and have been licensed in the USA and Europe since the 1960s [1, 10]. The WHO recommends annual vaccination of individuals at greatest risk of developing serious complications from seasonal influenza infection and lists older adults among the priority groups for vaccination [8]. Despite adults aged ≥ 65 years being identified as a high-risk group, there is disparate influenza vaccination coverage in this population globally [11]. Globally in 2019, influenza vaccination coverage rates among adults aged ≥ 65 years were estimated to range from the lowest in Turkey at 5.9% to highest in South Korea at 85.8% [11].
Egg-derived influenza vaccines are the most distributed influenza vaccines globally [12]. However, these traditional vaccines display limited vaccine effectiveness (VE), in terms of strength and longevity of immunogenicity, and breadth of protection across influenza strains [13]. This was evident in countries with widespread vaccination coverage of adults aged ≥ 65 years, such as 67.5% in the USA in 2020, where a substantial influenza-associated clinical burden persists [11, 12].
Suboptimal protection against influenza infection and high clinical burden may be partially explained by antigen mismatch between circulating influenza strains and seasonal influenza vaccines [14]. Circulating influenza strains vary by season and region, and influenza vaccines are produced annually based on predictions of the most prevalent circulating viruses for the coming season [15]. Influenza A(H3N2), which older adults are known to be more vulnerable to, is consistently one of the most common circulating strains and frequently mutates genetically and antigenically [15]. As such, many genetically distinct subtypes of influenza A(H3N2) co-circulate annually around the world [16]. Considerable antigenic drifting of A(H3N2) viruses, among others, results in variable influenza VE due to strain mismatch. For example, between 2012 and 2020, the US CDC estimated that influenza VE among adults aged ≥ 65 years ranged from 12% [95% confidence interval (CI) − 31 to 40] to 66% (95% CI 36–82] in the 2018–2019 and 2015–2016 seasons, respectively [17].
Egg-derived influenza vaccine production takes approximately 6 months and this time lapse exacerbates antigen mismatch between vaccines and circulating strains. The delay can result in poor protection against seasonal influenza, potentiating the clinical burden of disease in older adults [18] . A next generation of influenza vaccines that facilitate improved strain matching and rapid manufacture are necessary to improve influenza VE. Novel vaccine platforms, such as mRNA technology, are well placed to address limitations associated with traditional influenza vaccines. The mRNA manufacturing process allows the precise targeting of multiple influenza subtype strains and is not subject to egg-adapted antigenic changes. Moreover, mRNA vaccines can be rapidly manufactured, which facilitates strain matching nearer the start of the influenza season [19]. Currently, several promising multivalent mRNA influenza vaccines are undergoing early phase clinical trials [20, 21].
The aims of this review were threefold: to characterize the clinical burden of influenza in adults aged ≥ 65 years, to enhance our understanding of the strengths and limitations of currently used influenza vaccines, and to understand how the unique features of mRNA vaccines may be harnessed to reduce the clinical disease burden in this population.
Methods
Search Strategy
The search strategy was designed to capture publications reporting data that described the clinical burden of influenza in older adults (those ≥ 65 years of age) and that were published in English between January 1, 2012 and February 9, 2022. Recognizing that there are substantial differences in global influenza surveillance infrastructure, testing practices and reporting, healthcare services, and administration, we carefully selected countries in disparate regions to achieve a considered global overview—specifically limited to France, Germany, Italy, Spain, the United Kingdom (UK), USA, Canada, China, Japan, Brazil, Saudi Arabia, and South Africa.
An electronic database search was designed following guidance from the Cochrane Handbook for Systematic Reviews of Interventions [22] and conducted on February 9, 2022 in Embase, Medline, Econlit, PsycINFO, and Evidence-Based Medicine Reviews (EBMR) via the OVID® platform.
Bibliographies of relevant systematic literature reviews (SLRs), meta-analyses, and economic analyses identified in the database search were reviewed to ensure all studies that met study inclusion criteria had been captured. The SLR search strategy is presented in Appendix B in the supplementary material.
The database searches were supplemented by searching conference proceedings, and gray literature. Conferences were selected from current infectious disease congresses following critical review of the quantity and relevance of influenza data presented in the previous 2 years. Consequently, conference proceedings from the International Society for Pharmacoeconomic and Outcomes Research (ISPOR), European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), American Thoracic Society (ATS), and IDWeek (joint annual meeting of the Infectious Diseases Society of America (IDSA), Society for Healthcare Epidemiology of America (SHEA), the HIV Medical Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS), and the Society of Infectious Diseases Pharmacists (SIDP)) dating from the January 1, 2020 to February 9, 2022 were included in the search. Gray literature that reported the most recent epidemiological data from the WHO, ECDC, and the US CDC were included.
This review was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Study Eligibility Criteria
All retrieved studies were screened against the Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria outlined in Table 1. It was hypothesized that these outcomes would capture the most frequent and severe influenza-related outcomes, to comprehensively reflect the spectrum of clinical burden of influenza in adults ≥ 65 years. Studies that did not explicitly report data for ≥ 65 years and that reported aggregated data across age groups spanning from < 65 years to ≥ 65 years were excluded.
Table 1.
Eligibility criteria
| Topic | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population(s) | People aged ≥ 65 years with laboratory confirmed seasonal influenza or symptomatic ILI |
Studies reporting data from people aged < 65 years Studies reporting data from people without influenza or symptomatic ILI Studies reporting data from people with pandemic influenza Studies reporting data from people co-infected with influenza and COVID-19 where COVID-19 is not specifically stated as a secondary infection |
| Interventions | Any/none | N/A |
| Comparisons | All | N/A |
| Outcomes |
Prevalence and incidence Breakthrough cases Symptoms Morbidity and mortality Treatment, escalation, and long-term care Hospitalizations and ICU visits Complications and secondary infections Vaccine effectiveness (individuals aged ≥ 65 years who received their annual influenza vaccination) |
Studies reporting clinical outcomes not mentioned in inclusion criteria |
| Time | Studies published from January 2012 to February 2022 | N/A |
| Study design |
Randomized controlled trials Non-randomized interventional studies Observational studies SLRs and meta-analyses |
Editorials Case studies Letters to journals Non-systematic literature reviews Conference minutes |
| Other |
Human studies English language |
Animal studies Duplicates |
ICU intensive care unit, ILI influenza-like illness, N/A not applicable, SLR systematic literature review
The search date parameters spanned the outbreak and height of the COVID-19 pandemic. As the biology and literature relating to co-infection of influenza and SARS-CoV-2 pathogen has not yet been established, data reporting influenza and COVID-19 co-infection were excluded. Additionally, to ensure influenza was the primary study focus, data reporting respiratory infections such as pneumonia were only included if explicitly reported as secondary infection to influenza.
Study Selection and Data Extraction
Abstract and full-text screening were conducted by two independent reviewers, with any differences and uncertainties resolved by a third reviewer. Data extraction was conducted by one reviewer and verified by a second reviewer. Data were extracted into a concise data extraction form (DEF) developed within Microsoft Excel. For each of the included studies, publication information, study characteristics and methods, population and subgroup characteristics, as well as clinical outcomes of interest (Table 1) were extracted. Older adults are a heterogenous population and therefore clinical outcome data across relevant subgroups were captured where available (age, living arrangements, comorbidities, employment status, vaccination status). The DEF was designed to enable direct comparison of clinical outcomes between these subgroups. For the gray literature reporting epidemiological outcomes, prevalence and incidence rates, as well as influenza-associated hospitalization and mortality rates were extracted. A risk of bias assessment was performed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklists [23].
Results
Summary of Results
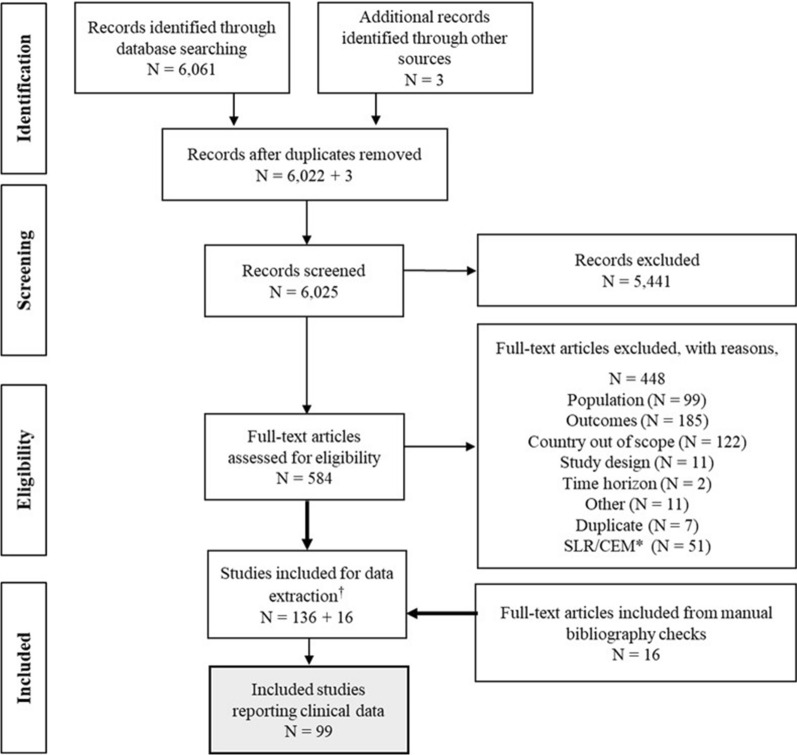
The SLR identified 99 publications that reported clinical burden data for adults aged ≥ 65 years (92 publications via database searches, five publications via manual bibliography search, and two conference abstracts). The number of eligible publications identified during the literature searches and screening process are presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Fig. 1).
Fig. 1.
PRISMA flowchart of publications included in the SLR. *SLRs and cost-effectiveness models captured in the database search that met inclusion criteria underwent a manual bibliography check, and data was extracted from studies that met inclusion criteria and were not already captured. †Search was designed to capture studies reporting the clinical, humanistic, and economic burdens of influenza. For this publication only studies reporting relevant clinical outcomes were included. CEM cost-effectiveness model, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SLR systematic literature review
Study Characteristics
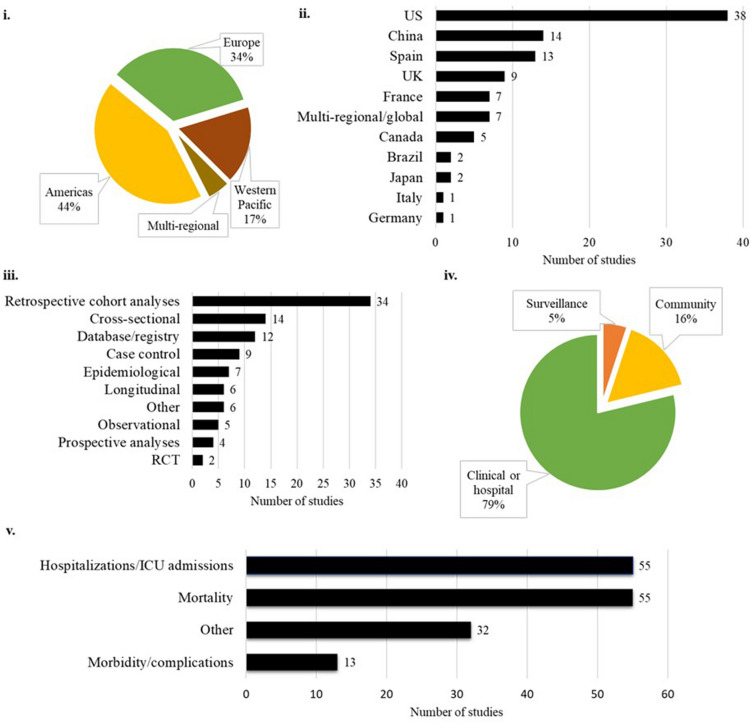
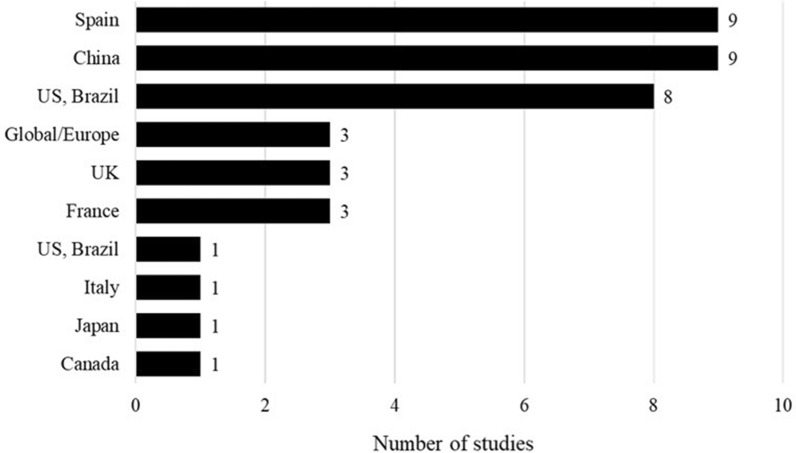
The clinical burden of influenza was reported across multiple regions, and a breakdown of included studies by the WHO regions and country are presented in Fig. 2. Most studies were conducted in the Americas (n = 43), followed by the European region (n = 34). Sixty-six studies were conducted in adults aged ≥ 65 years, while 20 studies were conducted in wider populations but stratified into an ≥ 65 years subgroup, and 13 studies were conducted in older age ranges (e.g., ≥ 80 years).
Fig. 2.
Included study characteristics. Breakdown of included studies by i. WHO region, ii. country, iii. type of study design, iv. study setting, and v. study outcome. ICU intensive care unit, RCT randomized controlled study, WHO World Health Organization
A breakdown of studies identified in the SLR by study setting and design is presented in Fig. 2. Specific clinical outcomes were identified, and a breakdown of the studies reporting clinical burden by outcome is also presented in Fig. 2. Influenza-related hospitalization or ICU admission, and mortality outcomes were equally reported (n = 55). Most publications reported multiple outcomes and therefore the number of studies by outcome is greater than the total number of publications included in the SLR.
Influenza-Related Hospitalization and Outpatient Visits
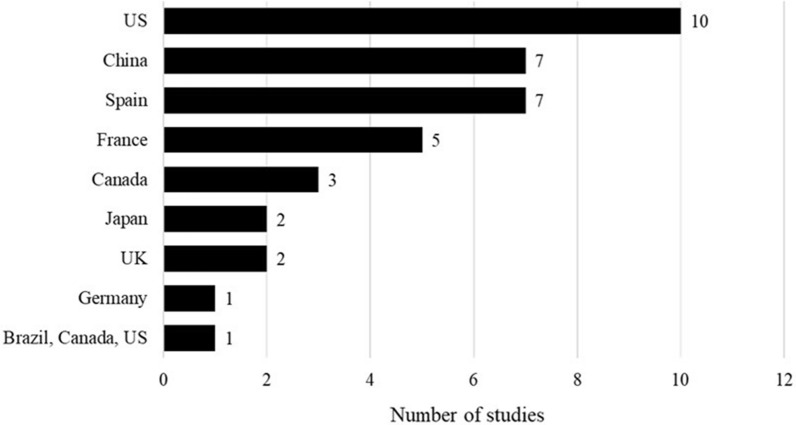
The SLR identified 30 studies reporting the burden exerted by influenza-associated hospitalizations, ICU admissions, emergency room (ER) visits, and outpatient visits. Most studies that reported the burden of influenza-associated hospitalization were conducted in the USA; Fig. 3 shows the breakdown across countries.
Fig. 3.
Studies reporting the burden of influenza-associated hospitalization by country. USA [24, 25, 26, 27, 28, 29, 30, 31, 32, 33], China [34, 35, 36, 37, 38, 39, 40], Spain [41, 42, 43, 44, 45, 46, 47], France [48, 49, 50, 51, 52], Canada [52, 53, 54], Japan [55, 56], UK [57, 58], Germany [59], Multinational (Brazil, Canada, and the USA) [60]
Impact of Age on Hospitalization
An overall trend of increasing hospitalization with increasing age was observed across five studies that reported hospitalization data stratified by age (Table 2) [29, 38, 39, 43, 50].
Table 2.
Studies reporting the impact of age on hospitalization rate
| Country | Author | Key clinical outcome(s) |
|---|---|---|
| USA | Chan, 2015 [39] | Between 1998 and 2012 across influenza strains (A and B), hospitalization rates were higher among adults aged ≥ 75 compared to 65–74 years |
| Matias, 2017 [61] | The mean annual rate of influenza-attributable hospitalizations was higher among patients aged ≥ 75 years than patients aged 65–74 | |
| Ortiz, 2013 [29] | The rates of hospitalization for patients aged 65–74, 75–84, and ≥ 85 years were 8.7 (95% CI 0.3–77.7), 16.5 (95% CI 1.5–126.2), and 27.9 (95% CI 3.2–170.4) per 100,000 population | |
| France | Lemaitre, 2022 [50] | Between 2010 and 2018, there was a higher overall hospitalization incidence among patients aged ≥ 75, but similar hospitalization incidence in the 75–84 years and ≥ 85 years age groups |
| Pivette, 2020 [48] |
Between 2012 and 2017 the mean annual rate of influenza hospitalization was 134 per 100,000 population Over the study period, hospitalization rate ranged between 28 and 358 per 100,000 population, during the 2013–2014 and 2016–2017 seasons, respectively |
|
| Spain | Ramos, 2016 [43] | During 2015, the proportion of influenza hospitalizations was greater among those aged > 80 years (n = 87, 4.9% of total number of patients admitted to study hospital) compared to those in the 65–79 years age group (n = 77, 3.0% of total number of patients admitted) |
| Germany | Pacis, 2022 [59] | Mean rate of weekly influenza-related hospitalizations among patients aged ≥ 80 years decreased from 5.8 in 2019, to 2.2 in 2020 (aIRR 0.4, 95% CI 0.3–0.5, p < 0.001) |
aIRR adjusted incidence rate ratio, CI confidence interval
Of note, Pacis et al. (2022) was a conference abstract that analyzed hospitalizations data between January 1, 2019 and May 31, 2020. The author stated that the decreased rate of hospitalizations was likely due to the national COVID-19 lockdown measures (social distancing and facemasks) that were introduced during this time period [59].
Impact of Living Arrangements
Gruneir et al. (2014) and Andrew et al. (2021) reported hospitalization outcomes by patients’ living arrangements [53, 54]. Gruneir et al. (2014) reported that Canadian long-term care residents aged 66–105 years were at greater risk of hospitalization than community residents in the same age range (322.5 vs 86.0, both per 100,000 population). Increased risk of influenza-related hospitalization among long-term care residents persisted following stratification into 66–85 years (319.5 vs 69.1, both per 100,000 population) and 86–105 years (325.4 vs 215.7, both per 100,000 population) age subgroups [54]. Frequency of influenza-related ER visits was also reported to be higher among long-term care residents than community-dwelling patients aged ≥ 65 years [54]. In another Canadian study, Andrew et al. (2021) reported that 15% of patients aged ≥ 65 years who were admitted to hospital with influenza-like illness (ILI) (n = 346) were admitted from long-term care facilities [53]. Neither study commented on the impact of vaccination status on hospitalization from long-term care.
Hospitalization by Circulating Strain
Six studies stratified hospitalization rate by influenza strain and reported data for influenza A strains A(H3N2) and A(H1N1), and influenza B [25, 30, 34, 36, 37, 38]. Of these strains, influenza A(H3N2) was the strain associated with the highest rate of influenza-related hospitalization across all studies [25, 34, 36, 37, 38], defined as A(H3) in one study [30]. All but one study [34] reported that influenza B was associated with more hospitalizations than A(H1N1) [25, 36, 37, 38] or A(H1) [30]. Li et al. (2021) reported opposing results [34]. The six studies were conducted in China [34, 36, 37] or the USA [25, 30, 38], and, when reviewed together, suggest that the A(H3N2) strain has been consistently associated with the highest rates of influenza-associated hospitalization among those aged ≥ 65 years over the past 20 years [25, 30, 34, 36, 37, 38]. High hospitalizations in A(H3N2)-dominated seasons demonstrate how specific dominant circulating strains such as A(H3N2) can further contribute to high levels of hospitalizations [41].
Chung et al. (2020) discussed that in the 2018–2019 US influenza season, the vaccine provided little protection over A(H3N2) due to antigenic drifting [25]. Moreover, while the A(H1N1)pdm09 viruses were genetically matched, only small proportions of hospitalizations in adults aged ≥ 65 years were prevented by the influenza vaccine in 2018–2019 [25]. The study therefore demonstrated the challenges with antigen mismatch and highlighted that protection against A(H1N1) alone does not prevent high levels of hospitalizations in those aged ≥ 65 years as A(H3N2) results in a greater burden in this population [25].
Hospitalization by Season
Seven studies identified in the SLR estimated the hospitalization burden of influenza by influenza season and highlighted the seasonal variation in clinical burden among those aged ≥ 65 years (Table 3) [26, 27, 40, 41, 48, 50]. Across the studies seasonal variation in the clinical burden of influenza was reported, with a trend toward increasing hospitalization of those aged ≥ 65 years in more recent years (ca. 2013–2014 onwards) [26, 27, 40, 41, 48, 50].
Table 3.
Studies reporting the hospitalization burden of influenza by season
| Country | Author | Key clinical outcome(s) |
|---|---|---|
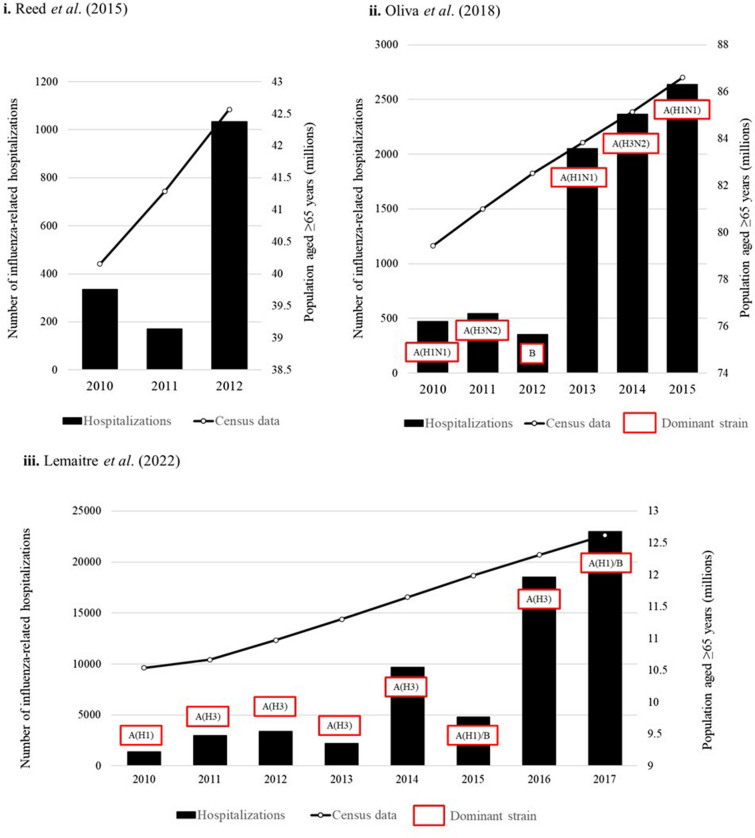
| Spain | Oliva, 2018 [41] |
Between 2010 and 2016, the rate of influenza-related hospitalizations was 16.5 (95% CI 15.7–17.4) per 100,000 population. Oliva et al. (2018) reported an increasing rate of hospitalization from 2013 to 2014 onward (Fig. 4) A(H1N1)2009 was the dominant strain in two of the three seasons with the highest numbers of hospitalizations (2013–2014 and 2015–2016) and A(H3N2) as the dominant strain in the other (2014–2015) Dominant strains in 2010–2011 and 2011–2012 were also A(H1N1)2009 and A(H3N2), respectively, but Oliva et al. (2018) reported much lower numbers of hospitalizations in these seasons |
| France | Lemaitre, 2022 [50] |
A peak in hospitalizations was reported in 2014–2015, with higher numbers of hospitalizations observed from 2016–2017 onwards Dominant strains in the seasons with the highest number of hospitalizations were A(H3) for 2016–2017 and A(H1)/B for 2017–2018. However, these strains were also dominant in previous seasons where considerably lower hospitalizations were reported (Fig. 4) |
| Pivette, 2020 [48] |
Between 2012 and 2017, mean annual rates of hospitalization among those aged ≥ 80 years ranged from 28 per 100,000 population in 2013–2014 to 358 per 100,000 population in 2016–2017. A peak in hospitalization was also reported in 2014–2015 at 174 per 100,000 population The 2014–2015 and 2016–2017 seasons were dominated by A(H3N2), resulting in poorer outcomes among the ≥ 80 years subgroup |
|
| USA | Reed, 2015 [26] | The rate of influenza-related hospitalizations per 100,000 population over the three seasons of study was 335 (95% CI 208–462) in 2010–2011, 170 (95% CI 96–245) in 2011–2012, and 1033 (95% CI 712–1355) in 2012–2013 |
| D’Mello, 2015 [40] | The overall rate of influenza-associated hospitalization was 258 per 100,000 population in 2014–2015 with the rate across the three previous seasons ranging from 30.2 to 183.2 per 100,000 population | |
| Appiah, 2015 [27] | The annual rate of influenza-related hospitalizations was 322.8 cases per 100,000 population during the 2014–2015 season, with a rate ranging from 30.2 to 183.2 between 2010 and 2014 |
CI confidence interval
As depicted in Fig. 4, Reed et al. (2015) (US-based study) reported a peak in the latter season of study with a higher hospitalization rate in 2012–2013 than in the previous two seasons [26]. However, as no further seasons were observed in this study, it was unclear whether this peak represents the start of a trend toward higher hospitalization in this population, or if hospitalization rates decreased in consequent seasons [26]. Interestingly, Oliva et al. (2018) (Spain-based study) and Lemaitre et al. (2022) (France-based study) did not observe the same increase from 2011–2012 to 2012–2013 as reported by Reed et al. (2015), which may suggest that the circulating influenza strains in the USA in 2012–2013 led to greater morbidity than in Europe (Fig. 4) [26, 41, 50]. However, Reed et al. (2015) did not comment on the circulating strain, and further direct comparisons of studies are limited because of heterogeneity across study populations and design. National census data for the population aged ≥ 65 years was also plotted in Fig. 4, and population aged ≥ 65 years increased over time across the three countries (USA, Spain, France) [62, 63, 64]. Across the studies, the number of influenza-related hospitalizations did not consistently increase with population age, indicating that an increasing older adult population was not the only determinant of influenza-related hospitalization frequency.
Fig. 4.
Trends in hospitalization for influenza in patients aged ≥ 65 years by season, reported by three publications [26, 41, 50], and national census data for population aged ≥ 65 years. i. Number of influenza-related hospitalizations among those aged ≥ 65 years by Reed et al. (US-study). Dominant strains not reported, US census data over study period [62]. ii. Number of severe hospitalized confirmed influenza cases among those aged ≥ 65 years reported by Oliva et al. (Spanish study, n of age subgroup not reported). Dominant strains [41], and Spanish census data over the same period [63]. iii. Number of influenza-related hospitalizations; average number of cases reported for ≥ 65 years subgroups (French study) (n of age subgroup not reported). Dominant strains [50] and French census data over the same period [64]
The burden of pandemic influenza was not included in the SLR. However, many studies presented pooled hospitalization data that comprised influenza seasonal strains and previously pandemic strains that circulate seasonally (e.g., A(H1N1)2009). Data captured in the SLR indicate that these circulating pandemic influenza strains contributed to the burden of influenza across these seasons [41]. High hospitalizations in A(H3N2)-dominated seasons also align with the finding that influenza strain impacts influenza hospitalization, with A(H3N2) contributing to high levels of hospitalization [41, 48]. Importantly, however, lower number of hospitalizations were also observed in A(H1N1)2009 and A(H3N2)-dominated seasons [41, 50].
While vaccination status was not reported, Pivette et al. (2020) commented on the 2014–2015 mismatch between the Northern Hemisphere seasonal vaccines and the circulating influenza A(H3N2) viruses, in addition to the considerable genetic diversification of the circulating A(H3N2) viruses in 2016–2017 [48]. D'Mello et al. (2015) similarly highlighted antigenic and genetic drifting of A(H3N2) in 2014–2015 and the reduced VE of Northern Hemisphere vaccines which likely contributed to the higher hospitalizations that season [40].
Readmittance to Hospital
Three studies reported hospital readmittance outcomes with conflicting results [24, 42, 50]. Gonzalez et al. [42] reported a 30-day all-cause hospitalization readmission rate of 4% among a Spanish population (N = 44) of patients aged ≥ 75 years with influenza A [42]. Similar rates were reported by Lee et al. (2021) in a US study (N = 78,668) where readmittance within 30 days of influenza-related hospitalization was higher among patients aged ≥ 80 years than those aged 65–79 years (5.8% vs 4.8%) [24]. A French study conducted by Lemaitre et al. (2022) assessed 3-month readmittance and conversely reported higher readmittance of those aged 65–74 vs 75–84 or ≥ 85 years (27.7% vs 26.0% and 21.9% for all-cause readmittance, respectively) [50]. In this study, similar proportions of patients were readmitted after 3 months for cardiac and respiratory causes [50]. None of the studies contained data on vaccination status and therefore the impact of the seasonal influenza vaccine on readmittance to hospital could not be determined.
Influenza-Related Mortality
Influenza-related mortality was reported by 39 studies in adults aged ≥ 65 years. A breakdown of study location is presented in Fig. 5. In-hospital mortality, post-hospital discharge, and ICU mortality were the most reported mortality-related outcomes (Fig. 5).
Fig. 5.
Studies reporting the burden of influenza-associated mortality by country
Similarly to hospitalization, the SLR identified studies that concluded that risk of influenza-related mortality increased among older patients (aged ≥ 75 years vs aged ≥ 65–74 years) [2, 24, 39, 44, 46, 50, 65, 66, 67, 68, 69], those aged ≥ 65 years with existing comorbidities [61, 70, 71], and when infected with influenza A(H3N2) [25, 34, 37, 72, 73, 74, 75, 76]. Both the proportion and risk of in-hospital mortality increased with age [46]. Lemaitre et al. (2022) and Czaja et al. (2019) reported higher risk of in-hospital mortality among older patients aged ≥ 85 years [50, 67]. Arrieta et al. (2021) reported a 64% influenza vaccination rate for the season of study and there was no significant difference in vaccination status between patients aged ≥ 85 years who were discharged alive or who died [46]. No studies were identified that compared the rate of mortality in patients admitted to hospital versus patients who died outside of a clinical setting.
When influenza-associated mortality rate was examined by influenza strain, influenza A(H3N2) resulted in more deaths than both influenza A(H1N1) and influenza B in eight studies [25, 34, 37, 72, 73, 74, 75, 76]. In particular, Jin et al. (2020) reported 97.02 deaths per 100,000 persons with influenza A(H3N2) compared to 23.4 deaths per 100,000 person in influenza A(H1N1) and 10.3 deaths per 100,000 persons with influenza B, between 2010 and 2015 [72]. Conversely, Qi et al. (2020) reported higher all-cause influenza-related mortality in patients with influenza B compared to A(H3N2) in a study in China (82.0 deaths per 100,000 persons aged ≥ 65 years compared to 13.8 deaths per 100,000 in the same age group, respectively), between 2012 and 2018 [77].
Influenza-attributable mortality rates by influenza season were reported in three studies [26, 78, 79]. Pebody et al. (2018) reported on the proportion of patients aged ≥ 65 years who died as a result of influenza across seven influenza seasons (2008–2016) [78]. In the 2008–2009 season, 14,261 persons died (95% CI 13,514–15,023) compared to the 2015–2016 season where a significant increase was reported (26,542; 95% CI 25,301–27,804). Similarly, a separate study reporting on influenza seasons from 2010 to 2013 found that more recent seasons had a higher overall mortality rate compared to the earlier seasons [22.8 per 100,000 population (95% CI 13.1–32.5) vs 54.6 per 100,000 (95% CI 36.2–73.0)] [26]. This upward trend was not found by Wu et al. (2018) where influenza seasons from 2007 to 2013 were assessed in China. The highest rate of influenza-associated deaths was reported in the earliest influenza season (2007–2008) with 164.1 deaths per 100,000 population (95% CI 101.2–499.9) compared to the most recent season (2012–2013) recording a rate of 75.6 deaths per 100,000 population (95% CI 15.2–349.5) [79].
As a result of the heterogeneity in study settings (USA, UK, and China) it is difficult to conclude on seasonal mortality trends; however, seasonal variability in the mortality burden of influenza was apparent.
Morbidity and Complications
Nine studies reported data on ICU admissions when characterizing the hospitalization burden of influenza in patients aged ≥ 65 years [26, 31, 41, 44, 45, 48, 50, 53, 56]. The proportion of hospitalized patients aged ≥ 65 years admitted to ICU ranged from approximately 4% to 27.3% across studies, with most studies reporting a rate of between 10% and 20% [26, 31, 45, 48, 50, 53, 56].
Chaves et al. (2015) reported that 62% of the study population (N = 6593) had received their seasonal influenza vaccine [31]. Considering all vaccinated and non-vaccinated patients, 13% required ICU admissions. Notably, extended care needs at hospital discharge were required in similar proportions of those vaccinated versus not vaccinated (82–84%) [31]. Extended care was defined as placement in a long-term care facility or rehabilitation facility [31].
Lemaitre et al. (2022) and Soldevila et al. (2021) assessed ICU admissions by age, and reported that increasing age was associated with decreasing ICU admissions [44, 50]. Lemaitre et al. found that the proportions of patients admitted to ICU were higher among those aged 65–74 years than 75–84 and ≥ 85 years. Similarly, Soldevila, et al. reported higher odds of ICU admission in patients aged 65–74 years than ≥ 75 years [44, 50].
Seasonal variability was reported in ICU admission rates, with a possible trend for increasing rates in more recent years. Reed et al. (2015) reported a higher ICU admission rate in 2012–2013 vs the previous two seasons [26]. Oliva et al. (2018) reported a marked increase in the annual number of influenza-attributable ICU admissions between 2010 and 2016 [41]. In the latter three seasons included in the study (2013–2014, 2014–2015, and 2015–2016), the dominant strains were A(H1N1)2009, A(H3N2), and A(H1N1)2009, respectively, which further suggests that the circulating pandemic strain and A(H3N2) result in increased ICU admissions in the older adult population [41]. Pivette et al. (2020) reported that the proportion of ICU admissions among those aged ≥ 80 years remained relatively stable between 2012 and 2016 (7–9%), decreasing to 4% in the 2016–2017 season. However, the lower proportion of ICU admissions in 2016–2017 was likely due to the considerably higher number of hospitalizations in 2016–2017 (13,277 vs 1034–6460 in the previous four seasons), as the number of ICU admissions in 2016–2017 was still higher than in previous seasons (597 vs 90–448) [48]. Similar to Oliva et al. (2018), the dominant strain in 2016–2017 was A(H3N2) which is known to cause a higher burden in patients aged ≥ 65 years. Pivette et al. (2020) also reported a peak in both hospitalizations and ICU admissions in 2014–2015, in which the dominant strain was also A(H3N2), suggesting that the circulation of this strain drives more severe influenza cases and ICU admissions in the older population [48].
The most severely affected patients with influenza infection may be admitted to an ICU and placed on a mechanical ventilator to support breathing. Only three studies included data on mechanical ventilation in those aged ≥ 65 years [31, 42, 46]. A US study by Chaves et al. (2015) found that 4% of patients with influenza aged ≥ 65 years required mechanical ventilation from 2010 to 2013 (N = 6593) [31]. In a Spanish study (N = 117) of patients aged ≥ 85 years, Arrieta et al. (2021) reported that only one patient required non-invasive mechanical ventilation [46]. Of note, only 54.7% of the 117 patients had received the current seasonal influenza vaccination [46]. This proportion of patients requiring mechanical ventilation is higher when considering only those admitted to ICU. Another Spanish study (N = 44) conducted by Gonzalez et al. (2016) found that 27% of patients aged ≥ 75 years admitted to ICU required invasive mechanical ventilation [42]. However, given the small sample size and limited data available for this study, results should be interpreted with caution.
Comorbidities
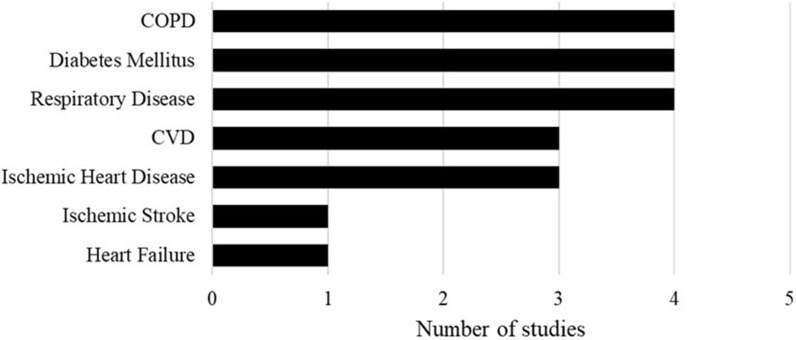
The presence of comorbidities was associated with increased rates of hospitalization, ICU admissions, and ER visits in patients aged ≥ 65 years (Table 4) [38, 80, 81]. The risk of influenza-related hospitalization was higher among adults aged ≥ 65 years with existing cardiovascular diseases (CVD), respiratory diseases, immunosuppressive conditions, diabetes mellitus, metabolic conditions, kidney diseases, and neurological conditions compared to those without these comorbidities [38, 80, 81]. Most studies reported more than one comorbidity, with the majority focusing on people with heart conditions (Fig. 6).
Table 4.
Studies reporting the burden of hospitalizations and ER visits among patients aged ≥ 65 years with existing comorbidities
| Country | Author | Key clinical outcome(s) | |
|---|---|---|---|
| Hospitalizations | USA | Owusu, 2020 [82] | Mean annual rate of influenza-related hospitalization was higher among patients with diabetes mellitus [276 (95% CI 230–330) per 100,000 population] than without [181 (95% CI 150–217) per 100,000 population] |
| Near, 2020 [80] |
The rate of influenza-related hospitalizations was higher among older patients [≥ 65 (9.4%] vs ≥ 75 years (25.7%)] The rate of influenza-related hospitalizations among patients aged ≥ 65 increased to 27.4% when patients presented with pre-existing COPD or CHF |
||
| Matias, 2017 [61] |
Between 1997 and 2009, annual mean hospitalization rates among patients aged 65–74 years and ≥ 75 years were 256 (SD 91, range 83–385) and 589 (SD 216, range: 173–864), per 100,000 population, respectively During the study period, hospitalization rate across the low- and high-risk cohorts was higher among patients aged ≥ 75 years compared to patients aged 65–74 years The highest hospitalization rate across risk and age groups was incurred by influenza A(H3N2) |
||
| Young-Xu, 2017 [81] |
The mean annual rate of influenza-attributed hospitalization was 71 (95% CI 60–83) per 100,000 Patients were stratified into high- and low-risk groups, with those defined as high risk having had at least one diagnosis code for chronic cardiac, pulmonary, renal, metabolic, liver, neurological diseases, diabetes, hemoglobinopathies, immunosuppressive conditions, or malignancy assigned during the influenza season The mean annual rate of influenza-attributed hospitalizations was higher in the high-risk group of patients [144 (95% CI 121–167) per 100,000 population] than the low-risk group [0 (95% CI 0–1) per 100,000] Of note, the high- and low-risk group sample sizes were 2029 and 4, respectively |
||
| China | Chair, 2020 [35] |
Between 1997 and 2017, 1199 patients were admitted for heart failure within 12 months of influenza-associated hospitalization The aOR for heart failure-related admittance was 1.1 (95% CI 1.0–1.2) |
|
| England | Cromer, 2014 [70] |
Patients with an acute respiratory illness code and with ICD-10 codes in other diagnostic fields for conditions indicated for seasonal influenza vaccination were flagged as being in a clinical risk group Among adults aged ≥ 65 years, being in a risk group increased the hospital admission rate by 1.8-fold (from 0.5 to 0.8/1000). Average annual admissions in those at clinical risk vs not at clinical risk were 368,489 vs 53,254, respectively |
|
| ER visits | USA | Near, 2020 [83] |
The presence of comorbidities in patients with influenza is associated with an increased rate of ER visits The proportion of patients visiting the ER with CKD stage 5/ESRD/dialysis and influenza was higher than CKD stage 5/ESRD/dialysis only (60 vs 28%, p < 0.5). Similar trends were observed for influenza with and without CAD (37 vs 14%, p < 0.5), COPD (44 vs 18%, p < 0.5), CHF (49 vs 23, p < 0.5) |
| Mortality | Spain | Soldevila, 2020 [71] | The comorbidity reported to result in the highest proportion of influenza deaths was congestive heart failure [50% (OR 2.5; 95% CI 1.5–4.2)] |
| China | Qi, 2020 [77] |
The annual influenza-associated mortality rate in patients with influenza A(H3N2) and B, and underlying COPD was 7.9 per 100,000 population (95% CI 6.3–9.5) and 23.1 per 100,000 population (95% CI 22.3–24.0), respectively The annual influenza-associated mortality rate in patients with influenza A(H3N2) and B, and underlying ischemic heart disease was 5.5 per 100,000 population (95% CI 4.5–6.5) and 11.1 per 100,000 population (95% CI 10.6–11.6), respectively |
aOR adjusted odds ratio, CAD coronary artery disease, CHF congestive heart failure, CI confidence interval, CKD chronic kidney disease, COPD chronic obstructive pulmonary disorder, ER emergency room, ESRD end-stage renal disease, ICD-10 International Classification of Disease 10th edition, OR odds ratios, SD standard deviation
Fig. 6.
Patient comorbidities in studies reporting clinical burden of influenza among patients with underlying conditions. COPD chronic obstructive pulmonary disease, CVD cardiovascular disease. COPD [24, 71, 72, 77], diabetes mellitus [24, 71, 75, 84], respiratory disease [71, 75, 76, 84], ischemic stroke [77], CVD [75, 76, 85], heart failure [24], ischemic heart disease [72, 77, 85]
Conflicting results were reported by Jin et al. (2020) and Qi et al. (2020) for people aged ≥ 65 years with laboratory-confirmed influenza in China. Jin et al. reported that among strains in people with ischemic heart disease or chronic obstructive pulmonary disease (COPD), influenza A(H3N2) resulted in a higher influenza-associated excess mortality rate than other influenza viruses (2010–2015) [72]. In contrast, Qi et al. reported that in people with ischemic heart disease or COPD, influenza A(H3N2) resulted in a lower mortality incidence rate than influenza B (2012–2018) [77].
The comorbidity reported to result in the highest proportion of influenza-related deaths was congestive heart failure [50% (OR 2.5; 95% CI 1.5–4.2)] [71]. Cromer et al. (2014) reported data for patients with comorbidities more broadly, by classifying the risk level of certain conditions using International Classification of Diseases (ICD)-10 codes [70]. The mortality rate of hospitalized patients and number of deaths per influenza-related hospital admission were reported in adults ≥ 65 years by clinical risk level. The annual prevalence of in-hospital mortality among adults ≥ 65 years not at clinical risk (without aforementioned diagnosis codes) was 378.0 deaths (SD ± 11) compared with those clinically at risk 1298 (SD ± 56). Mortality rates per 1000 influenza admissions were 185 (95% CI 179–192) vs 428 (95% CI 391–473) in the same populations, suggesting that those with comorbidities are at increased risk of influenza-related mortality [70].
Matias et al. (2014) also stratified patients by risk status, age, and influenza strain; high risk was defined as the presence of COPD, CVD, kidney disorders, diabetes, immunosuppression, liver disorders, stroke, or CNS disorders, while low risk was the absence of these comorbidities [61]. A(H3N2) was the strain associated with the highest number of deaths, and of patients with this strain, high-risk adults had higher mortality than low-risk adults [61]. A higher number of deaths was reported in those aged ≥ 75 years vs 65–74 years [61]. Additionally, high-risk subgroups had a higher influenza B-associated mortality rate vs low-risk subgroups for either age group [61].
Burden of Influenza by Region and Hemisphere
Global and Regional Differences
A modeling study by Iuliano et al. (2018) predicted a global annual mortality rate of 13.4–27.8 per 100,000 for adults aged 65–74 years and 51.3–99.4 per 100,000 for adults aged > 74 years [2]. By region/country, the lowest mortality rates (per 100,000) were reported for South Korea (3.8–24.9) and Japan (3.5–27.5), followed by Canada (6.1–44.5), the USA (8.6–49.4), Spain (6.8–54.7), the UK (17.3–66.6), Hong Kong (12.0–84.6), Brazil (19.8–111.1), China (19.1–112.7), and South Africa (37.4–123.3). These data suggested a higher mortality burden among upper-middle-income countries. Across all countries, mortality was highest in adults aged > 74 years vs 65–74 years.
In the Americas, Cheng et al. (2015) also reported that influenza-related mortality rates due to respiratory causes and/or circulatory causes were higher in Southern Brazil than in the USA [65]. Conversely, Palekar et al. (2019) found a lower burden of influenza in Brazil; influenza hospitalizations were lower in Brazil than Canada and the USA (2010–2015) [60]. Drivers for this were suggested, as there is a lower bed density in countries with lower income which may account for the lower hospitalization reported. Disparities in surveillance and reporting methodology and circulating strain may also have contributed.
In Europe, Paget et al. (2022) found that influenza respiratory mortality in 2002–2011 was similar across France, Germany, Italy, and Spain (ranging from 28.3 to 29.1 per 100,000) and slightly lower in the UK (25.5 per 100,000) [86].
Northern Hemisphere
All studies conducted in the Northern Hemisphere were in high-income countries, except for China. Despite socioeconomic and demographic differences, there was an increased risk of influenza-associated hospitalization [41, 42, 57] and mortality among adults aged ≥ 65 years across the regions [2, 48, 50, 65, 66, 87]. Seasonal variation in clinical burden was reported across France [48, 50], Spain [41], and the USA [26, 27, 40]. Influenza A(H3N2) was the strain responsible for the highest frequency of influenza-associated hospitalizations and mortality in adults aged ≥ 65 years across several seasons [25, 26, 34, 36, 37, 39]. No data were identified for Saudi Arabia.
Several studies commented on the mismatch between Northern Hemisphere vaccines and how this was driving high clinical burden in adults aged ≥ 65 years, notably in the 2014–2015 and 2016–2017 influenza seasons [40, 48, 88]. During the 2014–2015 influenza season, the influenza vaccine strains selected for the Northern Hemisphere were antigenically mismatched with the circulating A(H3N2) strains as a result of antigen mutation [89]. Genetic egg-adapted changes led to differences between H3N2 egg-derived vaccines and circulating strains during the 2016–2017 season [19].
Continental and seasonal climates can variably affect the seasonality and transmission dynamics of influenza [90]. Regions in the Northern Hemisphere with subtropical climates, such as China, can experience more variable seasonality than in temperate climates [90]. Feng et al. (2012) reported that mean annual incidence of all-cause influenza-associated mortality was high across both subtropical and temperate regions of China, with higher mortality reported in cities with temperate climates, possibly driven by differences socioeconomics and reporting [91]. A review of influenza burden and transmission across tropical regions by Ng and Gordon (2015) reported that, similar to data seen for temperate regions, levels of influenza-related hospitalization and mortality were high among adults aged ≥ 65 years [90].
Southern Hemisphere
All of the studies conducted in the Southern Hemisphere were in upper-middle-income countries and confined to Brazil and South Africa as per the study scope. Consistent with the Northern Hemisphere data, the risk of influenza-associated mortality increased with age, among adults aged ≥ 65 years [2, 65]. Brazil experiences equatorial, tropical, and subtropical climates which impacts influenza activity. The lack of winter in tropical climates such as Brazil means that year-round influenza circulation can occur, with multiple peaks of high disease burden [90].
Of the countries within our study scope, the SLR identified a clear gap in data available for adults aged ≥ 65 years for countries in the Southern Hemisphere.
Discussion
There are limited existing SLRs which focused on exploring the clinical burden of influenza in a single country or region with scope not limited to those aged ≥ 65 years and including other high-risk subpopulations. The focus of this SLR was to enhance our understanding of the current clinical burden of influenza among adults aged ≥ 65 years from a global perspective, and the potential clinical benefit of harnessing mRNA vaccine technology.
Data identified in this SLR reflect the substantial clinical burden of influenza among adults aged ≥ 65 years, characterized by high rates of hospitalizations, escalation of care (such as ICU admissions) and mortality. These outcomes were also reported to worsen with increasing age—people aged ≥ 75 years were more likely to be hospitalized for influenza than those aged ≥ 65–74 years [29, 38, 39, 50], with some indication that the hospitalization risk continues to increase with age [29, 43]. Furthermore, people with influenza aged ≥ 75 years were more likely to die than those aged 65–74 years [2, 24, 39, 44, 46, 50, 65, 66, 67, 68, 69]. Older adults (aged ≥ 65 years) are a growing subpopulation [92], and have greater healthcare needs, which frequently require hospitalization. These findings suggest that influenza infection exacerbates the existing clinical burden and highlights the need for improved influenza VE and vaccination coverage in this age group.
Older adults are a heterogenous population in terms of health and care requirements. Various comorbidities are common among adults aged ≥ 65 years [92], placing them at a higher risk for several conditions and contributing to their increased vulnerability to influenza. The literature identified in this SLR largely suggested that the burden of disease is exacerbated in older adults with comorbidities. Existing cardiovascular diseases, respiratory diseases, immunosuppressive conditions, diabetes mellitus, metabolic conditions, kidney diseases, and neurological conditions were all associated with increased risk of poor clinical outcomes among patients aged ≥ 65 years with influenza. The high prevalence of these comorbidities among older adults in the general population puts this population at risk of serious influenza infection, hospitalization, and death, highlighting the need for effective vaccination programs is this population [92]. Older adults also vary widely in terms of working and living arrangements. Although few data were identified, and were limited to Canada, the findings of this SLR suggested that long-term care residents with influenza were more likely to be hospitalized than community residents, even when stratified by age group [54]. These findings are consistent with CDC guidance, which states that people who live in nursing homes or other long-term care facilities are at higher risk of serious influenza complications [93]. This is an important trend, considering that from 2011 to 2026, there is a projected 71% increase in the number of Canadian residents aged ≥ 65 years who will require continuing care [94]. Therefore, greater understanding of the variable risk of influenza infection based on living arrangements in Canada and across regions would improve targeting of the most at-risk adults aged ≥ 65 years for influenza vaccination. No data characterizing differences in influenza clinical outcomes between minority and ethnic groups or employment status (e.g., retired, semi-retired, working) were identified. Therefore, further research across countries and older adult subpopulations may enhance the understanding of differential risk within the ≥ 65 years population.
While this SLR aimed to characterize the burden of disease across countries, the majority of identified studies were limited to North America and Europe, with a good representation of high-income countries in the Northern Hemisphere. Conversely, the data identified for Southern Hemisphere countries were limited and confined to Brazil and South Africa, both upper-middle-income countries. The high volume of burden data available for countries such as the USA could be due to the sophisticated influenza surveillance programs in these high-income countries. No differences between Northern and Southern hemisphere countries were identified, with all studies in this SLR reporting high clinical burden in the older population. However, as representative data for Southern Hemisphere countries was lacking, in-depth comparisons could not be made. Given the hemispheric differences in seasonality, circulating strains, and vaccination recommendations [95], further investigation into the differences in clinical burden of influenza across these regions is needed to characterize the specific burden of influenza among adults aged ≥ 65 years in the Southern Hemisphere.
The SLR was, however, able to consider the potential impact of income status on country-level differences in influenza burden. Disparities in disease burden by income status were reported by a global modeling study which reported the lowest mortality rates in high-income countries (South Korea and Japan) and the highest in the upper-middle-income countries included in this SLR (Brazil, China, and South Africa) [2]. Two further studies compared data for Southern Brazil and the USA; mortality was similarly higher in Southern Brazil than in the USA in the first [65], while the second reported fewer hospitalizations in Southern Brazil than the USA [60]. Drivers for the lower hospitalization in Brazil were suggested, including a lower bed density in countries with lower income [60]. This can contribute to fewer hospitalizations which may therefore skew the burden of disease data where hospitalization data is used as a proxy. Differences in surveillance and reporting methodology and circulating strain may also have contributed to country-level differences. Improved and standardized influenza surveillance infrastructure and reporting practices would facilitate better comparisons of the true burden of disease across countries.
Moreover, studies were often conducted across single or few cities within countries or regions, and therefore were unlikely to provide an accurate representation of the clinical burden at a national or regional level. This is due to population differences, variability in the level of available healthcare, and potential differences in climates across countries. Climatic differences in particular can affect the seasonality and transmission dynamics of influenza, thereby contributing to differences in influenza burden within countries or regions [90]. This is particularly relevant in countries where both temperate and tropical climates exist. The timing of the WHO influenza vaccination recommendations often does not correspond to the dynamics of the tropical regions in areas such as South America, China, and Africa as influenza activity is often out of phase with other dynamics in the hemispheric group [96]. Suboptimal recommendations and subsequent vaccine-induced protection across such regions may explain why these areas were found to be at high risk of clinical burden of influenza in the studies included in this SLR. This highlights the need to tailor vaccine formulation and timing of administration to each geographic area to better protect against influenza each season.
Understanding the seasonal nature and variability of influenza is vital to optimize vaccination strategies. This SLR emphasized the differential impact of influenza among adults aged ≥ 65 years between seasons with high levels of seasonal variability in clinical outcomes including hospitalizations and mortality identified across the literature. The seasonal differences are likely driven by a variety of factors including the level of natural and vaccine-induced immunity in the population, differential surveillance and reporting across seasons, and the seasonal dominant strain and its transmissibility. Influenza A(H3N2) is often associated with more severe flu seasons and is known to result in poor outcomes among the older adult population [25, 30, 34, 36, 37, 38, 41].
Multiple studies in this SLR found that seasons dominated by A(H3N2) were associated with high rates of hospitalization and mortality and more severe infection, resulting in ICU admissions [25, 30, 34, 36, 37, 38]. The substantial burden exerted by A(H3N2) may, in part, be due to the frequency that A(H3N2) mutates genetically and antigenically, which is higher than other common influenza subtypes [97]. The mutagenic nature of A(H3N2) increases the likelihood of antigen mismatch and suboptimal VE, which in turn increases the risk of influenza-associated hospitalization/ICU admission and mortality in seasons dominated by the A(H3N2) strain [97]. This challenge was discussed across several studies included in this SLR where the mismatched Northern Hemisphere vaccine in specific seasons was concluded to drive the peaks in clinical burden observed [40, 48, 88]. The clinical burden generated by influenza A(H3N2) may therefore persist despite vaccination in the ≥ 65 years population due to a mismatch between seasonal vaccines and circulating strains and subsequent suboptimal vaccine-induced immunity.
As a result of the increased risk of developing serious influenza-associated outcomes and complications among older adults, the WHO recommends that this population receive an annual influenza vaccination [8]. While vaccination coverage varies across regions, adults aged ≥ 65 years are a priority group for influenza vaccination, globally [7]. However, as found in this SLR, the clinical burden of influenza persists among older populations despite the availability of seasonal vaccine, which further suggests that there may be limitations in the current influenza vaccination programs and formulations. High-dose vaccines were developed to address reduced protection against severe influenza outcomes offered by standard-dose influenza vaccines in adults aged ≥ 65 years [7]. Currently available influenza vaccines have been shown to reduce the burden of influenza-related morbidity and mortality [98].
Despite the evolution of influenza vaccine formulations (first inactivated influenza vaccine [99], egg-derived and cell-based [100], addition of a fourth strain in recent years, and development of adjuvanted and recombinant vaccines [101, 102], and different dose formulations), we continue to see a significant clinical disease burden. Although currently available influenza vaccines have been shown to reduce the burden of influenza-related morbidity and mortality, the findings of this review demonstrate that a high clinical burden of influenza persists among older populations. The continued clinical burden of influenza disease and limitations of current vaccines support the entry and positioning of mRNA vaccine technology as a potential solution.
Seasonal variation observed in both high-dose and standard-dose influenza VE [103, 104, 105, 106] suggests that the differences are driven in part by seasonal factors, such as the prevalent circulating strain. In this SLR, the mismatch of seasonal influenza vaccines and circulating strains was reiterated across the findings of several studies, demonstrating suboptimal vaccine-induced immunity against dominant strains. Mismatch is particularly evident when genetic and antigenic variations occur after seasonal predictions have been made and vaccine manufacture has begun, which in some seasons can result in inadequate vaccine-induced protection. Moreover, studies reporting influenza VE for France, Germany, Italy, Spain, and UK have found a lower VE associated with increasing age and variable effectiveness against severe influenza-related outcomes in patients aged ≥ 65 years [45, 87, 107, 108, 109, 110]. These findings suggested that current influenza vaccines are suboptimal within a population with high morbidity and mortality. This was mirrored by the high rates of hospitalization and mortality in older adults seen across European countries included in this SLR. Limitations of current vaccines therefore persist despite strain prediction methods across a range of global regions, highlighting the need for more consistently effective seasonal vaccines. To overcome the limitations of antigen mismatch, the development of “universal” influenza vaccines that target antigenic determinants is needed [13]. New technologies may improve influenza VE by offering a solution for the challenges experienced with strain predictions and the time taken to develop traditional vaccines. Novel vaccine production methods such as using mRNA technology offers precise targeting of the antigenic determinants of multiple regional-specific influenza subtype strains and rapid manufacture. The manufacturing benefits of mRNA technology would facilitate strain prediction nearer the start of the influenza season, which is currently difficult considering the production demands for national antigen-based influenza vaccination schemes. Further, mRNA vaccines are not subject to limitations affecting traditional egg- and cell-based influenza vaccines, such as egg-adapted changes, which frequently alter immunogenicity and result in suboptimal VE [19].
The COVID-19 pandemic has generated an unprecedented wealth of data reporting the real-world safety and effectiveness of mRNA vaccines and has provided a clinical proof of concept to support mRNA-based influenza vaccines [111]. Post-authorization safety surveillance from the distribution of four billion doses of mRNA-based COVID-19 vaccines has not uncovered a major safety signal (only very rare reports of myocarditis in select age groups and anaphylaxis) and provides reassurance about the safety profile of this novel vaccine platform [111]. Several promising multivalent mRNA influenza vaccines are currently undergoing early phase clinical trials [20, 21] Although further research is required, early data considered in the context of the findings of this SLR suggest the novel mRNA vaccine technology may avoid some of the manufacturing limitations seen with current influenza vaccines, and attenuate the high clinical burden of seasonal influenza among adults aged ≥ 65 years.
A potential limitation of this SLR was the age restriction applied to the study population. The search strategy relied on relevant literature being appropriately indexed, with influenza and age stratification of outcomes present in the study title or abstract. Only data explicitly reported for adults aged ≥ 65 years was extracted. This may have led to the exclusion of data for the older adult population that was defined by a different threshold (e.g., 60–80 years). To refine the scope of the search, studies that made no reference to age stratification or older age/elderly patients were excluded at abstract screening stage. In addition, any studies that only referred to patient populations as “older” or “elderly” adults without specifying an age range of at least ≥ 65 years were excluded at full-text stage. It should be noted that co-infection data for influenza and other respiratory diseases (e.g., pneumonia, COVID-19, respiratory syncytial virus) were also excluded from this SLR to refine search scope and ensure that influenza remained the primary focus. However, as these respiratory infections often co-exist with influenza and are common among older adults, it is likely that the clinical burden of disease reported here is underestimated.
The lack of reported vaccination status in many of the included studies may have also limited interpretation of current vaccination efficacy on influenza-related outcomes captured in this SLR. Furthermore, limited available data for the countries within study scope (particularly for Southern Hemisphere countries) and disparate influenza vaccination surveillance and reporting practices limited direct comparison of clinical outcomes across different regions.
This SLR also did not identify any data reporting long-term sequelae of influenza in the included studies. While long-term complications of influenza may be present among those recovering from infection, this was not a focus of the studies reviewed. To date, there is no published literature reporting the clinical burden of long-term sequelae of influenza in older populations. Therefore, further empirical investigation into the prolonged clinical burden of influenza in this population is warranted.
Conclusion
Older adults are at high risk of influenza-associated hospitalizations and mortality, which is further exacerbated in the presence of comorbidities. A wealth of data highlighted the poor clinical outcomes in high-income countries, despite established national vaccination programs and relatively high influenza vaccination coverage in adults aged ≥ 65 years. Seasonal variation in disease burden was apparent, due in part to mismatched seasonal vaccines, suggesting that more accurate prediction and design of influenza vaccines to better match the circulating strains would improve VE in adults ≥ 65 years. mRNA vaccine technology developed for the COVID-19 pandemic may address the challenges in seasonal strain prediction, antigen mismatch, and manufacture time, and help reduce the overall clinical burden of influenza in adults aged ≥ 65 years.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Richard Macey for his help designing the search strategy and support during the systematic literature review process.
Funding
This study was funded by Pfizer Ltd. Open Access Fees and journal Rapid Service Fee were funded by Pfizer Ltd.
Author Contributions
All authors were involved in study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by Isabelle Whittle, Kristen Markus, Amy Sears and Ashley Enstone, and all authors provided comments on subsequent drafts. All authors read and approved the final manuscript.
Disclosures
Authors Jakob Langer, Verna L. Welch, Mary Moran, Alejandro Cane, Santiago M.C. Lopez are employees of Pfizer Inc and may hold stock or stock options. Amit Srivastava is employed by Orbital Therapeutics; he was a Pfizer employee at time of manuscript development and may hold stock or stock options. Ashley Enstone, Amy Sears, Kristen Markus, Maria Heuser, Rachel Kewley, and Isabelle Whittle are employees of Adelphi Values PROVE. Adelphi Values PROVE received funding from the study sponsor for the conduct of the review.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Moore KA, Ostrowsky JT, Kraigsley AM, et al. A Research and Development (R&D) roadmap for influenza vaccines: looking toward the future. Vaccine. 2021;39(45):6573–6584. doi: 10.1016/j.vaccine.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraigsley AM, Moore KA, Bolster A, et al. Barriers and activities to implementing or expanding influenza vaccination programs in low- and middle-income countries: a global survey. Vaccine. 2021;39(25):3419–3427. doi: 10.1016/j.vaccine.2021.04.043. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Flu disparities among racial and ethnic minority groups. 2021. https://www.cdc.gov/flu/highrisk/disparities-racial-ethnic-minority-groups.html#:~:text=Among%20adults%20(18%20years%20and,Indian%20or%20Alaska%20Native%20adults. Accessed 28 July 2022.
- 5.Ortiz JR, Neuzil KM. Influenza immunization in low- and middle-income countries: preparing for next-generation influenza vaccines. J Infect Dis. 2019;219(Suppl_1):S97–S106. [DOI] [PubMed]
- 6.Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Flu & people 65 years and older. 2021. https://www.cdc.gov/flu/highrisk/65over.htm. Accessed 22 June 2022.
- 8.World Health Organization. Influenza (seasonal). 2018. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 8 Aug 2022.
- 9.European Centre for Disease Prevention and Control. Factsheet about seasonal influenza. 2022. https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet. Accessed 9 Aug 2022.
- 10.Barberis I, Myles P, Ault SK, Bragazzi NL, Martini M. History and evolution of influenza control through vaccination: from the first monovalent vaccine to universal vaccines. J Prev Med Hyg. 2016;57(3):E115–E120. [PMC free article] [PubMed] [Google Scholar]
- 11.Organization for Economic Cooperation and Development database. Influenza vaccination rates. 2021. https://data.oecd.org/healthcare/influenza-vaccination-rates.htm. Accessed 30 June 2022.
- 12.Centers for Disease Control and Prevention. How influenza (flu) vaccines are made. 2021. https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm#:~:text=Cell%2Dbased%20flu%20vaccine%20production,the%20flu%20vaccine%20manufacturing%20process. Accessed 25 July 2022.
- 13.Pilkington EH, Suys EJA, Trevaskis NL, et al. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Antigenic characterization. 2021. https://www.cdc.gov/flu/about/professionals/antigenic.htm. Accessed 22 June 2022.
- 15.World Health Organization. Influenza laboratory surveillance information by the Global Influenza Surveillance and Response System (GISRS). 2022. https://apps.who.int/flumart/Default?ReportNo=6. Accessed 21 June 2022.
- 16.Centers for Disease Control and Prevention. Types of influenza viruses. 2021. https://www.cdc.gov/flu/about/viruses/types.htm. Accessed 22 June 2022.
- 17.Centers for Disease Control and Prevention. CDC seasonal flu vaccine effectiveness studies. 2022. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm. Accessed 9 Aug 2022.
- 18.Tricco AC, Chit A, Soobiah C, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci USA. 2017;114(47):12578–12583. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A study of mRNA-1010 seasonal influenza vaccine in healthy adults (NCT04956575). 2022. https://clinicaltrials.gov/ct2/show/NCT04956575. Accessed 19 July 2022.
- 21.A study to evaluate the safety, tolerability, and immunogenicity of a modified RNA vaccine against influenza (NCT05052697). ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT05052697. Accessed 19 July 2022.
- 22.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022): Cochrane; 2022. https://training.cochrane.org/handbook.
- 23.Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. 2020. https://synthesismanual.jbi.global. Accessed 29 July 2022.
- 24.Lee CC, Liu Y, Lu K-T, et al. Comparison of influenza hospitalization outcomes among adults, older adults, and octogenarians: a US national population-based study. Clin Microbiol Infection. 2021;27(3):435–42. [DOI] [PubMed]
- 25.Chung JRR, Flannery MA, Prasad B, et al. Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clin Infect Dis. 2020;71(8):E368–E376. [DOI] [PMC free article] [PubMed]
- 26.Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS ONE. 2015;10(3):e0118369. doi: 10.1371/journal.pone.0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appiah GD, Blanton L, D'Mello L, et al. Influenza activity - United States, 2014–15 season and composition of the 2015–16 influenza vaccine. MMWR Morb Mortal Wkly Rep. 64(21):583–90. [PMC free article] [PubMed]
- 28.Jules A, Grijalva CG, Zhu Y, et al. Age-specific influenza-related emergency department visits and hospitalizations in 2010–2011 compared with the pandemic year 2009–2010. Infect Dis Clin Pract. 2014;22(5):271–8.
- 29.Ortiz JR, Neuzil KM, Rue TC, et al. Population-based incidence estimates of influenza-associated respiratory failure hospitalizations, 2003–2009. Am J Respir Crit Care Med. 2013;188(6):710–5. [DOI] [PubMed]
- 30.Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis. 2012;54(10):1427–36. [DOI] [PMC free article] [PubMed]
- 31.Chaves SS, Perez A, Miller A, et al. Impact of prompt influenza antiviral treatment on extended care needs after influenza hospitalization among community-dwelling older adults. Clin Infect Dis. 2015;61(12):1807–14. [DOI] [PubMed]
- 32.Nguyen JL, Yang W, Ito K, et al. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1(3):274–81. [DOI] [PMC free article] [PubMed]
- 33.Machado MAA, Moura CS, Abrahamowicz M, et al. Relative effectiveness of influenza vaccines in elderly persons in the United States, 2012/2013–2017/2018 seasons. NPJ Vaccines. 2021;6(1):108. [DOI] [PMC free article] [PubMed]
- 34.Li J, Wang C, Ruan L, et al. Development of influenza-associated disease burden pyramid in Shanghai, China, 2010–2017: a Bayesian modelling study. BMJ Open. 2021;11(9):e047526. [DOI] [PMC free article] [PubMed]
- 35.Chair SY, Cheng HY, Choi KC, et al. Influenza-associated hospitalizations and risk of subsequent heart failure hospital admissions: a 20-year territorywide registry study in Hong Kong, China. Am J Epidemiol. 2021;190(5):779–85. [DOI] [PubMed]
- 36.Wang XL, Yang L, Chan KH, et al. Age and sex differences in rates of influenza-associated hospitalizations in Hong Kong. Am J Epidemiol. 2015;182(4):335–44. [DOI] [PubMed]
- 37.Wu P, Presanis AM, Bond HS, Lau EHY, Fang VJ, Cowling BJ. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998–2013. Sci Rep. 2017;7(1):929. doi: 10.1038/s41598-017-01021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matias GT, Haguinet R, Schuck-Paim F, Lustig C, Shinde RV. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health. 2017;17(1):271. doi: 10.1186/s12889-017-4177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan PKS, Tam WWS, Lee TC, et al. Hospitalization incidence, mortality, and seasonality of common respiratory viruses over a period of 15 years in a developed subtropical city. Medicine (United States). 2015;94(46):e2024. [DOI] [PMC free article] [PubMed]
- 40.D'Mello T, Brammer L, Blanton L, et al. Update: influenza activity–United States, September 28, 2014-February 21, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(8):206–212. [PMC free article] [PubMed] [Google Scholar]
- 41.Oliva J, Delgado-Sanz C, Larrauri A, et al. Estimating the burden of seasonal influenza in Spain from surveillance of mild and severe influenza disease, 2010–2016. Influenza Other Respir Viruses. 2018;12(1):161–70. [DOI] [PMC free article] [PubMed]
- 42.Gonzalez NFO, Sastre CR, Cambra AA,et al. Evaluation of influenza virus A in elderly hospitalized. Eur Geriatric Med. 2016;7(Suppl 1):S172–S173.
- 43.Ramos JM, García-Navarro MM, González de la Aleja MP, et al. Seasonal influenza in octogenarians and nonagenarians admitted to a general hospital: epidemiology, clinical presentation and prognostic factors. Rev Esp Quimioter. 2016;29(6):296–301. [PubMed]
- 44.Soldevila N, Acosta L, Martinez A, et al. Behavior of hospitalized severe influenza cases according to the outcome variable in Catalonia, Spain, during the 2017–2018 season. Sci Rep. 2021;11(1):13587. [DOI] [PMC free article] [PubMed]
- 45.Casado I, Domínguez A, Toledo D, et al. Effect of influenza vaccination on the prognosis of hospitalized influenza patients. Expert Rev Vaccines. 2016;15(3):425–32. [DOI] [PubMed]
- 46.Arrieta E, Lalueza A, Ayuso-Garcia B, et al. Influenza A-associated in-hospital mortality in very older people: does inflammation also play a role? Gerontology. 2022;68(7):780–88. [DOI] [PubMed]
- 47.Kestler Hernandez MJC, Burillo A, Catalán P, et al. Respiratory syncytial virus, an underestimated disease in the elderly population. ESCMID; 2020.
- 48.Pivette M, Nicolay N, de Lauzun V, Hubert B. Characteristics of hospitalizations with an influenza diagnosis, France, 2012–2013 to 2016–2017 influenza seasons. Influenza Other Respir Viruses. 2020;14(3):340–8. [DOI] [PMC free article] [PubMed]
- 49.Regis C, Voirin N, Escuret V, et al. Five years of hospital based surveillance of influenza-like illness and influenza in a short-stay geriatric unit. BMC Res Notes. 2014;7:99. [DOI] [PMC free article] [PubMed]
- 50.Lemaitre M, Fouad F, Carrat F, et al. Estimating the burden of influenza-related and associated hospitalizations and deaths in France: an eight-season data study, 2010–2018. Influenza Other Respir Viruses. 2022;10:10. [DOI] [PMC free article] [PubMed]
- 51.Cheysson F, Brun-Buisson C, Opatowski L, et al. Outpatient antibiotic use attributable to viral acute lower respiratory tract infections during the cold season in France, 2010–2017. Int J Antimicrob Agents. 2021;57(6):106339. [DOI] [PubMed]
- 52.Bernadou A, Fortin N, Hubert B, editors. Estimating the burden of influenza on hospitals using severe acute respiratory infections in metropolitan France, 2012–2018. ESCMID; 2020. [DOI] [PMC free article] [PubMed]
- 53.Andrew MK, MacDonald S, Godin J, et al. Persistent functional decline following hospitalization with influenza or acute respiratory illness. J Am Geriatrics Soc. 2021;69(3):696–703. [DOI] [PMC free article] [PubMed]
- 54.Gruneir A, Kwong JC, Campitelli MA, et al. Influenza and seasonal patterns of hospital use by older adults in long-term care and community settings in Ontario, Canada. Am J Pub Health. 2014;104(2):e141–7. [DOI] [PMC free article] [PubMed]
- 55.Yokomichi H, Mochizuki M, Lee JJ, et al. Incidence of hospitalisation for severe complications of influenza virus infection in Japanese patients between 2012 and 2016: a cross-sectional study using routinely collected administrative data. BMJ Open. 2019;9(1):e024687. [DOI] [PMC free article] [PubMed]
- 56.Sruamsiri R, Ferchichi S, Jamotte A, et al. Impact of patient characteristics and treatment procedures on hospitalization cost and length of stay in Japanese patients with influenza: a structural equation modelling approach. Influenza Other Respir Viruses. 2017;11(6):543–55. [DOI] [PMC free article] [PubMed]
- 57.Moss JWE, Davidson C, Mattock R, et al. Quantifying the direct secondary health care cost of seasonal influenza in England. BMC Public Health. 2020;20(1):1464. [DOI] [PMC free article] [PubMed]
- 58.Boddington NL, Verlander NQ, Pebody RG. Developing a system to estimate the severity of influenza infection in England: findings from a hospital-based surveillance system between 2010/2011 and 2014/2015. Epidemiol Infect. 2017;145(7):1461–1470. doi: 10.1017/S095026881700005X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pacis SM, Wilke U, Ghiani T, M. Social distancing and trends in influenza hospitalization during the COVID-19 outbreak: a difference-in-difference analysis of German claims data. Value Health. 2022;25(1 Suppl):S5.
- 60.Palekar RSR, Arriola MA, Acosta CS, et al. Burden of influenza-associated respiratory hospitalizations in the Americas, 2010–2015. PLoS ONE. 2019;14(9):e0221479. [DOI] [PMC free article] [PubMed]
- 61.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997–2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses. 2014;8(5):507–515. doi: 10.1111/irv.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The World Bank. Population ages 65 and above, total - United States. 2019. https://data.worldbank.org/indicator/SP.POP.65UP.TO?end=2013&locations=US&start=2010. Accessed 16 Aug 2022.
- 63.The World Bank. Population ages 65 and above, total - Spain. 2019. https://data.worldbank.org/indicator/SP.POP.65UP.TO?end=2016&locations=ES&start=2010&view=chart. Accessed 18 Aug 2022.
- 64.Institut national de la statistique et des études économiques. Demographic balance sheet 2021. https://www.insee.fr/en/statistiques/6040011?sommaire=6323335#titre-bloc-13. Accessed 16 Aug 2022.
- 65.Cheng PY, Palekar R, Azziz-Baumgartner E, et al. Burden of influenza-associated deaths in the Americas, 2002–2008. Influenza Other Respir Viruses. 2015;9(S1):13–21. [DOI] [PMC free article] [PubMed]
- 66.Czaja C, Miller L, Alden N, et al. Association of increasing age with hospitalization rates, clinical presentation, and outcomes among older adults hospitalized with influenza—US Influenza Hospitalization Surveillance Network (FluSurv-NET). Open Forum Infect Dis. 2018;5(Suppl 1):S750. [DOI] [PMC free article] [PubMed]
- 67.Czaja C, Miller L, Alden N, et al. Age-related differences in hospitalization rates, clinical presentation, and outcomes among older adults hospitalized with influenza—U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET). Open Forum Infect Diseases. 2019;6(7):01. [DOI] [PMC free article] [PubMed]
- 68.Gil de Miguel A, Eiros Bouza JM, Martinez Alcorta LL, et al. Direct medical costs of four vaccine-preventable infectious diseases in older adults in Spain. PharmacoEcon Open. 2022;6(4):509–18. [DOI] [PMC free article] [PubMed]
- 69.Rosano A, Bella A, Gesualdo F, et al. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14–2016/17 seasons). Int J Infect Dis. 2019;88:127–34. [DOI] [PubMed]
- 70.Cromer D, van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014;68(4):363–371. doi: 10.1016/j.jinf.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Soldevila N, Toledo D, Ortiz de Lejarazu R, et al. Effect of antiviral treatment in older patients hospitalized with confirmed influenza. Antiviral Res. 2020;178:104785. [DOI] [PubMed]
- 72.Jin S, Li J, Cai R, et al. Age- and sex-specific excess mortality associated with influenza in Shanghai, China, 2010–2015. Int J Infect Dis. 2020;98:382–9. [DOI] [PubMed]
- 73.Paget J, Spreeuwenberg P, Charu V, et al. Global mortality associated with seasonal influenza epidemics: new burden estimates and predictors from the GLaMOR Project. J Global Health. 2019;9(2):020421. [DOI] [PMC free article] [PubMed]
- 74.Wong JY, Goldstein E, Fang VJ, Cowling BJ, Wu P. Real-time estimation of the influenza-associated excess mortality in Hong Kong. Epidemiol Infect. 2019;147:e217. [DOI] [PMC free article] [PubMed]
- 75.Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis. 2012;206(12):1862–71. [DOI] [PMC free article] [PubMed]
- 76.Zhang H, Xiong Q, Peng W, Chen Y, Leung NHL, Cowling BJ. Influenza-associated mortality in Yancheng, China, 2011–15. Influenza Other Respir Viruses. 2018;12(1):98–103. [DOI] [PMC free article] [PubMed]
- 77.Qi L, Li Q, Ding XB, et al. Mortality burden from seasonal influenza in Chongqing, China, 2012–2018. Hum Vaccin Immunother. 2020;16(7):1668–74. [DOI] [PMC free article] [PubMed]
- 78.Pebody RG, Green HK, Warburton F, et al. Significant spike in excess mortality in England in winter 2014/15 - influenza the likely culprit. Epidemiol Infect. 2018;146(9):1106–13. [DOI] [PMC free article] [PubMed]
- 79.Wu S, Wei Z, Greene CM, et al. Mortality burden from seasonal influenza and 2009 H1N1 pandemic influenza in Beijing, China, 2007–2013. Influenza Other Respir Viruses. 2018;12(1):88–97. [DOI] [PMC free article] [PubMed]
- 80.Near AT, Young-Xu J, Hong Y, et al. Pin24 Incidence and costs of influenza-related hospitalizations by comorbidity in the United States. Value Health. 2020;23(Suppl 1):S172–3.
- 81.Young-Xu YVA, Russo R, Lee E, et al. The annual burden of seasonal influenza in the US Veterans Affairs population. PLoS ONE. 2017;12(1):e0169344. [DOI] [PMC free article] [PubMed]
- 82.Owusu D, Rolfes MA, Arriola Velezmoro CS, et al. Risk of influenza-associated hospitalization among older adults living with diabetes–United States, 2012–2017. Open Forum Infect Dis. 2020;7(Suppl 1):S754–5. [DOI] [PMC free article] [PubMed]
- 83.Near AT, Young-Xu J, Hong Y, et al. Health resource burden of influenza among the elderly with underlying conditions in the United States. Open Forum Infect Dis. 2020;7(Suppl 1):S174–5.
- 84.Guesneau CB, Bourigault AS, Berrut C, et al. Risk factors associated with 30-day mortality in older patients with influenza. J Clin Med. 2021;10(16):11. [DOI] [PMC free article] [PubMed]
- 85.Liu R, Liu X, Yang P, et al. Influenza-associated cardiovascular mortality in older adults in Beijing, China: a population-based time-series study. BMJ Open. 2020;10(11):e042487. [DOI] [PMC free article] [PubMed]
- 86.Paget J, Iuliano AD, Taylor RJ, Lone S, Viboud C, Spreeuwenberg P. Estimates of mortality associated with seasonal influenza for the European Union from the GLaMOR project. Vaccine. 2022; 40(9):1361–9. [DOI] [PMC free article] [PubMed]
- 87.Pebody RG, Warburton F, Andrews N, et al. Uptake and effectiveness of influenza vaccine in those aged 65 years and older in the United Kingdom, influenza seasons 2010/11 to 2016/17. Eurosurveillance. 2018;23(39):1800092. doi: 10.2807/1560-7917.ES.2018.23.39.1800092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilca R, Skowronski DM, Douville-Fradet M, et al. Mid-season estimates of influenza vaccine effectiveness against influenza A(H3N2) hospitalization in the elderly in Quebec, Canada, January 2015. PLoS ONE. 2015;10(7):e0132195. [DOI] [PMC free article] [PubMed]
- 89.Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of hemagglutinin residues responsible for H3N2 antigenic drift during the 2014–2015 influenza season. Cell Rep. 2015;12(1):1–6. doi: 10.1016/j.celrep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng S, Gordon A. Influenza burden and transmission in the tropics. Curr Epidemiol Rep. 2015;2(2):89–100. doi: 10.1007/s40471-015-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng L, Shay DK, Jiang Y, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003–2008. Bull World Health Organ. 2012;90(4):279–88B. [DOI] [PMC free article] [PubMed]
- 92.World Health Organization. Ageing and health 2021. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed 29 July 2022.
- 93.Centers for Disease Control and Prevention. People at higher risk of flu complications. 2021. https://www.cdc.gov/flu/highrisk/index.htm#:~:text=Chronic%20lung%20disease%20(such%20as,Kidney%20diseases. Accessed 10 Aug 2022.
- 94.Canadian Medical Association. Seniors care: 2015 Conference Board of Canada report. 2015. https://www.cma.ca/seniors-care. Accessed 19 July 2022.
- 95.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2022–2023 northern hemisphere influenza season. https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2022-2023-northern-hemisphere-influenza-season. Accessed 29 July 2022.
- 96.Alonso WJ, Yu C, Viboud C, et al. A global map of hemispheric influenza vaccine recommendations based on local patterns of viral circulation. Sci Rep. 2015;5:17214. doi: 10.1038/srep17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bedford T, Suchard MA, Lemey P, et al. Integrating influenza antigenic dynamics with molecular evolution. Elife. 2014;3:e01914. doi: 10.7554/eLife.01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Centers for Disease Control and Prevention. Vaccine effectiveness: how well do flu vaccines work? 2022. https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm. Accessed 29 July 2022.
- 99.Francis T, Salk JE, Pearson HE, Brown PN. Protective effect of vaccination against induced influenza A. J Clin Invest. 1945;24(4):536–546. doi: 10.1172/JCI101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Audsley JM, Tannock GA. Cell-based influenza vaccines: progress to date. Drugs. 2008;68(11):1483–1491. doi: 10.2165/00003495-200868110-00002. [DOI] [PubMed] [Google Scholar]
- 101.Soema PC, Kompier R, Amorij J-P, Kersten GFA. Current and next generation influenza vaccines: formulation and production strategies. Eur J Pharm Biopharm. 2015;94:251–263. doi: 10.1016/j.ejpb.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 102.Hampson AW. Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat. Ann Acad Med Singap. 2008;37(6):510–7. [PubMed]
- 103.Levin MJ, Divino V, Shah D, et al. Comparing the clinical and economic outcomes associated with adjuvanted versus high-dose trivalent influenza vaccine among adults aged >= 65 years in the US during the 2019–20 influenza season—a retrospective cohort analysis. Vaccines. 2021;9(10):1146. [DOI] [PMC free article] [PubMed]
- 104.Pelton SI, Divino V, Postma MJ, et al. Retrospective cohort study assessing relative effectiveness of adjuvanted versus high-dose trivalent influenza vaccines among older adults in the United States during the 2018–19 influenza season. Vaccine. 2021;39(17):2396–407. [DOI] [PubMed]
- 105.Lu Y, Chillarige Y, Izurieta HS, et al. Effect of age on relative effectiveness of high-dose versus standard-dose influenza vaccines among US medicare beneficiaries aged >=65 years. J Infect Dis. 2019;220(9):1511–20. [DOI] [PubMed]
- 106.Robison SG, Thomas AR. Assessing the effectiveness of high-dose influenza vaccine in preventing hospitalization among seniors, and observations on the limitations of effectiveness study design. Vaccine. 2018;36(45):6683–7. [DOI] [PubMed]
- 107.Rose AMC, Kissling E, Gherasim A, et al. Vaccine effectiveness against influenza A(H3N2) and B among laboratory-confirmed, hospitalised older adults, Europe, 2017–18: a season of B lineage mismatched to the trivalent vaccine. Influenza Other Respir Viruses. 2020;14(3):302–10. [DOI] [PMC free article] [PubMed]
- 108.Casado I, Dominguez A, Toledo D, et al. Repeated influenza vaccination for preventing severe and fatal influenza infection in older adults: a multicentre case-control study. CMAJ. 2018;190(1):E3–12. [DOI] [PMC free article] [PubMed]
- 109.Torner N, Navas E, Soldevila N, et al. Costs associated with influenza-related hospitalization in the elderly. Hum Vaccin Immunother. 2017;13(2):412–16. [DOI] [PMC free article] [PubMed]
- 110.Simpson CR, Lone N, Kavanagh K, et al. Seasonal Influenza Vaccine Effectiveness (SIVE): an observational retrospective cohort study—exploitation of a unique community-based national-linked database to determine the effectiveness of the seasonal trivalent influenza vaccine. Health Services and Delivery Research. Southampton (UK): NIHR J Library; 2013. [PubMed]
- 111.Chivukula S, Plitnik T, Tibbitts T, et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines. 2021;6(1):153. doi: 10.1038/s41541-021-00420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.