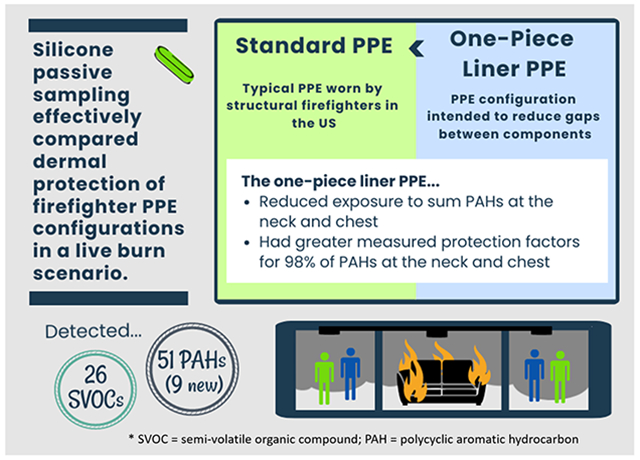
Abstract
A plethora of chemicals are released into the air during combustion events, including a class of compounds called polycyclic aromatic hydrocarbons (PAHs). PAHs have been implicated in increased risk of cancer and cardiovascular disease, both of which are disease endpoints of concern in structural firefighters. Current commercially available personal protective equipment (PPE) typically worn by structural firefighters during fire responses have gaps in interfaces between the ensemble elements (e.g., hood and jacket) that allow for ingress of contaminants and dermal exposure. This pilot study aims to use silicone passive sampling to assess improvements in dermal protection afforded by a novel configuration of PPE, which incorporates a one-piece liner to eliminate gaps in two critical interfaces between pieces of gear. The study compared protection against parent and alkylated PAHs between the one-piece liner PPE and the standard configuration of PPE with traditional firefighting jacket and pants. Mannequins (n=16) dressed in the PPE ensembles were placed in a Fireground Exposure Simulator for 10 minutes, and exposed to smoke from a combusting couch. Silicone passive samplers were placed underneath PPE at vulnerable locations near interfaces in standard PPE, and in the chamber air, to measure PAHs and calculate the dermal protection provided by both types of PPE. Silicone passive sampling methodology and analyses using gas chromatography with mass-spectrometry proved to be well-suited for this intervention study, allowing for the calculation and comparison of worker protection factors for 51 detected PAHs. Paired comparisons of the two PPE configurations found greater sum 2-3 ring PAH exposure underneath the standard PPE than the intervention PPE at the neck and chest, and at the chest for 4-7 ring PAHs (respective p-values: 0.00113, 0.0145, and 0.0196). Mean worker protection factors of the intervention PPE were also greater than the standard PPE for 98% of PAHs at the neck and chest. Notably, the intervention PPE showed more than 30 times the protection compared to the standard PPE against two highly carcinogenic PAHs, dibenzo[a,l]pyrene and benzo[c]fluorene. Nine of the detected PAHs in this study have not been previously reported in fireground exposure studies, and 26 other chemicals (not PAHs) were detected using a large chemical screening method on a subset of the silicone samplers. Silicone passive sampling appears to be an effective means for measuring dermal exposure reduction to fireground smoke, providing evidence in this study that reducing gaps in PPE interfaces could be further pursued as an intervention to reduce dermal exposure to PAHs, among other chemicals.
Keywords: Silicone passive sampling, fireground exposure simulator, polycyclic aromatic hydrocarbons (PAHs), structural firefighters, PPE, dermal exposure
Graphical Abstract
1. Introduction
Personal protective equipment (PPE) has long been developed to keep structural firefighters safe from thermal burns and smoke inhalation during the suppression of structural fires; the ensemble of PPE is referred to as turnout gear. Turnout gear provides a layer of protection against heat, moisture, and the self-contained breathing apparatus effectively protect the lungs from smoke inhalation during interior operations (Fent et al., 2014). However, even when wearing turnout gear, firefighters remain at risk of dermal chemical exposure due to gaps in protection at the interfaces between PPE components, demonstrated by the deposition of soot and polycyclic aromatic hydrocarbons (PAHs) on firefighters’ skin after a fire (Fent et al., 2017; Fent et al., 2014; Keir et al., 2017; Stec et al., 2018). There is evidence to suggest that dermal exposure to organic chemicals is a critical exposure pathway for firefighters, and is likely contributing to observed increases in firefighter cancer risk, as well as other disease endpoints (Fent et al., 2014; Gill and Britz-McKibbin, 2020). A retrospective cohort study from the National Institute for Occupational Safety and Health found that firefighters face a nine percent increase in cancer diagnoses and 14% increase in cancer-related deaths when compared to the general US population (Daniels et al., 2015). Furthermore, there is evidence for increased rates of specific cancers in firefighters. The International Agency for Research on Cancer recently used meta-analysis data to conclude that there is sufficient evidence that firefighting is associated with increased risk of mesothelioma (58% higher risk) and bladder cancer (16% higher risk). Additionally, there is limited evidence of increased risk for colon, prostate, and testicular cancers, and for non-Hodgkin lymphoma and melanoma (International Agency for Research on Cancer, 2022).
Combustion during a structure fire produces complex mixtures of chemicals, including some classified as known or suspected carcinogens (e.g., some PAHs, benzene, certain dioxins), endocrine disruptors (e.g., phthalates), or those associated with more diverse toxic endpoints in humans (e.g., halogenated organophosphorus flame retardants) (Fabian et al., 2014; Fent el al., 2020; Navrátil et al., 2017; Wakefield, 2010). Particularly vulnerable locations for dermal exposure to these chemicals include the neck and jawline (hood-jacket interface), groin and legs (boot-pant; pant-jacket interface), and the hands and forearms (glove-jacket interface) (Fent et al., 2014; Stec et al., 2018). Remediating dermal exposure to these compounds through innovations in PPE could be critical to reducing the elevated cancer rates and other disease endpoints in firefighters.
Quantifying the effectiveness of these intervention strategies in firefighters is crucial yet poses several unique sampling challenges given the hazards of fire suppression, high heat exposure, vigorous physical activity, and time sensitivity. Common technologies utilized in fireground sampling include active air samplers that pump air through a filter to capture particulate matter followed by a chemical sorbent, extractable wipes swiped over skin or gear, gear patches that can be extracted directly, or biological samples (e.g., urine, blood, feces, breath) (Bonner and Anderson, 2021; Engelsman et al., 2020). Each of these techniques has advantages and disadvantages when used on the fireground. Active air samplers for example, capture a known volume of air, which can be used to calculate ambient air concentrations. However, active air samplers are prone to failure in a burn scenario and can draw contaminants in under gear (Fent et al., 2014; Mayer et al., 2020). Skin wipes, coupled with corn oil as a lipophilic solvent, can lead to matrix complications during instrument analysis and dermal sampling can only collect chemicals that remain on the skin, excluding concentrations that were absorbed during the fire or volatilized prior to sampling (Fent et al., 2014; Mayer et al., 2020). Biological samples are informative but can be challenging to collect from firefighters in the field and often capture exposures beyond individual burns. Hence, intervention studies for reducing occupational exposures to chemicals would benefit from the development and adaptation of new sampling technologies for measuring protection. Silicone passive sampling is one such alternative tool in the evaluation of firefighter interventions to reduce dermal exposure. Silicone samplers capture chemical exposures for the duration of wear, provide consistent measurements, and are relatively easy to use (Dixon et al., 2018; O'Connell et al., 2014; Samon et al., 2022).
A useful property of silicone samplers is their ability to capture the bioavailable fraction of a chemical. This is the chemical fraction that is capable of passing through cellular membranes serving as the biological barriers between the skin or lungs, and the rest of the body (O'Connell et al., 2014; Ruby et al., 2016). The silicone’s selection of bioavailable chemicals makes it particularly well suited for determining which chemicals are available for dermal absorption, and their relative concentrations across samples. Silicone passive sampling was first introduced in the form of wristbands, which were worn by individuals to measure their chemical exposures (O'Connell et al., 2014). The validity of this technique has since been established via comparisons to other accepted sampling methodologies, such as active air monitoring, hand wipes, blood serum collection, or urine collection, in a multitude of studies (Dixon et al., 2018; Hammel et al., 2020; Hammel et al., 2018; Hammel et al., 2016; Hoffman et al., 2021; Levasseur et al., 2021). They have also been used successfully in several studies to investigate firefighter exposures (Baum et al., 2020; Levasseur et al., 2022; Poutasse et al., 2022; Poutasse et al., 2020).
In this pilot study, we investigate the feasibility of silicone sampling technology to assess differences in dermal exposure to PAHs between a novel intervention PPE configuration and a control configuration of standard PPE during smoke exposure testing. We will refer to the control configuration as standard PPE, a PPE design representative of that currently worn by most structural firefighters in the United States as of 2020. The standard PPE ensemble includes a turnout jacket, turnout pants, gloves, and hood elements that passively overlap each other (yet allows from some airflow), along with a helmet, boots and a self-contained breathing apparatus (National Fire Protection Association, 2020). These elements are worn over the firefighter’s clothing. The standard turnout jacket and pants are constructed with a thermal liner closest to skin, a moisture barrier, and outer shell (National Fire Protection Association, 2020). The intervention PPE configuration is an experimental prototype designed to eliminate the jacket-pant and jacket-hood interfaces and to reduce transfer through jacket-glove interfaces in the current standard PPE (pictured in Figure 1). The novel design joins the moisture barrier layers from the standard jacket and pants with an integrated hood into a one-piece liner that also employs a particle blocking material in the wrist cuffs. The remaining layers of the turnout jacket and pants, including the thermal liner and outer shell, are worn over the one-piece liner. This ensemble incorporating the one-piece liner is referred to as a “one-piece liner PPE” for the duration of this paper.
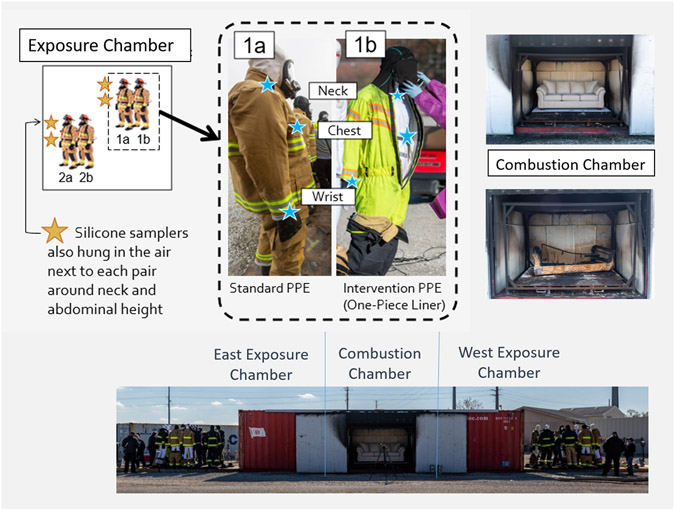
Figure 1.
The fireground exposure simulator (FES) consists of a central combustion chamber and two exposure chambers: East and West. For each burn, two mannequin pairs were staged on opposing sides of one exposure chamber (along with other mannequins wearing different PPE configurations outside the scope of this manuscript). Pairs consisted of one mannequin donning standard PPE (1a) and one mannequin donning the one-piece liner PPE (1b). Silicone samplers were fastened underneath PPE (blue stars) at the neck and chest with metal clips, and placed around the mannequins’ wrist, as well as next to each mannequin pair in the air at approximately breathing and abdominal height (gold stars).
To our knowledge, this is the shortest firefighter exposure duration tested with silicone passive sampling, including simulated exposures, trainings, and real occupational exposures. The combustion of fuel commonly found in a residential structure (a couch) in the study lasted only 6 minutes, the whole exposure experiment was 10 minutes, while other silicone sampling studies quantifying occupational exposure of firefighters to PAHs have ranged from 5.5 hours (Bakali et al., 2021) to a month (Poutasse et al., 2020). Bakali et al., 2021 also used a small subset of silicone samplers (n=8) to quantify ambient air concentrations of PAHs during a 30-minute ‘flashover’ simulation training (sudden and high intensity fires), providing support that silicone could capture detectable concentrations of PAHs in the air during a very short burn scenario (Bakali et al., 2021).
The two primary objectives of this study are (1) to test the feasibility of utilizing silicone sampling technology in short burn scenarios for assessing potential dermal exposures of firefighters (using mannequins), and (2) to compare dermal chemical protection provided by the standard PPE versus the novel one-piece liner PPE. Gas chromatography and mass spectrometry was used to quantify 63 PAHs and to screen for 1530 chemicals in the silicone sampling extracts. We hypothesize that protection against PAH dermal exposures is greater for the one-piece liner PPE configuration than standard PPE at three vulnerable locations (neck, chest, and wrist). Additionally, we anticipate that the chemical screening data will expand knowledge of chemicals that firefighters are exposed to dermally, and whether the one-piece liner PPE provides additional protection to these chemicals. Results from this study could support the feasibility of using silicone samplers in measuring dermal exposures of firefighters, extend our knowledge about the chemical exposures of firefighters, and inform innovations in PPE design that could significantly increase protection against PAHs and other chemicals.
2. Materials and Methods
2.1. Simulating firefighter exposures in a residential house fire
The Fireground Exposure Simulator (FES) utilized in this study was designed to mimic the conditions of a residential fire. The FES is constructed from a 2.4m x 2.9m x 12.2m intermodal shipping container, and contains a central combustion chamber with two adjacent exposure chambers as described in previous work, and as shown in Figure 1 (Horn et al., 2020). The protocol timing for ignition, ventilation, and suppression was standardized (Horn et al., 2020) based on previous fireground research (Horn et al., 2018). For this series of experiments, mannequins were staged in the exposure chambers to act as surrogates for firefighters in the burn scenarios, which allowed us to increase standardization while limiting exposure risk to firefighters.
2.2. Sampling design
The same type of residential couch was utilized in the combustion chamber for a total of four burns in this study. The couch meets California TB117 flammability standards, but no chemical flame retardants were added to the upholstery. After six minutes, the fire was suppressed with water, exterior doors from the exposure chambers were opened at eight minutes and the mannequins were extracted from the exposure chamber ten minutes after ignition. More details on the burn scenarios can be found in the SI (SI-Sampling Design). For every mannequin donning standard PPE (n=8), a mannequin dressed in the one-piece liner PPE (n=8) was paired by co-location in the chamber. Silicone passive samplers were fastened underneath each mannequin’s PPE, and over a cotton base layer (long sleeved t-shirt and long underpants) at the neck, chest, and wrist for the duration of the exposure (n=48 samplers). Sixteen total silicone samplers were also hung in the air near each mannequin pair at the neck and abdominal height (between the height of the chest and wrist samples) to allow for the calculation of worker protection factors (See Section 2.6). Field technicians handled silicone samplers with nitrile gloves before and after the burns and sealed the silicone samplers in labeled polytetrafluoroethylene (PTFE) bags immediately after removing mannequins from the chamber. Additionally, active ambient chamber air samples were deployed during each burn scenario in this study to measure PAH concentrations using methodology previously reported and summarized in the SI (SI-Active Air Samplers)(Mayer et al., 2020). Data from these active air samplers will only be discussed as it relates to the performance of the silicone passive samplers.
2.3. Silicone sample preparation, cleaning, and extraction
Silicone wristbands, 1.3 cm by 18.0 cm in circumference, were purchased from 24hourwristbands.com (Houston, TX, USA) to be used as silicone passive samplers. Prior to deployment, samplers were cleaned and conditioned in a vacuum oven at 300°C as previously reported (Anderson et al., 2017). After deployment, surface contaminants were removed and the silicone was solvent extracted using methods detailed in Anderson et al., 2017 (Anderson et al., 2017). More details can be found in the SI (SI- Silicone sample preparation, cleaning, and extraction).
2.4. Chemical Analyses
2.4.1. Polycyclic Aromatic Hydrocarbon Analysis
We analyzed silicone extracts for 63 parent and alkylated PAHs using a gas chromatograph equipped with an Agilent Select PAH column, paired with tandem mass spectrometry (Agilent 7890A GC; Agilent 7000 MS/MS) (Anderson et al., 2015). All target analytes in the method were calibrated with at least five points on the curve and a correlation of ≥0.99. Instrumental limits of detection (LOD) range from 0.24 to 6.44 ng per extract; all target PAHs and individual LODs for the method can be found in Table S2. All data quality objectives (DQOs) were met for this analysis (reported in SI – PAHs).
2.4.2. High-Throughput Chemical Screening
Silicone extracts from the first and fourth burn scenarios (half of all samples) were screened for 1530 organic chemicals using an Agilent 7890A GC coupled with an Agilent 5975 MS detector, and an Agilent DB-5MS column (30 m x 0.25 mm). The chemicals in this screen were chosen based on potential human health impacts, and include consumer product-related chemicals, flame retardants, industrial-related chemicals, pesticides, phthalates, PCBs/dioxins/furans, and PAHs. The analysis relies on an automated mass spectral deconvolution and identification system (AMDIS v. 2.66, National Institute of Standards and Technology) and deconvolution reporting software (DRS, Agilent) (Bergmann et al., 2018). All data quality objectives, detailed in SI – Chemical Screening, were met for this analysis. It should be noted that any PAHs occurring in both analytical methods will be discussed in terms of the PAH-specific method due to its increased quantitation capabilities.
2.5. Quality Assurance and Quality Control
Eight blank (non-deployed) silicone samplers were introduced at designated stages during sample preparation and processing in the lab: during sample construction (n=2), while packaging samples for transport (n=4), and during post deployment (n=2). A trip blank was also introduced; this silicone sampler was prepared and transported with all other samples in a sealed PTFE bag but remained un-opened for the duration of the study. No compounds in either GC-MS method were detected above the limit of quantitation in the trip blank. Four PAHs were detected in laboratory processing blanks; the detected concentrations were subtracted from all samples to correct for background signals (Table S4). Information on chemicals standards and solvents used can be found in the SI (SI - Chemical Standards and Solvents).
2.6. Statistical Analysis
We used R version 3.6.3 for all data visualization and statistical analyses. Analyses of the individual analytes and the high-throughput chemical screening were largely observational due to the small sample size chosen for conducting this pilot study.
PAH analyte concentrations below the method LOD were substituted with the LOD divided by the square root of two. High molecular weight (HMW) PAH (4-7 rings) and low molecular weight (LMW) PAH (2-3 rings) concentrations were separately summed for each sample prior to making paired comparisons for silicone samplers underneath standard PPE versus samplers underneath one-piece liner PPE. Summation of PAHs, rather than individual analytes were tested to minimize multiple comparisons for the limited sample size.
Normality of sum differences in concentrations between paired samples (adjacent standard and one-piece liner PPE samples at the same location) was assessed using Shapiro-Wilk tests with an alpha level of 0.05. Equal variance was assessed using Levene’s test. Assumptions for a paired t-test were met for all comparisons except for the LMW wrist samples due to one large outlying point; a non-parametric Wilcoxon signed rank test was used for that comparison. Paired t-tests were used for the other five comparisons. All tests were one-sided, with the alternative hypothesis being that the sum concentrations under the standard PPE would be greater than under the one-piece liner PPE. To account for multiple comparisons, a false discovery rate of 0.05 was implemented using a Benjamini-Holm correction.
Worker protection factors (WPFs) were calculated for each detected PAH for both PPE configurations at the neck, chest, and wrist. Air concentrations and concentrations underneath PPE were paired by proximity in height and location in the chamber; the “abdominal height” air samples approximated both the chest and wrist sample heights. WPFs represent the ratio of these two measures (outside vs. under gear) to indicate the level of dermal protection afforded by the PPE at that location.
3. Results
3.1. Variability in the Fireground Exposure Simulator
Variability in air concentrations of PAHs among burn scenarios and within the chambers in the FES were characterized in a previous publication (Horn et al., 2020). Briefly, the silicone air samples in this study showed consistently higher concentrations of PAHs on the left side of the exposure chamber than the right side in both the West and East exposure chambers (17% higher on average), suggesting bulk air flow from left to right in the chamber (Figure S1). In these experiments, air concentrations of PAHs also increased across the four burns, nearly doubling from the first to fourth burn (Table S5).
3.2. Polycyclic Aromatic Hydrocarbons
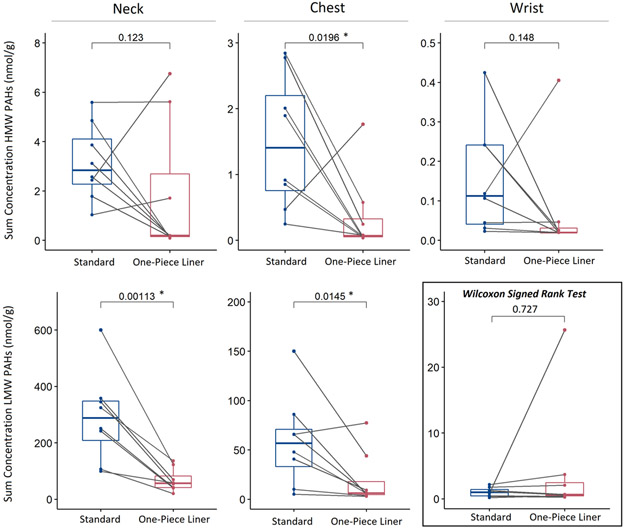
To our knowledge, this was the shortest exposure time for application of silicone passive samplers: 10 minutes with only six minutes of active combustion. Despite the brief exposure, the passive samplers were able to capture measures of 51 different PAHs (Table S6), exceeding the typical analyte scope for firefighter exposure studies. Furthermore, one-sided paired statistical analyses show a significant reduction in sum HMW PAH (4-7 rings) exposures with use of the one-piece liner PPE compared to standard PPE at the chest (p=0.0196), and in sum LMW PAH (2-3 rings) exposure at the neck (p=0.00113) and chest (p=0.0145; Benjamini-Hochberg, 0.05 FDR). The majority of paired sum HMW PAH concentrations were also higher under the standard PPE than the one-piece liner PPE at the neck and wrist, but these differences were not statistically significant (Figure 2).
Figure 2.
Boxplots show distributions of sum PAH concentrations (nmol/g) for samples under standard PPE versus one-piece liner PPE at the three locations (columns) for HMW PAHs (top) and LMW PAHs (bottom). Paired samples, based on collocation in the chamber, are linked with grey lines. Paired t-test p-values are shown for each comparison, with significant p-values following the Benjamini-Hochberg correction denoted with an asterisk. All tests were conducted using a paired t-test except for the comparison of wrist samples for LMW PAHs, which was conducted using a Wilcoxon Signed Rank test.
Eight of the 51 PAHs measured in this study had not previously been detected in firefighter exposure studies utilizing GC-MS or LC-MS Overlap between the detections in this study, and another silicone sampler study involving actual firefighters, utilizing the same PAH method are shown in a Venn diagram in the SI (Poutasse et al., 2020; Figure S2). Notably, 15 PAHs uniquely identified in the manuscript from Poutasse et al. are corroborated by this data (Poutasse et al., 2020).
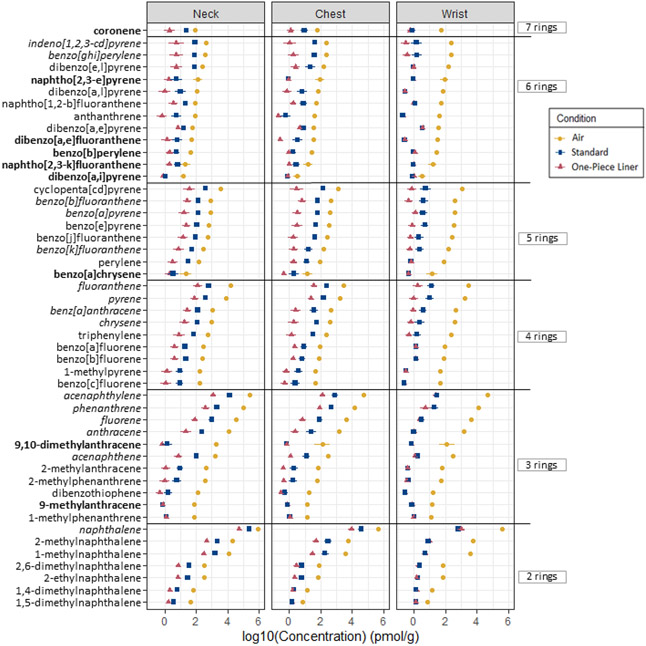
Out of the 63 PAHs analyzed using GC-MS, 51 were detected in at least one of all silicone samplers, and 49 were detected in samplers underneath PPE (Figure 3).
Figure 3.
Mean concentrations of PAHs under each condition. Parent and alkylated PAHs on the y-axis are sorted by number of rings, followed by air concentrations. The “priority PAHS” are italicized, and PAHs not previously detected in firefighter exposure studies are bolded. Each point represents the mean log10(concentration) of n=8 silicone samplers in picomoles per gram silicone; the error bars represent +/− log10(standard error).
There were 12 PAHs included in the analytical method that were not detected in any of the samplers: 1,3-dimethylnaphthalene, 1,6-dimethylnaphthalene, 2,3-dimethylanthracene, 2,6-diethylnaphthalene, 3,6-dimethylphenanthrene, 5-methylchrysene, 6-methylchrysene, 7,12-dimethylbenz[a]anthracene, dibenzo[a,h]anthracene, dibenzo[a,h]pyrene, naphtho[2,3-a]pyrene, naphtho[2,3-j]fluoranthene. Detection frequencies and concentration data of individual PAHs under each condition are documented in the supplementary information (Table S6; Table S7).
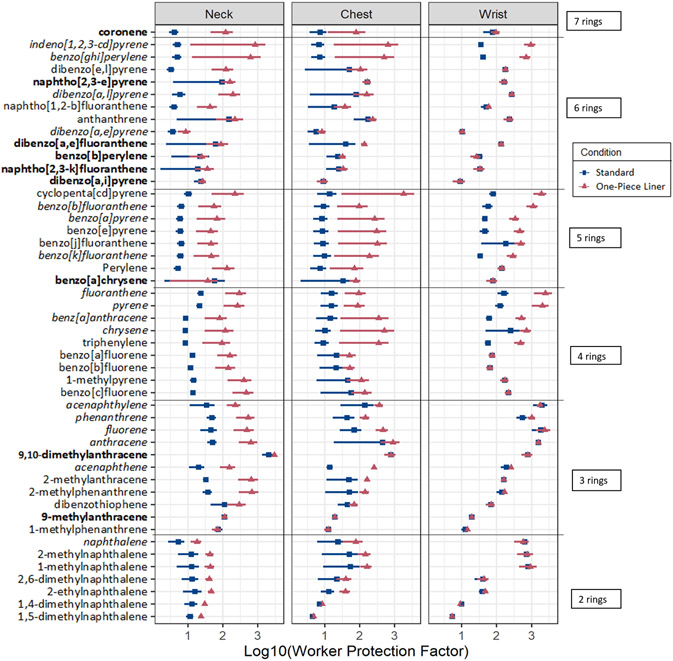
Mean WPF values for individual PAHs can be found in the SI (Table S8) and are presented visually in Figure 4. Mean WPFs were greater for the one-piece liner PPE relative to the standard PPE for 98% of detected compounds at the neck and chest locations. At the wrist, mean WPFs for the one-piece liner PPE were greater than for the standard PPE in 75% of detected PAHs. However, differences in protection at the wrist were generally much smaller, and in some cases were zero due to the chemical being below the limit of quantitation under both standard and one-piece liner PPE configurations.
Figure 4.
Mean WPFs for standard and one-piece liner PPE. PAHs are presented in the same order as in Figure 3: by number or rings, then air concentrations. The “priority PAHs” are italicized and PAHs not previously detected are bolded. Each point represents the mean log10(WPF) of n=8 silicone samplers; the error bars represent +/− log10(standard error).
The one-piece liner PPE provided greater protection than the standard PPE at the neck across all pairs for nine PAHs: naphthalene, 1,4-dimethylnaphthalene, 1,5-dimethylnaphthalene, 1-methylnaphthalene, 2,6-methylnaphthalene, 2-ethylnaphthalene, 2-methylnaphthalene, acenaphthene, acenaphthalene, and fluorene. Interestingly, many of these compounds with consistently better protection under the one-piece liner are alkylated naphthalenes.
Some of the PAH detections in this study can be compared with active air monitor sorbent beds that were deployed in the exposure chamber alongside the silicone passive samplers and analyzed for the 16 priority PAHs. With the exception of dibenzo[a,h]anthracene, which was not detected in any silicone sampler, the other 15 priority PAHs were detected in both types of air samplers, at all sampling locations in all samples.
3.3. Chemical Screen
Twenty six chemicals distinct from PAHs were detected in at least one of the silicone samplers. Detection frequencies and concentrations of each detected analyte can be found in the supplementary information (Table S9; Table S10). Biphenyl was detected in the highest concentrations and with the greatest frequency. Biphenyl was detected in 100% of air samples, 92% of samples under standard PPE, and 75% of samples under one-piece liner PPE, suggesting greater protection was afforded by the one-piece liner than the standard PPE configuration. Chemicals detected in three or more samples were further investigated for potential sources and adverse outcomes utilizing the PubChem and EPA’s CompTox databases (U.S. Environmental Protection Agency, 2019; U.S. National Institutes of Health, 2021). We also determined whether they had been detected in other firefighter exposure studies (Table 1). Two compounds that were detected in at least three samplers have not previously been reported in firefighter exposure studies to our knowledge: 4-chloroaniline and benzyl alcohol. 4-chloroaniline was detected in two air samples (25%) and only one sample under the standard PPE. Benzyl alcohol was only found underneath the PPE, not in air, suggesting that its source could be the PPE, base layer garment (long sleeved t-shirt and long underpants), or mannequin rather than the fire.
Table 1.
Exploration of detected chemicals from 1530 screening method. Only compounds with at least three detections are included in the table. For “Types of Samples,” air = A (n=8), standard PPE = S (n=12), and one-piece liner = O (n=12).
| Compound (CAS #) |
Detection Frequency (air = A, standard = S, one-piece liner = O) |
Relevant Sources | Detection in other firefighter exposure studies |
Associated Adverse Outcomes |
Airborne Permissible Exposure Level (8-hour workday) - OSHA |
|---|---|---|---|---|---|
| biphenyl (92-52-4) | A = 100% S = 92% O = 75% |
Combustion byproduct | Yes (Poutasse 2020 (10% detection); Hewitt 2017; Barboni 2006 for example) | Sensory irritant; liver, kidney, PNS effects from chronic exposure | 1 mg/m3 |
| dibenzofuran (132-64-9) | A = 100% S = 42% O = 8% |
Textiles/dyes, combustion byproduct | Yes (Poutasse 2020 (only 1 detection); Yiin 2012) | Predicted genotoxicity; hypothyroidism; chloracne | NA |
| quinoline (91-22-5) | A = 100% S = 25% O = 0% |
Commonly found in smoke | Yes (Poutasse 2020 (30% detection); Hewitt 2017) | Sensory irritant, chronic effects unknown, potential carcinogen | NA |
| 9-fluorenone (486-25-9) | A = 100% S = 0% O = 0% |
Combustion byproduct | Poutasse 2020 (only 1 detection) | Potential mutagen | NA |
| 4-methylphenol (p-cresol) (106-44-5) | A = 88% S = 0% O = 0% |
Flame retardants, detergents, dyes, plasticizers, wood preservatives; byproduct of wood combustion | Yes (Poutasse 2020 (only 2 detections); Barboni 2006; Edye 1991) | Sensory irritant; CVD, kidney disease (chronic) | 22 mg/m3 (for total cresols) |
| di(2-ethylhexyl)phthalate (DEHP) (117-81-7) | A = 75% S = 8% O = 17% |
Plasticizer for household goods (furniture upholstery; waterproof materials) | Yes (Poutasse 2020 (99% detection); Alexander 2012 (in all samples)) | Carcinogenic (hepatocellular carcinoma); CVD; reproductive disorders | 5 mg/m3 |
| coumarin (91-64-5) | A = 63% S = 8% O = 0% |
Wood; cleaning products | Poutasse 2020 (30% detection) | Irritant; carcinogenicity unknown | NA |
| amyl cinnamal (122-40-7) | A = 50% S = 0% O = 0% |
Cleaning products | Poutasse 2020 (84% detection) | Unknown | NA |
| dibenzothiophene (132-65-0) | A = 50% S = 0% O = 0% |
Combustion byproduct | Yes (Yiin 2012) | Sensory irritant; CNS and liver toxicity (repeated exposure) | NA |
| m-cresol (108-39-4) | A = 38% S = 0% O = 8% |
Wood combustion byproduct | Yes (Edye 1991) | Sensory irritant; reproductive effects; liver and kidney damage | 22 mg/m3 (for total cresols) |
| naphthalic anhydride (81-84-5) | A = 38% S = 0% O = 0% |
Pesticide, dyes, lubricant, potential combustion byproduct | Yes (Hewitt 2017) | Sensory irritant | NA |
| 4-chloroaniline (106-47-8) | A = 25% S = 8% O = 0% |
Found in furniture; pigments | No | Blood, liver, spleen, and kidney effects; possibly carcinogenic | NA |
| benzyl alcohol (100-51-6) | A = 0% S = 8% O = 17% |
Cleaning products, paints, adhesives, heat-sealing polyethylene films, dyes | No | Sensory irritant; potentially mutagenic | NA |
Citations in the table: (Alexander, 2012); (Barboni et al., 2006); (Edye and Richards, 1991); (Hewitt et al., 2017); (Poutasse et al., 2020); (Yiin et al., 2004). PEL’s from OSHA Occupational Chemical Database (U.S. Occupational Safety and Health Administration, 2020).
4. Discussion
4.1. PAH (Polycyclic Aromatic Hydrocarbons) Protection
The primary chemical exposures of concern in this study were to PAHs, which are abundant on the fireground due to their formation during combustion; they are also toxicologically relevant. PAHs are often discussed as a class rather than individual compounds, yet individual PAHs are associated with variable mechanisms of action and consequential toxicities (Chang et al., 2019; Patel et al., 2020; Siddens et al., 2012). Current PAH exposure studies are also commonly limited to the outdated “16 priority PAHs” as defined by the EPA in the 1970’s to represent the entire class of PAHs (Andersson and Achten, 2015). This list excludes a large number of PAHs, some of which are known to be highly toxic, or alternatively have unknown toxicity due to a lack of data (Andersson and Achten, 2015; Siddens et al., 2012). A total of 63 target parent and alkylated PAH analytes were included in this study, which extended the analyte list and detected PAHs from other firefighter exposure studies. Despite an extremely short exposure period of 10 minutes with only 6 minutes of active combustion, the silicone passive samplers, coupled with solvent extraction and our instrumental analyses effectively quantified 51 PAHs in at least one sample.
The silicone wristband and analytical methods allowed us to detect differences in exposure between PPE configurations in the short exposure. The data supports the hypothesis that protection against PAH exposures is greater for the intervention, a one-piece liner PPE configuration, than the standard PPE typically worn by firefighters, at the neck and chest specifically. As illustrated in Figure 2, there was a significant reduction in exposure to sum LMW PAHs at the neck and chest, and sum HMW PAHs at the chest. The majority of HMW PAH concentrations were also higher under the standard PPE than the one-piece liner PPE at the neck and wrist, but the differences were not statistically significant. This finding could be an artifact of the small sample size and or the variability of the data rather than the actual PPE performance. Having higher protection factors is likely more important at the neck and chest than the wrist because there is more ingress of contaminants at the neck and chest as evidenced he higher detection rate of PAHs at those locations. 32 PAHs were detected at the wrist, versus 48 and 45 AHs at the neck and chest respectively. The wrist location also had the greatest WPFs under both standard and one-piece liner PPE configurations. The reduced detections at the wrist is most likely due to elastic wristlets providing high protection for both configurations, but could also be influenced by the fact that concentrations of PAHs are greater at breathing height than abdominal height in the chamber (Figure S1). This information also has implications for other silicone sampling exposure studies involving firefighters; silicone dogtags that are worn around the neck could sample a more vulnerable location for dermal exposure than silicone wristbands as a result of chemical distribution in the environment and the varying levels of protection provided by firefighting PPE at different locations on the body (Poutasse et al., 2022; Poutasse et al., 2020).
Results also indicate increased protection against individual PAHs with the one-piece liner, although the degree of protection varies by compound. For example, in the first burn, the difference in mean WPFs between one-piece and standard PPE at the neck ranged from 0.05 (naphtho[2,3-k]fluoranthene) to 1280 (anthracene). For additional context, summed HMW PAHs at the neck has a mean WPF of 212 for the one-piece liner PPE, more than a magnitude larger than a mean WPF of 15.4 for the standard PPE. It is worth noting that there was no clear trend between PAH molecular weight, or volatility, and WPFs in Figure 4.
Out of the 51 detected PAHs, several are known to pose elevated risk, but have not been included in most previous fireground exposure studies. A couple specific examples are dibenzo[a,l]pyrene (also known as dibenzo[def,p]chrysene, or d[a,l]p) and benzo[c]fluorene (b[c]f), which were detected in the air, and under both PPE configurations. Despite their exclusion from the list of “priority PAHs,” d[a,l]p has a carcinogenic potential 30 times that of benzo[a]pyrene, and b[c]f has a carcinogenic potential 20 times that of benzo[a]pyrene based on available relative potency factors (International Agency for Research on Cancer, 2010). A study measuring carcinogenicity in mice following dermal exposure suggested that this relative potency factor is underestimating the toxicity of d[a,l]p, and that it may actually be more than 100 times as carcinogenic as benzo[a]pyrene (Siddens et al., 2012). Notably, in this experiment, the one-piece liner PPE showed much greater dermal protection against d[a,l]p than the standard PPE, providing more than 30 times the protection at the neck for d[a,l]p (WPFs 190 vs. 6) and two times the protection at the chest (WPFs 160 vs. 79). d[a,l]p was not detected at the wrist under either PPE configuration, indicating greater protection at this location. Similarly, the one-piece liner PPE provided 34 times the protection of standard PPE against b[c]f at the neck, and 2.5 times the protection at the chest (neck WPFs 470 vs. 14; chest WPFs 140 vs. 57). B[c]f was not detected underneath either PPE configuration at the wrist. Dibenzo[a,l]pyrene and benzo[c]fluorene showcase the value of expanding PAH analyses, and the positive impact the one-piece liner PPE could have on dermal exposure to highly carcinogenic PAHs.
4.2. Silicone Passive and Active Air Sorbent Sampling
Silicone passive sampling is a new technology relative to active air sampling, with the first silicone personal sampling paper published in 2014 by O’Connell et al (O'Connell et al., 2014). Since 2014, studies have shown that parent chemical concentrations found in silicone passive samplers correlate with metabolite concentrations in biological samples (i.e., urine) better than active air samplers, suggesting that silicone better approximate the fraction of a chemical entering the body (Dixon et al., 2018; Hammel et al., 2018; Hammel et al., 2016). However, the concentrations in the silicone are not directly comparable to air concentrations that occupational health limits are based on. Work is currently being done to allow for the modeling of environmental concentrations in relation to the concentrations of a chemical captured in the silicone sampler using sampling rates; this will allow for more direct comparisons to regulatory limits (O'Connell et al., 2021). Our data showed silicone passive sampling can be useful in assessing the chemical protective properties of PPE. The successful usage of silicone passive sampling in this six-minute burn scenario provides support for implementing this technology in future firefighter exposure analyses. The development of environmental concentration models for PAHs and other chemical classes of interest will make this tool even more useful. In this study, we used supplementary active air sample sorbents in the chamber air to provide some additional context to the silicone sampling results in terms of air concentrations and workplace standards.
Sorbents from active air samplers staged in the FES had overlapping detections of PAHs with silicone samplers in the chamber air, indicating that there was good agreement in the detection capabilities of these sampling technologies during very short exposures. For example, benzo[a]pyrene (b[a]p) was detected in all active air sampler sorbents and all silicone passive samplers in air; concentrations in the active air sorbent samplers ranged from 0.034 mg/m3 to 1.81 mg/m3. For context, this range of concentrations representing an exposure period of only 10-minutes for just b[a]p brackets all current recommended eight-hour exposure limits and threshold limit values for total PAHs in air (0.1 mg/m3 from NIOSH; 0.2 mg/m3 from ACGIH and OSHA), or “coal tar pitch volatiles” as they are currently classified for workplace standards and regulations (Agency for Toxic Substances and Disease Registry).
No PAH measurements from active air sampler sorbents are available underneath the PPE, however, WPFs for the summed detected priority PAHs from silicone samplers located at the neck, averaged across the four burns (6.8 for standard PPE; 24 for one-piece PPE at the neck) can help estimate the breakthrough PAH concentrations. Using these WPFs, we estimate that on average, 6.6 mg/m3 sum priority PAHs were underneath the standard PPE at the neck versus 1.9 mg/m3 underneath the one-piece PPE based on a mean sum air concentration for the 16 priority PAHs of 45.2 mg/m3 at breathing height. There are limitations of this back-of-the-envelope calculation; recall that the sum concentrations of 16 PAHs measured in the active air sampler sorbents only account for a fraction of PAHs present. 35 PAHs beyond the “16 priority PAHs” were quantified in the silicone passive samplers in this study. Because of this, the total PAH concentrations under the turnout gear are likely even higher.
4.3. Screening Data
Generally, large chemical screening data sets for firefighter exposures are sparse. In this study we screened for more than 1530 chemicals. While the exposure time was only 10 minutes, we detected 26 chemicals in the air and twenty were detected underneath one or both PPE conditions. Screened chemicals with the highest detection frequencies underneath PPE were biphenyl and dibenzofuran. Biphenyl and dibenzofuran are also two of five chemicals with lower detection frequencies under the one-piece liner PPE than under the standard PPE, suggesting greater protection. Quinolone, coumarin, and 4-chloroanaline were the other three chemicals (Table 1).
The majority of screened chemicals that were detected in three or more samples, summarized in Table 1, lacked permissible exposure limits (PELs) from OSHA, yet were associated with adverse effects in the CompTox or PubChem databases (U.S. Environmental Protection Agency, 2019; U.S. National Institutes of Health, 2021; U.S. Occupational Safety and Health Administration, 2020). Detected chemicals that did have PELs for an 8-hour work day included biphenyl (1 mg/m3), cresols (meta- and para- orthologs detected; 22 mg/m3), and DEHP (5 mg/m3) (U.S. Occupational Safety and Health Administration, 2020). Of these chemicals, biphenyl has the lowest PEL; it is a known combustion product, and has been included in other firefighter exposure studies (Barboni et al., 2006; Hewitt et al., 2017; Poutasse et al., 2020; U.S. National Institutes of Health, 2021).
Dibenzofuran, the second most frequently detected screened chemical under PPE (in 42% of samples under standard PPE) has no published occupational exposure limits but has shown systemic effects in rats and is predicted to be genotoxic (U.S. Occupational Safety and Health Administration, 2020). There are also relatively few publications that have analyzed for dibenzofuran exposures in firefighter population, with the exception of particulate matter analyses following the World Trade Center collapse (Yiin et al., 2004). Halogenated dibenzofurans are commonly screened for in firefighter exposure studies since they are common combustion byproducts associated with adverse health effects (Fent et al., 2020; Mayer et al., 2021; Piskorska-Pliszczyńska and Maszewski, 2014; Shaw et al., 2013).
One chemical of particular interest is 4-chloroaniline, detected in 25% of air samples, 8% of standard PPE samples, and no one-piece liner PPE samples. It is recognized to be acutely toxic to a variety of target organs, and is classified as potentially carcinogenic, but it has not been included in any fireground exposure studies to our knowledge (U.S. Environmental Protection Agency, 2019; U.S. National Institutes of Health, 2021). Hence, the chemicals detected with this screening method could inform an expansion of analytes in future exposure studies on the fireground.
There were a few compounds detected underneath one or both PPE configurations, but not in the air: benzyl alcohol (detected in three samples), dimethyl phthalate (detected in two samples), and benzotriazole (detected in one sample). Notably, all of these detections were at the neck location. The detection of these chemicals is infrequent, but they could be originating from the thermal liner and/or moisture barrier in the PPE, the base layer garment worn under the firefighting PPE, or potentially the mannequin. The moisture barrier is important to consider as a chemical source because in the one-piece liner PPE prototype, dermal contact with the moisture barrier was increased. There is also evidence of toxicologically relevant chemicals being found in the moisture barrier. For example, perfluorinated compounds are abundant in common moisture barriers used in PPE worn in the United States and dermal exposure risks have been identified (Muensterman et al., 2022; Peaslee et al., 2020). Ideally, the physical barrier that the one-piece liner PPE achieves could be created with a material that does not add to a firefighter’s total dermal exposure.
4.4. Strengths and Limitations
One anticipated limitation to the silicone sampling, was that silicone uptake of organic chemicals from atmospheric exposure varies by chemical, and some chemicals may not have a fast enough uptake rate to be detected in a short, 10-minute exposure. That being said, the PAHs detected in this exposure spanned from the lightest (naphthalene) to heaviest (coronene) molecular weight compounds in the quantitation method. For this application, silicone passive sampling was demonstrated to be fit-for-purpose for describing exposure during firefighting activity. It also allowed for comparing relative dermal exposure potential for one-piece liner PPE versus standard PPE by calculating WPFs of bioavailable chemicals. The side-by-side operation of active air samplers and silicone passive samplers in this experiment illustrated operational advantages of the silicone samplers: they do not require batteries or calibration of pumps and are significantly smaller than traditional sampling trains. Several pumps in the active air samplers failed during sampling due to overloading and/or overheating, whereas passive sampling did not present this challenge. One current challenge for silicone passive sampling is a lack of established methodology for calculating air concentrations (mass per volume air), but that is an area of ongoing research.
Limitations of the study include the high variability in air concentrations across burns and within the chamber, and the overall small sample size. Since the one-piece liner PPE was a prototype, there were some challenges with closing the zipper around the neck; this could explain the greater variability in samples under the one-piece liner than the standard PPE. This is a design issue that could be addressed in future iterations. We accounted for this variability by limiting statistical analyses to paired comparisons, with pairs based on co-location within the same burn. However, a study with more samples would afford a more robust analysis.
While this pilot study has shown that limiting PPE interfaces through modifications to traditional turnout gear designs such as the one-piece liner holds promising for reducing exposure risk, more research is needed including human subject studies to test impact of firefighter operations that may force air movement in and out of PPE. Furthermore, tradeoffs between increased protection from fireground contaminants with impacts on firefighter heat stress and ergonomics should be evaluated.
5. Conclusion
In this pilot study, silicone passive sampling and analyses using gas chromatography with mass-spectrometry proved to be an effective means for estimating a firefighters’ dermal exposure to fireground smoke. There is compelling evidence that the passive sampling technology is capable of capturing relevant time-weighted concentrations of PAHs and other semi-volatile organic compounds even within the extremely short (10-minute) exposure window of a burn with relatively simple fuel. The silicone passive sampling technology enabled more comprehensive characterization of chemicals present in a live burn than previously reported; newly reported chemicals included nine alkylated and parent PAHs, 4-chloroaniline, and benzyl alcohol, all of which have known or suspected adverse health effects. The findings of this study also strongly support the implementation of PPE configurations that reduce or eliminate gaps between interface elements of the PPE as a way to reduce contaminant ingress. Quantitative comparisons in dermal protection between an intervention PPE and standard PPE, across 51 detected PAHs, found significant reduction in the summation of both high and low molecular weight PAH concentrations at multiple locations when the one-piece liner intervention PPE was worn. The one-piece liner PPE resulted in greater dermal worker protection factors for 98% of 51 detected PAHs at the neck and chest during smoke exposure. The one-piece liner also provided more than 30 times the dermal protection at the neck than the standard PPE against two highly carcinogenic, but not “priority” PAHs: dibenzo[a,l]pyrene and benzo[c]fluorene. Future fireground and fire training exposure studies would benefit from the inclusion of these PAHs (and other high risk PAHs beyond the 16 priority PAHs) in their list of analytes. Given the results of this study, future studies should explore the potential for creating a one-piece liner or other dermal barrier with different materials, paying special attention to the chemical constituents of the barrier as well as potential impacts on wearability and heat stress for the firefighter.
Supplementary Material
Highlights.
Silicone passive sampling and analyses using gas chromatography with mass-spectrometry proved to be an effective means for measuring dermal exposure reduction in live-fire studies.
Nine of the detected PAHs in this study have not been previously reported in fireground exposure studies, and 26 other semi-volatile chemicals (not PAHs) were detected using a screening method for 1530 chemicals on a subset of the silicone samplers.
An intervention using newly designed PPE with a one-piece liner resulted in a reduced dermal exposure risk to sum low molecular weight PAHs relative to standard PPE at the neck and chest, and high molecular weight PAHs at the chest.
The one-piece liner PPE resulted in greater dermal worker protection factors for 98% of 51 detected PAHs at the neck and chest during live-fire smoke exposure.
The one-piece liner PPE provided >30 times more dermal protection at the neck than the standard PPE against two highly carcinogenic PAHs: dibenzo[a,l]pyrene and 21 benzo[c]fluorene.
Acknowledgements:
The authors would like to thank Clarisa Caballero-Ignacio, Caoilinn Haggerty, and Kaci Graber for contributing to the laboratory processing and extraction of samples, Peter Hoffman for administrative support, and Brian Smith for statistical consultation. We would also like to express gratitude for access to the burn chambers used in this study, the Illinois Fire Service Institute (IFSI) Research program and those that assisted in facilitating the burn, particularly Richard Kesler and Jeffery Lattz.
Funding:
Research reported in this publication was in part supported by the UL Research Institutes, Fire Safety Research Institute, and the FEMA Assistance to Firefighters Grant Program under award numbers EMW-2015-FP-00646 and EMW-2016-FP-000754, as well as the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Numbers P42ES016465, P30ES030287, and P30ES000210. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- B[a]p
Benzo[a]pyrene
- BLOD
Below the Limit of Detection
- DQO
Data Quality Objective
- EPA
US Environmental Protection Agency
- FES
Fireground Exposure Simulator
- GCMS
Gas Chromatography Mass Spectrometry
- LCMS
Liquid Chromatography Mass Spectrometry
- LOD
Limit of Detection
- LOQ
Limit of Quantitation
- OSHA
Occupational Safety and Health Administration
- PAH
Polycyclic Aromatic Hydrocarbon
- PPE
Personal Protective Equipment
- PTFE
Polytetrafluoroethylene
- WPF
Worker Protection Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
Kim A. Anderson, an author of this research, discloses a financial interest in MyExposome, Inc., which is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by OSU in accordance with its policy on research conflicts of interest. The authors have no other disclosures.
References
- Agency for Toxic Substances and Disease Registry, Standards and Regulations for Polycyclic Aromatic Hydrocarbons Exposure, Environmental Health and Medicine Education. https://www.atsdr.cdc.gov/csem/polycyclic-aromatic-hydrocarbons/standards_and_regulations_for_exposure.html (accessed 22.08.24).
- Alexander BM, 2012. Contamination of Firefighter Personal Protective Gear. University of Cincinnati. [Google Scholar]
- Anderson KA, Points GL 3rd, Donald CE, Dixon HM, Scott RP, Wilson G, Tidwell LG, Hoffman PD, Herbstman JB, O'Connell SG, 2017. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J Expo Sci Environ Epidemiol 27, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Szelewski MJ, Wilson G, Quimby BD, Hoffman PD, 2015. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. Journal of Chromatography A 1419, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JT, Achten C, 2015. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycyclic aromatic compounds 35, 330–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakali U, Baum JL, Killawala C, Kobetz EN, Solle NS, Deo SK, Caban-Martinez AJ, Bachas LG, Daunert S, 2021. Mapping carcinogen exposure across urban fire incident response arenas using passive silicone-based samplers. Ecotoxicology and environmental safety 228, 112929. [DOI] [PubMed] [Google Scholar]
- Barboni T, Chiaramonti N, Leoni E, Desjobert J-M, Santoni P-A, 2006. Analysis of smoke during prescribed fires, 2006 First International Symposium on Environment Identities and Mediterranean Area. IEEE, pp. 18–23. [Google Scholar]
- Baum JLR, Bakali U, Killawala C, Santiago KM, Dikici E, Kobetz EN, Solle NS, Deo S, Bachas L, Daunert S, 2020. Evaluation of silicone-based wristbands as passive sampling systems using PAHs as an exposure proxy for carcinogen monitoring in firefighters: Evidence from the firefighter cancer initiative. Ecotoxicol Environ Saf 205, 111100. [DOI] [PubMed] [Google Scholar]
- Bergmann AJ, Scott RP, Wilson G, Anderson KA, 2018. Development of quantitative screen for 1550 chemicals with GC-MS. Analytical and bioanalytical chemistry 410, 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner EM, Anderson KA, 2021. Fireground Exposures: Why the Sample Matters, Fire Engineering. [Google Scholar]
- Chang Y, Siddens LK, Heine LK, Sampson DA, Yu Z, Fischer KA, Löhr CV, Tilton SC, 2019. Comparative mechanisms of PAH toxicity by benzo [a] pyrene and dibenzo [def, p] chrysene in primary human bronchial epithelial cells cultured at air-liquid interface. Toxicology and applied pharmacology 379, 114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RD, Bertke S, Dahm MM, Yiin JH, Kubale TL, Hales TR, Baris D, Zahm SH, Beaumont JJ, Waters KM, Pinkerton LE, 2015. Exposure-response relationships for select cancer and non-cancer health outcomes in a cohort of U.S. firefighters from San Francisco, Chicago and Philadelphia (1950-2009). Occup Environ Med 72, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HM, Scott RP, Holmes D, Calero L, Kincl LD, Waters KM, Camann DE, Calafat AM, Herbstman JB, Anderson KA, 2018. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal Bioanal Chem 410, 3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edye LA, Richards GN, 1991. Analysis of condensates from wood smoke. Components derived from polysaccharides and lignins. Environmental science & technology 25, 1133–1137. [Google Scholar]
- Engelsman M, Toms LL, Banks APW, Wang X, Mueller JF, 2020. Biomonitoring in firefighters for volatile organic compounds, semivolatile organic compounds, persistent organic pollutants, and metals: A systematic review. Environ Res 188, 109562. [DOI] [PubMed] [Google Scholar]
- Fabian TZ, Borgerson JL, Gandhi PD, Baxter CS, Ross CS, Lockey JE, Dalton JM, 2014. Characterization of firefighter smoke exposure. Fire Technology 50, 993–1019. [Google Scholar]
- Fent KW, Alexander B, Roberts J, Robertson S, Toennis C, Sammons D, Bertke S, Kerber S, Smith D, Horn G, 2017. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. Journal of occupational and environmental hygiene 14, 801–814. [DOI] [PubMed] [Google Scholar]
- Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil JD, Stiegel MA, Mueller C, Horn GP, Dalton J, 2014. Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Annals of occupational hygiene 58, 830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, LaGuardia M, Luellen D, McCormick S, Mayer A, Chen IC, Kerber S, Smith D, Horn GP, 2020. Flame retardants, dioxins, and furans in air and on firefighters' protective ensembles during controlled residential firefighting. Environ Int 140, 105756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill B, Britz-McKibbin P, 2020. Biomonitoring of smoke exposure in firefighters: A review. Current Opinion in Environmental Science & Health 15, 57–65. [Google Scholar]
- Hammel SC, Hoffman K, Phillips AL, Levasseur JL, Lorenzo AM, Webster TF, Stapleton HM, 2020. Comparing the use of silicone wristbands, hand wipes, and dust to evaluate children’s exposure to flame retardants and plasticizers. Environmental science & technology 54, 4484–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM, 2016. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ Sci Technol, 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Phillips AL, Hoffman K, Stapleton HM, 2018. Evaluating the use of silicone wristbands to measure personal exposure to brominated flame retardants. Environmental science & technology 52, 11875–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt F, Christou A, Dickens K, Walker R, Stec AA, 2017. Release of volatile and semi-volatile toxicants during house fires. Chemosphere 173, 580–593. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Levasseur JL, Zhang S, Hay D, Herkert NJ, Stapleton HM, 2021. Monitoring human exposure to organophosphate esters: comparing silicone wristbands with spot urine samples as predictors of internal dose. Environmental Science & Technology Letters 8, 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn GP, Kesler RM, Kerber S, Fent KW, Schroeder TJ, Scott WS, Fehling PC, Fernhall B, Smith DL, 2018. Thermal response to firefighting activities in residential structure fires: impact of job assignment and suppression tactic. Ergonomics 61(3), 404–419 [DOI] [PubMed] [Google Scholar]
- Horn GP, Kerber S, Lattz J, Kesler RM, Smith DL, Mayer A, Fent KW, 2020. Development of fireground exposure simulator (FES) prop for PPE testing and evaluation. Fire technology 56, 2331–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2010. Some Non-Heterocyclic PAHs and Some Related Exposures. World Health Organization, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- International Agency for Research on Cancer, 2022. Occupational exposure as a firefighter, IARC Monogr Identif Carcinog Hazards Hum. Volume 132. Lyon, France; June 7-14, 2022. [Google Scholar]
- Kaifie A, Schettgen T, Bertram J, Lohndorf K, Waldschmidt S, Felten MK, Kraus T, Fobil JN, Kupper T, 2020. Informal e-waste recycling and plasma levels of non-dioxin-like polychlorinated biphenyls (NDL-PCBs) - A cross-sectional study at Agbogbloshie, Ghana. Sci Total Environ 723, 138073. [DOI] [PubMed] [Google Scholar]
- Keir JL, Akhtar US, Matschke DM, Kirkham TL, Chan HM, Ayotte P, White PA, Blais JM, 2017. Elevated exposures to polycyclic aromatic hydrocarbons and other organic mutagens in Ottawa firefighters participating in emergency, on-shift fire suppression. Environmental science & technology 51, 12745–12755. [DOI] [PubMed] [Google Scholar]
- Levasseur JL, Hammel SC, Hoffman K, Phillips AL, Zhang S, Ye X, Calafat AM, Webster TF, Stapleton HM, 2021. Young children’s exposure to phenols in the home: associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environment international 147, 106317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur JL, Hoffman K, Herkert NJ, Cooper E, Hay D, Stapleton HM, 2022. Characterizing firefighter's exposure to over 130 SVOCs using silicone wristbands: A pilot study comparing on-duty and off-duty exposures. Sci Total Environ 834, 155237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AC, Fent KW, Chen IC, Sammons D, Toennis C, Robertson S, Kerber S, Horn GP, Smith DL, Calafat AM, Ospina M, Sjodin A, 2021. Characterizing exposures to flame retardants, dioxins, and furans among firefighters responding to controlled residential fires. Int J Hyg Environ Health 236, 113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AC, Horn GP, Fent KW, Bertke SJ, Kerber S, Kesler RM, Newman H, Smith DL, 2020. Impact of select PPE design elements and repeated laundering in firefighter protection from smoke exposure. Journal of Occupational and Environmental Hygiene 17, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muensterman DJ, Titaley IA, Peaslee GF, Minc LD, Cahuas L, Rodowa AE, Horiuchi Y, Yamane S, Fouquet TNJ, Kissel JC, Carignan CC, Field JA, 2022. Disposition of Fluorine on New Firefighter Turnout Gear. Environ Sci Technol 56, 974–983. [DOI] [PubMed] [Google Scholar]
- National Fire Protection Association, 2020. Standard on Selection, Care, and Maintenance of Protective Ensembles for Structural Fire Fighting and Proximity Fire Fighting, Codes and Standards. [Google Scholar]
- Navrátil J, Sadovská V, Švarcová I, 2017. Health risk assessment of combustion products from simulated residential fire, Mathematical-Statistical Models and Qualitative Theories for Economic and Social Sciences. Springer, pp. 15–23. [Google Scholar]
- O'Connell SG, Anderson KA, Epstein MI, 2022. Determining chemical air equivalency using silicone personal monitors. J Expo Sci Environ Epidemiol 32, 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell SG, Kincl LD, Anderson KA, 2014. Silicone wristbands as personal passive samplers. Environ Sci Technol 48, 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D, 2020. Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Frontiers in Microbiology 11, 562813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaslee GF, Wilkinson JT, McGuinness SR, Tighe M, Caterisano N, Lee S, Gonzales A, Roddy M, Mills S, Mitchell K, 2020. Another Pathway for Firefighter Exposure to Per- and Polyfluoroalkyl Substances: Firefighter Textiles. Environmental Science & Technology Letters 7, 594–599. [Google Scholar]
- Piskorska-Pliszczyńska J, Maszewski S, 2014. Brominated dioxins: little-known new health hazards-a review. Journal of Veterinary Research 58, 327–335. [Google Scholar]
- Poutasse CM, Haddock CK, Poston WS, Jahnke SA, Tidwell LG, Bonner EM, Hoffman PD, Anderson KA, 2022. Firefighter exposures to potential endocrine disrupting chemicals measured by military-style silicone dog tags. Environment international 158, 106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutasse CM, Poston WSC, Jahnke SA, Haddock CK, Tidwell LG, Hoffman PD, Anderson KA, 2020. Discovery of firefighter chemical exposures using military-style silicone dog tags. Environ Int 142, 105818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby MV, Lowney YW, Bunge AL, Roberts SM, Gomez-Eyles JL, Ghosh U, Kissel JC, Tomlinson P, Menzie C, 2016. Oral Bioavailability, Bioaccessibility, and Dermal Absorption of PAHs from Soil-State of the Science. Environ Sci Technol 50, 2151–2164. [DOI] [PubMed] [Google Scholar]
- Samon SM, Hammel SC, Stapleton HM, Anderson KA, 2022. Silicone wristbands as personal passive sampling devices: current knowledge, recommendations for use, and future directions. Environment International, 107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SD, Berger ML, Harris JH, Yun SH, Wu Q, Liao C, Blum A, Stefani A, Kannan K, 2013. Persistent organic pollutants including polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans in firefighters from Northern California. Chemosphere 91, 1386–1394. [DOI] [PubMed] [Google Scholar]
- Siddens LK, Larkin A, Krueger SK, Bradfield CA, Waters KM, Tilton SC, Pereira CB, Löhr CV, Arlt VM, Phillips DH, 2012. Polycyclic aromatic hydrocarbons as skin carcinogens: comparison of benzo [a] pyrene, dibenzo [def, p] chrysene and three environmental mixtures in the FVB/N mouse. Toxicology and applied pharmacology 264, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec AA, Dickens KE, Salden M, Hewitt FE, Watts DP, Houldsworth PE, Martin FL, 2018. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Scientific reports 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency, 2019. CompTox Chemicals Dashboard, Research Triangle Park, NC. https://comptox.epa.gov/dashboard/ (accessed 22.07.20). [Google Scholar]
- U.S. National Institutes of Health, 2021. PubChem. National Library of Medicine, Bethesda, MD. https://pubchem.ncbi.nlm.nih.gov/ (accessed 22.07.20). [Google Scholar]
- U.S. Occupational Safety and Health Administration, 2020. OSHA Occupational Chemical Database. United States Department of Labor. https://www.osha.gov/chemicaldatabase (accessed 22.07.29). [Google Scholar]
- Wakefield J, 2010. A toxicological review of the products of combustion. Health Protection Agency, Centre for Radiation, Chemical and Environmental Hazards. [Google Scholar]
- Yiin L-M, Millette JR, Vette A, Ilacqua V, Quan C, Gorczynski J, Kendall M, Chen LC, Weisel CP, Buckley B, 2004. Comparisons of the dust/smoke particulate that settled inside the surrounding buildings and outside on the streets of southern New York City after the collapse of the World Trade Center, September 11, 2001. Journal of the Air & Waste Management Association 54, 515–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.