Abstract
Fever of unknown origin (FUO) is a serious challenge for physicians. The aim of the present study was to consider epidemiology and dynamics of FUO in countries with different economic development. The data of FUO patients hospitalized/followed between 1st July 2016 and 1st July 2021 were collected retrospectively and submitted from referral centers in 21 countries through ID-IRI clinical research platform. The countries were categorized into developing (low-income (LI) and lower middle-income (LMI) economies) and developed countries (upper middle-income (UMI) and high-income (HI) economies). This research included 788 patients. FUO diagnoses were as follows: infections (51.6%; n = 407), neoplasms (11.4%, n = 90), collagen vascular disorders (9.3%, n = 73), undiagnosed (20.1%, n = 158), miscellaneous diseases (7.7%, n = 60). The most common infections were tuberculosis (n = 45, 5.7%), brucellosis (n = 39, 4.9%), rickettsiosis (n = 23, 2.9%), HIV infection (n = 20, 2.5%), and typhoid fever (n = 13, 1.6%). Cardiovascular infections (n = 56, 7.1%) were the most common infectious syndromes. Only collagen vascular disorders were reported significantly more from developed countries (RR = 2.00, 95% CI: 1.19–3.38). FUO had similar characteristics in LI/LMI and UMI/HI countries including the portion of undiagnosed cases (OR, 95% CI; 0.87 (0.65–1.15)), death attributed to FUO (RR = 0.87, 95% CI: 0.65–1.15, p-value = 0.3355), and the mean duration until diagnosis (p = 0.9663). Various aspects of FUO cannot be determined by the economic development solely. Other development indices can be considered in future analyses. Physicians in different countries should be equally prepared for FUO patients.
Keywords: Economic development, Fever, Fever of unknown origin, FUO, ID-IRI
Introduction
Fever of unknown origin (FUO) is a clinical and diagnostic challenge in routine medical practice and the potential causes of FUO may involve more than 200 diseases [1, 2]. Apart from diarrheal diseases, respiratory infections, and skin lesions, FUO is one of the most common health problems in travelers [3, 4]. In 1961, Petersdorf and Beeson published the first fundamental research on FUO. In 1991, Durack and Street revised previous definition of FUO and suggested that the diagnostic period should be as short as three days with the improving diagnostic capacities of the hospitals [5].
Although there are economic inequalities, social and political tensions around the world, lifesaving technologies have improved the diagnostic capacity greatly. There has been an increase in the life expectancy leading to aging of populations, which is more visible in countries with high economic and social development compared to countries with limited resources [6]. On the other hand, lifesaving technologies, innovation, and ongoing researches have improved the quality of human life greatly. There has been a recent increase in the life expectancy leading to aging of populations, which is more visible in countries with high economic and social development compared to the countries with limited resources. Accordingly, chronic diseases like hypertension, diabetes, and malignant diseases increased with advancing age [7] and this trend has the potential to result in more FUO cases in the community, particularly the elders. Thus, we aimed to show the alterations in FUO epidemiology by including the economic statuses of the countries. To the best of our knowledge, no such research has been conducted.
Materials and methods
The diagnoses of diseases were established in this case series according to the common concepts elsewhere. FUO was defined as follows: (a) febrile illness of more than 3 weeks; (b) fever higher than 38.3 °C on several occasions; (c) absence of diagnosis after three inpatient days or three outpatient visits to physician.
The inclusion criteria:
Adults > 18 years of age
Patients hospitalized/followed between 1st July 2016 – 1st July 2021
Patients with the main clinical symptom – fever
The exclusion criteria:
Known immunodeficiency
Pregnancy
Data collection and participants
This study was performed through ID-IRI international clinical research platform (https://infectdisiri.com/). ID-IRI has members worldwide as clinical researchers and they voluntarily join ID-IRI research projects. Demographic parameters, clinical presentation, laboratory results, and clinical outcomes of all participants were obtained from electronic medical records retrospectively. Axillary temperature was measured in all study participants. For the thermometerization process, it used validated and licensed thermometers approved by the referral medical centers (hospitals) which included in this research. The participants in the study were divided in two groups on admission: “Non-late elderly” — persons aged 75 years and below; and “Late elderly” — persons over 75 years of age [8].
Stratification of the economic status
Countries were stratified as “low-income economies” — Gross National Income (GNI) per capita of US $1045 or less; “lower middle-income economies” — GNI per capita between US $1046 and $4095; “upper middle-income economies” — GNI per capita between US $4096 and $12,695; and “high-income economies” — GNI per capita of $12,696 or more [9]. Countries where participating centers were located were categorized as lower income (LI) (Afghanistan), lower-middle income (LMI) (Egypt, India, Iran, Pakistan, Tunisia), upper-middle income (UMI) (Albania, Bosnia and Herzegovina, Bulgaria, Kazakhstan, North Macedonia, Romania, Russian Federation, Turkey), and high income (HI) countries (Croatia, Cyprus, Hungary, Italy, Saudi Arabia, Slovakia, United Arab Emirates) (Fig. 1).
Fig. 1.
The countries where participant centers are located
Statistical analysis
Statistical analysis was performed using descriptive statistics to present results as frequencies and percentages. To present quantitative variables, we obtained median and interquartile ranges or mean and standard deviations as appropriate. One-way analysis of variance was used to examine whether the mean values of inflammatory markers differ across various fever of unknown origin diagnoses. Relative risks and 95% confidence intervals were obtained to determine associations. p-value of less than 0.05 was statistically significant.
Ethics
The present survey was performed in accordance with the ethical principles and recommendations of the Declaration of Helsinki (June 1964, last revision in October 2013). All medical procedures in this research were performed according to the national legislation of the country in which the medical center (hospital) is located. The ethical approval of the study was taken from The Ethical Counsel of Istanbul Medeniyet University, Faculty of Medicine, Istanbul (4 August 2021/0411).
Results
We included 788 patients to our survey. The median (IQR) duration until diagnosis after hospital admission was 12 (8–21) days. The mean age of the patients was 46.8 ± 18.1 years and 345 (43.8%) were females, 744 (94.4%) were adults (18–75 years), and 44 (5.6) were late elders (≥ 76 years). Economically, 74 (9.4%) patients were from HI, 1 (0.1%) was from LI, 297 (37.7%) were from LMI, and 416 (52.8%) were from UMI countries.
Distribution of the cases by the countries
The data of patients were submitted from 21 countries: Afghanistan (n = 1; 0.1%), Albania (n = 31; 3.9%), Bosnia and Herzegovina (n = 55; 6.9%), Bulgaria (n = 37; 4.7%), Croatia (n = 3; 0.4%), Cyprus (n = 2; 0.3%), Egypt (n = 75; 9.5%), Hungary (n = 1; 0.1%), India (n = 50; 6.3%), Iran (n = 50; 6.3%), Italy (n = 59; 7.5%), Kazakhstan (n = 5; 0.6%), North Macedonia (n = 18; 2.3%), Pakistan (n = 7; 0.9%), Romania (n = 33; 4.3%), Russia (n = 5; 0.6%), Saudi Arabia (n = 3; 0.4%), Slovakia (n = 1; 0.1%), Tunisia (n = 117; 14.8%), Turkey (n = 232; 29.3%), and United Arab Emirates (n = 5; 0.6%).
A. Reasons for FUO
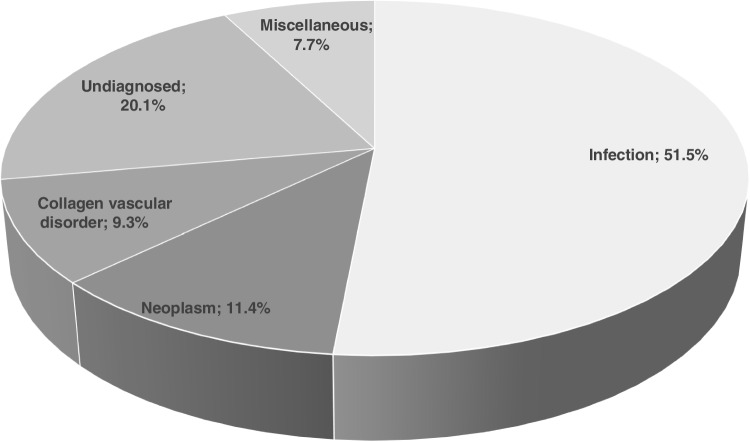
The distribution of FUO diagnoses were as follows: infections (n = 407, 51.6%), neoplasms (n = 90, 11.4%), collagen vascular disorders (n = 73, 9.3%), undiagnosed (n = 158, 20.1%), miscellaneous diseases (n = 60, 7.7%) (Fig. 2).
Fig. 2.
Distribution of FUO diagnoses
I. Infections (n = 407)
Zoonoses (n = 88): (Brucellosis (n = 39) {no organ involvement (n = 31); prostatitis (n = 1); sacroiliitis (n = 1); spondylodiscitis (n = 6)}, rickettsiosis (n = 23) (Q fever (n = 11); untyped (n = 6); Marseilles fever (n = 4); Rickettsia conorii infection (n = 2)), lyme disease (n = 5), visceral leishmaniasis (n = 4), CCHFV (n = 3), toxoplasmosis (n = 3), cat scratch disease (n = 2), malaria (n = 2), hantavirus pulmonary syndrome (n = 1), hydatid disease (liver, n = 1), toxocariasis (n = 2), tularemia (n = 1), typhus (n = 1), WNV infection (n = 1)).
Returning travelers: Two Malaria cases returning from Africa to Türkiye (Plasmodium ovale) and Romania (P. falciparum) (0.5% of all infections) were associated to international travel.
Cardiovascular infections (n = 56): native valve endocarditis (n = 44), brucellar endocarditis (n = 3), pericarditis (n = 3), prosthetic valve endocarditis (n = 2), myocarditis (n = 2), pace-maker endocarditis (n = 1), culture negative endocarditis (n = 1).
Respiratory tract infections (n = 36): (pneumonia (n = 18); bronchopneumonia (n = 3); atypical pneumonia (Mycoplasma pneumoniae n = 3; Chlamydia pneumoniae n = 1; Chlamydia psittaci n = 1; untyped n = 2); empyema (n = 2); actinomycosis (n = 1); Pneumocystis jiroveci pneumonia (n = 1); pulmonary tuberculosis (n = 1); maxillary sinusitis (n = 1); atypical measles (n = 1); obliterating bronchiolitis (n = 1); tonsillopharyngitis (n = 1)).
Urogenital infections (n = 35): urosepsis (n = 11); lower urinary tract infections (n = 11); pyelonephritis (n = 8); chronic cystitis (n = 1, Ureaplasma urealyticum); hydro/pyonefrosis (n = 1); pelvic infection (n = 1); perinephritis (n = 1), gonorrhea (n = 1).
Intestinal infections (n = 23): typhoid fever (n = 13), neutropenic colitis (n = 3), Clostridioides difficile colitis (n = 2), enterocolitis, untyped (n = 1), diverticulitis (n = 2), colon perforation (n = 1), typhlitis (n = 1).
Viral infections (n = 39): (HIV/AIDS (n = 20); EBV infection (n = 8); CMV infection (n = 7); COVID-19 (n = 2); parvovirus infection (n = 1); retroviral infection (n = 1)).
Tuberculosis (n = 45): (pulmonary (n = 15); miliary (n = 11); lymphadenitis (n = 7); peritoneal (n = 4); pleurisy (n = 3); hepatic (n = 2); intestinal (n = 2); mediastinal (n = 1)),
Central nervous system infections (n = 17): meningitis, untyped (n = 7); tuberculous meningitis (n = 1); brucellar meningitis (n = 1); thoraco-lumbar myelitis (n = 1); HIV encephalitis (n = 1); ventriculoperitoneal shunt infection (n = 1); viral encephalitis (untyped (n = 3); Toscana virus (n = 1)), cryptococcal meningitis (n = 1).
Bacteremia of unidentified origin (n = 10): Staphylococcus aureus (n = 4); Klebsiella pneumoniae (n = 2); Enterococcus faecalis (n = 1); Streptococcus constellatus (n = 1); Pseudomonas aeruginosa (n = 1); Acinetobacter baumannii (n = 1); Escherichia coli (n = 1).
Bone and joint infections (n = 9): (spondylodiscitis (n = 7); skull base osteomyelitis (n = 1); sacroiliitis (n = 1)).
Skin and soft tissue infections (n = 5): (bedsore infection (n = 3); lymphadenitis (n = 1); lymphangitis (n = 1)).
Hepatobiliary infections (n = 4): cholecystitis (n = 4).
Fungal diseases (n = 4): hepato-splenic candidiasis (n = 2), mucormycosis (n = 1), fusariasis (n = 1).
Periodontitis (n = 1).
Abscess formations (n = 33)
• Intra-abdominal abscesses (n=24): liver (n=7), intraabdominal (n=4), renal (n=4), pericecal (n=2), amebic liver (n=1), diverticular (n=1), gall bladder (n=1), iliopsoas (n=1), perianal (n=1), uterine (n=1), subhepatic (n=1).
• CNS abscesses (n=3): epidural brucellar (n=1), cerebral (n=1), epidydimal (n=1).
• Pulmonary abscesses (n=2): lungs (n=2).
• Other abscesses (n=4): paravertebral (n=2), dental (n=1), subcutaneous (n=1).
II. Neoplasms (n = 90)
Solid cancers (n = 27): lung (n = 8), colorectal (n = 3), adrenal gland (n = 3), neuroendocrine (n = 2), renal (n = 2), pancreas (n = 2), liver (n = 1), endometrium (n = 1), head and neck (n = 1), primary unknown (n = 1), prostate (n = 1), bone (n = 1), right atrium myxoma (n = 1).
Hematological malignancies (n = 60):
Lymphomas (n = 50): non-Hodgkin lymphoma (n = 25), Hodgkin lymphoma (n = 19), T-cell lymphoma (n = 4), anaplastic lymphoma (n = 2).
Leukemias (n = 13): acute lymphocytic leukemia (n = 4), acute myeloid leukemia (n = 5), chronic myeloid leukemia (n = 2), multiple myeloma (n = 2).
III. Collagen vascular disorders (n = 73)
Adult-onset Still's disease (n = 24), systemic lupus erythematosus (n = 8), polymyalgia rheumatica (n = 6), polyarteritis nodosa (n = 5), rheumatoid arthritis (n = 4), temporal arteritis (n = 4), large-vessel vasculitis (n = 3), reactive arthritis (n = 3), Behcet’s disease (n = 2), myelitis (n = 1), familial Mediterranean fever (n = 1), giant cell arteritis (n = 1), gout arthritis (n = 1), Henoch Schoenlein purpura (n = 1), inflammatory myositis (n = 1), juvenile rheumatoid arthritis (n = 1), autoimmune hepatitis (n = 1), lupus nephropathy (n = 1), polymyositis (n = 1), seronegative arthropathy (n = 1), small vessel vasculitis (n = 1), Takayasu disease (n = 1), Wegener granulomatosis (n = 1).
IV. Miscellaneous diseases
Thyroiditis (n = 12), histiocytosis (n = 7), Crohn’s disease (n = 5), macrophage activation syndrome (n = 4), familial Mediterranean fever (n = 4), sarcoidosis (n = 3), embolic events (n = 3), hemophagocytic syndrome (n = 3), Kikuchi disease (n = 3), ulcerative colitis (n = 2), aplastic anemia (n = 1), polycythemia vera (n = 1), autoimmune thyroiditis (n = 1), chronic fatigue syndrome (n = 1), cholecystitis (n = 1), cirrhosis (n = 1), primary biliary cirrhosis (n = 1), colon perforation (n = 1), Churg-Strauss syndrome (n = 1), endometriosis (n = 1), drug induced fever (n = 1), hepatic arterial thrombosis (n = 1), Horton disease (n = 1), neuroleptic malignant syndrome (n = 1).
B. Invasive diagnostic procedures
Overall, 201 (25.5%) patients were performed invasive sampling for FUO diagnosis. Invasive diagnostic sampling of FUO patients in accordance with the economic statuses are presented in Table 1. Diagnostic biopsies were done in 22 (29.7%) of HI country patients, in 90 (n = 21.6%) UMI country patients, and in 89 (29.9%) LMI country patients. There was a significant difference between the three economic statuses and performing biopsies (chi-square = 7.07, p = 0.029).
Table 1.
Invasive diagnostic sampling in accordance with economic statuses
| HI (n = 74) | UMI (n = 416) | LMI (n = 297) | LI (n = 1) | |
|---|---|---|---|---|
| Lymph node bx (n = 188) | 21 (28.4%) | 85 (20.4%) | 82 (27.6%) | |
| Bone marrow bx (n = 57) | 9 (4.7%) | 28 (6.7%) | 20 (6.7%) | |
| Liver bx (n = 13) | 3 (0.7%) | 10 (3.4%) | ||
| Colon bx (n = 11) | 3 (4.1%) | 5 (1.2%) | 3 (1%) | |
| Skin bx (n = 11) | 1 (1.4%) | 4 (1%) | 6 (2%) | |
| Temporal artery bx (n = 7) | 3 (0.7%) | 4 (1.3%) | ||
| Kidney bx (n = 5) | 5 (1.7%) | |||
| Lung bx (n = 5) | 2 (0.5% %) | 3 (1%) | ||
| Pleura bx (n = 2) | 1 (0.2%) | |||
| Thyroid bx (n = 2) | 1 (0.2%) | 1 (0.3%) | ||
| Prostate bx (n = 1) | 1 (0.3%) | |||
| İleum bx (n = 1) | 1 (0.2%) | |||
| Gastric bx (n = 1) | 1 (1.4%) |
HI high income, LI low-income, LMI low-middle income, UMI upper-middle income, Bx biopsy
C. FUO and economic status
Compared to residents of LI and LMI countries, having FUO among residents of HI and UMI countries does not significantly predict the diagnosis of infections (RR = 0.92, 95% CI: 0.80–1.05), and neoplasms (RR = 1.10, 95% CI: 0.73–1.66). Similarly, comparing HI vs UMI vs LMI countries, having FUO does not significantly predict the diagnosis of infection (χ2 = 0.5046, p = 0.777), and neoplasms (χ2 = 2.4270 p = 0.297). However, collagen vascular disorders (RR = 2.00, 95% CI: 1.19–3.38) were more likely to be reported from HI and UMI countries compared to LMI and LI countries. When HI vs UMI vs LMI countries were compared, FUO significantly predicts the diagnosis of collagen vascular disorders (χ2 = 7.5526, p = 0.023).
D. Clinical FUO associations
Compared to non-late elderly age group, having FUO among the late elderly population does not significantly predict the diagnoses of infection (RR = 1.11, 95% CI: 0.85–1.45), neoplasms (RR = 1.43, 95% CI: 0.70–2.89), and collagen vascular disorders (RR = 0.48, 95% CI: 0.12–1.88) (Tables 2 and 3). The relationships between inflammatory markers and FUO diagnoses are shown in Table 4; the outcomes of FUO in accordance with economic statuses are presented in Table 5. We could not disclose any significant difference for death attributable to FUO when HI vs UMI vs LMI countries were compared (χ2 = 1.62, p = 0.440). Similarly, the mean duration of days until diagnosis after hospitalization did not differ across the economic statuses (p = 0.9663).
Table 2.
FUO categories and economic status
| Diagnoses | High-income countries (n = 74) | Upper-middle income countries (n = 416) | Low-middle income countries (n = 297) | Low income countries (n = 1) |
|---|---|---|---|---|
| Infections (n = 407) | 36 | 209 | 162 | 0 |
| Neoplasms (n = 90) | 5 | 53 | 32 | 0 |
| CVD (n = 73) | 7 | 49 | 17 | 0 |
| Undiagnosed (n = 158) | 14 | 79 | 64 | 1 |
| Other (n = 60) | 12 | 26 | 22 | 0 |
CVD collagen vascular disorders
Table 3.
Comparison of FUO distributions in accordance with the economic statuses
| Etiology of FUO | Relative risk (95% CI) | Pearson chi-square χ2 | p-value | ||
|---|---|---|---|---|---|
| Infections | Non-infections | ||||
| HI/UMI | 245/407 | 245/381 | 0.92 (0.80–1.05) | 1.41 | 0.2347 |
| LMI/LI | 162/407 | 136/381 | |||
| Neoplasms | Non-neoplasms | ||||
| HI/UMI | 58/90 | 432/698 | 1.10 (0.73–1.66) | 0.22 | 0.633 |
| LMI/LI | 32/90 | 266/698 | |||
| CVDs | Non-CVDs | ||||
| HI/UMI | 56/73 | 434/715 | 2.00 (1.19–3.38) | 7.22 | 0.0072 |
| LMI/LI | 17/73 | 281/715 | |||
| Undiagnosed | All Others | ||||
| HI/UMI | 93/158 | 397/630 | 0.87 (0.65–1.15) | 0.93 | 0.3355 |
| LMI/LI | 65/158 | 233/630 | |||
| Aging and FUO | |||||
| Infections | Non—infections | ||||
| Late elderly | 25/407 | 19/381 | 1.11 (0.85–1.45) | 0.50 | 0.4802 |
| Non-late elderly | 382/407 | 362/381 | |||
| Neoplasms | Non-neoplasms | ||||
| Late elderly | 7/90 | 37/699 | 1.43 (0.70–2.89) | 0.93 | 0.3355 |
| Non-late elderly | 83/90 | 661/699 | |||
| CVDs | Non-CVDs | ||||
| Late elderly | 2/73 | 42/715 | 0.48 (0.12–1.88) | 1.23 | 0.2666 |
| Non-late elderly | 71/73 | 673/715 | |||
| Undiagnosed | All Others | ||||
| Late elderly | 9/158 | 35/630 | 1.02 (0.56–1.86) | 0.00 | 0.9451 |
| Non-late elderly | 149/158 | 595/630 | |||
CVD collagen vascular disorders, HI high income, LI low-income, LMI low-middle income, UMI upper-middle income
Table 4.
Examining for the relationships between inflammatory markers and FUO diagnoses
| Infections | Neoplasms | CVD | Undiagnosed | Other diseases | F test | p | |
|---|---|---|---|---|---|---|---|
| CRP (mg/l) | n = 392, 120.9 ± 108.6 | n = 88, 129.2 ± 103.7 | n = 71, 108.4 ± 84.1 | n = 148, 120.6 ± 95.6 | n = 61, 108.5 ± 121.5 | 0.58 | 0.6762 |
| WBC (/μL) | n = 406, 9868.0 ± 6807.0 | n = 90, 7602.3 ± 6183.9 | n = 73, 11,129.8 ± 5610.1 | n = 158, 8804.9 ± 5833.9 | n = 60, 11,012.7 ± 21,297.9 | 2.69 | 0.0300 |
| ESR (mm/h) | n = 349, 66.06 ± 52.49 | n = 80, 66.9 ± 35.7 | n = 70, 8.41 ± 33.5 | n = 125, 61.8 ± 39.2 | n = 54, 60.0 ± 39.1 | 1.59 | 0.1765 |
| Ferritin (ng/ml) | n = 185, 875.2 ± 2574.2 | n = 61, 2102.9 ± 4191.4 | n = 63, 2788.5 ± 6451.3 | n = 72, 1815.1 ± 4195.2 | n = 36, 3477.4 ± 8707.5 | 3.82 | 0.0046 |
| Cr (mg/dl) | n = 402, 1.0 ± 0.6 | n = 87, 0.9 ± 0.3 | n = 72, 0.8 ± 0.5 | n = 153, 0.9 ± 0.8 | n = 59, 0.9 ± 0.4 | 1.18 | 0.3195 |
CRP C-reactive protein, PCT procalcitonin, WBC white blood cell count, ESR erythrocyte sedimentation rate, Cr creatinine, CVD collagen vascular disorders
Table 5.
FUO outcomes in accordance with economic statuses
| HI (n = 74) | UMI (n = 416) | LMI (n = 297) | LI (n = 1) | |
|---|---|---|---|---|
| Died; attributed to FUO (n = 50, 6.3%) | 2 | 29 | 19 | 0 |
| Died; NOT attributable to FUO (n = 17, 2.1%) | 1 | 11 | 5 | 0 |
| Transferred to another unit (n = 71, 9%) | 5 | 36 | 29 | 1 |
| Still at hospital (n = 7, 0.9%) | 0 | 2 | 5 | 0 |
| Discharged with cure (n = 386, 49%) | 47 | 198 | 141 | 0 |
| Discharged with sequelae (n = 129, 16.4%) | 17 | 36 | 76 | 0 |
| Discharged as she/he is (n = 120, 15.2%) | 2 | 97 | 21 | 0 |
| No information (n = 8, 1%) | 0 | 7 | 1 | 0 |
CVD collagen vascular disease, HI high income, LI low-income, LMI low-middle income, UMI upper-middle income
Discussion
FUO cases are a critical group of patients with 6.3% attributable mortality according to our data. Traditionally numerous causes of classic FUO fall within five categories: infections, neoplasms, connective tissue diseases, miscellaneous other disorders, and undiagnosed illnesses [10]. Infections had long been the leading causes of classical FUO [1, 2]. Accordingly, febrile conditions are the optimum timings of consultations from infectious diseases departments and increase the workloads of these services [11]. In recent FUO papers from LMI countries, infections ranged from 43 to 63% establishing the majority of FUO cases while neoplasms comprised 1–22%, and collagen vascular disorders made up 13–30%, miscellaneous diseases comprised 2–14%, and undiagnosed FUO patients had a share of 2–12% [12–15]. The distributions of FUO diagnoses in FUO reports from richer (UMI and HI) countries were infections 15–49%, neoplasms 7–18%, collagen vascular disorders 19–47%, miscellaneous diseases 1–13%, and undiagnosed 8–30% [16–23]. Thus, there has been an understanding that FUO due to infections was most likely to be related to countries with limited resources, and developed or richer countries have predilections for noninfectious subsets of FUO diagnoses like neoplasms or collagen vascular disorders [2, 24]. However, we could not disclose such relationships for the entire FUO groups other than collagen vascular disorders, which were more frequently reported from richer countries. Although it appears that the disseminated knowledge and improving health infrastructures worldwide have a tendency to uniform the diagnoses for infections and neoplasms, the collagen vascular disorders were not equally identified and less commonly detected in country groups with lower economic incomes. Since our study pooled relatively new FUO patients followed in the last 5 years, this datum appears to be the new trend in the context of FUO diagnoses. In addition, either the duration of diagnosis for FUO cases or patients without diagnosis did not differ between the richer countries and those with the limited resources showing a degree of standardization.
Actually, economic welfare may not always be translated as a high Human Development Index, which is a statistic composite index of life expectancy, education, and per capita income [25]. In addition, wide geographical distribution of the participating centers may have resulted in diverse epidemiological exposures. Hence, other developmental parameters may disclose variations in FUO epidemiology, rather than the economic status. Accordingly, there had been an understanding that infections were less common in the elderly population compared to non-elderly [10]. But it was not the case in this study. Moreover, attributable FUO mortality was not significantly different between richer countries and those with limited resources. There seemed to be a uniformity in the distribution and outcomes of FUO diagnoses in the participating centers, and the delayed diagnosis is likely to be due to subtle nature of FUO causes rather than the economic prosperity.
In this study, half of FUO diagnoses were infections, which can have fatal outcomes when timely and rational antimicrobial treatment are not provided [26]. Although infections as the agents of FUO tend to vary in incidence according to locale, the leading community-acquired infections for classical FUO were tuberculosis, brucellosis, rickettsiosis, HIV infection, and typhoid fever in this survey. Tuberculosis has long been one of the common causes of FUO [24, 27] and brucellosis is the most frequent zoonotic infection worldwide [28] so that they were two of the common infections causing FUO. Since zoonoses are a heterogenous group of syndromes and cardiovascular infections are a uniform clinical entity, we can say that cardiovascular infections are the most common infectious syndromes among our FUO cases. We found that native valve endocarditis in particular, comprising more than two thirds of cardiovascular infections in routine medical practice [29], was the most common infectious syndromes among our FUO cases. The other common cause of FUO “bacteremia of unidentified origin” in this study may represent cardiovascular infections where the diagnosis could not be well established, too. Hence, any type of bacteremia in a FUO patient should warrant investigation for cardiovascular infections. In addition, pulmonary infections with atypical patterns, urogenital infections, and central nervous system infections with rare presentations were the other common syndromic infectious FUO presentations. Abscesses, intraabdominal locations as the most prominent, were not rare suppurative foci compelling the need of well-established radiological diagnosis in this study. Since infections imposes serious challenges and high mortality when untreated [26], early diagnosis in which even invasive procedures are shown to be needed in one-fourth of FUO patients in our study. Interestingly, biopsies were performed more commonly in LMI countries according to our data.
Returning travelers is a certain subset of patients in FUO series in which malaria, typhoid fever, and acute HIV infection were commonly recorded [10]. In routine medical practice, intestinal and respiratory infections including pneumonia and tuberculosis followed by malaria, visceral leishmaniasis, and hemorrhagic fevers are the common etiologies in severely infected travelers and migrants [3, 30]. We detected malaria only as the causes of FUO in this subgroup of patient population. This is the most common mosquito-borne disease with major epidemiological outbreaks in the equatorial, tropical, and subtropical climate zones of mainly in Africa and to a lesser extent in Asia, Central America, and Southern America. Another potential reason for FUO in this group of persons could be lymphatic filariasis (elephantiasis) which is endemic to Africa. In fact, the reasons for FUO in returning travelers are too many as with other people who have not had a trip. Here, too, the potential and most likely cause is an infectious disease caused by bacteria or parasites. Therefore, in FUO patients coming from countries with a high prevalence of any infection, it is appropriate to investigate the most common infections found in the country they have visited. The patient’s contact history for any type of infected patients, skin rashes, bites including vectors, water, and food consumption history should be questioned in detail.
The next common FUO category comprises neoplastic diseases, most commonly hematological malignancies including lymphomas and leukemias followed by solid cancers. The third leading cause of classical FUO is collagen vascular disorders where adult-onset Still’s disease is the most common followed by systemic lupus erythematosus, polymyalgia rheumatica, and polyarteritis nodosa in this study. When the inflammatory markers are compared among the diagnostic categories, ferritin and leucocyte count was significantly higher in the FUO patients without an established diagnosis, and this result may likely to stress the presence of non-infectious inflammatory diseases or collagen vascular disorders in patients without a diagnosis. Histopathologic examination of tissues, which appears to be the last bullet for definitive diagnosis, can provide a final diagnosis in FUO patients in fewer than half of cases[31], and thus one fourth of our patients were performed histopathological examination to reach a definitive diagnosis.
The current research has some limitations that need to be addressed. First, the study has a retrospective design, although we included only patients followed in the last 5 years. Second, the medical centers participating in this survey have heterogenous diagnostic capacities due to the different economic developments of their countries. Third, because of heterogeneity of FUO cases, the number of patients included in the study is very low for particular subsets of diagnoses. Finally, the numbers of patients included from each country were variable, and thus, the patients may not be representative of all patients for FUO in their particular countries. However, as a strength, we categorized and analyzed the patients according to the economic level of the countries they belong. Despite the aforementioned limitations, this work represents the first research on patients with FUO from countries with different economic stages of development.
In conclusion, the diagnosis of FUO should be tailored according to the common disorders causing FUO. In this regard, it is a serious difficulty to implement diagnostic protocols for patients with FUO and a potential FUO protocol cannot cover all possible causes of this medical problem. Our results and data from other studies show that regardless of the economic development of the countries, the leading causes of FUO are similar and still conventional. Hence, the clinicians worldwide should be aware of the current FUO epidemiology, which is unaffected from economic status to ease clinical decision making.
Author contribution
Hakan Erdem: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing, and supervision. Magdalena Baymakova: conceptualization, formal analysis, investigation, methodology, and writing. Sevil Alkan: conceptualization, data curation, and investigation. Farouq Dayyab: conceptualization, statistical analysis, and writing. Jordi Rello: conceptualization, writing and editing, and supervision. All other authors agreed the design of the study, collected and submitted the data of patients followed/treated in their centers, reviewed the paper, and obtained IRBs, if necessary. All authors have read and agreed to the submitted version of the manuscript.
Data availability
It will available on request.
Code availability
None applicable.
Declarations
Ethics approval
The ethical approval of the study was taken from The Ethical Counsel of Istanbul Medeniyet University, Faculty of Medicine, Istanbul (4 August 2021/0411).
Consent to participate
None applicable.
Consent for publication
None applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haidar G, Singh N. Fever of unknown origin. N Engl J Med. 2022;386:463–477. doi: 10.1016/B978-0-323-05405-8.00056-5. [DOI] [PubMed] [Google Scholar]
- 2.Kucukardali Y, Oncul O, Cavuslu S, Danaci M, Calangu S, Erdem H, et al. The spectrum of diseases causing fever of unknown origin in Turkey: a multicenter study. Int J Infect Dis. 2008;12:71–79. doi: 10.1016/j.ijid.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Erdem H, Ak O, Elaldi N, Demirdal T, Hargreaves S, Nemli SA, et al. Infections in travellers returning to Turkey from the Arabian peninsula: a retrospective cross-sectional multicenter study. Eur J Clin Microbiol Infect Dis. 2016;35:903–910. doi: 10.1007/s10096-016-2614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korzeniewski K, Gaweł B, Krankowska D, Wasilczuk K. Fever of unknown origin in returning travellers. Int Marit Health. 2015;66:77–83. doi: 10.5603/IMH.2015.0019. [DOI] [PubMed] [Google Scholar]
- 5.Durack DT, Street AC. Fever of unknown origin–reexamined and redefined. Curr Clin Top Infect Dis. 1991;11:35–51. [PubMed] [Google Scholar]
- 6.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med (Baltim) 2012;54:S29–37. doi: 10.1016/j.ypmed.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly”. Geriatr Gerontol Int. 2006;6:149–158. doi: 10.1111/j.1447-0594.2006.00341.x. [DOI] [Google Scholar]
- 9.World Bank Country and Lending Groups. Available at https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 10 May 2022
- 10.Wright W. Fever of Unknown Origin. In: Bennett JE, Dolin R, Blaser Martin J, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Philadelphia: Elsevier Co; 2020. pp. 790–803. [Google Scholar]
- 11.Erdem H, Kurtaran B, Arun O, Ylmaz H, Çelebi G, Özkaya HD, et al. The place and the efficacy of infectious disease consultations in the hospitals. Infect Dis Clin Pract. 2012;20:131–136. doi: 10.1097/IPC.0b013e31823c4b25. [DOI] [Google Scholar]
- 12.Mahmood K, Akhtar T, Naeem M, Talib A, Haider I. Siraj-Us-Salikeen Fever of unknown origin at a teritiary care teaching hospital in Pakistan. Southeast Asian J Trop Med Public Health. 2013;44:503–11. [PubMed] [Google Scholar]
- 13.Kabapy AF, Kotkat AM, Shatat HZ, El-Wahab A. Clinico-epidemiological profile of fever of unknown origin in an Egyptian setting: a hospital-based study (2009–2010) J Infect Dev Ctries. 2016;10:030–042. doi: 10.3855/jidc.7198. [DOI] [PubMed] [Google Scholar]
- 14.Rupali P, Garg D, Viggweswarupu S, Sudarsanam T, Jeyaseelan V, Abraham O. Etiology of classic fever of unknown origin (FUO) among immunocompetent Indian adults. J Assoc Physicians India. 2019;67(1):21–26. [PubMed] [Google Scholar]
- 15.Pannu AK, Golla R, Kumari S, Suri V, Gupta P, Kumar R. Aetiology of pyrexia of unknown origin in north India. Trop Doct. 2021;1(51):34–40. doi: 10.1177/0049475520947907. [DOI] [PubMed] [Google Scholar]
- 16.Letertre S, Fesler P, Zerkowski L, Picot M-C, Ribstein J, Guilpain P, et al. Clinical medicine place of the 18 F-FDG-PET/CT in the diagnostic workup in patients with classical fever of unknown origin (FUO) J Clin Med. 2021 doi: 10.3390/jcm10173831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang J, Yan L, Du L, Liang L, Zhou Q, Liang T, et al. Recent trends in the distribution of causative diseases of fever of unknown origin. Wien Klin Wochenschr. 2017;129:201–207. doi: 10.1007/s00508-016-1159-6. [DOI] [PubMed] [Google Scholar]
- 18.Naito T, Tanei M, Ikeda N, Ishii T, Suzuki T, Morita H, et al. Key diagnostic characteristics of fever of unknown origin in Japanese patients: a prospective multicentre study. BMJ. 2019;9:e032059. doi: 10.1136/bmjopen-2019-032059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosilkovski M, Dimzova M, Cvetkova M, Poposki K, Spasovska K, Vidinic I. The changing pattern of fever of unknown origin in the Republic of North Macedonia. Rom J Intern Med. 2019;57:248–253. doi: 10.2478/rjim-2019-0007. [DOI] [PubMed] [Google Scholar]
- 20.Zhou G, Zhou Y, Zhong C, Ye H, Liu Z, Liu Y, et al. Retrospective analysis of 1,641 cases of classic fever of unknown origin. Ann Transl Med. 2020;8:690. doi: 10.21037/atm-20-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yenilmez E, Kakalicoglu D, Bozkurt F, Filiz M, Akkol Camurcu A, Damar Midik EO, et al. Fever of unknown origin (FUO) on a land on cross-roads between Asia and Europa; a multicentre study from Turkey. Int J Clin Pract. 2021;75:1–12. doi: 10.1111/ijcp.14138. [DOI] [PubMed] [Google Scholar]
- 22.Buchrits S, Gafter-Gvili A, Eynath Y, Bernstine H, Guz D, Avni T. The yield of F18 FDG PET-CT for the investigation of fever of unknown origin, compared with diagnostic CT. Eur J Intern Med. 2021;93:50–56. doi: 10.1016/j.ejim.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Schönau V, Vogel K, Englbrecht M, Wacker J, Schmidt D, Manger B, et al. The value of 18F-FDG-PET/CT in identifying the cause of fever of unknown origin (FUO) and infammation of unknown origin (IUO): data from a prospective study. Ann Rheum Dis. 2018;77:70–77. doi: 10.1136/annrheumdis-2017-211687. [DOI] [PubMed] [Google Scholar]
- 24.Wright WF, Yenokyan G, Simner PJ, Carroll KC, Auwaerter PG. Geographic variation of infectious disease diagnoses among patients with fever of unknown origin: a systematic review and meta-analysis. Open Forum Infect Dis. 2021;9:151. doi: 10.1093/ofid/ofac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Human Development Index [Internet]. Wikipedia [cited 2022 23] https://en.wikipedia.org/wiki/Human_Development_Index
- 26.Erdem H, Tetik A, Arun O, Besirbellioglu BA, Coskun O, Eyigun CP. War and infection in the pre-antibiotic era: the Third Ottoman Army in 1915. Scand J Infect Dis. 2011;43:690–695. doi: 10.3109/00365548.2011.577801. [DOI] [PubMed] [Google Scholar]
- 27.Erdem H, Akova M. Leading infectious diseases problems in Turkey. Clin Microbiol Infect. 2012;18:1056–1067. doi: 10.1111/1469-0691.12000. [DOI] [PubMed] [Google Scholar]
- 28.Saydam FN, Erdem H, Ankarali H, El-Arab Ramadan ME, El-Sayed NM, Civljak R, et al. Vector-borne and zoonotic infections and their relationships with regional Asia and socioeconomic statuses: an ID-IRI survey in 24 countries of Europe, Africa and Asia. Travel Med Infect Dis. 2021;44:102174. doi: 10.1016/j.tmaid.2021.102174. [DOI] [PubMed] [Google Scholar]
- 29.Erdem H, Puca E, Ruch Y, Santos L, Ghanem-zoubi N, Argemi X. Portraying infective endocarditis: results of multinational ID-IRI study. Eur J Clin Microbiol Infect Dis. 2019;38:1753–1763. doi: 10.1007/s10096-019-03607-x. [DOI] [PubMed] [Google Scholar]
- 30.Alp E, Erdem H, Rello J. Management of septic shock and severe infections in migrants and returning travelers requiring critical care. Eur J Clin Microbiol Infect Dis. 2016;35:527–533. doi: 10.1007/s10096-016-2575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnow PM, Flaherty JP. Fever of unknown origin. Lancet. 1997;350:575–580. doi: 10.1016/S0140-6736(97)07061-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
It will available on request.
None applicable.